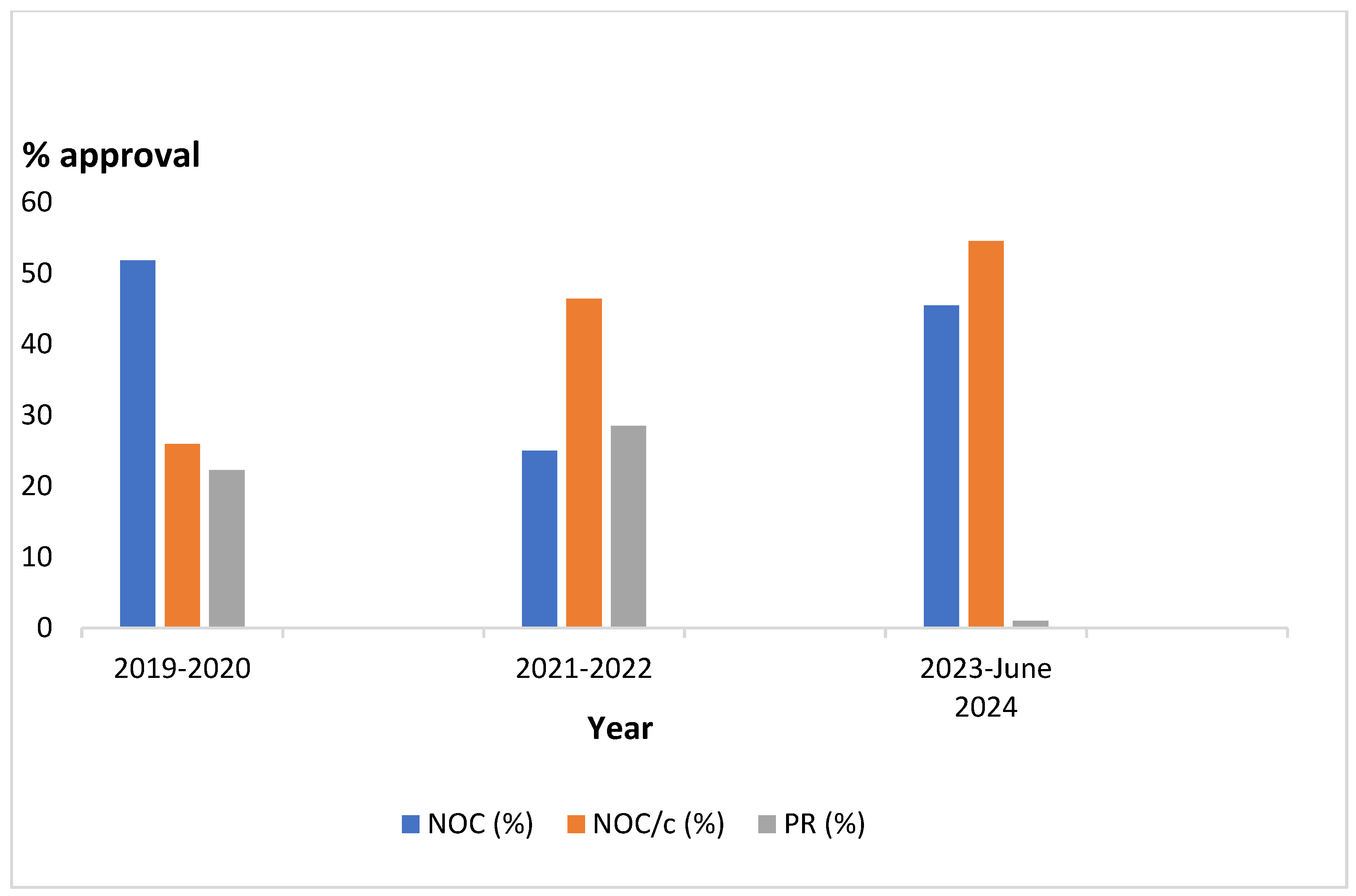1. Introduction
The rapid evolution of targeted therapies for cancer patients in the past decade has put tremendous pressure on regulatory agencies globally to manage critical approval decisions based on early promising data rather than data confirmed by the gold-standard, randomized controlled trial (RCT) [
1,
2,
3]. Novel small molecules, monoclonal antibodies and their antibody-drug-conjugates are powerful tools for targeting proteins responsible for the mutagenesis leading to cancer. In the past decade, targeted therapies have proliferated with now close to 40 for lung cancer and 20 for breast cancer [
4,
5]. Similar to chemotherapy regimens (often a cocktail of toxic and nonspecific chemicals), combinations of targeted therapies are delivering even more impressive results in cancer care [
6].
