Submitted:
27 August 2024
Posted:
27 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
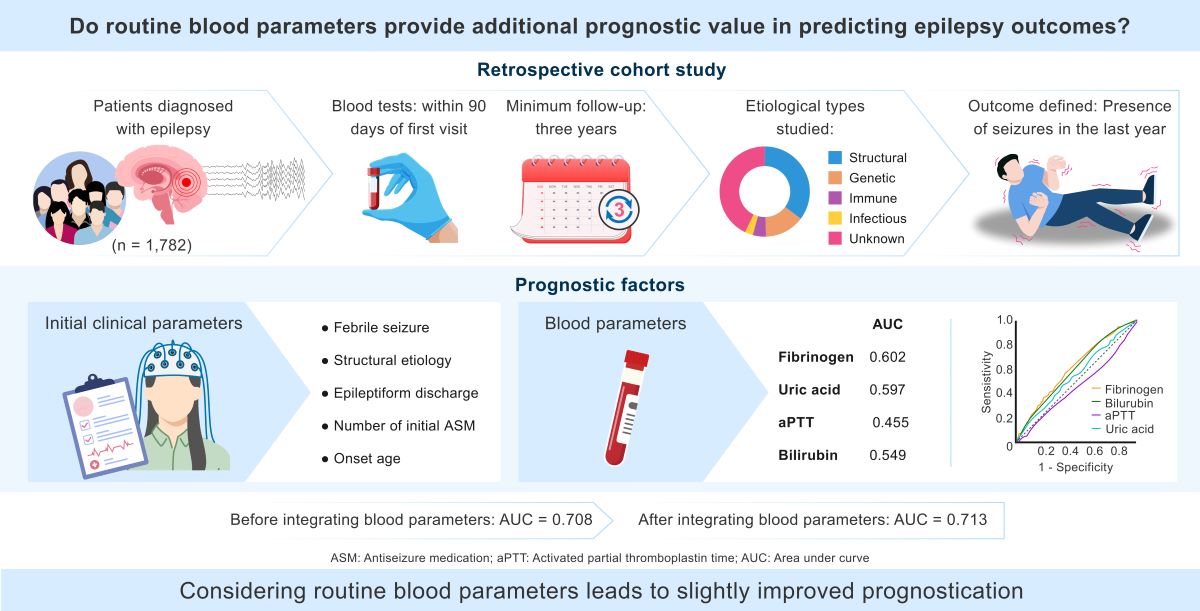
2.1. The Study Population
2.2. Collected Data Characteristics
2.3. Statistical Analysis
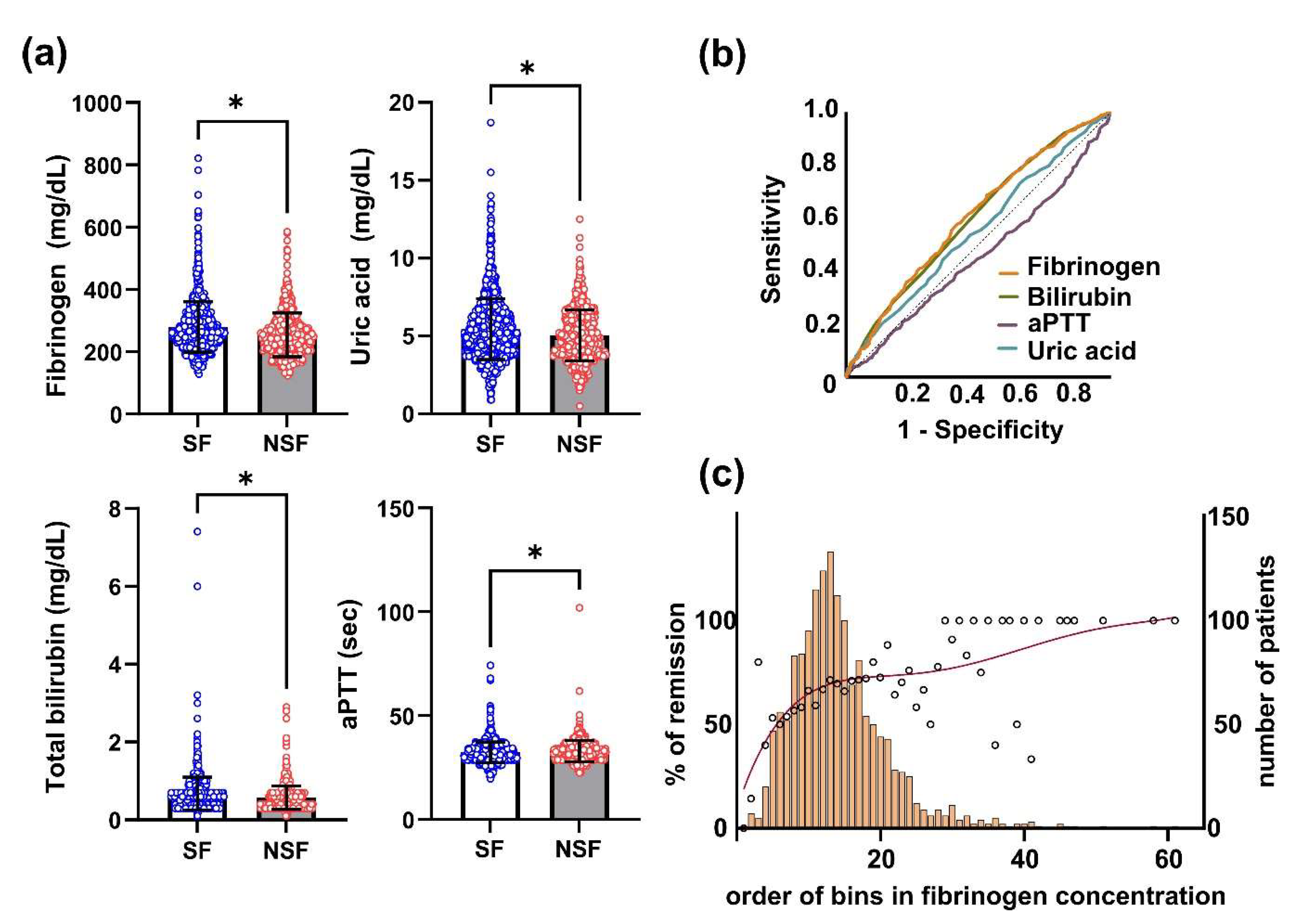
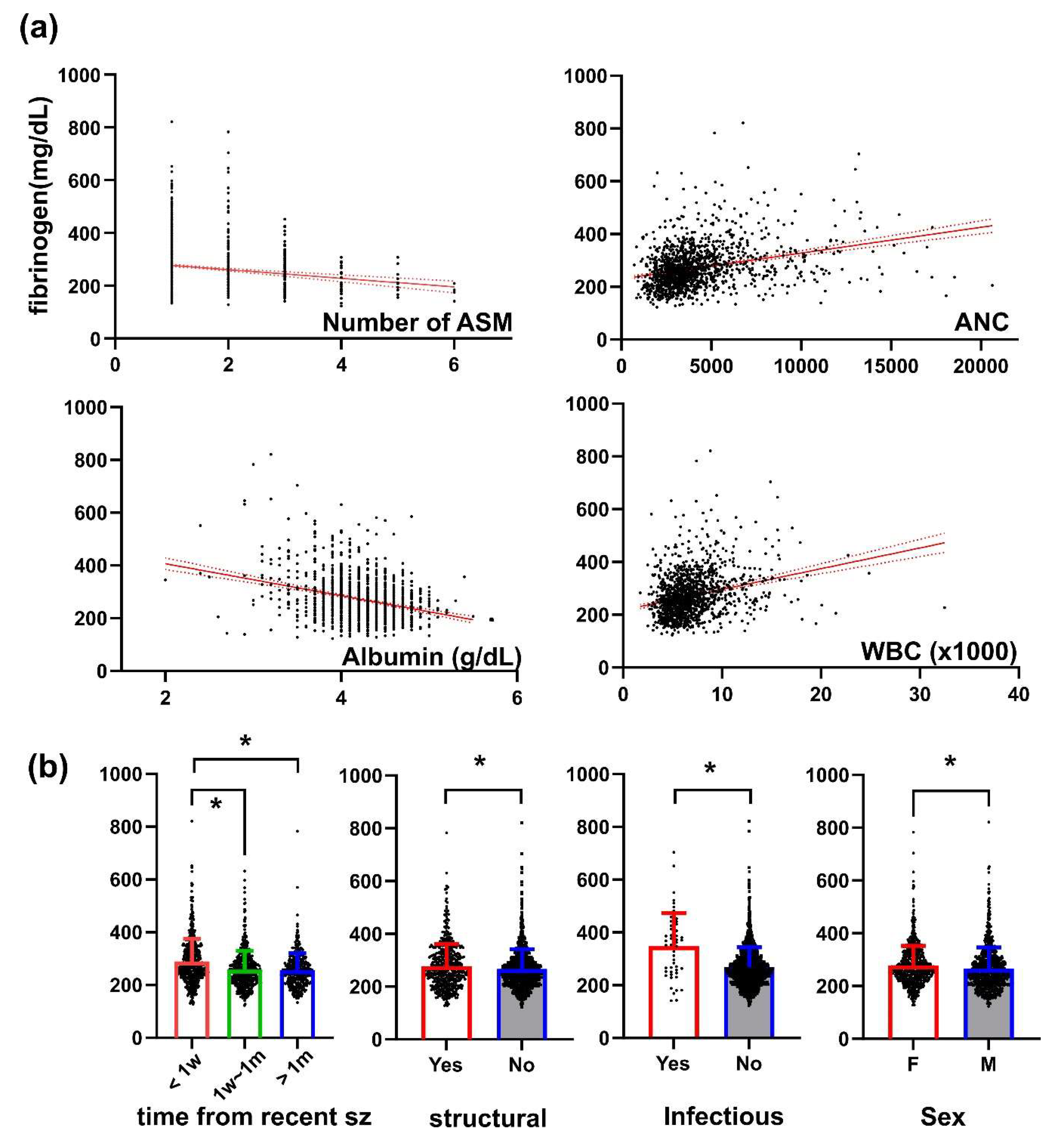
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, K. I.; Lee, S. K.; Chu, K.; Jung, K. H.; Bae, E. K.; Kim, J. S.; Lee, J. J.; Lee, S. Y.; Chung, C. K. Withdrawal of antiepileptic drugs after neocortical epilepsy surgery. Ann Neurol 2010, 67(2), 230–238. