Submitted:
27 August 2024
Posted:
28 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Soybean Crop Harvest
2.2. Protein Analysis
2.3. ‘Free’ Amino Acid Analysis
2.4. Fatty Acid Analysis
2.5. Elemental Analysis
2.6. Iron Oxidation State Analysis
2.7. Phytic Acid Analysis
2.8. Statistical Analysis
3. Results
4. Discussion
Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soybeans: chemistry, production, processing, and utilization; Edited by Johnson, L.A.; White P.J.; Galloway, R. AOCS Press, Urbana, Illinois, USA, 2008.
- Michelfelder, A.J. Soy: A complete source of protein. American Family Physician, 2009, 79(1), 43–47.
- Living, O. Enjoy soy: Plant protein complete with all 9 essential amino acids. HuffPost. 2015. Available online: https://www.huffpost.com/entry/enjoy-soy-plant-protein-c_b_6509712 (accessed on 18 July 2024).
- Thrane, M.; Paulsen, P.V.; Orcutt, M.W.; Krieger, T.M. Soy protein: Impacts, production, and applications (Ch. 2). in Sustainable Protein Sources, (Edited by Nadathur, S.R.; Wanasundara, J.P.D.; L. Scanlin), Academic Press, Cambridge, MA, USA, 2017, p. 23-45.
- Messina, M.J. Legumes and soybeans: Overview of their nutritional profiles and health effects. American Journal of Clinical Nutrition 1999, 70, 439s–450s. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Baroni, L. Soy, soy foods and their role in vegetarian diets. Nutrients 2018, 10(1), 43. [Google Scholar] [CrossRef]
- “Global soybean production forecast to reach record levels in 2023/2024.” in Oils and Fats International News, Global soyabean production forecast to reach record levels in 2023/24 (ofimagazine.com) (accessed on 20 July 2024).
- Lernoud, J.; Potts, J.; Sampson, G.; Schlatter, B.; Huppe, G.; Voora, V.; Willer, H.; Wozniak, J.; Dang, D. The state of sustainable markets 2018: Statistics and emerging trends. 2018, International Trade Centre., Geneva, Switzerland, http://www. intracen.org/uploadedFiles/intracenorg/Content/Publications/Sustainibility%202018%20layout-FIN-web2.pdf (accessed 17 July 2024).
- Meier, C.; Sampson, G.; Larrea, C.; Schlatter, B.; Voora, V.; Dang, D.; Bermudez, S.; Wozniak, J.; Willer, H. The State of Sustainable Markets 2020: Statistics and emerging trends. 2020, International Trade Centre, Geneva, Switzerland. Available online: https://api.pageplace.de/preview/DT0400.9789210054065_A42152460/preview-9789210054065_A42152460.pdf (accessed on 17 July 2024).
- United Nations. DESA Report 2017. World Population Prospects; United Nations: New York, NY, USA, 2017. [Google Scholar]
- Baldwin, I.T.; Benning, C.; Burke, A.; Caicedo, A.; Carpita, N.; Dilworth, M.; Horsch, R.; Kutchan, T.; Last, R.; Mackenzie, S.; et al.; Unleashing a Decade of Innovation in Plant Science—A Vision for 2015–2025 Plant Science Research Summit Held 2013 at the Howard Hughe Medical Institute and Sponsored by the American Society of Plant Biology the Howard Hughes Medical Institute, the National Science Foundation, the U.S. Department of Agriculture, and the U.S. Department of Energy. Available online: https://aspb.org/wp-content/uploads/2016/05/plantsciencedecadalvision10-18-13.pdf (accessed on 4 March 2024).
- Transparency Market Research. Soybean market size, share, trends, growth, export value, volume & trade, sales, pricing forecast. 2018. Available online: https://www.transparencymarketresearch.com/soybean-market.html (accessed on 17 July 2024).
- Ritchie, H.; Roser, M. Meat and seafood production & consumption. Our World in Data. 2018. Available online: https://ourworldindata.org/meat-and-seafood-production-consumption (accessed on 17 July 2024).
- Transparency Market Research. Food & beverages (Section 7.2.1). In Soybean Market. 2017, p. 235. Available online: https://www.transparencymarketresearch.com/soybean-market.html (accessed on 18 July 2024).
- Chatham House. Agricultural commodity supply chains: Trade, consumption and deforestation. 2016. Available online: https://www.chathamhouse.org/publication/agricultural-commodity-supply-chains-trade-consumption-and-deforestation (accessed on 15 July 2024).