Instead of being enabling, regulatory and reimbursement platforms have fallen short of aligning with the advancements in biology and innovative treatment strategies [
7,
8,
9]. The US Food and Drug Administration (FDA) was the first to introduce regulatory reforms to accelerate reviews of targeted oncology therapies, including Real Time Oncology Review, fast-track designation and breakthrough designation [
10,
11]. More recently, through the Project Orbis initiative, FDA has been working with global counterparts to enable earlier approvals of these life-saving therapies worldwide [
12].
Several recent publications documented significant delays for cancer patients in accessing these critical targeted therapies in Canada [
7,
8,
13]. Unlike the FDA, which launched regulatory reforms to ensure accelerated approval of new cancer treatments, Health Canada made minor modifications to one of its two existing pathways – Notice of Compliance with Conditions (NOC/c) – to approve new cancer therapies with promising clinical evidence, usually based on a small single-arm trial [
1,
2,
13]. While NOC/c was originally designed to approve rare, life-threatening diseases with no treatment available, it has now been used for the onslaught of targeted oncology therapies, surpassing Notice of Compliance (NOC) approvals for the same drug category [
13]. Until the condition is removed by a confirmatory study (phase III RCT), NOC/c approvals are deemed to carry a high level of uncertainty [
14], which translates to a high percentage of negative reimbursement decisions from health technology assessment (HTA) agencies, including the Canadian Agency for Drugs and Technologies in Health (CADTH) – now being renamed Canada’s Drug Agency (CDA) – and the Institut national excellence en santé et services sociaux (INESSS) (Quebec only).
The next step is the negotiation between manufacturers on pricing and funding conducted between the government drug plans’ pan-Canadian Pharmaceutical Alliance (pCPA) and manufacturers based on a positive recommendation from CADTH or INESSS and a price deduction suggested by the HTA agency [
15]. A negative recommendation denies further negotiation for most therapies. The high percentage of negative decisions, especially for NOC/c drugs, reported in several recent publications is responsible for the significant delay in patient accessibility to treatment in Canada compared to many European countries with similar universal health system structures [
16,
17,
18].
Canada’s complex approval and reimbursement landscape led to substantial delays in cancer patients’ access to appropriate treatments. A recent Targeted Literature Review (TLR) [
7] reveals that important clinical endpoints, such as life years lost, overall survival and progression-free survival are most impacted by cancer patients’ delays in accessing new treatments. Physicians and patients are calling for system sea-changes to save the lives of cancer patients and prevent the deterioration of their quality of life [
13].
This manuscript evaluates data from 2019 to June 2024 to assess any changes or modifications that might suggest different stakeholders are working towards a better solution for Canadian cancer patients.
2. Methods
The assessment targets were the New Active Substances (NAS) listed in the Health Canada submissions under review data completed between 2019 and June 30, 2024 [
19,
20]. Only antineoplastic drugs were recorded and reviewed (
Supplementary Table S1). Corresponding dates of submission to CADTH and dates of recommendations and dates of any pCPA negotiations or decisions not to negotiate were also captured.
Summary basis of decisions (SBD) or Regulatory Decision Summaries (RDS) for all listed products (
Supplementary Table S1) were reviewed [
21] to categorize products into NOC, NOC/c or Priority Reviews (PR).
Section 3 of SBDs was reviewed to assess whether Project ORBIS was part of the review process. Sections 2 (why was the drug approved) and 7 (what are the scientific rationale of Health Canada’s decision) were examined to ascertain whether RWE was used for regulatory decisions based on search criteria with the keywords “real,” “historical,” “history,” “observation,” “natural,” “experience,” “registry,” “world” and “safety” as used in a previous publication [
22]. Five categories of RWE-use were created from the information derived to grade the RWD/E or historical data utilized by Health Canada for regulatory decision-making [
22].