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S. W.; Lee, S. K.; Hong, K. S.; Kim, K. K.; Chung, C. K.; Kim, H. Prognostic factors for the surgery for mesial temporal lobe epilepsy: longitudinal analysis. Epilepsia 2005, 46(8), 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Park, K. I.; Shin, D. S.; Moon, J.; Lee, S. T.; Jung, K. H.; Jung, K. Y.; Chu, K.; Lee, S. K. Lesion Detection Through MRI Postprocessing in Pathology-Proven Focal Cortical Dysplasia: Experience at a Single Institution in the Republic of Korea. J Clin Neurol 2023, 19(3), 288–295. [Google Scholar] [CrossRef] [PubMed]
- Roy, P. L.; Ronquillo, L. H.; Ladino, L. D.; Tellez-Zenteno, J. F. Risk factors associated with drug resistant focal epilepsy in adults: A case control study. Seizure 2019, 73, 46–50. [Google Scholar] [CrossRef]
- Tripathi, M.; Padhy, U. P.; Vibha, D.; Bhatia, R.; Padma Srivastava, M. V.; Singh, M. B.; Prasad, K.; Chandra, S. P. Predictors of refractory epilepsy in north India: a case-control study. Seizure 2011, 20(10), 779–783. [Google Scholar] [CrossRef]
- Stroup, D. F.; Berlin, J. A.; Morton, S. C.; Olkin, I.; Williamson, G. D.; Rennie, D.; Moher, D.; Becker, B. J.; Sipe, T. A.; Thacker, S. B. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283 (15), 2008-2012.
