Submitted:
27 August 2024
Posted:
28 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Quantum Chemical Computations
3. Discussion
3.1. Ionisation Tolerance and Electronic Excitation
3.2. Higher Electronic Multiplicity
4. Conclusion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pinzbach, H et al. Gas-phase production and photoelectron spectroscopy of the smallest fullerene, C20. Nature 2000, 407, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Jarrold, Martin F. The smallest fullerene. Nature 2000, 407, 26–27. [Google Scholar] [CrossRef]
- Wang, Zhenxia et al. A new carbon solid made of the world’s smallest caged fullerene C20. Physics Letters A 2001, 280, 351–356. [Google Scholar] [CrossRef]
- Iqbal, Z et al. Evidence for a solid phase of dodecahedral C20. Eur. Phys. J. B 2003, 31, 509–515. [Google Scholar] [CrossRef]
- Torrisi, L. , Torrisi, A., Cutroneo, M. Fullerene C20 carbon nucleation induced by IR ns pulsed laser-generated plasma. FNCN 2024, 32, 783–790. [Google Scholar]
- Parker, Q.A. , Bojicic, I.S., Frew, D. HASH: the Hong Kong/AAO/Strasbourg Hα planetary nebula database. J. Phys.: Conf. Ser 2016, 728, 032008. [Google Scholar]
- Kwok, S. The mystery of unidentified infrared emission bands. Astrophys. Space. Sci 2022, 367, 16. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, F. , García-Hernández, D. A., Manchado, Arturo. Petroleum, coal and other organics in space. Astrophys. Space. Sci 2020, 365, 81. [Google Scholar] [CrossRef]
- Wood, P. The discovery of cosmic fullerenes. Nat Astron 2020, 4, 299–305. [Google Scholar] [CrossRef]
- Cami, J et al. Detection of C60 and C70 in a Young Planetary Nebula. Science 2010, 329, 1180–1182. [Google Scholar] [CrossRef]
- Zhang, Y. , Sadjadi, SA., Hsia, C-H. Hydrogenated fullerenes (fulleranes) in space. Astrophys. Space. Sci 2020, 365, 67. [Google Scholar] [CrossRef]
- Diaz-Luis, J.J. A search for hydrogenated fullerenes in fullerene-containing planetary nebulae. J. Phys.: Conf. Ser 2016, 728, 052005. [Google Scholar]
- Adjizian, Jean-Joseph et al. Ab initio infrared vibrational modes for neutral and charged small fullerenes (C20, C24, C26, C28, C30 and C60). Phil. Trans. R. Soc. A 2016, 374, 201550323. [Google Scholar]
- Castro, A et al. Can optical spectroscopy directly elucidate the ground state of C20? J. Chem. Phys 2002, 116, 1930–1933. [Google Scholar] [CrossRef]
- Sadjadi, SA et al. A theoretical investigation of the possible detection of C24 in space. FNCN 2020, 28, 637–641. [Google Scholar]
- McGuire, Brett A. 2021 Census of Interstellar, Circumstellar, Extragalactic, Protoplanetary Disk, and Exoplanetary Molecules. ApJS 2022, 29, 30. [Google Scholar]
- Chan, B et al. From C60 to Infinity: Large-Scale Quantum Chemistry Calculations of the Heats of Formation of Higher Fullerenes. J. Am. Chem. Soc. 2016, 138, 1420–1429. [Google Scholar] [CrossRef] [PubMed]
- Chan, B. Fullerene Thermochemical Stability: Accurate Heats of Formation for Small Fullerenes, the Importance of Structural Deformation on Reactivity, and the Special Stability of C60. J. Phys. Chem. A 2020, 124, 6688–6698. [Google Scholar] [CrossRef]
- Marcos, P.A. Simulating the thermal stability and phase changes of small carbon clusters and fullerenes. Eur. Phys. J. D 1999, 6, 221–233. [Google Scholar] [CrossRef]
- Davydov, I. V. , Podlivaev, A. I., Openov, L. A. Anomalous thermal stability of metastable C20 fullerene. Physics of the Solid State 2005, 47, 778–784. [Google Scholar] [CrossRef]
- Bhardwaj, V. R, Corkum, P. B., Rayner, D. M. Internal Laser-Induced Dipole Force at Work in C60 molecule. Phys. Rev. Lett 2003, 91, 203004. [Google Scholar] [CrossRef] [PubMed]
- Sadjadi, SA. , Parker, Q. A. It remains a cage: Ionization tolerance of C60 fullerene in planetary nublae. FNCN 2021, 29, 620–625. [Google Scholar]
- Lin, Fei et al. C20, theSmallest Fullerene. In Handbook of Nanophysics: Clusters and Fullerenes; Sattler, Klaus D., Ed.; Publishing House: Boka Raton, US, 2010; pp. 552–563. [Google Scholar]
- Granovsky, Alex A. Firefly version 8.2.0. Availableonline: URL (http://classic.chem.msu.su/gran/firefly/ index.html).
