Submitted:
26 August 2024
Posted:
28 August 2024
You are already at the latest version
Abstract
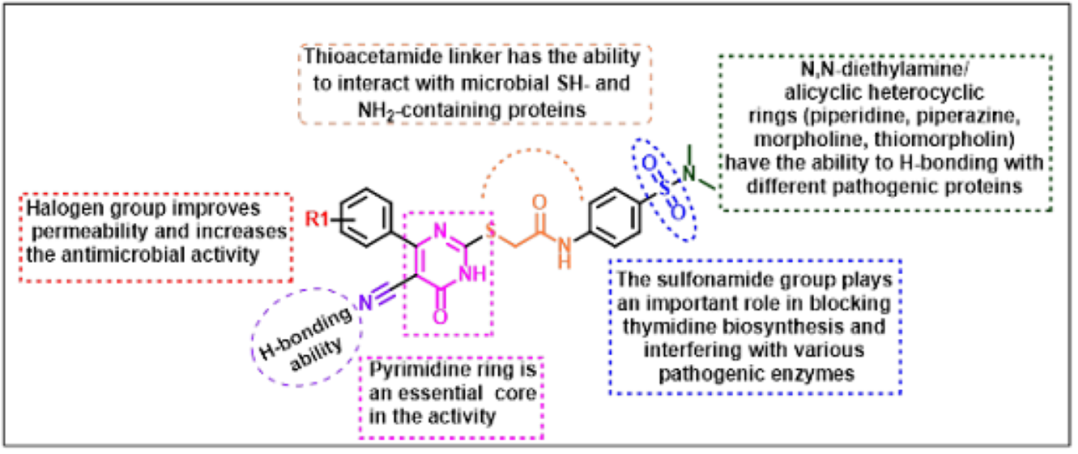
Keywords:
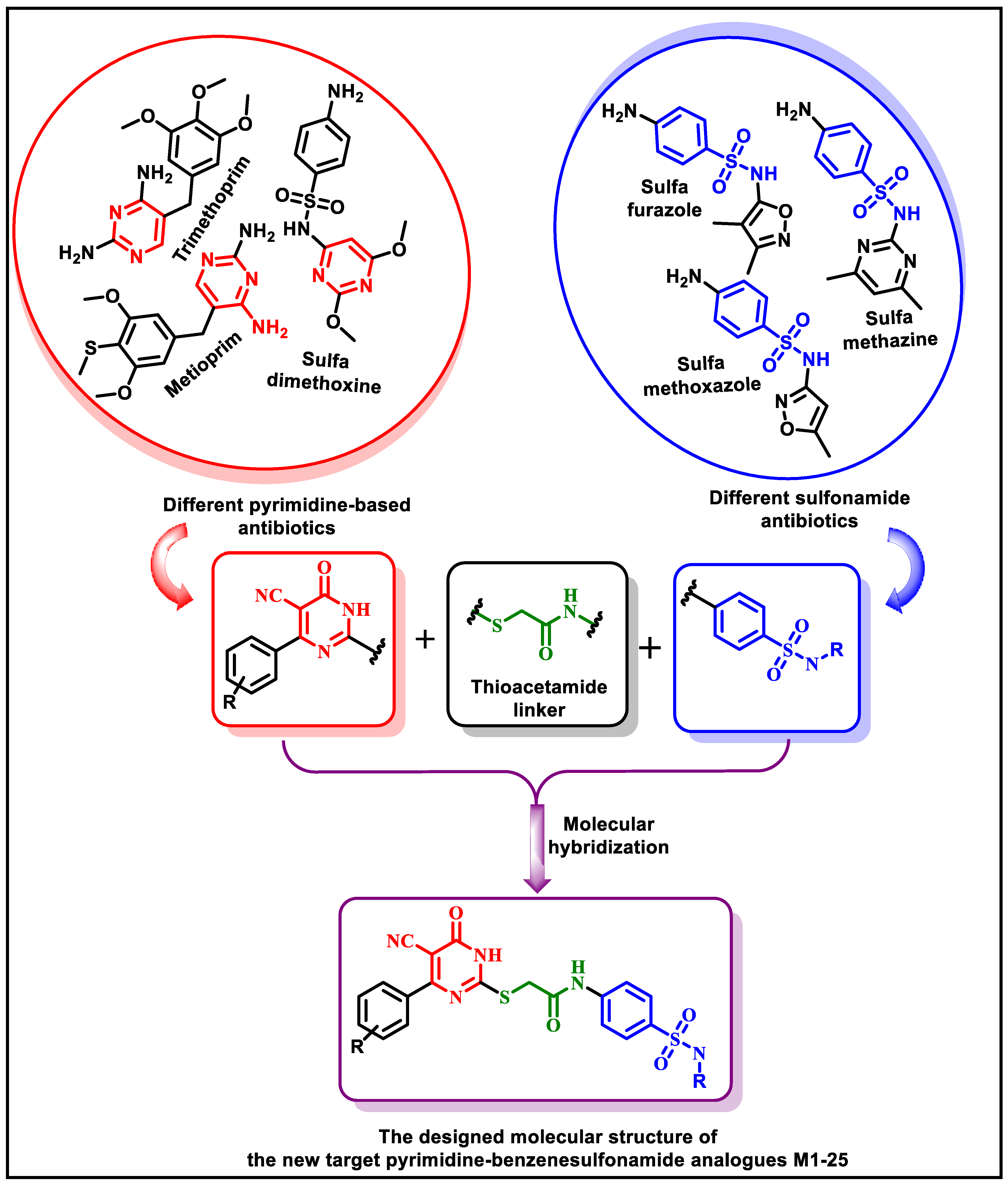
1. Introduction
2. Results and Discussion
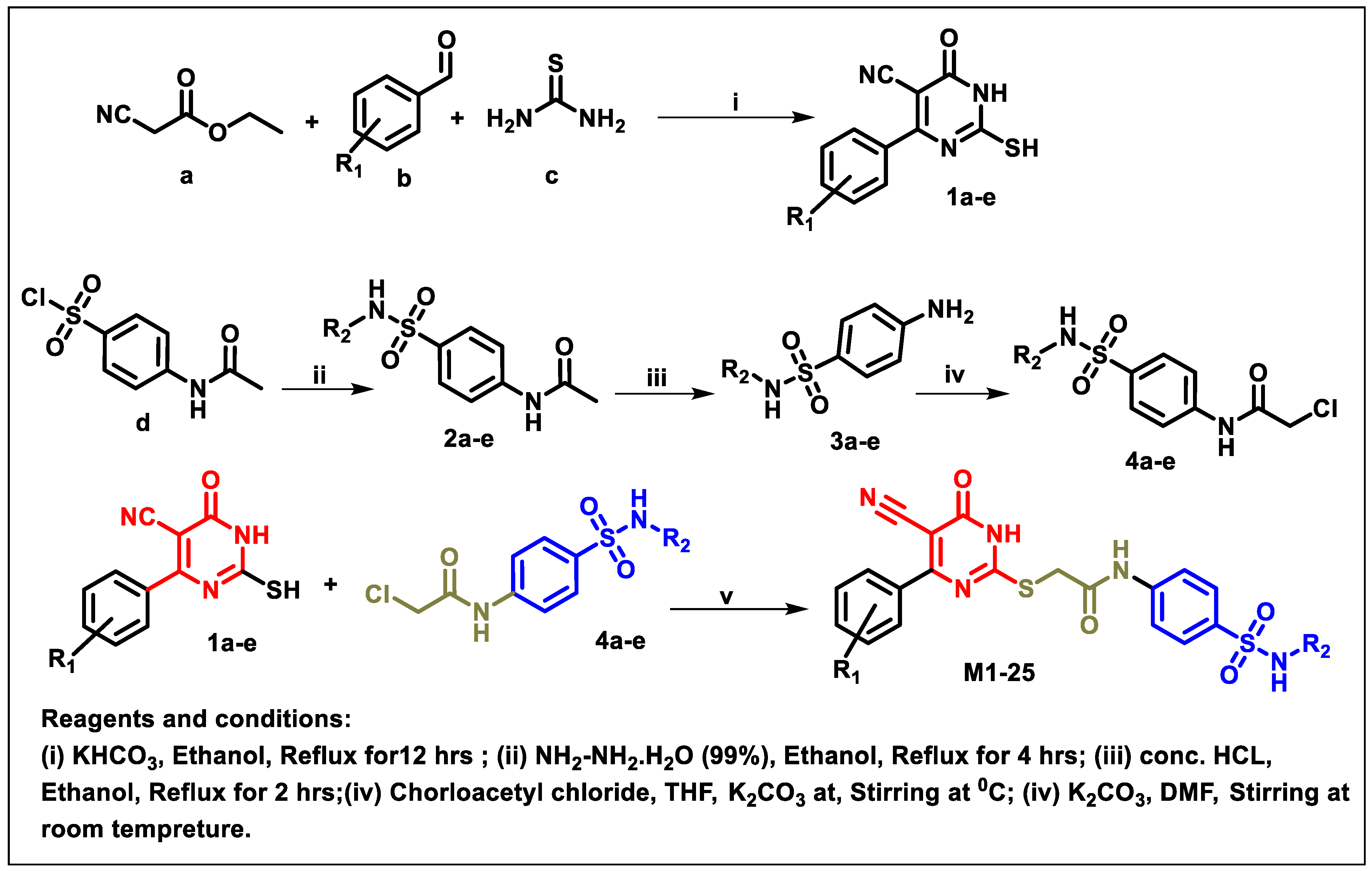
2.1. Chemistry
2.2. Biological Evaluation
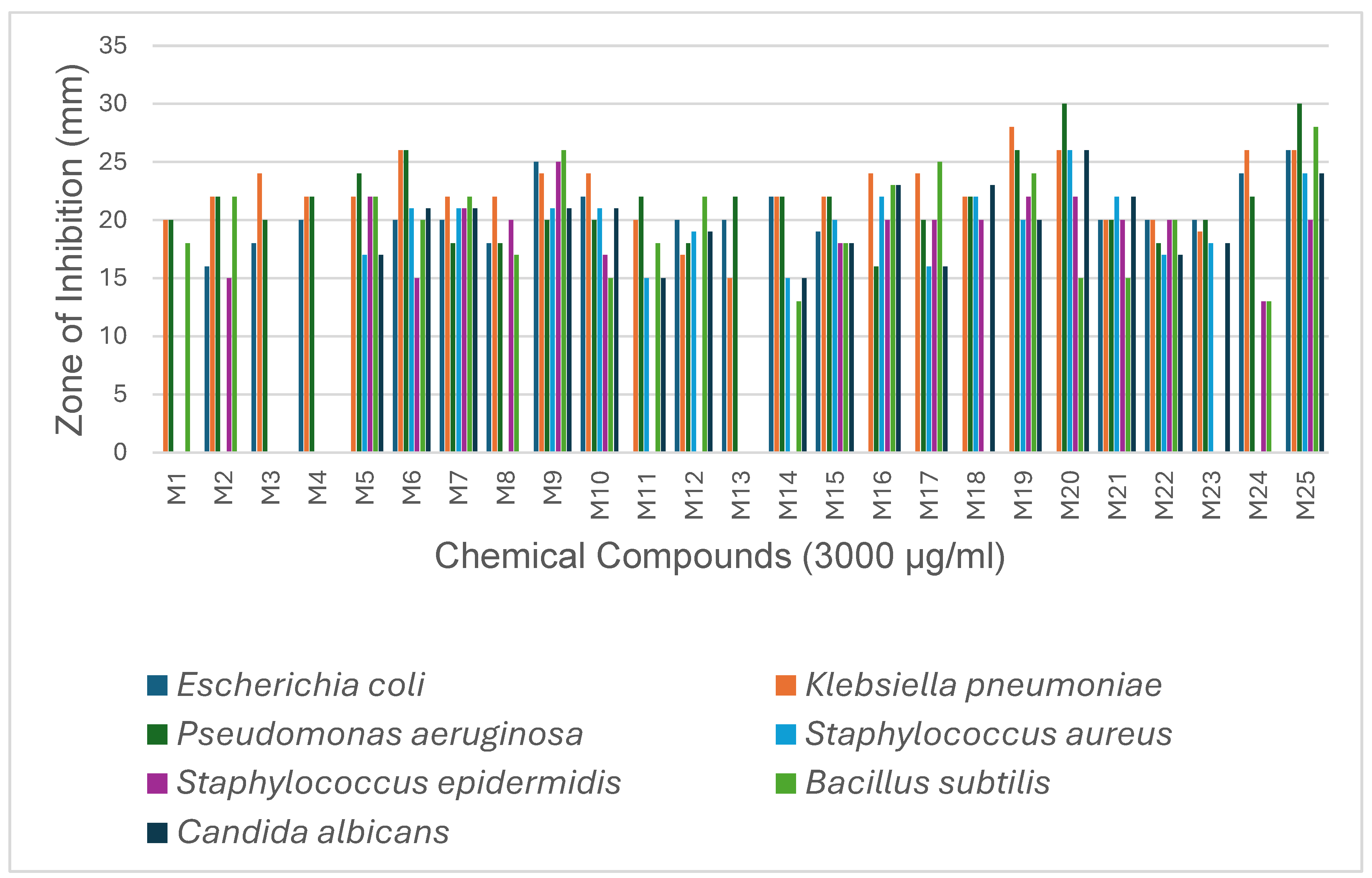
2.2.1. Antimicrobial Activity Determination
2.2.2. MIC and MBC of Selected Compounds against More Susceptible Bacteria
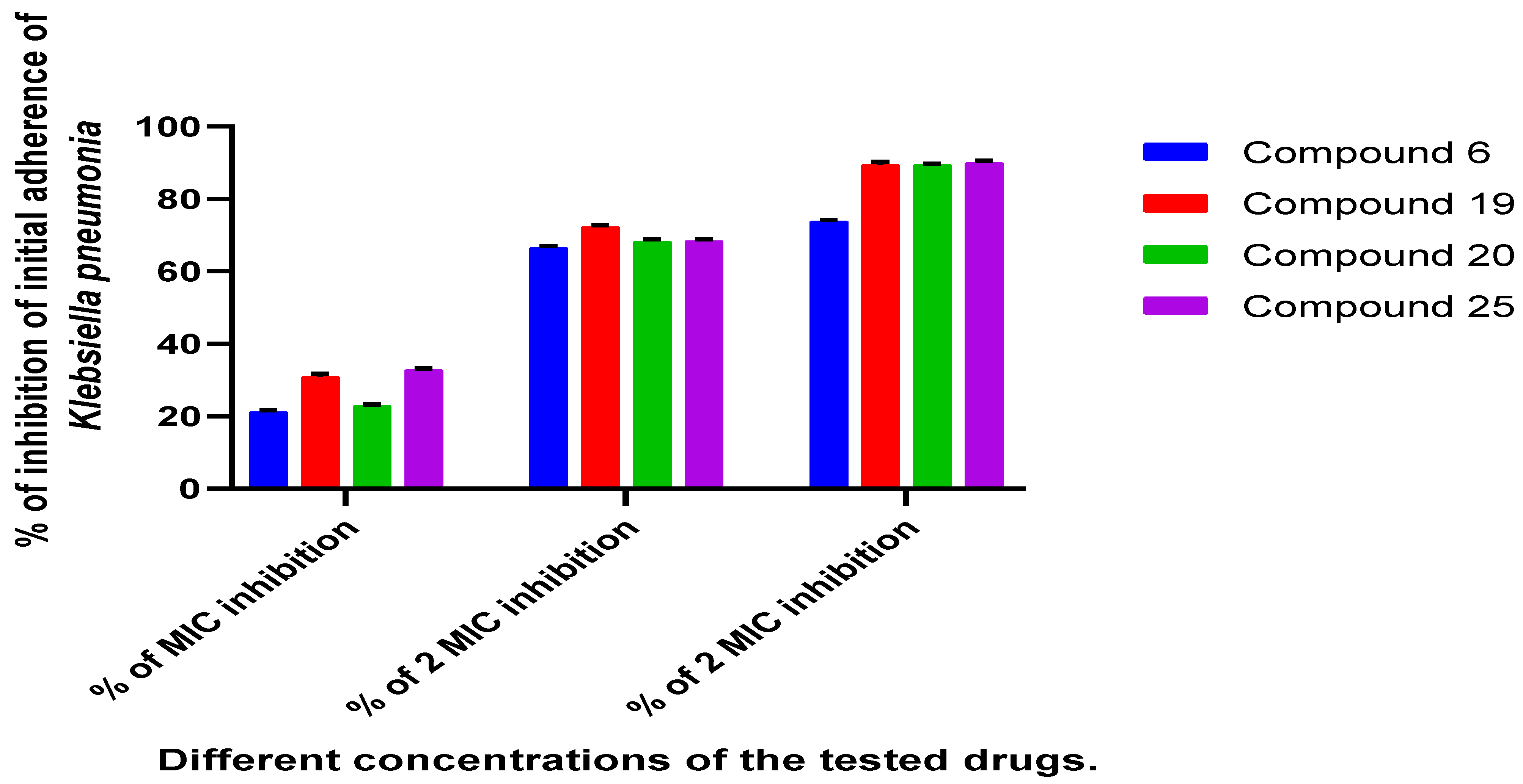
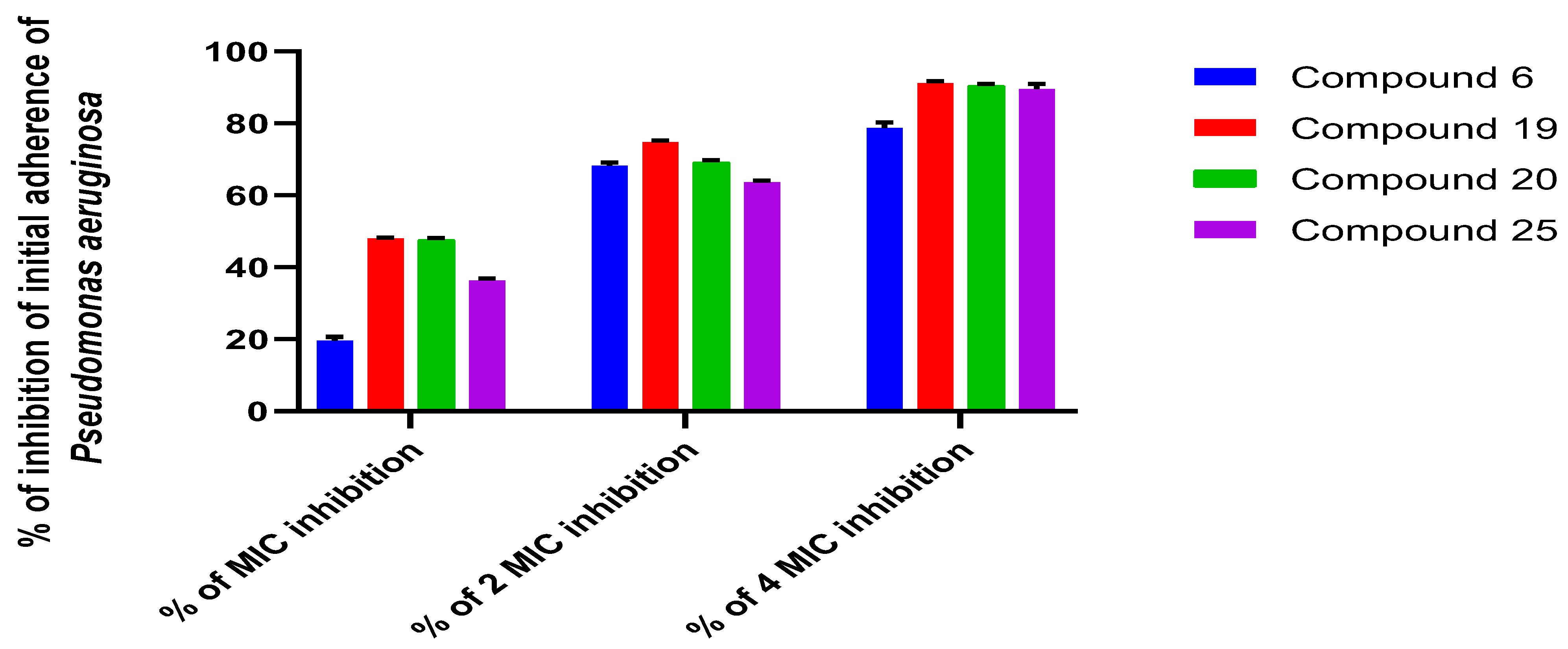
2.2.3. Determination of the Antibiofilm Effect of the Most Promising Compounds Using TCP Method
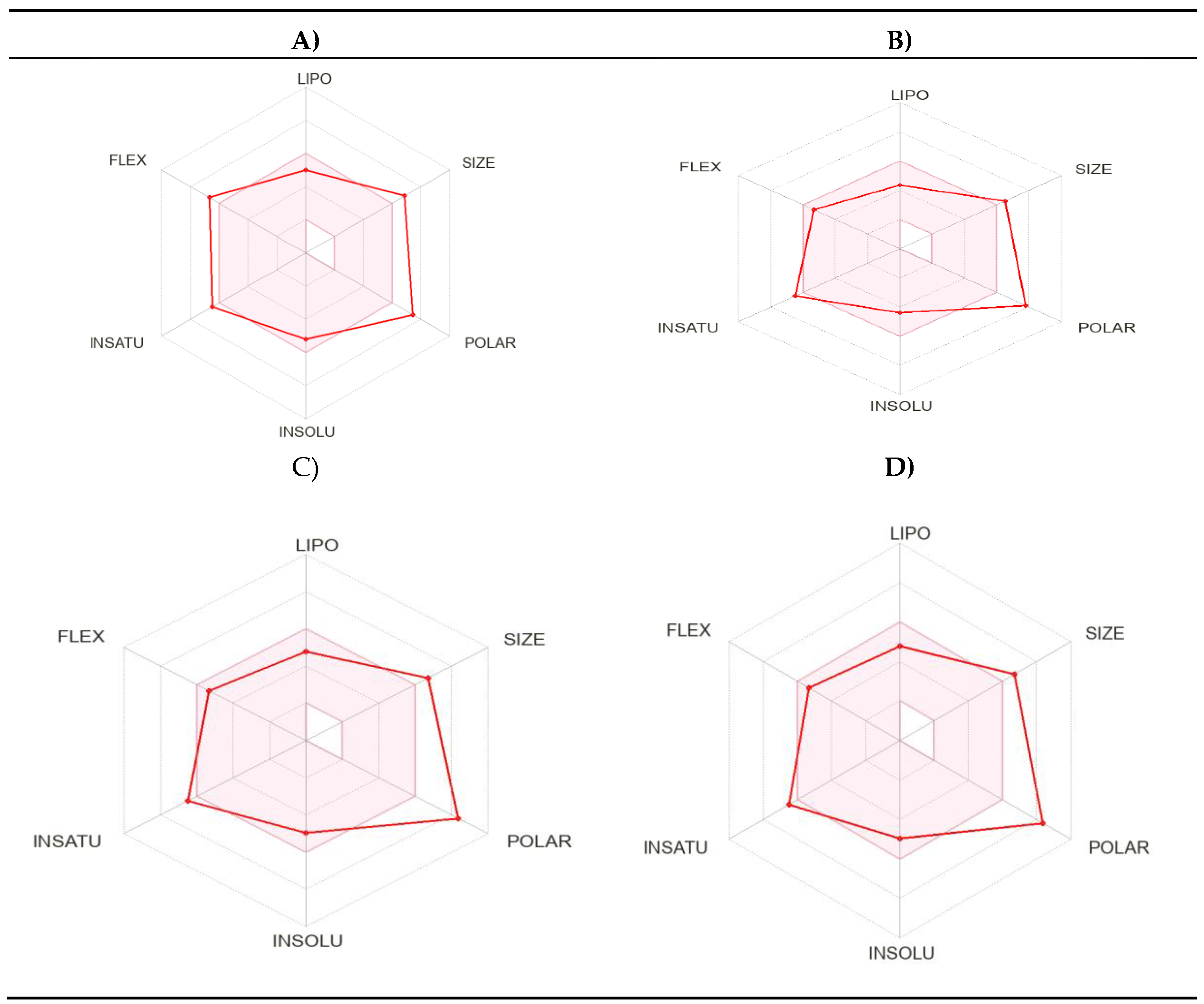
2.3. Physicochemical Properties and ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicity) Studies
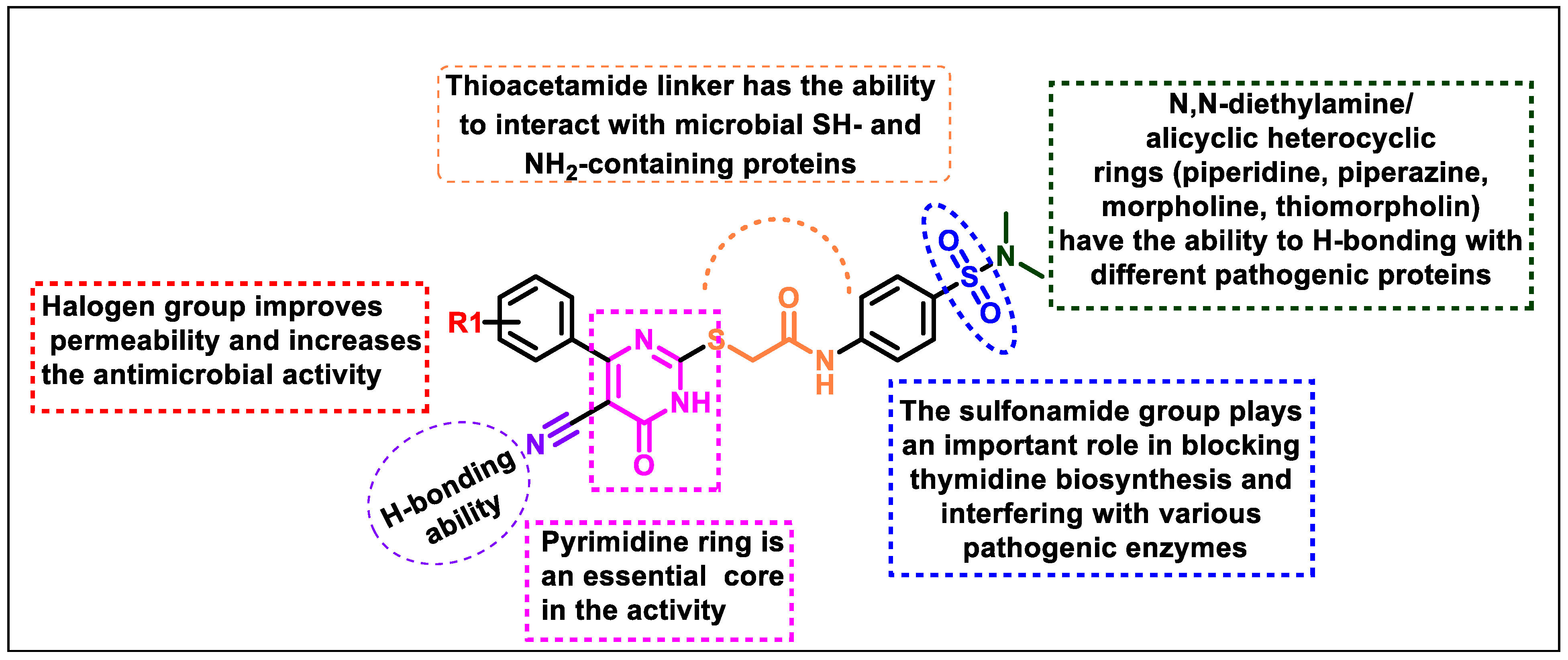
3. Conclusion
4. Experimental
4.1. Chemistry