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects, and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- da Silva Porto, F.G.; Campos, A.D.; Garcia, I.T.S. Distilled pyroligneous liquor obtained from Eucalyptus grandis and chitosan: Physicochemical properties of the solution and films. Environ. Sci. Pollut. Res. 2019, 26, 672–683. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Z.; Meki, K.; Wang, X.; Liu, B.; Zheng, H.; You, X.; Li, F. Effect of co-application of wood vinegar and biochar on seed germination and seedling growth. J. Soils Sediments 2019, 19, 3934–3944. [Google Scholar] [CrossRef]
- Cândido, N.R.; Duarte Pasa, V.M.; de Oliveira Vilela, A.; Campos, A.D.; de Fátima, A.; Modolo, L.V. Understanding the multifunctionality of pyroligneous acid from waste biomass and the potential applications in agriculture. Sci. Total Environ. 2023, 881, 163519. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.P.; Ku, C.S. Pyrolysis GC-MS analysis of tars formed during the aging of wood and bamboo crude vinegars. J. Wood Sci. 2009, 56, 47–52. [Google Scholar] [CrossRef]
- Mungkunkamchao, T.; Kesmala, T.; Pimratch, S.; Toomsan, B.; Jothityangkoon, D. Wood vinegar and fermented bioextracts: Natural products to enhance growth and yield of tomato (Solanum lycopersicum L.). Sci. Hortic. 2013, 154, 66–72. [Google Scholar] [CrossRef]
- Ofoe, R.; Gunupuru, L.R.; Qin, D.; Thomas, R.H.; Abbey, L. Pyroligneous acid increases productivity and nutritional quality of greenhouse tomato. Plants 2022, 11, 1650. [Google Scholar] [CrossRef]
- Ofoe, R.; Mousavi, S.M.N.; Thomas, R.H.; Lord, A. Foliar application of pyroligneous acid acts synergistically with fertilizer to improve the productivity and phytochemical properties of greenhouse-grown tomato. Sci. Rep. 2024, 14, 1934. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, J.; Luo, T.; Zhang, K.; Khan, Z.; Zhou, Y.; Cheng, T.; Yuan, B.; Peng, X.; Hu, L. Wood vinegar impact on the growth and low-temperature tolerance of rapeseed seedlings. Agronomy 2022, 12, 2453. [Google Scholar] [CrossRef]
- Zeng, L.; Sun, X.; Zhou, W.; Li, J.; Guo, Y.; Liu, X.; Cui, D. Combined treatment of a pyroligneous solution and soluble calcium enhances cotton growth through improving soil quality in saline-alkali soils. J. Soil Sci. Plant Nutr. 2022, 22, 25–35. [Google Scholar] [CrossRef]
- Fedeli, R.; Vannini, A.; Guarnieri, M.; Monaci, F.; Loppi, S. Bio-based solutions for agriculture: Foliar application of wood distillate alone and in combination with other plant-derived corroborants results in different effects on lettuce (Lactuca sativa L.). Biology 2022, 11, 404. [Google Scholar] [CrossRef]
- Noel, R.; Schueller, M.J.; Ferrieri, R.A. Radiocarbon flux measurements provide insight into why a pyroligneous acid product stimulates plant growth. Int. J. Mol. Sci. 2024, 25, 4207. [Google Scholar] [CrossRef]
- Wu, X.; Xiong, E.; Wang, W.; Scali, M.; Cresti, M. Universal sample preparation method integrating trichloroacetic acid/acetone precipitation with phenol extraction for crop proteomic analysis. Nature Protocols 2014, 9, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Qu, W.; Robert, C.A.M.; Erb, M.; Hibbard, B.E.; Paven, M.; Gleede, T. Dynamic precision phenotyping reveals mechanisms of crop tolerance to root herbivory. Plant Physiology 2016, 172, 776–788. [Google Scholar] [CrossRef]
- Ichihara, K.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. Journal of Lipid Research 2010, 51, 635–640. [Google Scholar] [CrossRef]
- Atkins, R.C. Colorimetric determination of iron in vitamin supplement tablets. A general chemistry experiment. J Chem. Educ. 1975, 52(8), 550. [Google Scholar] [CrossRef]
- Vaintraub, A.; Lapteva, N. A. Colorimetric determination of phytate in unpurified extracts of seeds and the products of their processing. Anal. Chem. 1988, 175, 227–230. [Google Scholar] [CrossRef]
- Agostinho, A.J.; de Souza Oliveira, W.; Anunciação, D.S.; Santos, J.C.C. Simple and sensitive spectrophotometric method for phytic acid determination in grains. Food Anal. Methods 2016, 9, 2087–2096. [Google Scholar] [CrossRef]
- Wolf, W.J. Soybean protein nomenclature: A progress report. Cereal Science Today. Number 3. 1969, 14, 75–129. [Google Scholar]
- Meinke, D.W.; Chen, J.; Beachy, R.N. Expression of storage-protein genes during soybean seed development. Planta 1981, 153, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Uematsu, Y.; Kashiwaba, K.; Yagasaki, K.; Hajika, M.; Matsunaga, R. Accumulation of high levels of free amino acids in soybean seeds through integration of mutations conferring seed protein deficiency. Planta. 2003, 217(4), 577–586. [Google Scholar] [CrossRef] [PubMed]