- Schmidt, M. W et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Keith, Todd A. TK Gristmill Software, Overland Park KS, USA. URL (aim.tkgristmill.com).
- Xu, Yu-He et al. An all-metal fullerene: [K@Au12Sb20]5-. Science 2023, 382, 840–843. [Google Scholar] [CrossRef] [PubMed]
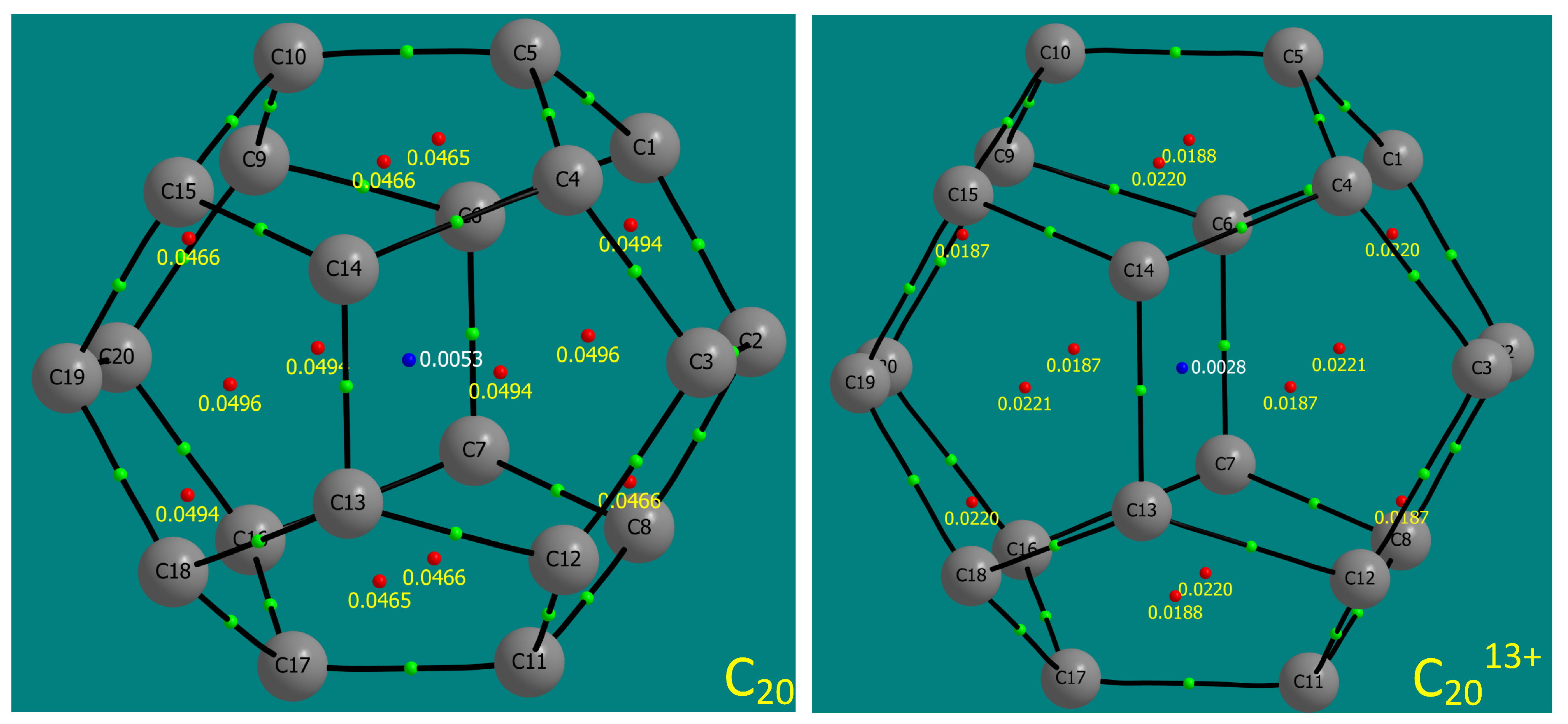
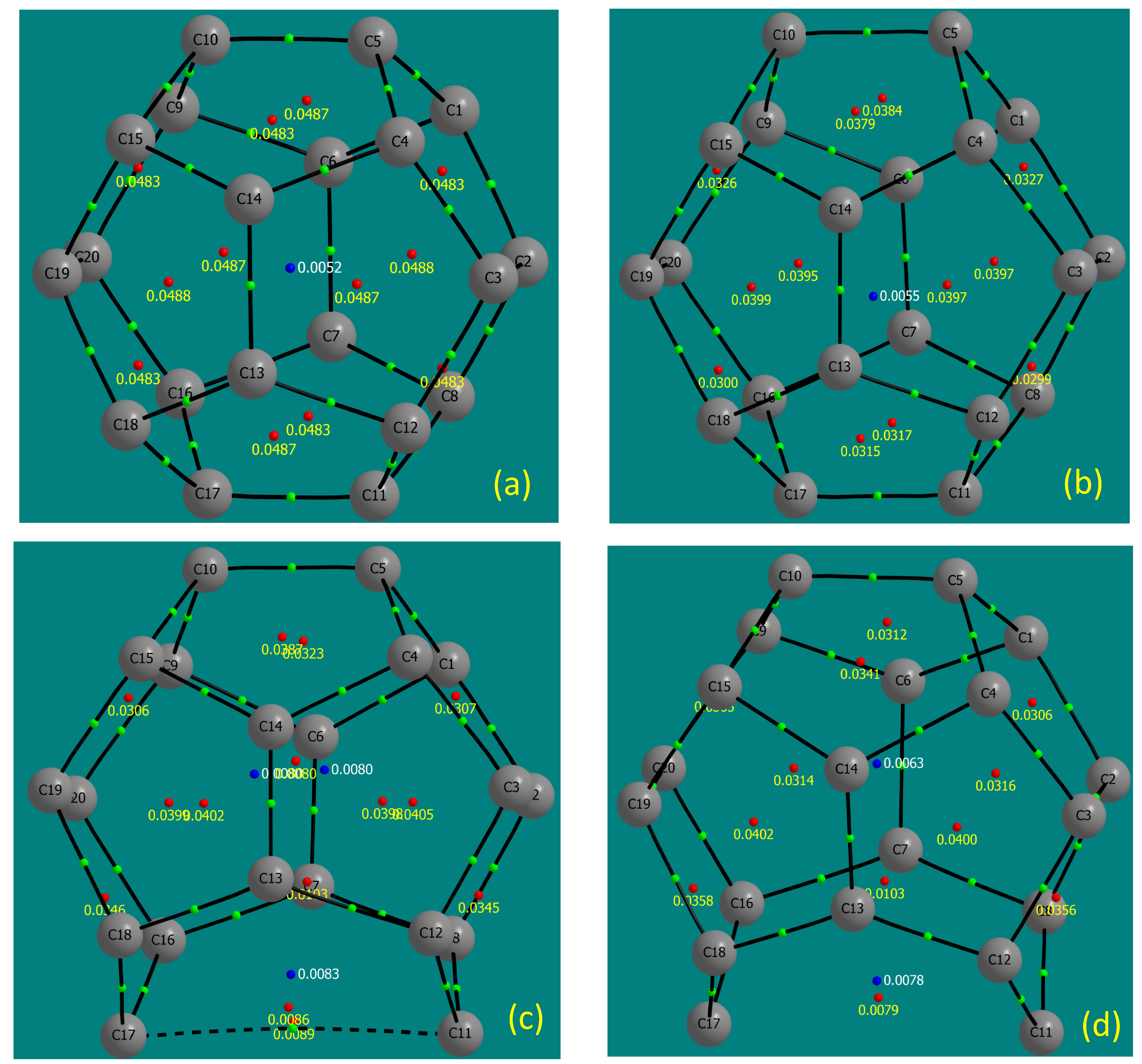
| Species | Electronic Multiplicity | Dipole Moment (Debye) | Lowest unscaled frequency (cm) |
|---|---|---|---|
| C | 1 (s)1 | 0.000319 | 158.33 |
| C | 2 (d)1 | 0.000126 | 175.23 |
| C | 1 | 0.001072 | 526.73 |
| C | 2 | 0.000679 | 373.11 |
| C | 1 | 0.001060 | 448.18 |
| C | 2 | 0.000092 | 215.31 |
| C | 1 | 0.000107 | 216.82 |
| C | 2 | 0.000318 | 320.59 |
| C | 1 | 0.000697 | 90.43 |
| C | 2 | 0.000588 | 283.19 |
| C | 1 | 0.000186 | 326.28 |
| C | 2 | 0.000230 | 311.93 |
| C | 1 | 0.000130 | 324.16 |
| C | 2 | 0.000527 | 80.99 |
| Species | Electronic Multiplicity | Dipole Moment (Debye) | Lowest unscaled frequency (cm) | Energy difference to lower multiplicity state (kcal/mol)1 |
|---|---|---|---|---|
| C | 3 (t)2 | 0.000152 | 254.56 | 2.64 |