4.1.1. General Procedure for Preparation of 6-Substituted-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitriles [30].
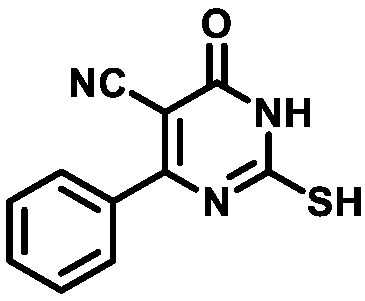
4.1.1.1. 2-Mercapto-6-oxo-4-phenyl-1,6-dihydropyrimidine-5-carbonitrile (1a)
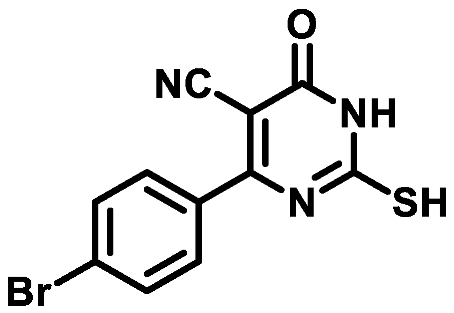
4.1.1.2. 4-(4-Bromophenyl)-2-mercapto-6-oxo-1,6-dihydropyrimidine-5-carbonitrile (1b)
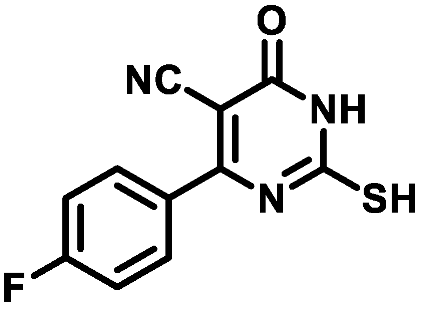
4.1.1.3. 4-(4-Fluorophenyl)-2-mercapto-6-oxo-1,6-dihydropyrimidine-5-carbonitrile (1c)
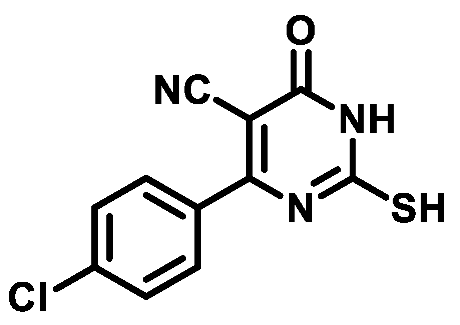
4.1.1.4. 4-(4-Chlorophenyl)-2-mercapto-6-oxo-1,6-dihydropyrimidine-5-carbonitrile (1d)
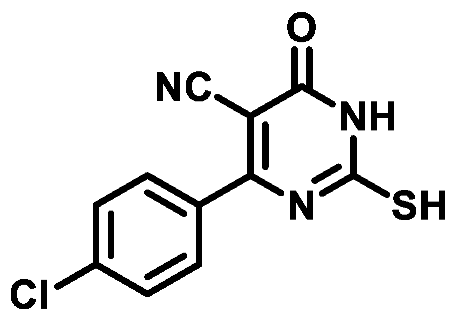
4.1.1.5. 4-(3-Chlorophenyl)-2-mercapto-6-oxo-1,6-dihydropyrimidine-5-carbonitrile (1e)
4.1.2. General Procedure for Preparation of N-substituted sulfonyl phenyl acetamides 2
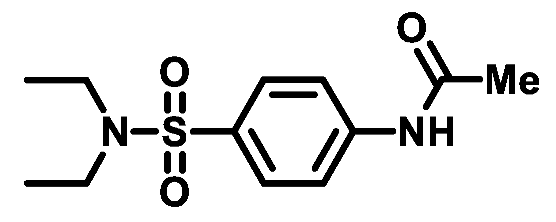
4.1.2.1. N-(4-(N,N-diethylsulfamoyl)phenyl)acetamide (2a)