- Dwivedi, R.; Ramanujam, B.; Chandra, P. S.; Sapra, S.; Gulati, S.; Kalaivani, M.; Garg, A.; Bal, C. S.; Tripathi, M.; Dwivedi, S. N.; et al. Surgery for Drug-Resistant Epilepsy in Children. N Engl J Med 2017, 377(17), 1639–1647. [Google Scholar] [CrossRef]
- Kwan, P.; Brodie, M. J. Early identification of refractory epilepsy. N Engl J Med 2000, 342(5), 314–319. [Google Scholar] [CrossRef]
- Brodie, M. J. Outcomes in newly diagnosed epilepsy in adolescents and adults: Insights across a generation in Scotland. Seizure 2017, 44, 206–210. [Google Scholar] [CrossRef]
- Park, K. M.; Shin, K. J.; Ha, S. Y.; Park, J.; Kim, S. E.; Kim, S. E. Response to antiepileptic drugs in partial epilepsy with structural lesions on MRI. Clin Neurol Neurosurg 2014, 123, 64–68. [Google Scholar] [CrossRef]
- Brodie, M. J.; Barry, S. J.; Bamagous, G. A.; Norrie, J. D.; Kwan, P. Patterns of treatment response in newly diagnosed epilepsy. Neurology 2012, 78(20), 1548–1554. [Google Scholar] [CrossRef]
- Berg, A. T.; Shinnar, S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology 1991, 41(7), 965–972. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, R.; Brodie, M. J. Early predictors of outcome in newly diagnosed epilepsy. Seizure 2013, 22(5), 333–344. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, J.; Guo, H.; Ding, L.; Zhang, Y.; Xu, Y. High Mobility Group Protein B1 (HMGB1) and Interleukin-1beta as Prognostic Biomarkers of Epilepsy in Children. J Child Neurol 2018, 33(14), 909–917. [Google Scholar] [CrossRef] [PubMed]
- Chang, C. C.; Lui, C. C.; Lee, C. C.; Chen, S. D.; Chang, W. N.; Lu, C. H.; Chen, N. C.; Chang, A. Y.; Chan, S. H.; Chuang, Y. C. Clinical significance of serological biomarkers and neuropsychological performances in patients with temporal lobe epilepsy. BMC Neurol 2012, 12, 15. [Google Scholar] [CrossRef]
- Davalos, D.; Akassoglou, K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol 2012, 34(1), 43–62. [Google Scholar] [CrossRef]
- Sulimai, N.; Brown, J.; Lominadze, D. The Role of Nuclear Factor-Kappa B in Fibrinogen-Induced Inflammatory Responses in Cultured Primary Neurons. Biomolecules 2022, 12(12), 1741. [Google Scholar] [CrossRef]
- Peng, S.; Lv, K. The role of fibrinogen in traumatic brain injury: from molecular pathological mechanisms to clinical management. Eur J Trauma Emerg Surg 2022, 49, 1665–1672. [Google Scholar] [CrossRef]
- Vasse, M.; Paysant, J.; Soria, J.; Collet, J. P.; Vannier, J. P.; Soria, C. Regulation of fibrinogen biosynthesis by cytokines, consequences on the vascular risk. Haemostasis 1996, 26 Suppl 4, 331–339. [Google Scholar] [CrossRef]
- Morganti-Kossman, M. C.; Lenzlinger, P. M.; Hans, V.; Stahel, P.; Csuka, E.; Ammann, E.; Stocker, R.; Trentz, O.; Kossmann, T. Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol Psychiatry 1997, 2(2), 133–136. [Google Scholar] [CrossRef]