Times in calendar days of reviews by Health Canada, reviews by CADTH and negotiations with pCPA, and time between CADTH recommendation and pCPA decision on whether to negotiate were calculated (data not shown).
RWE information was reviewed in CADTH reports according to the method described previously [
23]. Briefly, the determination was made after a complete review of CADTH review reports that the assessment of fit-for-purpose RWE submitted by sponsors could be found in the clinical and pharmacoeconomic combined reports or the final clinical guidance reports. Section 1.2 (key results and interpretations) and Section 7 (supplemental questions) were reviewed in detail to understand how CADTH critically appraised RWE information submitted by sponsors. CADTH’s comments in its clinical reviews on parameters critical for the validity of RWE were extracted and comparisons were made between positive and negative reimbursement recommendations (
Supplementary Table S2).
3. Results
Health Canada Review of Oncology Products: 2019 to June 2024
Between 2019 and June 2024, 66 oncology drugs were approved by Health Canada.
Table 1 lists the number of oncology drugs approved by year and by approval category. The total number of approvals peaked around 2019 and more products were approved between 2019 to 2021 than 2022 to June 2024.
Figure 1 illustrates 2-year approval intervals showing NOC approvals were consistent over the period with NOC/c approvals increasing rapidly and PR approvals diminishing drastically in 2023 to June 2024.
Health Canada joined the Project Orbis review initiative in 2019 [
24]. The actual joint review process started around mid-year 2019 with the highest focus on NOC/c reviews. All but 2 NOC/c approved on or after June 30, 2021, were part of the Project Orbis review. The time used to review products under Project Orbis (median 277 days; IQ range 236-494 days) is similar to the average review time of NOC/c products from 2019 to June 2024 (median 279 days; IQ range 236-494 days) (
Table 1 and
Supplementary Table S1).
Details of clinical reviews captured in
Section 2 and Section 7 were reviewed to search for the use of RWE in regulatory decision-making. Only 7 reviews mentioned real-world evidence (Mylotarg, Enhertu, Tepmetko, Minjuvi, Pemazyre, Lumakras and Tecartus) (
Supplementary Table S1 and ref. [
22]). All products approved using RWE were approved in 2020 and 2021. No RWE was mentioned in the review of products approved between 2022 to June 2024.
CADTH Review and pCPA Negotiations of Oncology Drugs Approved by Health Canada between 2019-June 2024
Of the 66 new oncology drugs that received approval from Health Canada between 2019 and the end of June 2024: 26 received an NOC, 26 an NOC/c and 14 had a priority review (
Table 1).
All but two NOC drugs were submitted to CATH and two of the 24 submitted are currently being reviewed. Of the remaining 22 drugs, CADTH recommended 18 (81.8%) be reimbursed with or without conditions and/or criteria. No drug was reviewed within CADTH’s “typical timeline” of 180 calendar days, but 77.3% were reviewed within CADTH’s 190 business days (270 calendar days) target (
Table 2). The pCPA’s decision regarding negotiation was made within its stated target of 40 business days (60 calendar days) or less following the reimbursement recommendation for only 22.7% of the drugs. Price negotiations between the pCPA and the drug developer were ongoing for three of the 22 drugs. The pCPA decided not to pursue a negotiation for three of the other 19 medicines, all of which received a negative recommendation from CADTH. Negotiations were completed within the pCPA’s stated target of 90 business days (130 calendar days) or less for 37.5% of the drugs.
Two of the 26 NOC/c drugs were not submitted to CADTH and two were currently being reviewed. Twelve of the 22 drugs (54.5%) with a completed CADTH review received a positive reimbursement recommendation. Few reviews (13.6%) were completed within CADTH’s “typical timeline,” while 59.1% were completed within 270 days. One drug (Epkinly) was reviewed under CADTH’s new time-limited reimbursement recommendation process for NOC/c drugs [
25], which was completed in 199 days – still longer than the claimed “typical timeline.” The pCPA’s decision about whether to negotiate was within its target time for only 11.1% of the NOC/c drugs and completed negotiations were within its target for 54.5% of the negotiated drugs. Remarkably, the pCPA engaged with Epkinly’s developer before CADTH’s recommendation was issued, which it has not done previously.