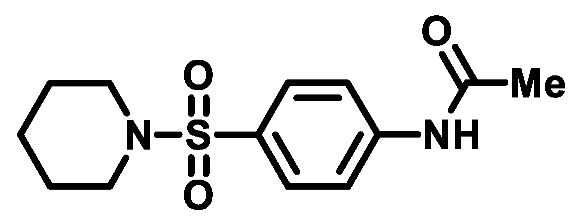
4.1.2.2. N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamide (2b)
4.1.2.3. N-(4-((4-methylpiperazin-1-yl)sulfonyl)phenyl)acetamide (2c)
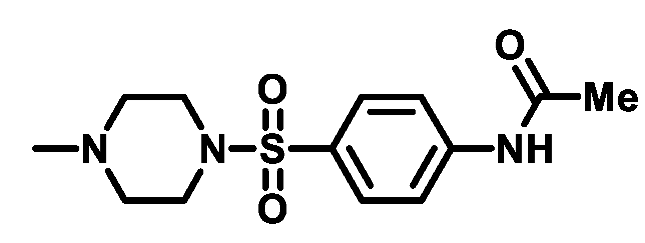
4.1.2.4. N-(4-(morpholinosulfonyl)phenyl)acetamide (2d)
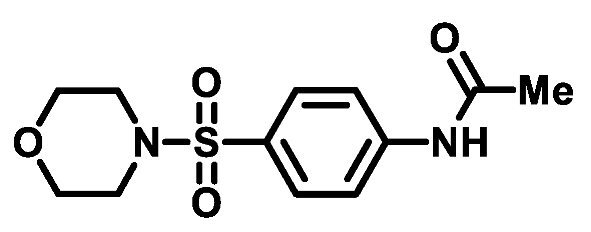
4.1.2.5. N-(4-(thiomorpholinosulfonyl)phenyl)acetamide (2e)
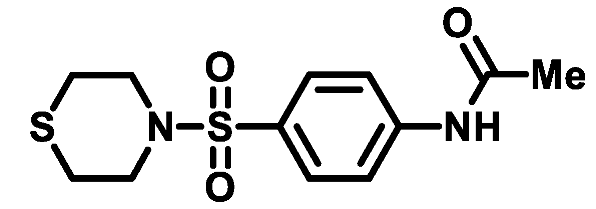
4.1.3. General Procedure for Preparation of 4-amino-benzenesulfonamides 3
4.1.3.1. 4-Amino-N,N-diethylbenzenesulfonamide (3a)
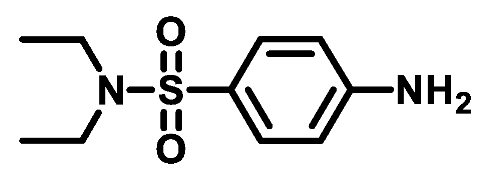
4.1.3.2. 4-(Piperidin-1-ylsulfonyl)aniline (3b)
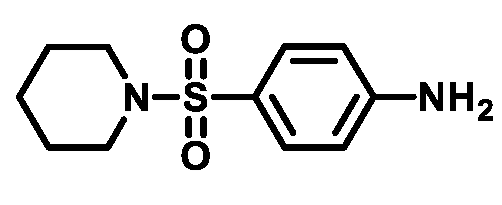
4.1.3.3. 4-((4-Methylpiperazin-1-yl)sulfonyl)aniline (3c)
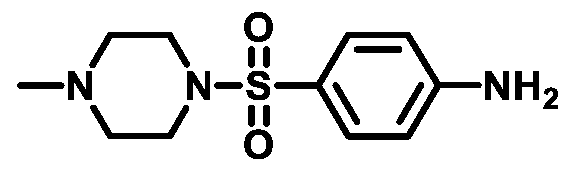
4.1.3.4. 4-(Morpholinosulfonyl)aniline (3d)
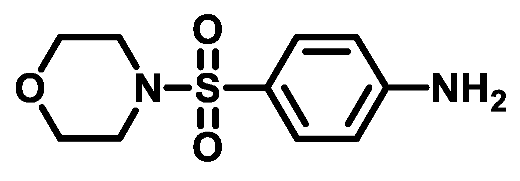
4.1.3.5. 4-(Thiomorpholinosulfonyl)aniline (3e)