- Singer, W.M.; Zhang, B.; Rouf Mian, M.A.; Huang, H. “Soybean Amino Acids in Health, Genetics, and Evaluation.” published in Soybean for Human Consumption and Animal Feed (Aleksandra Sudarić, ed.), 2020, IntechOpen. [CrossRef]
- Wallis, J.G.; Bengtsson, J.D.; Browse, J. Molecular approaches reduce saturates and eliminate trans fats in food oils. Front. Plant Sci. 2022, 13, 908608. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renewable and Sustainable Energy Reviews, 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Abdelghany, A.M.; Zhang, S.; Azam, M.; Shaibu, A.S.; Feng, Y.; Li, Y.; Tian, Y.; Hong, H.; Li, B.; Sun, J. Profiling of seed fatty acid composition in 1025 Chinese soybean accessions from diverse ecoregions. The Crop Journal 2020, 8, 635–644. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol. 2015, 52(2), 676–84. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R.; Fernie, A.R. The regulation of essential amino acid synthesis and accumulation in plants Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef]
- Angelovici, R.; Fait, A.; Fernie, A.R.; Galili, G. A seed high-lysine trait is negatively associated with the TCA cycle and slows down Arabidopsis seed germination. New Phytol., 2011, 189, 148–159. [Google Scholar] [CrossRef]
- Xing, A.; Last, R.L. A regulatory hierarchy of the Arabidopsis branched-chain amino acid metabolic network. Plant Cell 2017, 29, 1480–1499. [Google Scholar] [CrossRef]
- Fait, A.; Fromm, H.; Walter, D.; Galili, G.; Fernie, A.R. Highway or byway: the metabolic role of the GABA shunt in plants. Trends Plant Sci. 2008, 13, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Sebastia, C.; Marsolais, F.; Saravitz, C.; Israel, D.; Dewey, R.E.; Huber, S.C. Free amino acid profiles suggest a possible role for asparagine in the control of storage-product accumulation in developing seeds of low- and high-protein soybean lines. J. Exp. Bot. 2005, 56, 1951–1963. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Plant Biotechnol. J. 2013, 11, 211–222. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R.; Hoefgen, R.; Hesse, H. Improving the levels of essential amino acids and sulfur metabolites in plants. Biol. Chem. 2005, 386, 817–831. [Google Scholar] [CrossRef]
- Ufaz, S.; Galili, G. Improving the content of essential amino acids in crop plants: goals and opportunities. Plant Physiol. 2008, 147, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Wenefrida, I.; Utomo, H.S.; Blanche, S.B.; Linscombe, S.D. Enhancing essential amino acids and health benefit components in grain crops for improved nutritional values. Recent Patents on DNA & Gene Sequencing 2009, 3, 219–225. [Google Scholar]
- World Health Organization (WHO), Protein and amino acid requirements in human nutrition. In: Report of a joint WHO/FAO/UNU. 2007, WHO Press, Geneva, Switzerland. http://whqlibdoc.who.int/trs/WHO_TRS_935_eng.pdf. (accessed on 23 July 2024).
- Pires, S.M.G.; Reis, R.S.; Cardoso, S.M.; Pezzani, R.; Paredes-Osses, E.; Seilkhan, A. Phytates as a natural source for health promotion: A critical evaluation of clinical trials. Front. Chem. 2023, 11, 1174109. [Google Scholar] [CrossRef]
- Nahapetian, A.; Young, V.R. Metabolism of 14C-phytate in rats: Effect of low and high dietary calcium intakes. J. Nutr. 1980, 110, 1458–1472. [Google Scholar] [CrossRef]
- Vucenik, I.; Shamsuddin, A.M. Cancer inhibition by inositol hexaphosphate (IP6) and inositol: From laboratory to clinic. J. Nutr. 2003, 133, 3778s–3784s. [Google Scholar] [CrossRef]
- Vucenik, I.; Shamsuddin, A.M. Protection against cancer by dietary IP6 and inositol. Nutr. Cancer 2006, 55, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Simonet, B.M.; Vucenik, I.; Prieto, R.M.; Costa-Bauzá, A.; March, J.G. Absorption and excretion of orally administered inositol hexaphosphate (IP(6) or phytate) in humans. Biofactors 2001, 15, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Park, H.-J.; Chun, H.-K.; Cho, S.-Y.; Cho, S.-M.; Lillehoj, H.S. Dietary phytic acid lowers the blood glucose level in diabetic KK mice. Nutr. Res. 2006, 26, 474–479. [Google Scholar] [CrossRef]
- USDA Foreign Agricultural Service, 2024 World Production, Market and Trade Report, “Oilseeds: World Markets and Trade” (Washington DC: USDA FAS). Available online: https://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf (accessed on 23 July 2024).