- Pyun, J. M.; Ryoo, N.; Park, Y. H.; Kim, S. Fibrinogen Levels and Cognitive Profile Differences in Patients with Mild Cognitive Impairment. Dement Geriatr Cogn Disord 2020, 49(5), 489–496. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, H.; Zhang, S.; Fan, X.; Liu, X. Plasma fibrinogen is associated with cognitive decline and risk for dementia in patients with mild cognitive impairment. Int J Clin Pract 2008, 62(7), 1070–1075. [Google Scholar] [CrossRef]
- Ishikawa, N.; Kobayashi, Y.; Fujii, Y.; Kobayashi, M. Increased interleukin-6 and high-sensitivity C-reactive protein levels in pediatric epilepsy patients with frequent, refractory generalized motor seizures. Seizure 2015, 25, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Fabene, P. F.; Navarro Mora, G.; Martinello, M.; Rossi, B.; Merigo, F.; Ottoboni, L.; Bach, S.; Angiari, S.; Benati, D.; Chakir, A.; et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med 2008, 14(12), 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Varvel, N. H.; Neher, J. J.; Bosch, A.; Wang, W.; Ransohoff, R. M.; Miller, R. J.; Dingledine, R. Infiltrating monocytes promote brain inflammation and exacerbate neuronal damage after status epilepticus. Proc Natl Acad Sci U S A 2016, 113(38), E5665–5674. [Google Scholar] [CrossRef] [PubMed]
- Shin, H. R.; Chu, K.; Lee, W. J.; Lee, H. S.; Kim, E. Y.; Son, H.; Moon, J.; Kim, N.; Jung, K. Y.; Jung, K. H.; et al. Neuropsychiatric symptoms and seizure related with serum cytokine in epilepsy patients. Sci Rep 2022, 12(1), 7138. [Google Scholar] [CrossRef] [PubMed]
- Zattoni, M.; Mura, M. L.; Deprez, F.; Schwendener, R. A.; Engelhardt, B.; Frei, K.; Fritschy, J. M. Brain infiltration of leukocytes contributes to the pathophysiology of temporal lobe epilepsy. J Neurosci 2011, 31(11), 4037–4050. [Google Scholar] [CrossRef]
- Lv, K.; Yuan, Q.; Fu, P.; Wu, G.; Wu, X.; Du, Z.; Yu, J.; Li, Z.; Hu, J. Impact of fibrinogen level on the prognosis of patients with traumatic brain injury: a single-center analysis of 2570 patients. World J Emerg Surg 2020, 15(1), 54. [Google Scholar] [CrossRef]
- Sikka, M.; Sodhi, R.; Kotru, M.; Singh, G. Markers of Fibrinolysis in Indian Patients with Isolated Head Trauma. Asian J Neurosurg 2019, 14(1), 118–121. [Google Scholar] [CrossRef]
- Pronto-Laborinho, A. C.; Lopes, C. S.; Conceição, V. A.; Gromicho, M.; Santos, N. C.; de Carvalho, M.; Carvalho, F. A. γ’ Fibrinogen as a Predictor of Survival in Amyotrophic Lateral Sclerosis. Front Cardiovasc Med 2021, 8, 715842. [Google Scholar] [CrossRef]
- Liguori, C.; Romigi, A.; Izzi, F.; Placidi, F.; Nuccetelli, M.; Cordella, A.; Bernardini, S.; Biagio, M. N. Complement system dysregulation in patients affected by Idiopathic Generalized Epilepsy and the effect of antiepileptic treatment. Epilepsy Res 2017, 137, 107–111. [Google Scholar] [CrossRef]