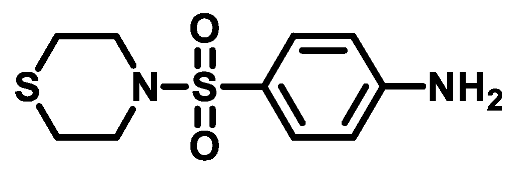
4.1.4. General Procedure for Preparation of N-substituted sulfonyl phenyl chloro- acetamides [31].
4.1.4.1. 2-Chloro-N-(4-(N,N-diethylsulfamoyl)phenyl)acetamide (4a)
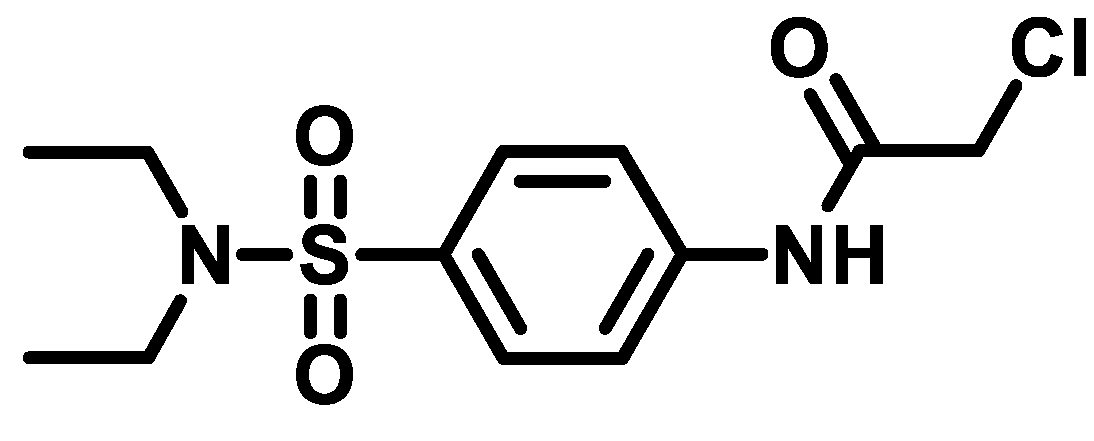
4.1.4.2. 2-Chloro-N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamide (4b)
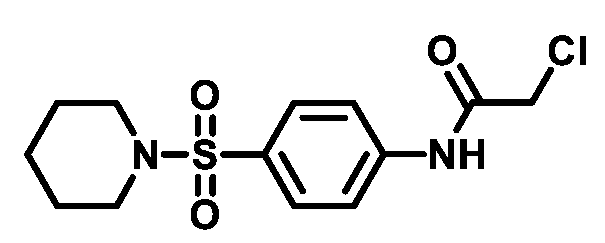
4.1.4.3. 2-Chloro-N-(4-((4-methylpiperazin-1-yl)sulfonyl)phenyl)acetamide (4c)
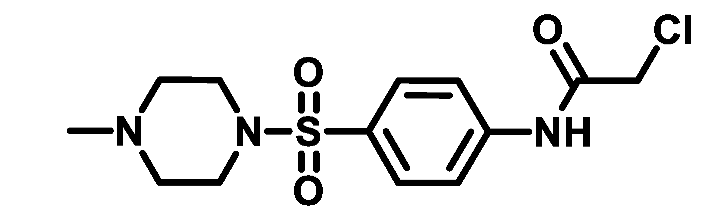
4.1.4.4. 2-Chloro-N-(4-(morpholinosulfonyl)phenyl)acetamide (4d)
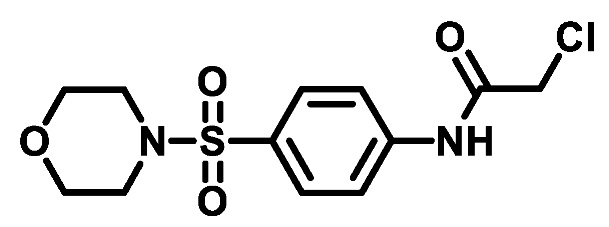
4.1.4.5. 2-Chloro-N-(4-(thiomorpholinosulfonyl)phenyl)acetamide (4e)
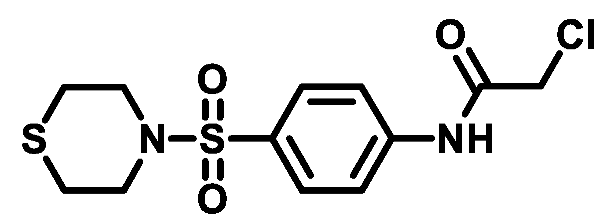
4.1.5. General Procedure for Preparation of Final Target Compounds M1-25
4.1.5.1. 2-((5-Cyano-6-oxo-4-phenyl-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(N,N- diethylsulfamoyl)phenyl)acetamide (M1)
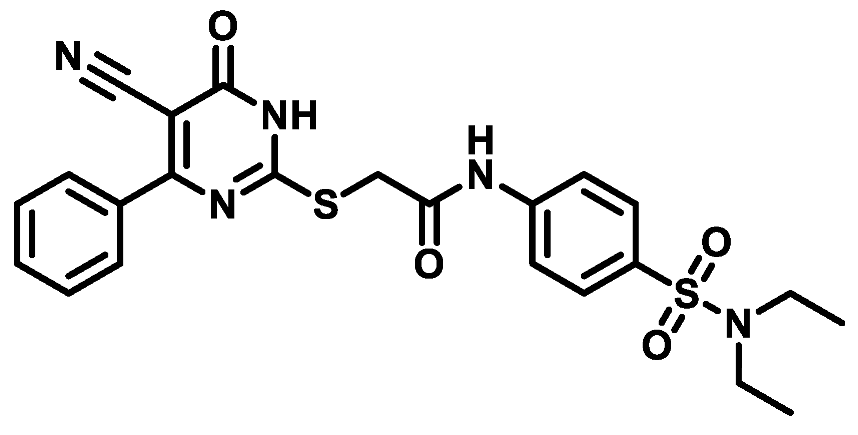
4.1.5.2. 2-((5-Cyano-6-oxo-4-phenyl-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamide (M2)
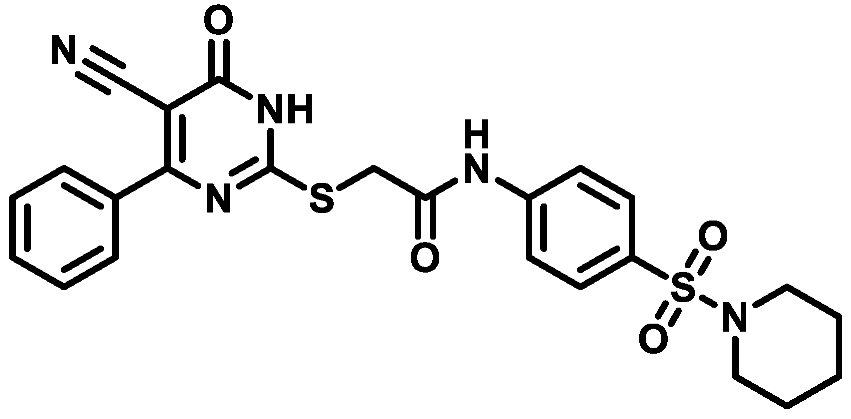
4.1.5.3. 2-((5-Cyano-6-oxo-4-phenyl-1,6-dihydropyrimidin-2-yl)thio)-N-(4-((4-methylpiperazin-1-yl)sulfonyl)phenyl)acetamide (M3)
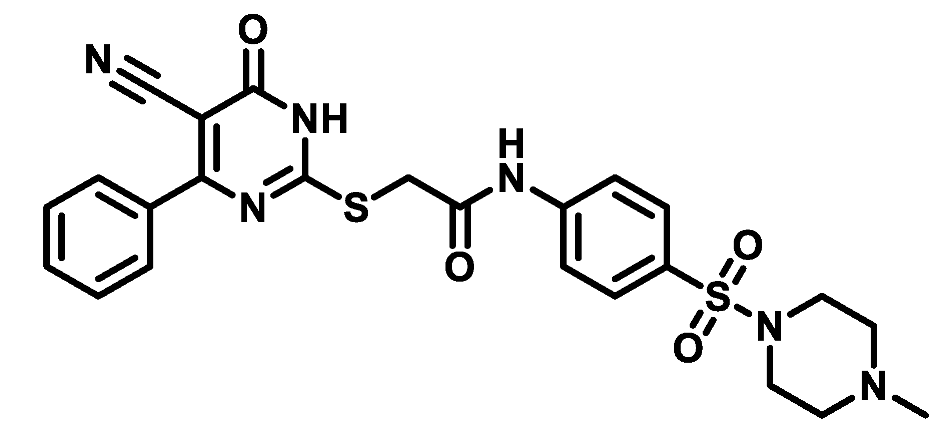
4.1.5.4. 2-((5-Cyano-6-oxo-4-phenyl-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(morpholinosulfonyl)phenyl)acetamide (M4)
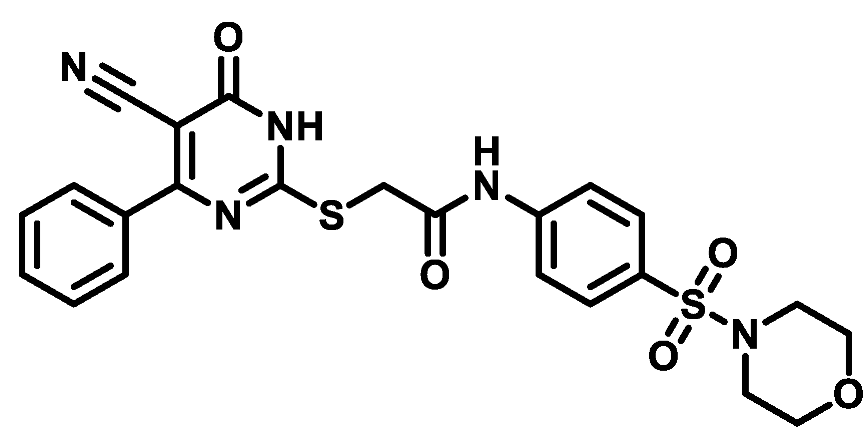
4.1.5.5. 2-((5-Cyano-6-oxo-4-phenyl-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(thiomorpholinosulfonyl)phenyl)acetamide (M5)
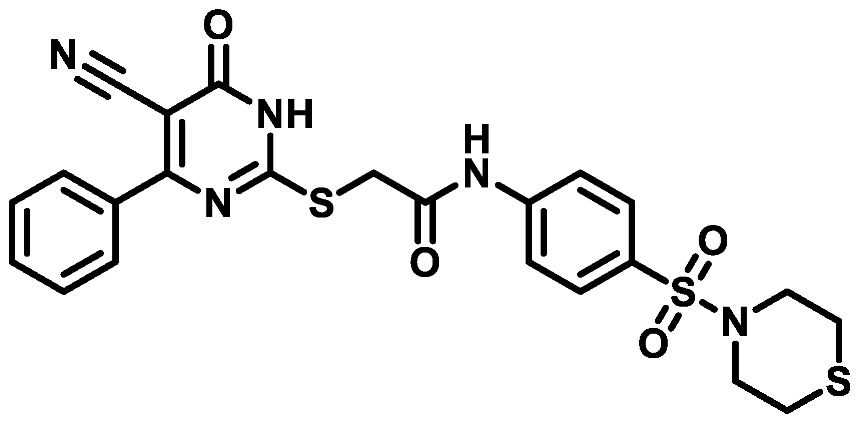
4.1.5.6. 2-((4-(4-Bromophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(N,N-diethylsulfamoyl)phenyl)acetamide (M6)
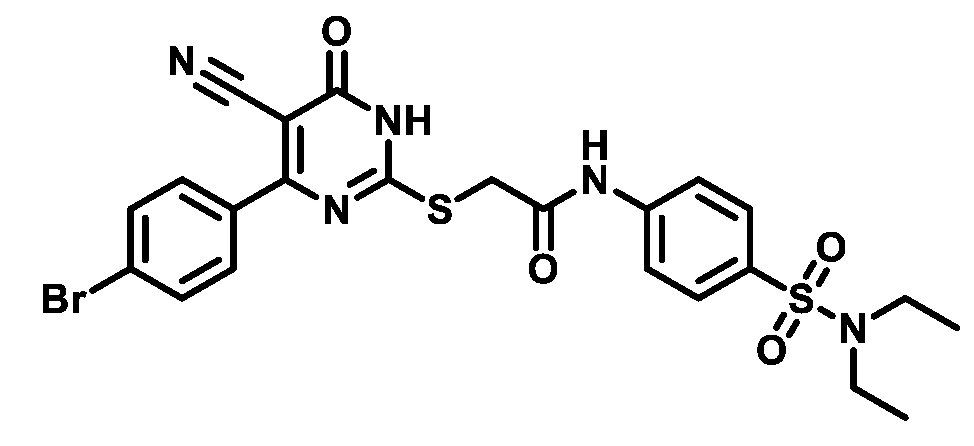
4.1.5.7. 2-((4-(4-Bromophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamide (M7)
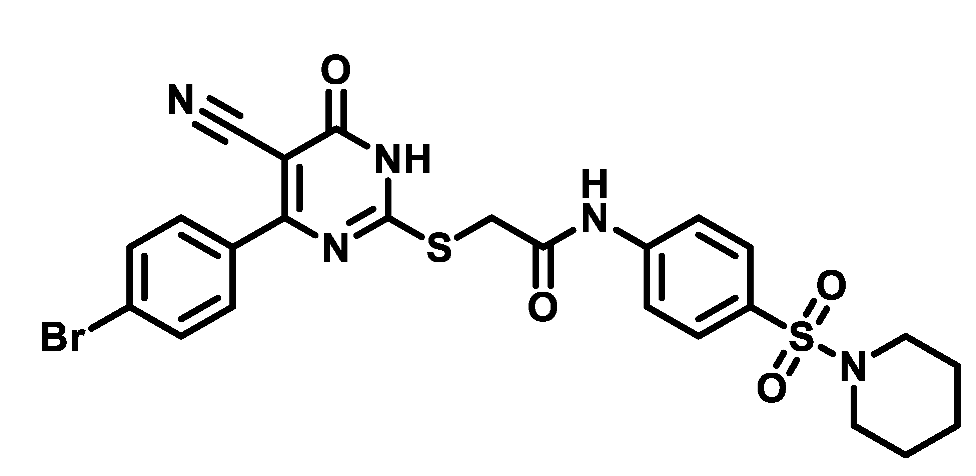
4.1.5.8. 2-((4-(4-Bromophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-((4-methylpiperazin-1-yl)sulfonyl)phenyl)acetamide (M8)
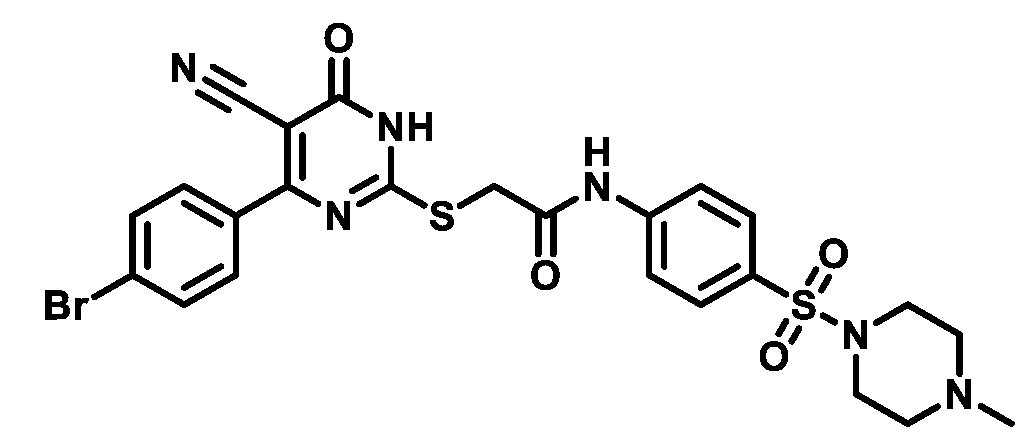
4.1.5.9. 2-((4-(4-Bromophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(morpholinosulfonyl)phenyl)acetamide(M9)
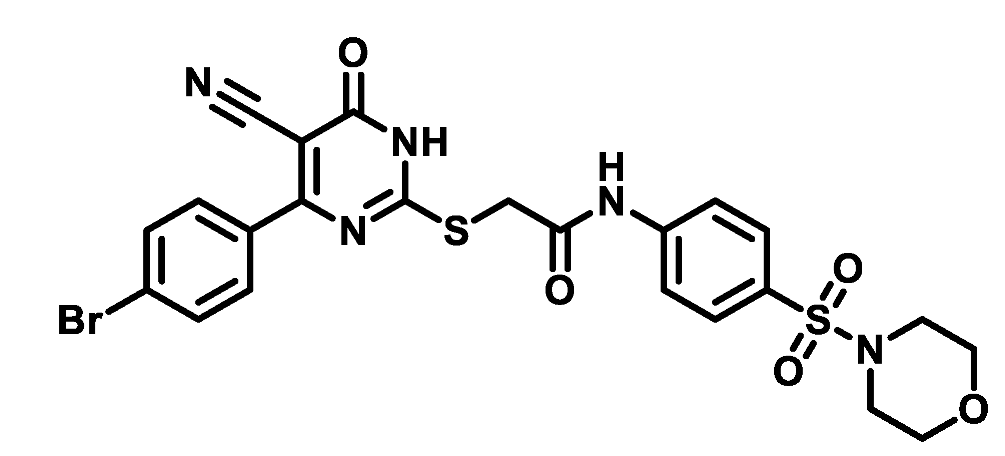
4.1.5.10. 2-((4-(4-Bromophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(thiomorpholinosulfonyl)phenyl)acetamide (M10)
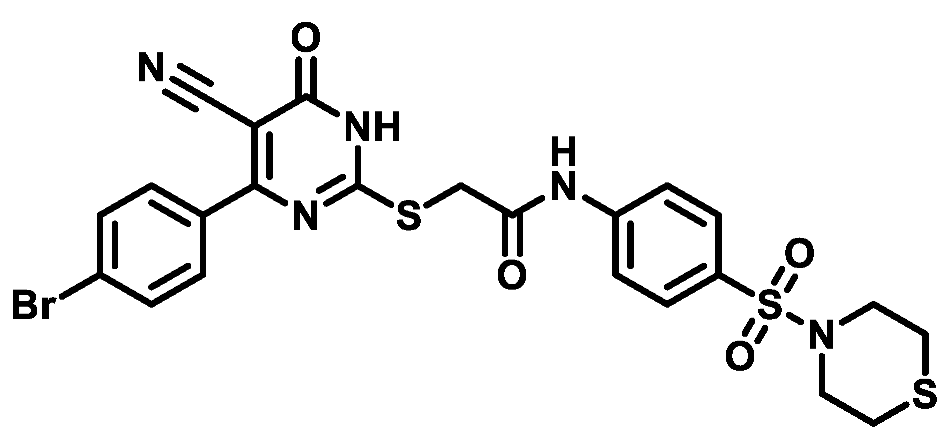
4.1.5.11. 2-((4-(4-Fluorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(N,N-diethylsulfamoyl)phenyl)acetamide (M11)