- US Centers for Disease Control, “Health, United States 2020-2021”, CDC, Atlanta, USA. https://www.cdc.gov/nchs/hus/topics/nutrition.htm (accessed on 23 July 2024).
- Hilditch, T.P.; Williams, P.N. The Chemical Constitution of Natural Fats. (4th ed.), Chapman & Hall Publishing, London, England, 1964.
- Gunstone, F. Movements towards tailor-made fats. Prog. Lipid Res. 1998, 37, 277–305. [Google Scholar] [CrossRef] [PubMed]
- Ohlrogge, J.; Browse, J. Lipid biosynthesis. Plant Cell, 1995, 7, 957–970. [Google Scholar]
- Weiss, T.J. Food Oils and Their Uses. 2nd Edition. AVI Publishing, Westport, CT, USA, 1983.
- Gurr, M.I. Role of Fats in Food and Nutrition. Elsevier Applied Science, New York City, NY, USA, 1992.
- Palomer, X.; Pizarro-Delgado, J.; Emma Barroso, E.; Vázquez-Carrera, M. Palmitic and oleic acid: the Yin and Yang of fatty acids in Type 2 diabetes Mellitus. Trends in Endocrinology & Metabolism 2018, 29, 178–190. [Google Scholar]
- Vessby, B.; Uusitupa, M.; Hermansen, K.; Riccardi, G.; Rivellese, A.A.; Tapsell, L.C., et. al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia, 2001, 44, 312–319. [Google Scholar] [CrossRef]
- “Carefully consider feeding dairy cattle high oleic soybeans,” Farm and Dairy News, 2023, https://www.farmanddairy.com/columns/carefully-consider-feeding-dairy-cattle-high-oleic-soybeans/794068.html (accessed on 19 July 2024).
