Submitted:
27 August 2024
Posted:
27 August 2024
You are already at the latest version
Abstract
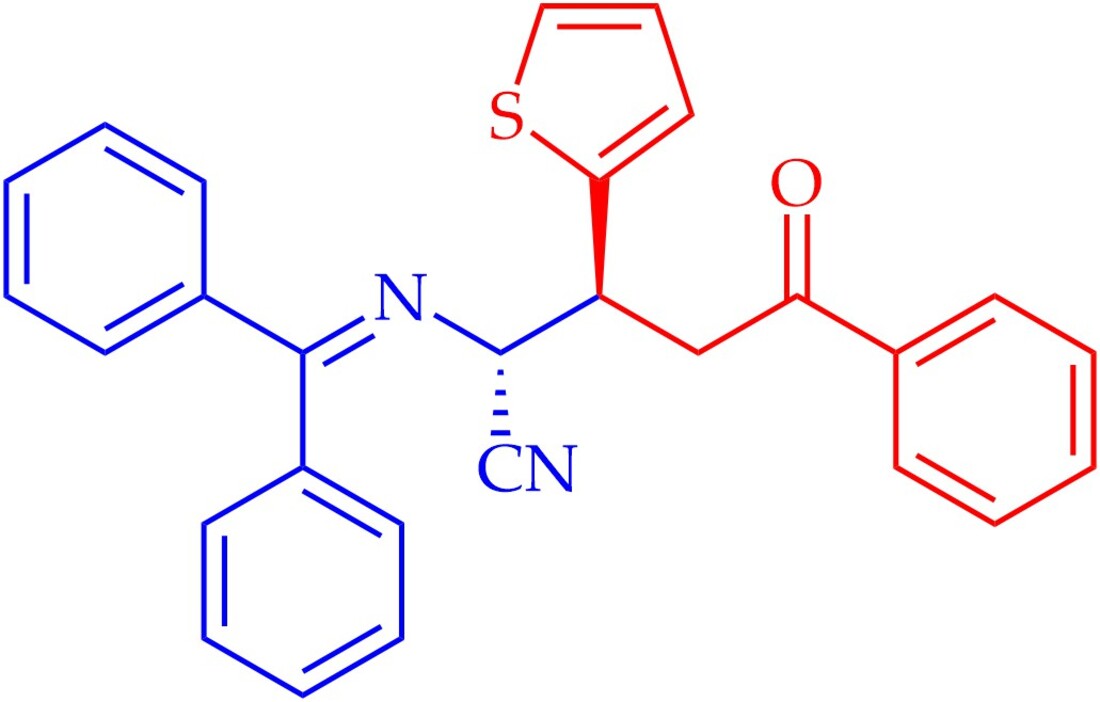
Keywords:
1. Introduction
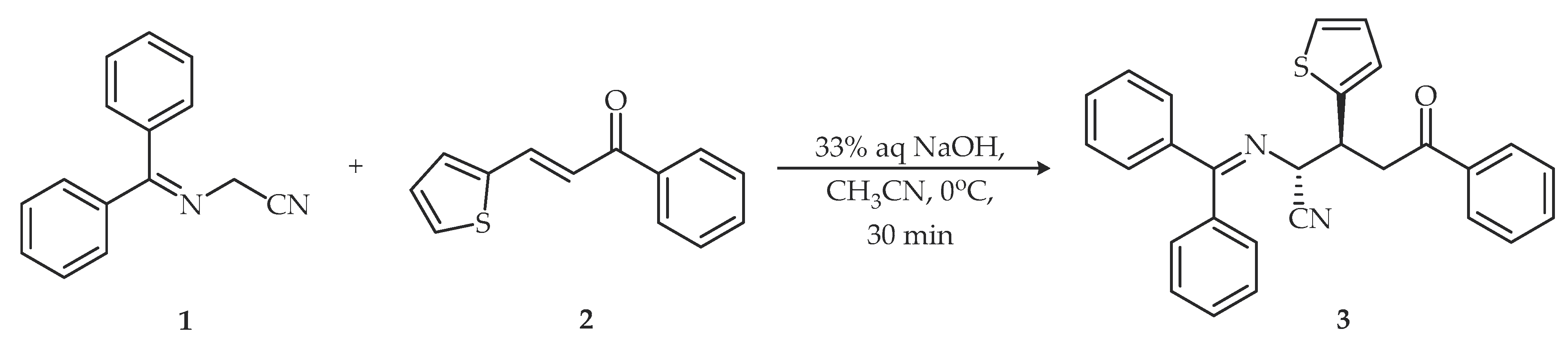
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of rel-(2R,3S)-2-((Diphenylmethylene)amino)-5-oxo-5-phenyl-3-(thiophen-2-yl)pentanenitrile
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunt, S. The Non-Protein Amino Acids. In Chemistry and Biochemistry of the Amino Acids, 1st ed.; Barrett, G.C. Ed.; Chapman and Hall: London, UK, 1985; pp. 55–85.