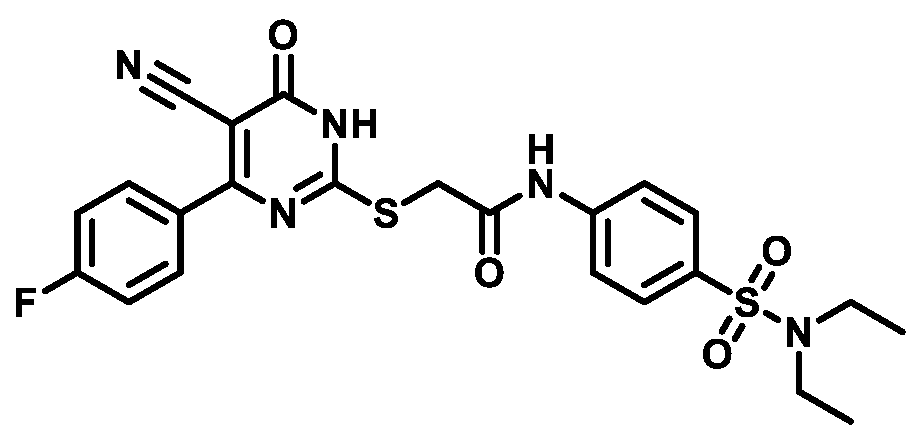
4.1.5.12. 2-((4-(4-Fluorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamide (M12)
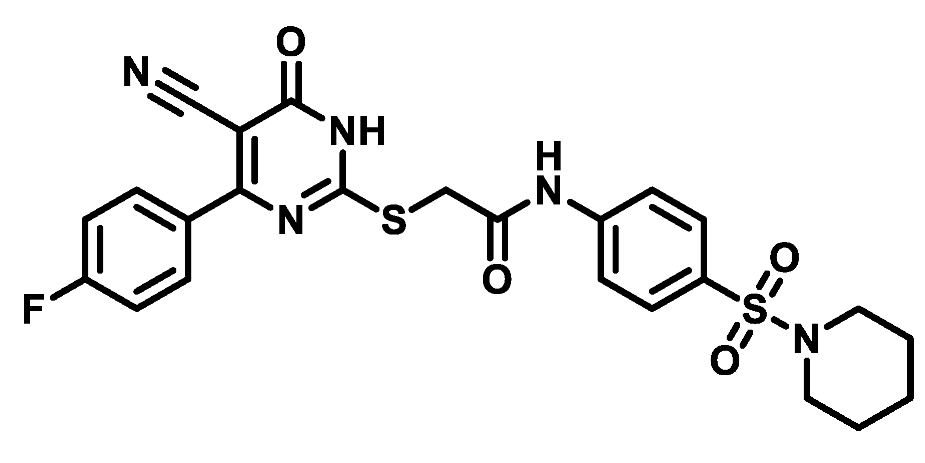
4.1.5.13. 2-((4-(4-Fluorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-((4-methylpiperazin-1-yl)sulfonyl)phenyl)acetamide (M13)
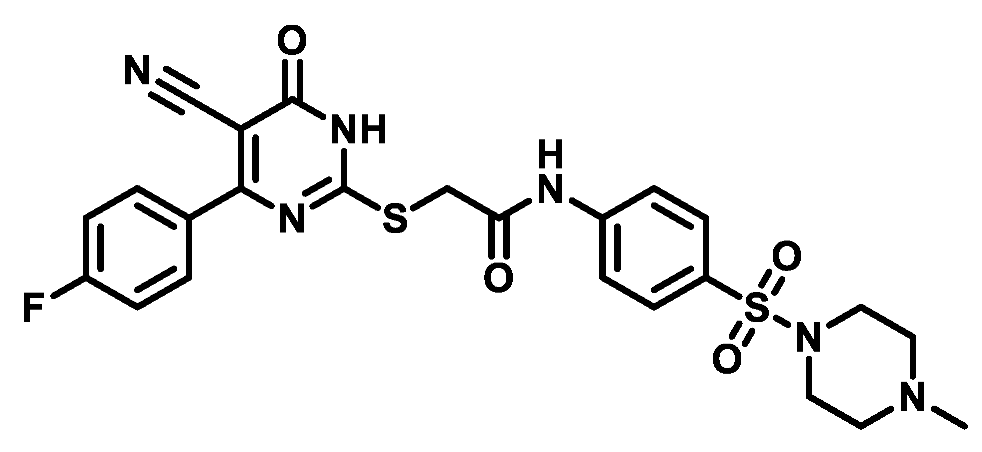
4.1.5.14. 2-((4-(4-Fluorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(morpholinosulfonyl)phenyl)acetamide (M14)
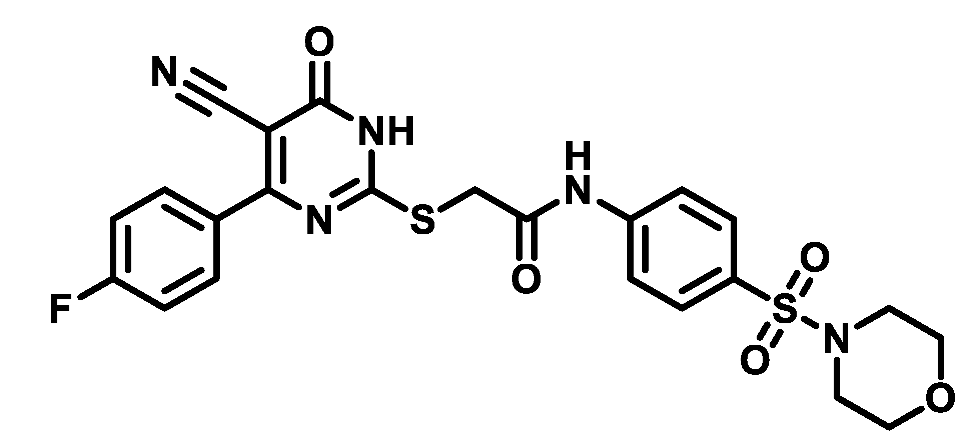
4.1.5.15. 2-((4-(4-Fluorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(thiomorpholinosulfonyl)phenyl)acetamide (M15)
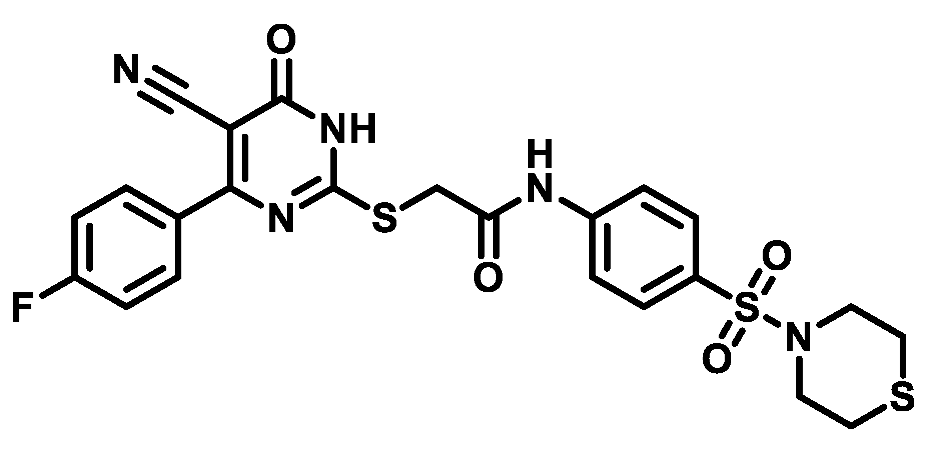
4.1.5.16. 2-((4-(4-Chlorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(N,N-diethylsulfamoyl)phenyl)acetamide (M16)
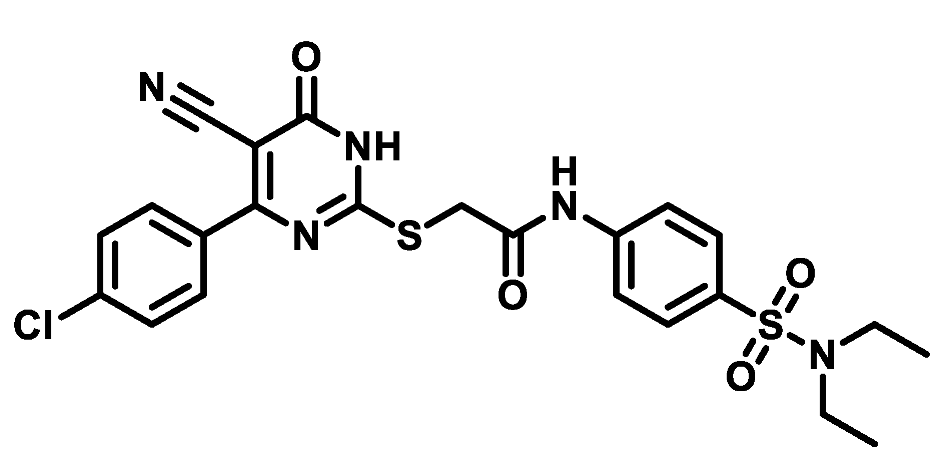
4.1.5.17. 2-((4-(4-Chlorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamide (M17)
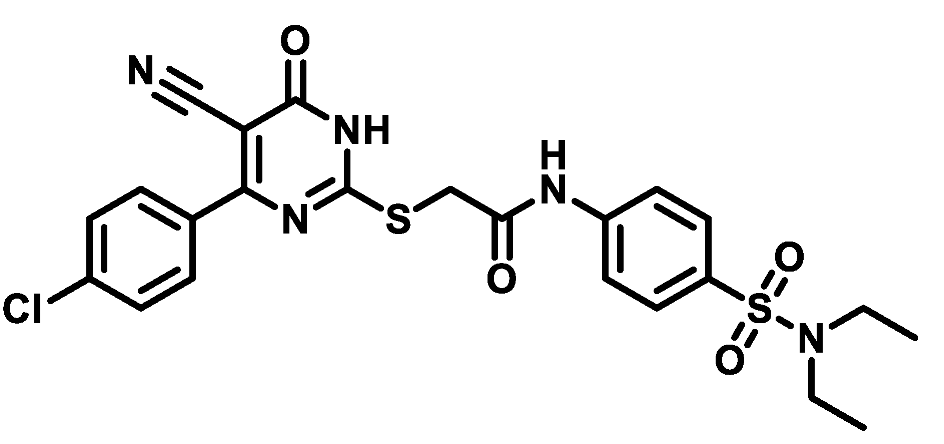
4.1.5.18. 2-((4-(4-Chlorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-((4-methylpiperazin-1-yl)sulfonyl)phenyl)acetamide (M18)
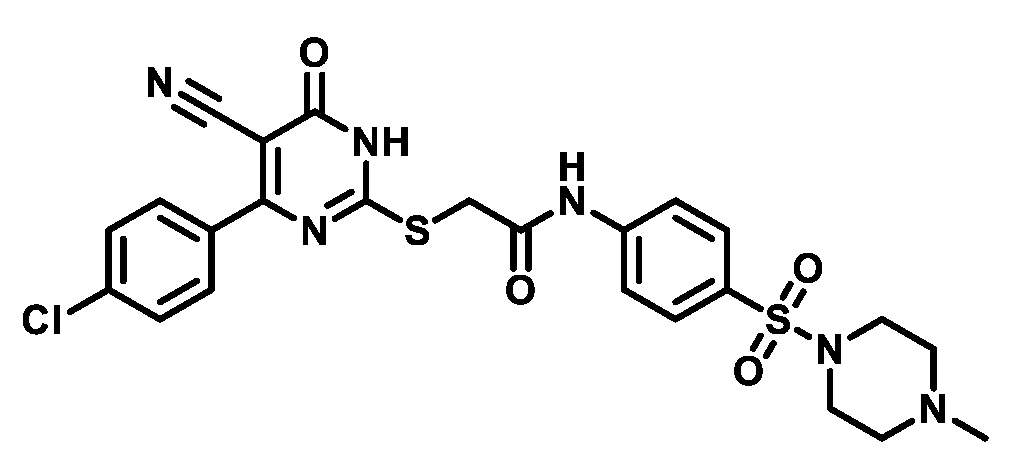
4.1.5.19. 2-((4-(4-Chlorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(morpholinosulfonyl)phenyl)acetamide (M19)
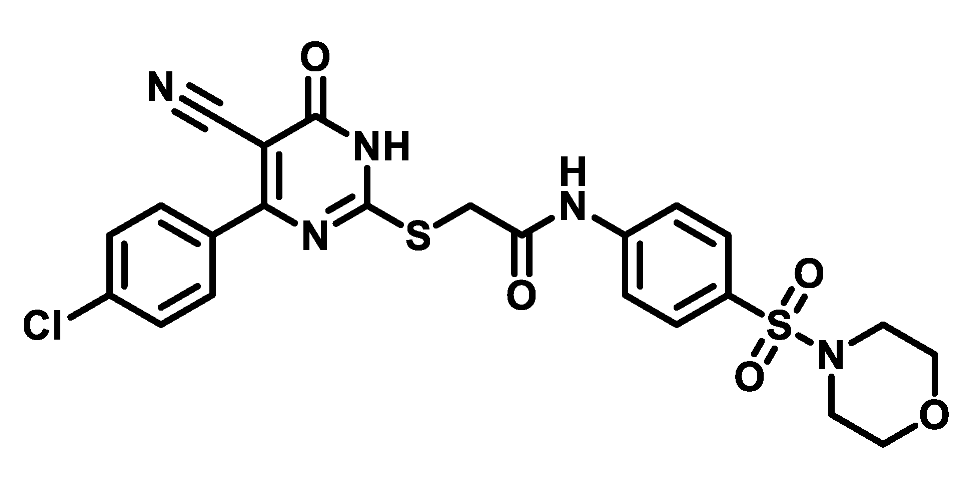
4.1.5.20. 2-((4-(4-Chlorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(thiomorpholinosulfonyl)phenyl)acetamide (M20)
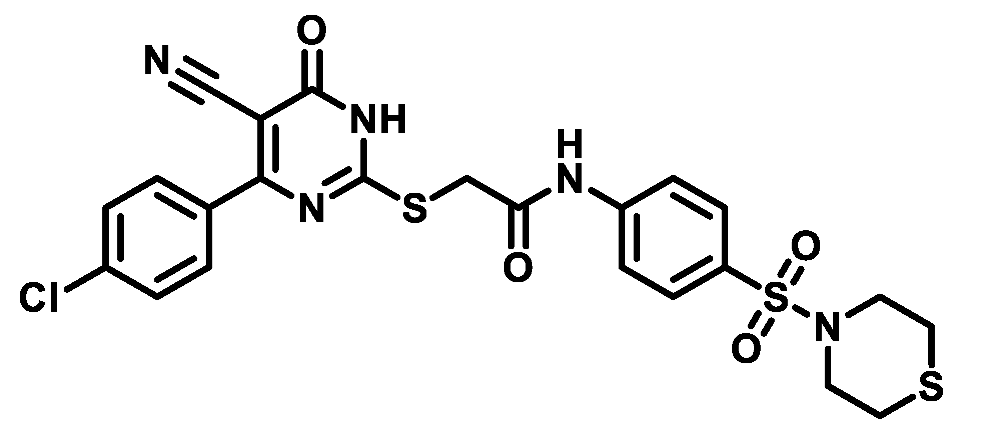
4.1.5.21. 2-((4-(3-Chlorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(N,N-diethylsulfamoyl)phenyl)acetamide (M21)
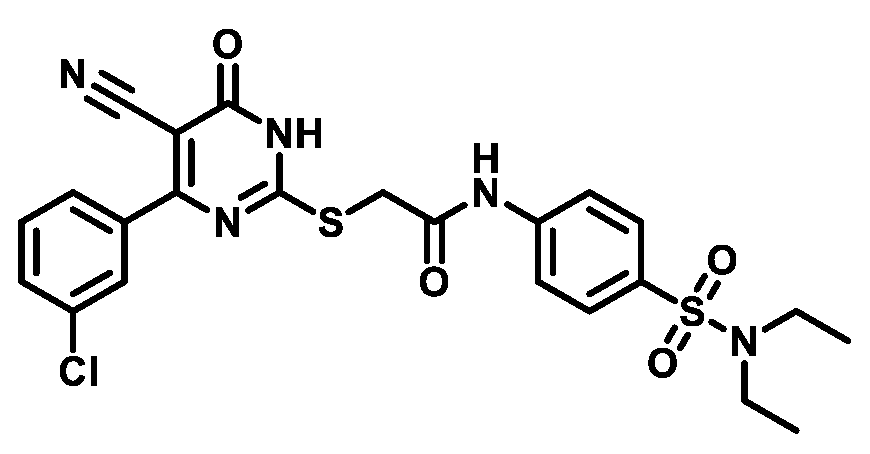
4.1.5.22. 2-((4-(3-Chlorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(piperidin-1-ylsulfonyl)phenyl)acetamide (M22)
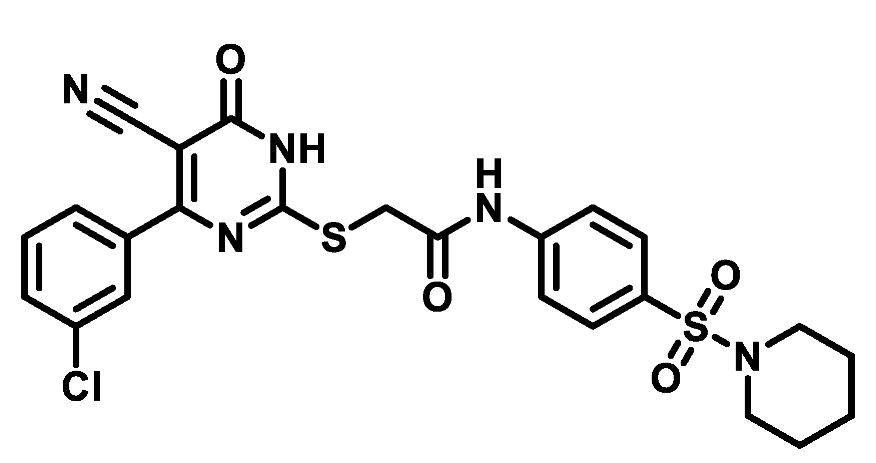
4.1.5.23. 2-((4-(3-Chlorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-((4-methylpiperazin-1-yl)sulfonyl)phenyl)acetamide (M23)
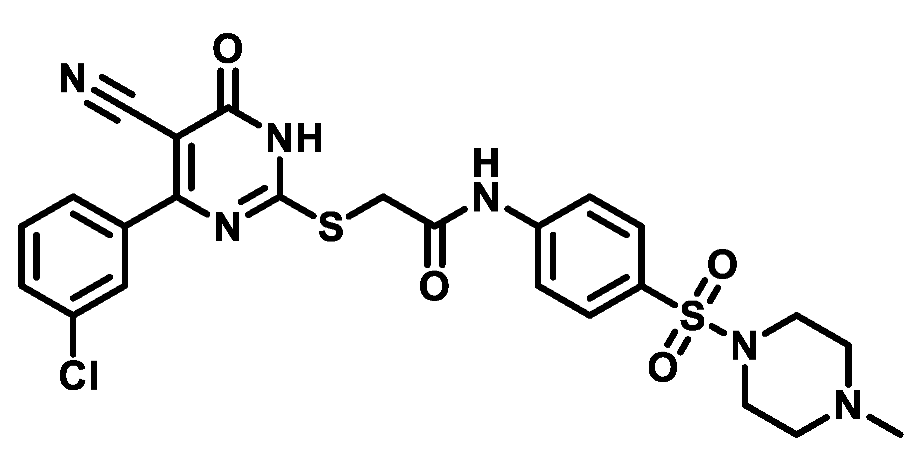
4.1.5.24. 2-((4-(3-Chlorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(morpholinosulfonyl)phenyl)acetamide (M24)
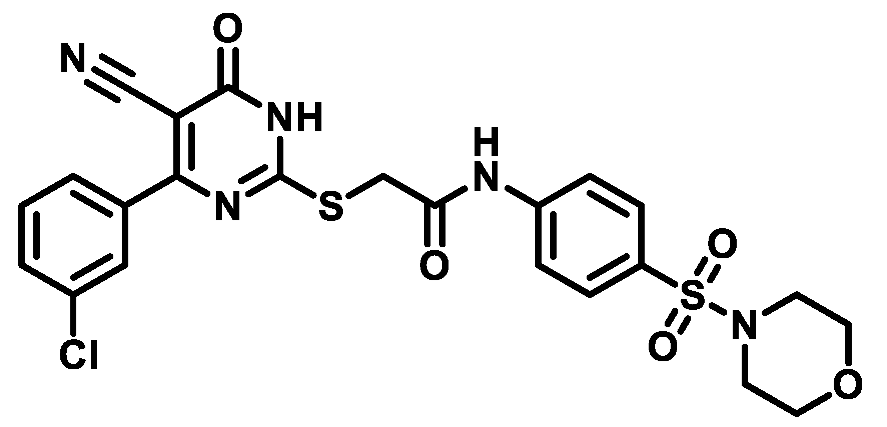
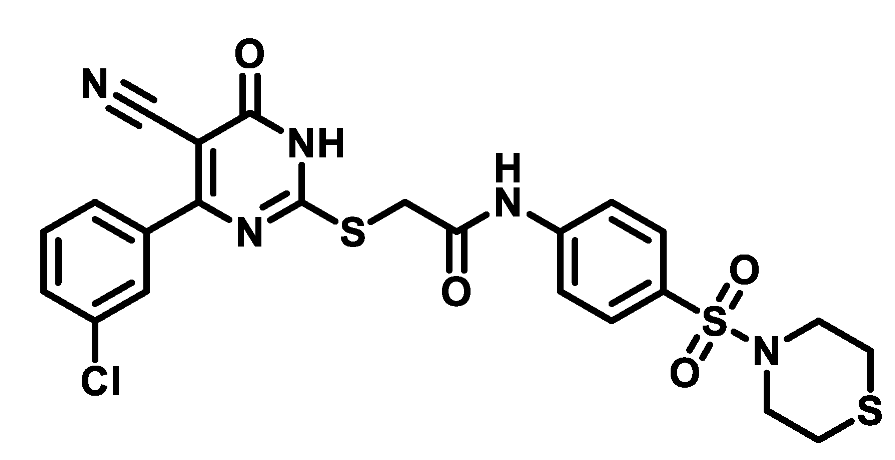
4.1.5.25. 2-((4-(3-Chlorophenyl)-5-cyano-6-oxo-1,6-dihydropyrimidin-2-yl)thio)-N-(4-(thiomorpholinosulfonyl)phenyl)acetamide (M25)
4.2. Biological Evaluation
4.2.1. Materials
4.2.2. Bacterial Strains
4.2.3. Agar Diffusion-Based Screening of Antimicrobial Activity