- “Precious metals and other important minerals for health.” Harvard Medical School, Harvard Health Publishing, 2021, Cambridge, MA, USA, https://www.health.harvard.edu/staying-healthy/precious-metals-and-other-important-minerals-for-health (accessed on 3 August, 2024).
- Underwood, E.J.; Suttle, N.F. 3rd ed. “The Mineral Nutrition of Livestock.” CABI Publishing, Boston, MA, USA, 2022.
- Allen, L.; de Benoist, B.; Dary, O.; Hurrell, R., eds. “Guidelines on Food Fortification with Micronutrients,” 2006, WHO and FAO, Geneva, Switzerland. https://www.who.int/publications/i/item/9241594012 (accessed on 23 July 2024).
- Brabin, B.J.; Premji, Z.; Verhoeff, F. An analysis of anemia and child mortality. J Nutr. 2001, 131(2S-2), 604S-614S, discussion 614S-615S.
- Institute of Medicine. Food and Nutrition Board. “Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academy Press, Washington, DC, USA, 2001.
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef]
- Hess, S.Y. National risk of zinc deficiency as estimated by national surveys. Food Nutr. Bull. 2017, 38, 3–17. [Google Scholar] [CrossRef]
- National Institutes of Health Office of Dietary Supplements: Manganese Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/Manganese-HealthProfessional/ (accessed on 22 July 2024).