- O'Donnell, M.J. The Enantioselective Synthesis of α-Amino Acids by Phase-Transfer Catalysis with Achiral Schiff Base Esters. Acc. Chem. Res. 2004, 37, 506–517. [CrossRef]
- Nájera, C.; Sansano, J.M. Catalytic Asymmetric Synthesis of α-Amino Acids. Chem. Rev. 2007, 107, 4584–4671. [CrossRef]
- Shirakava, S.; Maruoka, K. Recent Developments in Asymmetric Phase-Transfer Reactions. Angew. Chem. Int. Ed. 2013, 52, 4312–4348. [CrossRef]
- O’Donnell, M. J.; Polt, R. L. A mild and efficient route to Schiff base derivatives of amino acids. J. Org. Chem. 1982, 47, 2663–2666. [CrossRef]
- Meyer, N.; Werner, F.; Opatz, T. One-Pot Synthesis of Polysubstituted Pyrrolidines from Aminonitriles. Synthesis 2005, 6, 945–956. [CrossRef]
- He, W.; Wang, Q.; Wang, Q.; Zhang, B.; Sun, X.; Zhang, S. Synthesis of Novel Chiral Phase-Transfer Catalysts and Their Application to Asymmetric Synthesis of α-Amino Acid Derivatives. Synlett 2009, 8, 1311–1314. [CrossRef]
- Waser, M.; Gratzer, K.; Herchl, R.; Müller, N. Design, synthesis, and application of tartaric acid derived N-spiroquaternary ammonium salts as chiral phase-transfer catalysts. Org. Biomol. Chem. 2012, 10, 251–254. [CrossRef]
- Schettini, R.; De Riccardis, F.; Della Sala, G.; Izzo, I. Enantioselective Alkylation of Amino Acid Derivatives Promoted by Cyclic Peptoids under Phase-Transfer Conditions. J. Org. Chem. 2016, 81, 2494–2505. [CrossRef]
- Dryanska, V. Phase-Transfer Catalyzed Additions. VI. Reaction of N-Diphenylmethyleneaminoacetonitrile with Aromatic Aldehydes. Synth. Commun. 1990, 20, 1055–1061. [CrossRef]
- Dryanska, V.; Tasheva, D. Phase-transfer catalyzed additions. VII. Preparation of 3-aryl-3-arylamino-2-(N-diphenylmethyleneamino)propanenitriles. Synth. Commun. 1992, 22, 63–71. [CrossRef]
- Ooi, T.; Kameda, M.; Taniguchi, M.; Maruoka, K. Development of Highly Diastereo- and Enantioselective Direct Asymmetric Aldol Reaction of a Glycinate Schiff Base with Aldehydes Catalyzed by Chiral Quaternary Ammonium Salts. J. Am. Chem. Soc. 2004, 126, 9685–9694. [CrossRef]
- Tsuge, O.; Ueno, K.; Kanemasa, S.; Yorozu, K. Michael Addition and Alkylation of 2-Azaallyl Anions Derived from N-(1-Cyanoalkyl)imines, and Stereoselective Cyclization of Imine Esters or Ketones Leading to 1-Pyrrolines. Bull. Chem. Soc. Jpn. 1987, 60, 3347–3358. [CrossRef]
- Meyer, N.; Opatz, T. A Short Synthesis of Polysubstituted Pyrrolidines via α-(Alkylideneamino)nitriles. Synlett 2004, 5, 787–790. [CrossRef]
- Tasheva, D.; Petrova, A.; Simova, S. Convenient Synthesis of Some Substituted 5-Oxonitriles under Aqueous Conditions: Synthesis of 3,4-Dihydro-2H-pyrrole-2-carbonitriles. Synth. Commun. 2007, 37, 3971–3979. [CrossRef]
- Ma, T.; Fu, X.; Kee, C.W.; Zong, L.; Pan, Y.; Huang, K.-W.; Tan, C.-H. Pentanidium-Catalyzed Enantioselective Phase-Transfer Conjugate Addition Reactions. J. Am. Chem. Soc. 2011, 133, 2828–2831. [CrossRef]
- Nie, J.; Hua, M.-Q.; Xiong, H.-Y.; Zheng, Y.; Ma, J.-A. Asymmetric Phase-Transfer-Catalyzed Conjugate Addition of Glycine Imine to Exocyclic α,β-Unsaturated Ketones: Construction of Polycyclic Imines Containing Three Stereocenters. J. Org. Chem. 2012, 77, 4209–4216. [CrossRef]
- Konno, T.; Watanabe, S.; Takahashi, T.; Tokoro, Y.; Fukuzawa, S. Silver/ThioClickFerrophos Complex as an Effective Catalyst for Asymmetric Conjugate Addition of Glycine Imino Ester to Unsaturated Malonates and α-Enones. Org. Lett. 2013, 15, 4418–4421. [CrossRef]
- Timofeeva, D. S.; Ofial, A. R.; Mayr, H. Nucleophilic reactivities of Schiff base derivatives of amino acids. Tetrahedron 2019, 75, 459–463. [CrossRef]
- Lee, H.; Nam, H.; Lee, S.Y. Enantio- and Diastereoselective Variations on α-Iminonitriles: Harnessing Chiral Cyclopropenimine-Thiourea Organocatalysts. J. Am. Chem. Soc. 2024, 146, 3065−3074. [CrossRef]
- Li, J.-T.; Yang, W.-Z.; Wang, S.-X.; Li, S.-H.; Li, T.-S. Improved synthesis of chalcones under ultrasound irradiation. Ultrason. Sonochem. 2002, 9, 237–239. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