| C | 4 (qr)2 | 0.000937 | 299.29 | 40.59 |
| C | 3 | 0.000353 | 396.04 | 39.98 |
| C | 4 | 0.000734 | 311.31 | 31.01 |
| C | 3 | 0.000073 | 311.46 | -2.05 |
| C | 4 | 0.000161 | 333.78 | -3.32 |
| C | 3 | 0.000269 | 160.93 | -1.61 |
| C | 4 | 0.000815 | 215.47 | -2.46 |
| C | 3 | 0.000185 | 227.84 | -1.47 |
| C | 4 | 0.000294 | 316.46 | -7.68 |
| C | 3 | 0.000469 | 247.25 | -1.34 |
| C | 4 | 0.000393 | 226.00 | 35.94 |
| C | 3 | 0.000199 | 200.39 | 36.28 |
| C | 4 | 0.000118 | 151.37 | 26.27 |
| step | Electronic Multiplicity | IP (eV)2 | (nm) | spectrum region |
|---|---|---|---|---|
| C→C | s→d | 7.343 | 168.98 | vacuum uv |
| C→C | d→s | 12.02 | 103.13 | vacuum uv |
| C→C | s→d | 18.88 | 65.66 | extreme uv |
| C→C | d→t | 23.55 | 52.64 | extreme uv |
| C→C | d→s | 23.64 | 52.44 | extreme uv |
| C→C | t→qr | 28.45 | 43.59 | extreme uv |
| C→C | s→d | 28.50 | 43.50 | extreme uv |
| C→C | qr→t | 33.42 | 37.10 | extreme uv |
| C→C | d→s | 33.34 | 37.19 | extreme uv |
| C→C | t→qr | 38.05 | 32.59 | extreme uv |
| C→C | s→d | 38.08 | 32.55 | extreme uv |
| C→C | qr→t | 42.94 | 28.88 | extreme uv |