4.2.4. Measurement of Inhibition Zones
4.2.5. Determination of Minimum Inhibitory Concentration (MIC)
4.2.6. Determination of Minimum Bactericidal Concentration (MBC)
4.2.7. Antibiofilm Assay of the Selected Compounds by Tissue Culture Plate Method (TCP):
4.3. Physicochemical Properties and ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicity) Studies
Supplementary Materials
Funding
Declaration of Competing Interest
Data availability
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xue, Y.J., et al., Design, synthesis and evaluation of carbazole derivatives as potential antimicrobial agents. J Enzyme Inhib Med Chem, 2021. 36(1): p. 295-306. [CrossRef]
- Singh, K., et al., Design, Synthesis, DFT, docking Studies, and antimicrobial evaluation of novel benzimidazole containing sulphonamide derivatives. Bioorg Chem, 2024. 149: p. 107473. [CrossRef]
- Almalki, A.J., et al., Synthesis, Antimicrobial, Anti-Virulence and Anticancer Evaluation of New 5(4H)-Oxazolone-Based Sulfonamides. Molecules, 2022. 27(3): p. 671.
- García Altares, M., Structural Diversity of Microalgal Marine Toxins. 2017. p. 35-88.
- Bsharat, I., et al., Synthesis, characterization, antibacterial and anticancer activities of some heterocyclic imine compounds. Journal of Molecular Structure, 2023. 1289: p. 135789. [CrossRef]
- Wu, P., et al., Synthesis and biological evaluation of pentacyclic triterpenoid derivatives as potential novel antibacterial agents. Bioorg Chem, 2021. 109: p. 104692. [CrossRef]
- Vornhagen, J., et al., Kinase Inhibitors that Increase the Sensitivity of Methicillin Resistant Staphylococcus aureus to β-Lactam Antibiotics. Pathogens, 2015. 4(4): p. 708-21. [CrossRef]
- Krátký, M., et al., Improving the antimicrobial activity of old antibacterial drug mafenide: Schiff bases and their bioactivity targeting resistant pathogens. Future Med Chem, 2023. 15(3): p. 255-274. [CrossRef]
- Zhang, X.J., et al., Topical Sulfamylon cream inhibits DNA and protein synthesis in the skin donor site wound. Surgery, 2006. 139(5): p. 633-9. [CrossRef]
- Krátký, M., Novel Sulfonamide Derivatives as a Tool to Combat Methicillin-Resistant Staphylococcus Aureus. Future Medicinal Chemistry, 2024. 16(6): p. 545-562. [CrossRef]
- Liu, X.-Y., et al., Novel carbazole derivatives designed by an ortho-linkage strategy for efficient phosphorescent organic light-emitting diodes. Journal of Materials Chemistry C, 2018. 6(15): p. 4300-4307. [CrossRef]