- de la Fuente, C.; Monreal, L.; Ceron, J.; Pastor, J.; Viu, J.; Anor, S. Fibrinolytic activity in cerebrospinal fluid of dogs with different neurological disorders. J Vet Intern Med 2012, 26(6), 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. F.; Xu, L. P.; Luo, Z. Y.; Yu, Z. Q.; Li, Z. Y.; Cui, Q. Y.; Qin, L. M.; Ren, Y. Y.; Shen, H. S.; Tang, J. Q.; et al. Valproic acid-associated low fibrinogen and delayed intracranial hemorrhage: case report and mini literature review. Drug Des Devel Ther 2013, 7, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Goerdt, C.; Rubins, H. B.; Swaim, W.; Folsom, A. Can phenytoin lower plasma fibrinogen concentrations? Thromb Res 1995, 79(3), 231–236. [Google Scholar] [CrossRef] [PubMed]
- Bavoux, F.; Fournier-Perhilou, A. I.; Wood, C.; Francoual, C.; Boccara, J. F. Neonatal fibrinogen depletion caused by sodium valproate. Ann Pharmacother 1994, 28(11), 1307. [Google Scholar] [CrossRef]
- Dale, B. M.; Purdie, G. H.; Rischbieth, R. H. Fibrinogen depletion with sodium valproate. Lancet 1978, 1(8077), 1316–1317. [Google Scholar] [CrossRef]
- Koh, S.; Lee, D. Y.; Cha, J. M.; Kim, Y.; Kim, H. H.; Yang, H. J.; Park, R. W.; Choi, J. Y. Association between pre-diagnostic serum uric acid levels in patients with newly diagnosed epilepsy and conversion rate to drug-resistant epilepsy within 5 years: A common data model analysis. Seizure 2024, 118, 103–109. [Google Scholar] [CrossRef]
- Parfenova, H.; Leffler, C. W.; Basuroy, S.; Liu, J.; Fedinec, A. L. Antioxidant roles of heme oxygenase, carbon monoxide, and bilirubin in cerebral circulation during seizures. J Cereb Blood Flow Metab 2012, 32(6), 1024–1034. [Google Scholar] [CrossRef]
| Clinical parameters | Number of patients (%) |
|---|---|
| Sex (Female : Male) | 814 : 968 (45.7% : 54.3%) |
| Onset age | |
| mean ± SD (range) | 31.0 ± 20.2, (0-91) |
| Missing, N | 32 |
| Age of first visit (range) | 37.5 ± 18.0 (6-91) |
| Follow-up duration, month (range) | 104.5 ± 30.5 (36-161) |
| Epilepsy duration, year | |
| mean ± SD (range) | 6.3 ± 9.5 (0-56) |
| Missing, N | 32 |
| Newly diagnosed | 1026 (57.6%) |
| Unknown | 166 (9.3%) |
| Seizure types | |
| Focal | 1476 (82.9%) |
| Generalized | 281 (15.8%) |
| Unknown | 23 (1.3%) |
| Missing, N | 2 |
| Epilepsy classification | |
| Focal | 1387 (77.8%) |
| Generalized | 275 (15.4%) |
| Combined | 98 (5.5%) |
| Unknown | 22 (1.2%) |
| Etiology | |
| Structural | 626 (35.1%) |
| Genetic | 253 (14.2%) |
| Immune | 84 (4.7%) |
| Infectious | 51 (2.9%) |
| Hypoxic | 5 (0.3%) |
| metabolic | 3 (0.2%) |
| Unknown | 760 (42.6%) |
| History of febrile seizure | 138 (7.7%) |
| Family history of epilepsy | 55 (3.1%) |
| Presence of epileptiform discharge on EEG | |
| N (%) | 429 (26.3%) |
| Missing, N | 151 |
| Lesion on MRI | |
| N (%) | 764 (49.2%) |
| Missing, N | 229 |
| Hippocampal sclerosis | |
| N (%) | 101 (6.5%) |
| Missing, N | 229 |