| C→C | d→s | 42.89 | 28.91 | extreme uv |
| C→C | t→qr | 47.23 | 26.55 | extreme uv |
| C→C | s→d | 47.50 | 26.10 | extreme uv |
| C→C | qr→t | 52.31 | 23.70 | extreme uv |
| C→C | d→s | 52.03 | 23.83 | extreme uv |
| C→C | t→d | 56.54 | 21.93 | extreme uv |
| C→C | s→d | 56.48 | 21.95 | extreme uv |
| C→C | d→s | 60.52 | 20.49 | extreme uv |
| C→C | s→d | 65.99 | 18.79 | extreme uv |
| Species | Unpaired Electrons | Electronic Multiplicity | PG 1 | Dipole Moment (Debye) | Lowest unscaled frequency (cm) | Energy difference to singlet state (kcal/mol)2 | Energy difference to singlet state (eV)2 |
|---|---|---|---|---|---|---|---|
| C | 4 | 5 | C | 0.000256 | 286.57 | 37.87 | 1.64 |
| 6 | 7 | C | 0.000023 | 303.66 | 83.29 | 3.61 | |
| 8 | 9 | C | 0.000681 | 311.33 | 137.86 | 5.98 | |
| 10 | 11 | C | 0.137266 | 94.47 | 187.80 | 8.14 | |
| 12 | 13 | C | 0.006378 | 332.16 | 237.11 | 10.28 | |
| 14 | 15 | C | 0.245907 | 185.37 | 344.60 | 14.94 | |
| 16 | 17 | C | 0.792567 | 290.08 | 452.92 | 19.64 | |
| 18 | 19 | C | 0.591260 | 228.76 | 521.17 | 22.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