- Ghorbani-Choghamarani, A., P. Moradi, and B. Tahmasbi, Ni-SMTU@boehmite: as an efficient and recyclable nanocatalyst for oxidation reactions. RSC Advances, 2016. 6(61): p. 56458-56466. [CrossRef]
- Keypour, H., et al., Cadmium (II) macrocyclic Schiff-base complexes containing piperazine moiety: Synthesis, spectroscopic, X-ray structure, theoretical and antibacterial studies. Journal of Molecular Structure, 2018. 1155: p. 196-204. [CrossRef]
- Zayed, E.M., et al., Synthesis, Characterization, DFT, Docking, Antimicrobial and Thermal study of Pyrimidine - Carbonitrile ligand and its Metal Complexes. Journal of Molecular Structure, 2023. [CrossRef]
- Sharma, V., N. Chitranshi, and A.K. Agarwal, Significance and biological importance of pyrimidine in the microbial world. Int J Med Chem, 2014. 2014: p. 202784. [CrossRef]
- Robinson, P.K., Enzymes: principles and biotechnological applications. Essays Biochem, 2015. 59: p. 1-41. [CrossRef]
- Rajabi, F., S. De, and R. Luque, An Efficient and Green Synthesis of Benzimidazole Derivatives Using SBA-15 Supported Cobalt Nanocatalysts. Catalysis Letters, 2015. 145: p. 1566-1570. [CrossRef]
- Lal, K., J.P. Lalitmohan, and B.B. Mahendra, A Green Protocol for One-Pot Biginelli Condensation Catalyzed by Para Toulene Sulfonic Acid under Microwave Irradiation. Letters in Organic Chemistry, 2016. 13(4): p. 255-262.
- Kalčic, F., et al., Polysubstituted Pyrimidines as Potent Inhibitors of Prostaglandin E(2) Production: Increasing Aqueous Solubility. ChemMedChem, 2021. 16(18): p. 2802-2806. [CrossRef]
- Kasralikar, H.M., et al., Design and Synthesis of Novel 1,2,3-triazolyl-pyrimidinone Hybrids as Potential Anti-HIV-1 NNRT Inhibitors. Journal of Heterocyclic Chemistry, 2018. 55(4): p. 821-829.
- Zayed, E.M., M.A. Zayed, and M. El-Desawy, Preparation and structure investigation of novel Schiff bases using spectroscopic, thermal analyses and molecular orbital calculations and studying their biological activities. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2015. 134: p. 155-164. [CrossRef]
- Zayed, E.M., et al., Synthesis, characterization, antimicrobial, and docking study of novel 1-(furanyl)-3-(pyrrolyl)propenone-based ligand and its chelates of 3d-transition metal ions. Applied Organometallic Chemistry, 2022. 36(1): p. e6489.
- Kamal, R., et al., Synthesis, Anthelmintic and Antimicrobial Evaluation of New 2-Arylidene-1-(4-methyl-6-phenylpyrimidin-2-yl)hydrazines. ChemistrySelect, 2019. 4(2): p. 713-717.