| Final seizure-free | 609 (34.2%) |
| Number of initial ASMs | Range 1-6 (median 1) |
| Sampling time from a recent seizure | |
| <1 week | 608 (39.8%) |
| 1 week~1 month | 539 (35.3 %) |
| >1 month | 379 (24.8%) |
| B | SE | Wald | P value | Exp (B) | |
|---|---|---|---|---|---|
| Sex (Male = 1) | -0.331 | 0.125 | 6.976 | 0.008 | 0.719 |
| Febrile seizure | 0.438 | 0.228 | 3.692 | 0.055 | 1.550 |
| Epileptiform discharge | 0.456 | 0.138 | 10.981 | <0.001 | 1.577 |
| MRI lesion | 0.070 | 0.155 | 0.203 | 0.653 | 1.072 |
| Focal seizure | 0.917 | 0.842 | 1.185 | 0.276 | 2.501 |
| generalized epilepsy | 1.292 | .846 | 2.334 | 0.127 | 3.640 |
| Structural etiology | 0.428 | 0.158 | 7.311 | 0.007 | 1.533 |
| Genetic etiology | -0.680 | 0.504 | 1.823 | 0.177 | 0.507 |
| Hippocampal sclerosis | 0.436 | 0.257 | 2.883 | 0.090 | 1.546 |
| Number of initial ASM | 0.505 | 0.085 | 35.420 | <0.001 | 1.657 |
| Age at first visit | -0.002 | 0.008 | 0.052 | 0.819 | 0.998 |
| Onset age | -0.029 | 0.007 | 17.266 | <0.001 | 0.971 |
| Follow-up duration | 0.002 | 0.002 | 1.000 | 0.317 | 1.002 |
| Constant | -1.680 | 0.898 | 3.494 | 0.062 | 0.186 |
| B | SE | Wald | P value | Exp (B) | |
|---|---|---|---|---|---|
| Clinical parameters | |||||
| Sex (Male = 1) | -0.045 | 0.171 | 0.068 | 0.794 | 0.956 |
| Febrile seizure | 0.511 | 0.246 | 4.301 | 0.038 | 1.666 |
| Epileptiform discharge | 0.538 | 0.153 | 12.340 | <0.001 | 1.712 |
| MRI lesion | 0.008 | 0.174 | 0.002 | 0.963 | 1.008 |
| Focal seizure | 1.654 | 1.191 | 1.929 | 0.165 | 5.229 |
| Generalized epilepsy | 0.925 | 0.943 | 0.962 | 0.327 | 2.522 |
| Structural etiology | 0.562 | 0.178 | 10.016 | 0.002 | 1.755 |
| Genetic etiology | -0.417 | 0.601 | 0.481 | 0.488 | 0.659 |
| Hippocampal sclerosis | 0.400 | 0.280 | 2.039 | 0.153 | 1.492 |
| Number of initial ASM | 0.415 | 0.093 | 19.946 | <0.001 | 1.514 |
| Age at first visit | 0.003 | 0.009 | 0.139 | 0.709 | 1.003 |
| Onset age | -0.026 | 0.008 | 10.428 | 0.001 | 0.975 |
| Follow-up duration | 0.000 | 0.002 | 0.015 | 0.902 | 1.000 |
| Blood parameters | |||||
| WBC | -0.048 | 0.127 | 0.141 | 0.707 | 0.953 |
| Neutrophil | 0.026 | 0.025 | 1.142 | 0.285 | 1.027 |
| Lymphocyte | 0.022 | 0.023 | 0.906 | 0.341 | 1.022 |
| ANC | 0.000 | 0.000 | 0.158 | 0.691 | 1.000 |
| albumin | -0.059 | 0.216 | 0.076 | 0.783 | 0.942 |
| bilirubin | -0.570 | 0.245 | 5.431 | 0.020 | 0.565 |
| AST | 0.000 | 0.003 | 0.003 | 0.956 | 1.000 |
| glucose | -0.002 | 0.002 | 0.571 | 0.450 | 0.998 |
| BUN | -0.026 | 0.019 | 1.874 | 0.171 | 0.974 |
| creatinine | 0.294 | 0.229 | 1.646 | 0.199 | 1.342 |
| Uric acid | -0.118 | 0.046 | 6.540 | 0.011 | 0.888 |
| aPTT | 0.045 | 0.015 | 8.532 | 0.003 | 1.046 |
| fibrinogen | -0.004 | 0.001 | 11.815 | <0.001 | 0.996 |
| constant | -3.705 | 2.626 | 1.991 | 0.158 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