All the oncology drugs with a priority review were evaluated by CADTH, with 78.6% being reviewed within 270 days; none were reviewed within the agency’s 180-day “typical timeline.” One of the priority review drugs was under consideration for negotiation by the pCPA. The decision regarding negotiation was made within the pCPA’s target for only 7.7% of the medicines. Two negotiations were currently in progress, while 36.4% of the completed negotiations were accomplished within the pCPA’s target for negotiations.
For products approved by Health Canada between 2019 and 2021, 76.7% with completed CADTH reviews were given a positive reimbursement recommendation, while 100% of products approved in 2022 and 2023 received a positive reimbursement recommendation. For NOC/c approvals, 37.5% and 100% of products received a positive reimbursement recommendation respectively.
Sponsors of nine drugs (Piqray, Abecma, Tepmetko, Minjuvi, Pemazyre, Zepzelca, Lumakras, Jemperli and Rybrevant) requested reconsideration of CADTH’s recommendation for major revisions and a procedural review was also requested for Minjuvi and Pemazyre. All but Rybrevant received a final negative recommendation. Reconsideration was requested for major revisions for Rylaze by government drug plans. The impact on the time required for reimbursement reviews due to reconsiderations and procedural reviews is unknown because the extent of any delay is not recorded by CADTH. A re-analysis excluding these drugs did not demonstrate a significant change in the overall results. It is also unknown whether the reconsideration for Rybrevant changed its recommendation from negative to positive. However, based on the final recommendations for the other seven drugs being negative, it appears that reconsiderations and procedural reviews rarely change CADTH’s recommendations.
All oncology drugs that received a positive reimbursement review had a pCPA price negotiation and 82% of the negotiations were successful. In contrast, only three (30%) of the drugs with a negative reimbursement recommendation were negotiated and only two were successful (
Supplementary Table S1).
The use of RWE in CADTH recommendations for NOC/c products approved by Health Canada from 2019 to June 2024 not included in a previous publication [
23] was reviewed (
Supplementary Table S2). Four products Gavreto, Carvykti, Tecvayli and Elrexfio (approved in 2023) with positive reimbursement recommendations received a moderately positive review mentioning that the RWE data were generally consistent with the expectations of clinical experts or better than comparators. Products with do not reimburse recommendations all received negative reviews of the RWE information and two with positive recommendations (Columvi and Epkinly) (
Supplementary Table S2) received negative RWE reviews.
4. Discussion
The availability of new medications to patients is a long and arduous process in Canada. A recent speech by Doug Ford, premier of Ontario summarized it crisply, “Currently, patients in Canada wait almost two years to access life-saving breakthrough medicines, a year longer than in other developed countries, placing us last in the G7” [
26]. For this message to reach the level of a premier who boldly announced that one of his focuses as the chair of the Council of Federation is to ensure Canadian patients have the same timely access to life-saving treatments as patients in the rest of the world are monumental, if this recognition results in positive change. In recent years, evidence supporting delayed access to new and life-saving medications for Canadian patients has become stronger and louder, as witnessed by the rising number of peer-reviewed publications by Canadian and international authors [
16,
27].
Canadian regulatory bodies, HTA organizations and reimbursement agencies are aware of the issues but are deterred by fear that high drug prices could bankrupt the drug budget. These agencies are open to discussion regarding ways to improve drug access. However, resource constraints especially from review divisions, layers of uncertainties with data packages, economic evaluations and value for money hold back decisions to make new and life-prolonging medications available to Canadian patients [
27,
28].