- Heighton, L.; Schmidt, W.F.; Siefert, R.L. Kinetic and equilibrium constants of phytic acid and ferric and ferrous phytates derived from nuclear magnetic resonance spectroscopy. J. Agric. Food Chem., 2008, 56, 9543–9547. [Google Scholar] [CrossRef]
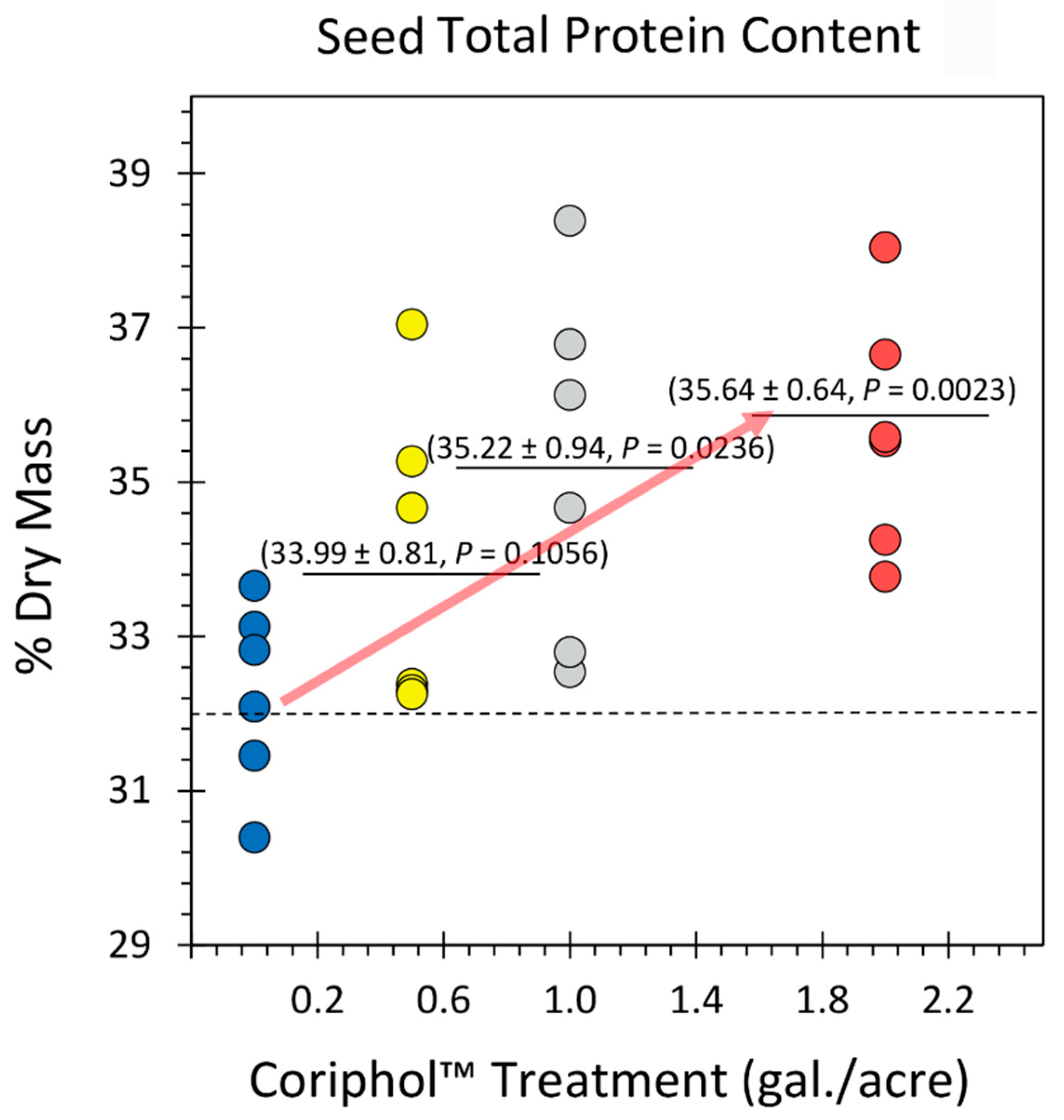
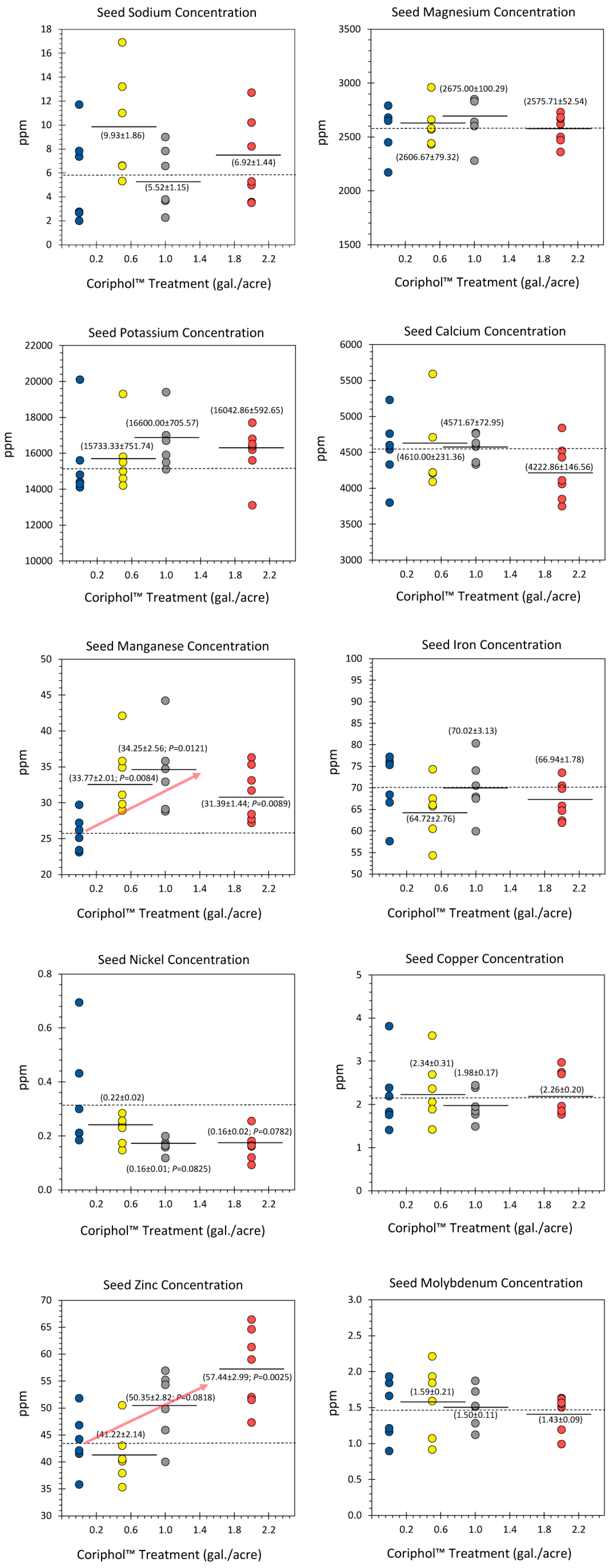
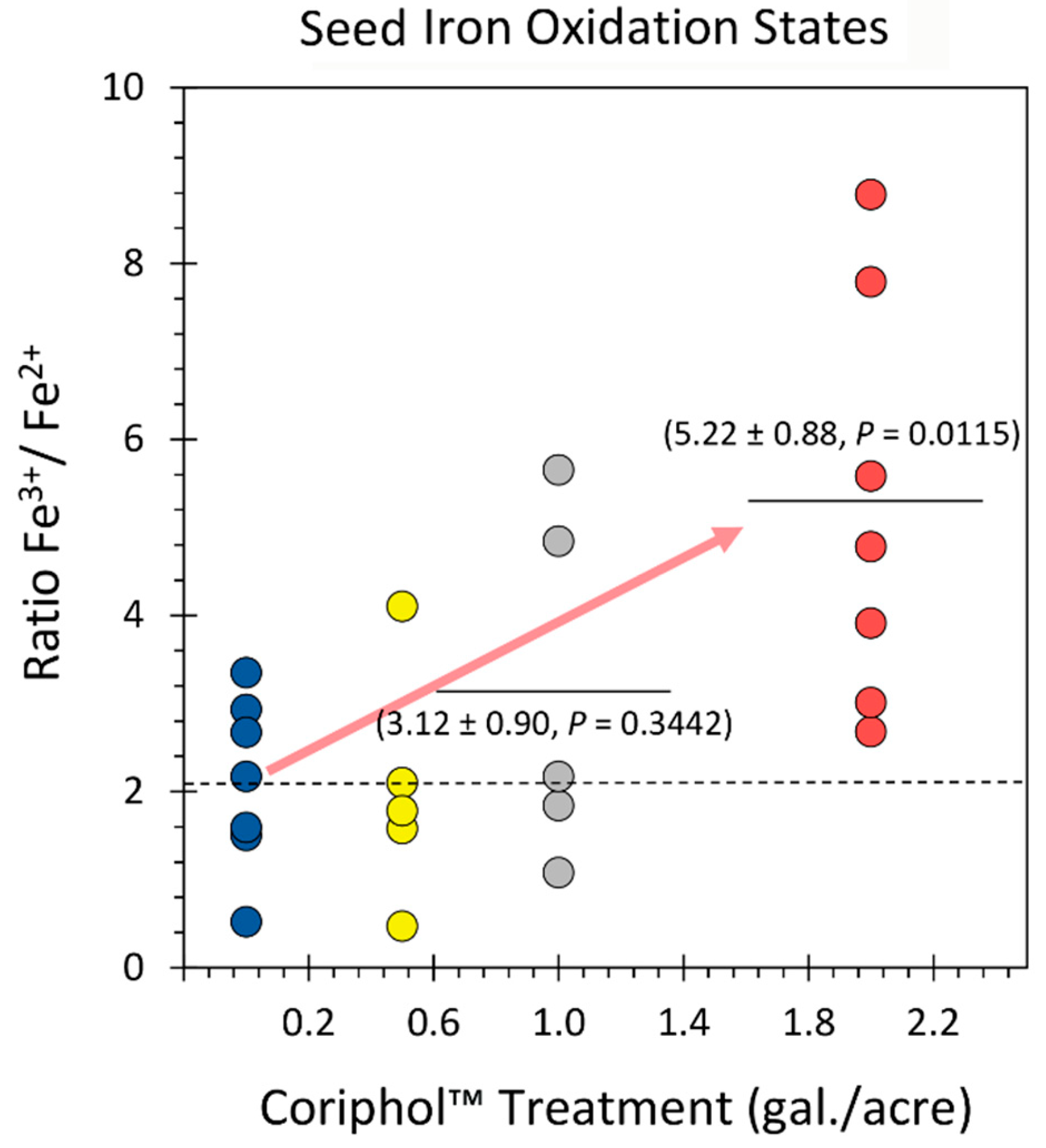
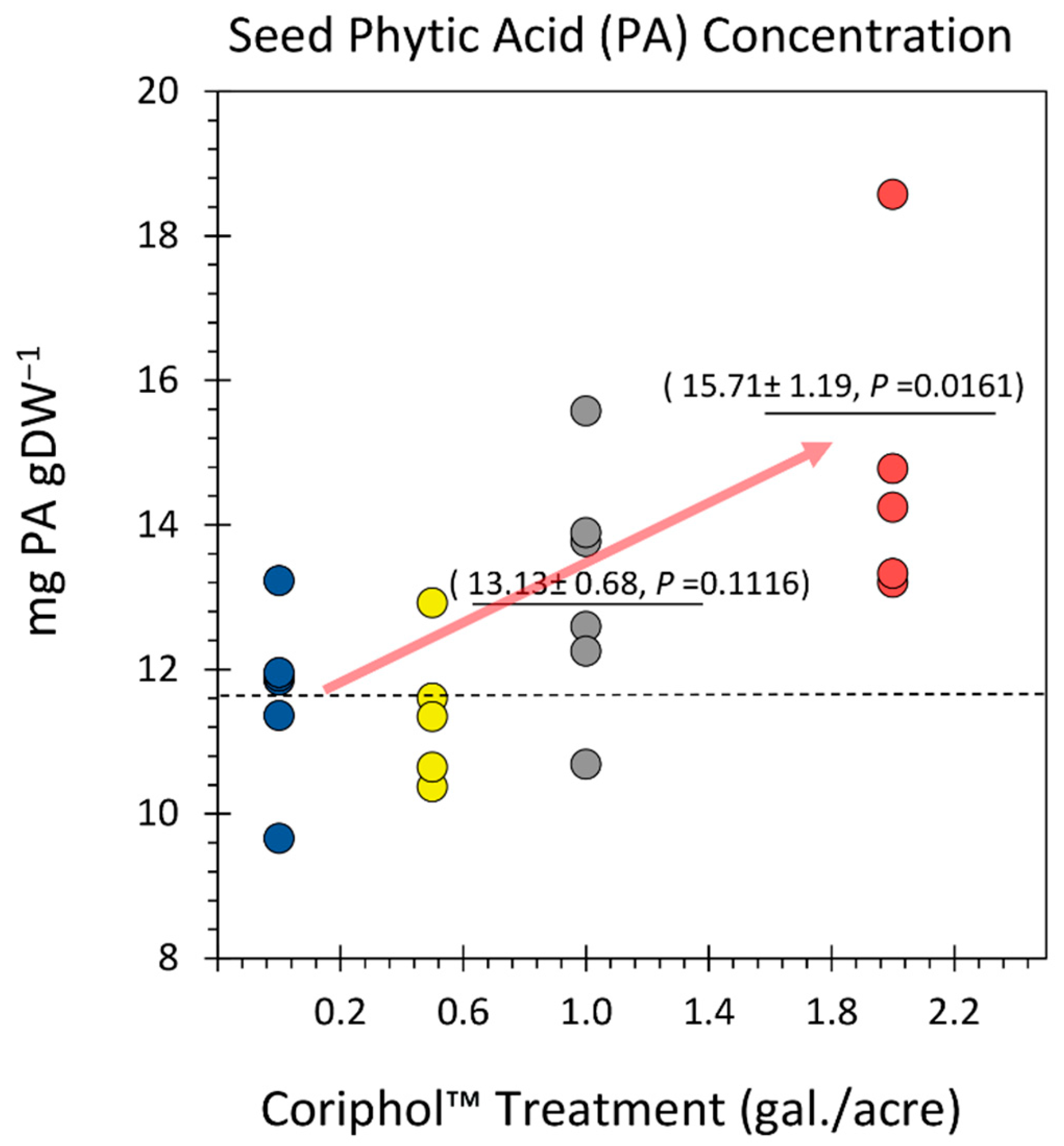
| Amino Acid | Control (N=4) | 0.5 gal./acre (N=4) | 1.0 gal/acre (N=4) | 2.0 gal/acre (N=5) | ||||
|---|---|---|---|---|---|---|---|---|
| Norm. Peak Areaa. | % Distribution |
Norm. Peak Areaa. |
% Distribution |
Norm. Peak Areaa. |
% Distribution | Norm. Peak Areaa. |
% Distribution | |
| Asp | 530±142 | 3.78±0.71 | 848±369 | 7.28±1.95 | 432±147 | 9.28±2.72 | 392±73 | 6.81±1.25 |
| Glu | 357±150 | 2.67±1.08 | 1178±169 | 11.86±2.35 | 283±46 | 6.28±0.96 | 312±73 | 5.21±0.75 |
| GABA | 346±72 | 2.72±0.44 | 207±59 | 2.28±0.72 | 136±32 | 3.15±0.84 | 139±36 | 2.32±0.48 |
| Asn | 2984±1011 | 22.16±5.60 | 936±181 | 9.39±2.11 | 295±97 | 6.63±2.23 | 515±106 | 8.51±1.02 |
| Ser | 1394±486 | 9.87±3.27 | 842±122 | 8.06±0.32 | 261±31 | 5.72±0.38 | 289±44 | 4.97±0.57 |
| Gln | 379±136 | 2.84±0.97 | 403±80 | 3.92±0.82 | 149±22 | 3.29±0.44 | 371±68 | 6.13±0.65 |