- Farag, A.M., et al., Removal of hazardous pollutants using bifunctional hydrogel obtained from modified starch by grafting copolymerization. Int J Biol Macromol, 2018. 120(Pt B): p. 2188-2199. [CrossRef]
- Kalčic, F., et al., Polysubstituted Pyrimidines as mPGES-1 Inhibitors: Discovery of Potent Inhibitors of PGE2 Production with Strong Anti-inflammatory Effects in Carrageenan-Induced Rat Paw Edema. ChemMedChem, 2020. 15(15): p. 1398-1407. [CrossRef]
- Mahmoudi-Gom Yek, S., et al., Heterogenized magnetic graphene oxide-supported N-Schiff base Cu (II) complex as an exclusive nanocatalyst for synthesis of new pyrido [2,3-d]pyrimidine-7-carbonitrile derivatives. Applied Organometallic Chemistry, 2020. 34(12): p. e5989.
- Zhao, C., et al., Pharmaceutical and medicinal significance of sulfur (S(VI))-Containing motifs for drug discovery: A critical review. Eur J Med Chem, 2019. 162: p. 679-734.
- Dharuman, S., et al., Synthesis and Structure-Activity Relationship of Thioacetamide-Triazoles against Escherichia coli. Molecules, 2022. 27(5). [CrossRef]
- Ghorab, M.M., et al., Novel N-(Substituted) Thioacetamide Quinazolinone Benzenesulfonamides as Antimicrobial Agents. Int J Nanomedicine, 2020. 15: p. 3161-3180. [CrossRef]
- Khalifa, A., et al., Isatin-pyrimidine hybrid derivatives as enoyl acyl carrier protein reductase (InhA) inhibitors against Mycobacterium tuberculosis. Bioorg Chem, 2023. 138: p. 106591. [CrossRef]
- Altamimi, A.-M.S., et al., Symmetric molecules with 1,4-triazole moieties as potent inhibitors of tumour-associated lactate dehydrogenase-A. Journal of Enzyme Inhibition and Medicinal Chemistry, 2018. 33(1): p. 147-150. [CrossRef]
- Jondle, C.N., et al., Klebsiella pneumoniae infection of murine neutrophils impairs their efferocytic clearance by modulating cell death machinery. PLoS Pathog, 2018. 14(10): p. e1007338. [CrossRef]
- Aghamohammad, S., et al., First Report of Extended-Spectrum Betalactamase-Producing Klebsiella pneumoniae Among Fecal Carriage in Iran: High Diversity of Clonal Relatedness and Virulence Factor Profiles. Microb Drug Resist, 2020. 26(3): p. 261-269. [CrossRef]
- Bodey, G.P., et al., Infections caused by Pseudomonas aeruginosa. Rev Infect Dis, 1983. 5(2): p. 279-313.
- Aspatwar, A., et al., Mycobacterium tuberculosis β-Carbonic Anhydrases: Novel Targets for Developing Antituberculosis Drugs. Int J Mol Sci, 2019. 20(20). [CrossRef]
- Faleye, O.S., et al., Halogenated Antimicrobial Agents to Combat Drug-Resistant Pathogens. Pharmacological Reviews, 2024. 76(1): p. 90-141. [CrossRef]
- Khalaf, H.S., et al., Synthesis, Docking, Computational Studies, and Antimicrobial Evaluations of New Dipeptide Derivatives Based on Nicotinoylglycylglycine Hydrazide. Molecules, 2020. 25(16): p. 3589. [CrossRef]
- Princiotto, S., et al., The antimicrobial potential of adarotene derivatives against Staphylococcus aureus strains. Bioorganic Chemistry, 2024. 145: p. 107227. [CrossRef]
- Tangadanchu, V.K.R., Y.F. Sui, and C.H. Zhou, Isatin-derived azoles as new potential antimicrobial agents: Design, synthesis and biological evaluation. Bioorg Med Chem Lett, 2021. 41: p. 128030. [CrossRef]
- Mirghani, R., et al., Biofilms: Formation, drug resistance and alternatives to conventional approaches. AIMS Microbiol, 2022. 8(3): p. 239-277. [CrossRef]
- Riquelme, Sebastian A., D. Ahn, and A. Prince, Pseudomonas aeruginosa and Klebsiella pneumoniae Adaptation to Innate Immune Clearance Mechanisms in the Lung. Journal of Innate Immunity, 2018. 10(5-6): p. 442-454. [CrossRef]
- Mohsenipour, Z. and M. Hassanshahian, The Effects of Allium sativum Extracts on Biofilm Formation and Activities of Six Pathogenic Bacteria. Jundishapur J Microbiol, 2015. 8(8): p. e18971. [CrossRef]
- Mounir, R., et al., Unlocking the Power of Onion Peel Extracts: Antimicrobial and Anti-Inflammatory Effects Improve Wound Healing through Repressing Notch-1/NLRP3/Caspase-1 Signaling. Pharmaceuticals, 2023. 16(10): p. 1379.
- Roskoski, R., Jr., Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol Res, 2019. 144: p. 19-50. [CrossRef]
- López-López, E., J.J. Naveja, and J.L. Medina-Franco, DataWarrior: an evaluation of the open-source drug discovery tool. Expert Opin Drug Discov, 2019. 14(4): p. 335-341. [CrossRef]
- Pires, D.E.V., T.L. Blundell, and D.B. Ascher, pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. Journal of Medicinal Chemistry, 2015. 58(9): p. 4066-4072. [CrossRef]
- Parvekar, P., et al., The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater Investig Dent, 2020. 7(1): p. 105-109. [CrossRef]
- Vollaro, A., et al., PYED-1 Inhibits Biofilm Formation and Disrupts the Preformed Biofilm of Staphylococcus aureus. Antibiotics (Basel), 2020. 9(5). [CrossRef]
- Delafield, F.P., et al., Decomposition of poly-beta-hydroxybutyrate by pseudomonads. J Bacteriol, 1965. 90(5): p. 1455-66.
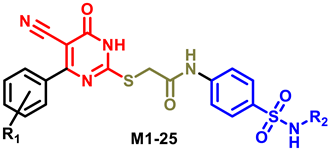 | |||||
| Compounds | R1 | R2 | Compounds | R1 | R2 |
| M1 | H | 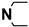 |
M14 | 4-Cl |  |
| M2 | H |  |
M15 | 4-Cl |  |
| M3 | H | 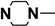 |
M16 | 4-F | 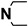 |
| M4 | H |  |
M17 | 4-F |  |
| M5 | H |  |
M18 | 4-F |  |
| M6 | 4-Br |  |
M19 | 4-F |  |
| M7 | 4-Br |  |
M20 | 4-F |  |
| M8 | 4-Br | 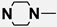 |
M21 | 3-Cl | 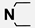 |
| M9 | 4-Br |  |
M22 | 3-Cl |  |
| M10 | 4-Br |  |
M23 | 3-Cl |  |
| M11 | 4-Cl | 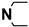 |
M24 | 3-Cl |  |
| M12 | 4-Cl |  |
M25 | 3-Cl |  |
| M13 | 4-Cl |  |
|||
| Microbial organisms | M 1 |
M2 | M3 | M 4 |
M 5 |
M 6 |
M 7 |
M 8 |
M 9 |
M 10 |
M 11 |
M 12 |
M 13 |
M 14 |
M 15 |
M 16 |
M 17 |
M 18 |
M 19 |
M 20 |
M 21 |
M 22 |
M 23 |
M 24 |
M 25 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
E. coli ATCC-25922 |
0 | 16 | 18 | 20 | 0 | 20 | 20 | 18 | 25 | 22 | 0 | 20 | 20 | 22 | 19 | 0 | 0 | 0 | 0 | 20 | 0 | 20 | 20 | 24 | 26 |
| K. pneumoniae | 20 | 22 | 24 | 22 | 22 | 26 | 22 | 22 | 24 | 24 | 20 | 17 | 15 | 22 | 22 | 24 | 24 | 22 | 28 | 26 | 20 | 20 | 19 | 26 | 26 |
|
P. aeruginosa ATCC 27853 |
20 | 22 | 20 | 22 | 24 | 26 | 18 | 18 | 20 | 20 | 22 | 18 | 22 | 22 | 22 | 16 | 20 | 22 | 26 | 30 | 20 | 18 | 20 | 22 | 30 |
|
S. aureus ATCC 6538 |
0 | 0 | 0 | 0 | 17 | 21 | 21 | 0 | 21 | 21 | 15 | 19 | 0 | 15 | 20 | 22 | 16 | 22 | 20 | 26 | 22 | 17 | 18 | 0 | 24 |
|
S. epidermidis ATCC 35984 |
0 | 15 | 0 | 0 | 22 | 15 | 21 | 20 | 25 | 17 | 0 | 0 | 0 | 0 | 18 | 20 | 20 | 20 | 22 | 22 | 20 | 20 | 0 | 13 | 20 |
|
B. subtilis ATCC 6633 |
18 | 22 | 0 | 0 | 22 | 20 | 22 | 17 | 26 | 15 | 18 | 22 | 0 | 13 | 18 | 23 | 25 | 0 | 24 | 15 | 15 | 20 | 0 | 13 | 28 |
|
C. albicans ATCC-10231 |
0 | 0 | 0 | 0 | 17 | 21 | 21 | 0 | 21 | 21 | 15 | 19 | 0 | 15 | 18 | 23 | 16 | 23 | 20 | 26 | 22 | 17 | 18 | 0 | 24 |
| Chemical Compounds | Bacterial strains | MIC μg/mL | MBC μg/mL |
|---|---|---|---|
| M6 | Klebsiella pneumoniae | 375 + 0.00 | 1500 + 0.45 |
| Pseudomonas aeruginosa | 375 + 0.00 | 1500 + 0.00 | |
| M19 | Klebsiella pneumoniae | 375 + 0.00 | 1500 + 0.29 |
| Pseudomonas aeruginosa | 375 + 0.00 | 1500 + 0.00 | |
| M20 | Klebsiella pneumoniae | 375 + 0.00 | 7500 + 0.00 |
| Pseudomonas aeruginosa | 375 + 0.00 | 1500 + 0.00 | |
| M25 | Klebsiella pneumoniae | 375 + 0.00 | 7500 + 0.00 |
| Pseudomonas aeruginosa | 375 + 0.00 | 1500 + 0.00 |
| Compound No. | MW | HBA | HBD | logP (o/w) | TPSA Å2 |
Num. rotatable bonds | Lipinski |
|---|---|---|---|---|---|---|---|
| M6 | 576.49 | 6 | 2 | 3.04 | 169.7 | 8 | Yes; 1 violation: MW>500 |
| M9 | 546.02 | 7 | 2 | 1.72 | 178.9 | 6 | Yes; 1 violation: MW>500 |
| M20 | 562.09 | 6 | 2 | 2.35 | 195 | 6 | Yes; 1 violation: MW>500 |
| M25 | 562.09 | 6 | 2 | 2.39 | 195 | 6 | Yes; 1 violation: MW>500 |
| Compound M6 | Compound M9 | Compound M20 |
Compound M25 |
|
|---|---|---|---|---|
|
Absorption Water solubility (log mol/L) Intestinal absorption Skin permeability (log Kp) |
-4.0 82.4 -2.7 |
-3.8 78.7 -2.7 |
-3.8 85.1 -2.7 |
-3.8 85.2 -2.7 |
|
Distribution Blood brain permeability(log BB) CNS permeability (log PS) |
-1.46 -2.78 |
-1.43 -3.45 |
-1.42 -2.73 |
-1.42 -2.73 |
|
Metabolism CYP2D6 substrate CYP3A4 substrate CYP1A2 inhibitor |
No Yes No |
No Yes No |
No Yes No |
No Yes No |
|
Excretion Total clearance (log ml/min/Kg) Renal OCT2 substrate |
0.1 NO |
0.2 No |
0.1 No |
0.1 No |
|
Toxicity AMES toxicity hERG inhibitor Tumorigenic Irritant |
No No No No |
No No No No |
No No No No |
No No No No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