The arrival of a tsunami of targeted therapies offering potent efficacies against cancer and with better safety profiles is not unexpected. The FDA realized that waiting for definitive results, such as overall survival or progression-free survival from multi-year-long randomized clinical studies, deprive cancer patients of life-saving treatments. The FDA will approve targeted oncology drugs with novel mechanisms of action after a single-arm trial and, in some cases, supported by historical data or RWE in comparable patient populations [
29]. Health Canada decided to use the existing NOC/c pathway to provide access to these promising new therapies. Confirmatory studies aligned with the indications have to be ongoing for this conditional approval to be granted [
2,
14]. Health Canada also issued two guidance policies for the use of RWE in regulatory decision-making in April 2019 [
30,
31] focusing on requirements for regulatory approvals. A study conducted to evaluate the use of RWE by Health Canada in the approval of oncology and rare disease drugs showed that Health Canada used much less RWE in regulatory decision-making than the FDA and EMEA [
22] for approvals between 2020 to 2021. Of the 29 oncology drugs approved, only 7 (2 in 2020 and 5 in 2021) mentioned RWE. The current study evaluated the use of RWE in oncology drug approvals from 2019 to June 2024 and compared to the previous publication [
22], only one more drug approved in 2021 was reviewed using RWE historical data. None of the drugs approved between 2022 to June 2024 mentioned the use of RWE as part of the regulatory decision-making. This can potentially put Health Canada out of step with the FDA, which has been incorporating more RWE into regulatory decision-making, especially for validating whether an “external control arm” can serve as the comparator arm of a single-arm trial. The FDA is exploring whether using a well-validated external control arm with the treatment arm could yield results comparable to RCTs [
29].
Health Canada has been endorsing and collaborating with CADTH on RWE initiatives [
32]. CADTH was active both in revising the RWE guidance document and engaging stakeholders in panel discussions on how to incorporate RWE into CADTH submissions. The guidance document was finalized in 2023 [
33] had a long stakeholder consultation period, suggesting that CADTH is actively interested in improving the review of RWE submitted to them. The current study also evaluated CADTH review reports for the use of RWE and found that instead of providing the same negative reviews of RWE for all drugs regardless of the recommendations [
23], at least 4 drugs reviewed between 2022-2023 received positive appraisals regarding the provided RWE information. Although some drugs with positive recommendations still received negative comments on submitted RWE, this is a step in the positive direction (
Supplementary Table S2). More recently, CADTH published a report from its time-limited Industry Task Force including a summary report from the Post Marketing Drug Evaluation (PMDE) committee, a multi-stakeholder panel advising on the best collaborative approach for industry and HTA to incorporate RWE into PMDE [
34]. Several feasibility initiatives will be launched, and longer-term data will be needed to assess the value of these programs.
For oncology products approved between 2019 to June 2024, review times by CADTH and pCPA and time to start negotiation by pCPA showed inadequate alignment with the agencies’ stated targets. While some delays could originate from the sponsors, online accessible times in review are not detailed enough to reveal where the delays took place. Sponsors of several drugs requested CADTH’s reconsideration of negative decisions. It is not clear whether reconsiderations impacted CADTH timelines, but a reanalysis of CADTH review times did not show significant differences with or without these products. The time required for Epkinly which received a positive recommendation under the CADTH new time-limited reimbursement pathway took 199 days, closer to the target of 190 days, and if sustainable and governments list the drugs quickly will enable cancer patients to receive their treatments promptly.
5. Conclusions
While the availability of new treatments to cancer patients is still a challenge in Canada, collaboration among key stakeholders, revising current regulatory platforms and encouraging openness to accepting new data formats will be critical parameters for long-term benefits of cancer patients.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org.
References
- Agrawal, S.; Arora, S.; Amiri-Kordestani, L.; Fashoyin-Aje, L.; Gormley, N.; Kim, T.; Lemery, S.; Mehta, G.M.; Scott, E.C.; Singh, H; Tang, S.; Theoret, M.R.; Pazdur, R.; Kluetz, P.G.; Beaver, J.A. Use of single-arm trials for US Food and Drug Administration drug approval in oncology, 2002-2021. JAMA Oncol. 2023, 9, 266-272. [CrossRef]
- Martin, A.; Hunt, M.; Blommaert, S.; Udayakumar, S.; Lu, B.; Chatterjee, S.; Sathiyabalan, G., Brun, J.; Kampman, M.; Lambert, L.; Mittmann, N.; Robinson, K.; Chan, K.K. Oncology drug approvals under Health Canada’s Notice of Compliance with Conditions policy: a retrospective cohort analysis. Can. J. Health Technol. 2024, 4. https://canjhealthtechnol.ca/index.php/cjht/article/view/MG0024/MG0024 (accessed on 20 Aug 2024). [CrossRef]
- Mishra-Kalyani, P.S.; Amiri Kordestani, L.; Riveral, D.R.; Concato, R.; Pazdur, R.; Beaver, J.A. External control arm in oncology: current use and future directions. Ann. Oncol. 2022, 33, 376-383. [CrossRef]
- Targeted therapy to treat cancer. National Cancer Institute, 2022. https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies (accessed on 20 Aug 2024).