| Thr | 162±34 | 1.51±0.50 | 142±24 | 1.42±0.30 | 49±8 | 1.13±0.26 | 38±5 | 0.65±0.07 |
| Gly | 907±122 | 7.15±0.87 | 549±76 | 5.36±0.69 | 283±18 | 6.45±0.89 | 445±54 | 7.62±0.81 |
| Cyc | 342±46 | 2.81±0.57 | 229±39 | 2.16±0.07 | 85±25 | 1.82±0.45 | 142±26 | 2.35±0.18 |
| Ala | 2871±684 | 22.78±5.05 | 2766±790 | 25.16±3.81 | 1580±226 | 34.68±3.32 | 1684±361 | 27.69±4.04 |
| Tyr | 81±23 | 0.73±0.30 | 261±122 | 2.21±0.69 | 59±9 | 1.34±0.25 | 95±14 | 1.74±0.43 |
| Val | 114±267 | 16.95±2.42 | 877±276 | 9.12±3.11 | 218±32 | 4.80±0.49 | 469±111 | 7.93±1.62 |
| Met | 452±139 | 3.20±0.90 | 158±29 | 1.62±0.26 | 172±19 | 3.80±0.29 | 272±36 | 4.69±0.53 |
| Trp | 98±23 | 0.86±0.30 | 148±37 | 1.54±0.50 | 72±7 | 1.64±0.28 | 67±9 | 1.18±0.18 |
| Phe | 189±45 | 1.71±0.63 | 135±28 | 1.37±0.37 | 80±9 | 1.84±0.35 | 82±10 | 1.42±0.17 |
| Lys | 13±3 | 0.09±0.01 | 94±21 | 0.97±0.30 | 53±13 | 1.22±0.32 | 85±17 | 1.49±0.34 |
| iLeu | 81±10 | 0.72±0.19 | 120±33 | 1.08±0.12 | 39±13 | 0.83±0.24 | 45±12 | 0.84±0.24 |
| Leu | 365±147 | 2.65±1.05 | 379±33 | 5.45±2.13 | 299±30 | 6.76±0.98 | 496±83 | 8.45±1.05 |
| Total | 13666±1976 | 100.00 | 10270±170 | 100.00 | 4544±314 | 100.00 | 5937±756 | 100.00 |
| Treatment Type |
FA Content (% Dry Mass) |
FA | Measured Distribution (%) |
P-Value | Literature Range (%) |
Measured % Sat. FA |