- Aggarwal, D.; Yang, J.; Abdus Salam, M; Sengupta, S.; Al-Amin, Y.; Mustafa, S.; Aasif Khan, M.; Huang, X.; Singh Pawar, J. Antibody-drug conjugates: the paradigm shifts in the targeted cancer therapy. Front. Immunol. 2023, 14, 1203073. [CrossRef]
- Oncology regulatory expertise and early guidance (OREEG). Food and Drug Administration, 2024. https://www.fda.gov/about-fda/oncology-center-excellence/oncology-regulatory-expertise-and-early-guidance-oreeg (accessed on 20 Aug 2024).
- Sehdev, S.; Gotfrit, J.; Elias, M.; Stein, B.D. Impact of systemic delays for patient access to oncology drugs on clinical, economic, and quality of life outcomes in Canada: a call to action. Curr. Oncol. 2024, 31, 1460–1469. [CrossRef]
- Rawson, N.S.B.; Stewart, D.J. Timeliness of health technology assessments and price negotiations for oncology drugs in Canada. Clinicoecon. Outcomes Res. 2024, 16, 437–445. [CrossRef]
- Post, H.C.; Schutte, T.; van Oijen M.G.H.; van Laarhoven, H.W.M.; Hollak, C.E.M. Time to reimbursement of novel anticancer drugs in Europe: a case study of seven European countries. ESMO Open. 2023, 8, 101208. [CrossRef]
- Fast track, breakthrough therapy, accelerated approval, priority review. Food and Drug Administration, 2023. https://www.fda.gov/patients/learn-about-drug-and-device-approvals/fast-track-breakthrough-therapy-accelerated-approval-priority-review (accessed on 20 Aug 2024).
- Real-time oncology review. Food and Drug Administration, 2023. https://www.fda.gov/about-fda/oncology-center-excellence/real-time-oncology-review (accessed on 20 Aug 2024).
- Project Orbis. Food and Drug Administration, 2024. https://www.fda.gov/about-fda/oncology-center-excellence/project-orbis (accessed on 20 Aug 2024).
- Sehdev, S.R.; Rawson, N.S.B.; Aseyev, O.J.; Buick, C.J.; Butler, M.O.; Edwards, S.; Gill, S.; Gotfrit, J.M.; Hsia, C.C.; Juergens, R.A.; Manna, M.; McCarthy, J.S.; Mukherjee, S.D.; Snow, S.L.; Spadafora, S.; Stewart, D.J.; Wentzell, J.R.; Wong, R.P.W.; Zalewski, P.G. Access to oncology medicines in Canada: consensus forum for recommendations for improvement Curr. Oncol. 2024, 31, 1803-1816.
- Notice of Compliance with Conditions. Health Canada, 2024. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/notice-compliance/conditions.html (accessed on 20 Aug 2024).
- About pCPA. Pan-Canadian Pharmaceutical Alliance, 2024. https://www.pcpacanada.ca/about (accessed on 20 Aug 2024).
- Gotfrit, J.; Shin, J.J.W.; Stewart, D.J.; Mallick, R.; Wheatley-Price, P. Determinants of the cancer drug funding process in Canada. Curr. Oncol. 2022, 29, 1997–2007. [CrossRef]
- Hoskyn, S.L. Patient access to new medicines in Canada: an international comparison of launch and public reimbursement performance. Innovative Medicines Canada, 2019. https://innovativemedicines.ca/wp-content/uploads/2019/04/2019-CADTH-Poster-EN-1.pdf (accessed on 20 Aug 2024).