P-Value | Literature Range (%) |
Measured Ratio Poly-to-Mono UnSat. FA |
P-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Control (N=6) |
21.61 ± 0.70 | PA | 15.61 ± 2.03 | - | 10-15 | 19.96 ± 1.96 | - | 12-22 | 2.39 ± 0.45 | - |
| SA | 4.34 ± 0.28 | - | 2-7 | |||||||
| OA | 25.72 ± 3.26 | - | 13-26 | |||||||
| LA | 44.82 ± 5.33 | - | 40-64 | |||||||
| α-LA | 9.51 ± 1.37 | - | 4-13 | |||||||
| 0.5 gal./acre (N=6) |
20.41 ± 1.28 | PA | 12.59 ± 0.64 | 0.2054 | - | 15.36 ± 1.04 | 0.0703 | - | 3.09 ± 0.20 | 0.1953 |
| SA | 2.77 ± 0.38 | 0.0083 | - | |||||||
| OA | 20.27 ± 0.47 | 0.1587 | - | |||||||
| LA | 57.98 ± 1.25 | 0.0530 | - | |||||||
| α-LA | 6.40 ± 0.64 | 0.0789 | - | |||||||
| 1.0 gal./acre (N=6) |
20.71 ± 1.40 | PA | 8.86 ± 0.55 | 0.0184 | - | 10.96 ± 1.22 | 0.0040 | - | 1.03 ± 0.32 | 0.0309 |
| SA | 2.10 ± 0.39 | 0.0012 | - | |||||||
| OA | 45.85 ± 4.24 | 0.0044 | - | |||||||
| LA | 34.39 ± 4.58 | 0.1689 | - | |||||||
| α-LA | 8.14 ± 0.92 | 0.4274 | - | |||||||
| 2.0 gal./acre (N=6) |
20.26 ± 1.21 | PA | 11.36 ± 0.64 | 0.0924 | - | 13.33 ± 0.74 | 0.0194 | - | 0.54 ± 0.10 | 0.0104 |
| SA | 2.19 ± 0.21 | 0.0002 | - | |||||||
| OA | 57.31 ± 3.69 | 0.0001 | - | |||||||
| LA | 21.54 ± 3.51 | 0.0054 | - | |||||||
| α-LA | 7.61 ± 0.85 | 0.2723 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