- Bartol, A.; Dressler, K.; Kaskel, P.; Landsberg, C.; Lechner, C.; Petschulies, M. Ten years of AMNOG from an oncological perspective: new horizons and continuing expansion. J. Cancer Res. Clin. Oncol. 2022, 149, 2637–2645. [CrossRef]
- Drug and health product submissions under review (SUR). Health Canada, 2024. https://www.canada.ca/en/health-canada/services/drug-health-product-review-approval/submissions-under-review.html#a1 (accessed on 20 Aug 2024).
- Drug and health product submissions under review (SUR): new drug submissions under review. Health Canada, 2024. https://www.canada.ca/en/health-canada/services/drug-health-product-review-approval/submissions-under-review/new-drug-submissions-under-review.html (accessed on 20 Aug 2024).
- Summary basis of decision (SBD). Health Canada, 2024. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/summary-basis-decision.html (accessed on 20 Aug 2024).
- Lau, C.Y.; Jamali, F.; Loebenberg, R. Health Canada usage of real world evidence (RWE) in regulatory decision making compared with FDA/EMA usage based on publicly available information. J. Pharm. Pharm. Sci. 2022, 25, 227-236. [CrossRef]
- Lau, CY.; Dranitsaris, G.; Impact of regulatory approval status on CADTH reimbursement of oncology drugs and role of real-world evidence on conditional approvals from 2019 to 2021. Curr. Oncol. 2022, 29, 8031–8042.
- Project Orbis. Health Canada, 2022. https://www.canada.ca/en/health-canada/services/drugs-health-products/international-activities/project-orbis.html (accessed on 20 Aug 2024).
- CADTH’s Time-Limited Reimbursement category aims to support earlier access to promising drugs. Canada’s Drug Agency, 2024. https://www.cda-amc.ca/news/cadths-time-limited-recommendation-category-aims-support-earlier-access-promising-drug (accessed on 20 Aug 2024).
- Rushowy, K. Ford to focus on medication approval at premier’s conference. Toronto Star, 2024 July 14. https://www.thestar.com/politics/doug-ford-to-focus-on-getting-medications-approved-faster-at-annual-premiers-conference/article_5e8af906-3ef1-11ef-980e-5fedf2bc45f7.amp.html (accessed on 20 Aug 2024).
- Binder, L.; Ghadban, M.; Sit, C.; Barnard, K. Health technology assessment process for oncology drugs: impact of CADTH changes on public payer reimbursement recommendations. Curr. Oncol. 2022, 29, 1514-1526. [CrossRef]
- Real-world evidence and health technology assessment: past, present and future. Canada’s Drug Agency, 2024. https://www.cda-amc.ca/real-world-evidence-and-health-technology-assessment-past-present-and-future (accessed on 20 Aug 2024).
- Jahanshahi, M; Gregg, K.; Davis, G.; Ndu, A.; Miller, V.; Vockley, J.; Ollivier, C.; Franolic, T.; Sakai, S. The use of external controls in FDA regulatory decision making. Ther. Innov. Regul. Sci. 2021, 55, 1019-1035. [CrossRef]
- Optimizing the use of real world evidence to inform regulatory decision-making. Health Canada, 2019. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/announcements/optimizing-real-world-evidence-regulatory-decisions.html (accessed on 20 Aug 2024).
- Elements of real world data/evidence quality throughout the prescription drug product life cycle. Health Canada, 2019. https://www.canada.ca/en/services/health/publications/drugs-health-products/real-world-data-evidence-drug-lifecycle-report.html (accessed on 20 Aug 2024).
- Health Canada’s position on the CADTH Guidance for Reporting RWE to Support Decision-making. Health Canada, 2023. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/announcements/health-canada-position-guidance-reporting-real-world-evidence-supporting-decision-making.html (accessed on 20 Aug 2024).
- Guidance for reporting real-world evidence. Canada’s Drug Agency, 2023. https://www.cda-amc.ca/guidance-reporting-real-world-evidence (accessed on 20 Aug 2024).
- Canada’s Drug Agency taking steps to expand access to real-world evidence. Canada’s Drug Agency, 2024. https://www.cda-amc.ca/news/canadas-drug-agency-taking-steps-expand-access-real-world-evidence (accessed on 20 Aug 2024).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).