Submitted:
26 August 2024
Posted:
28 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
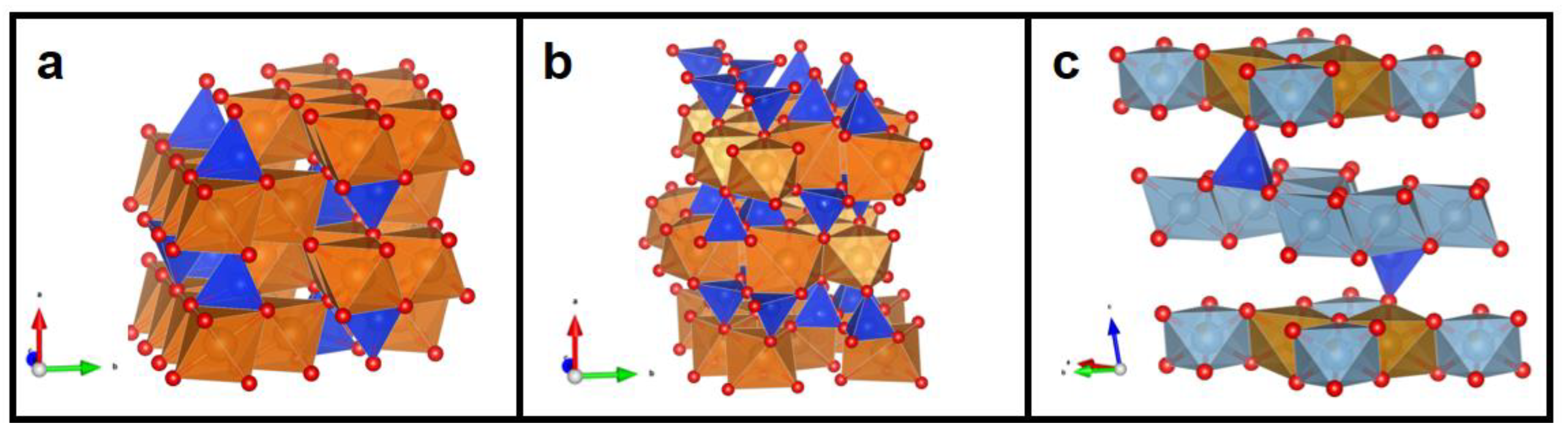
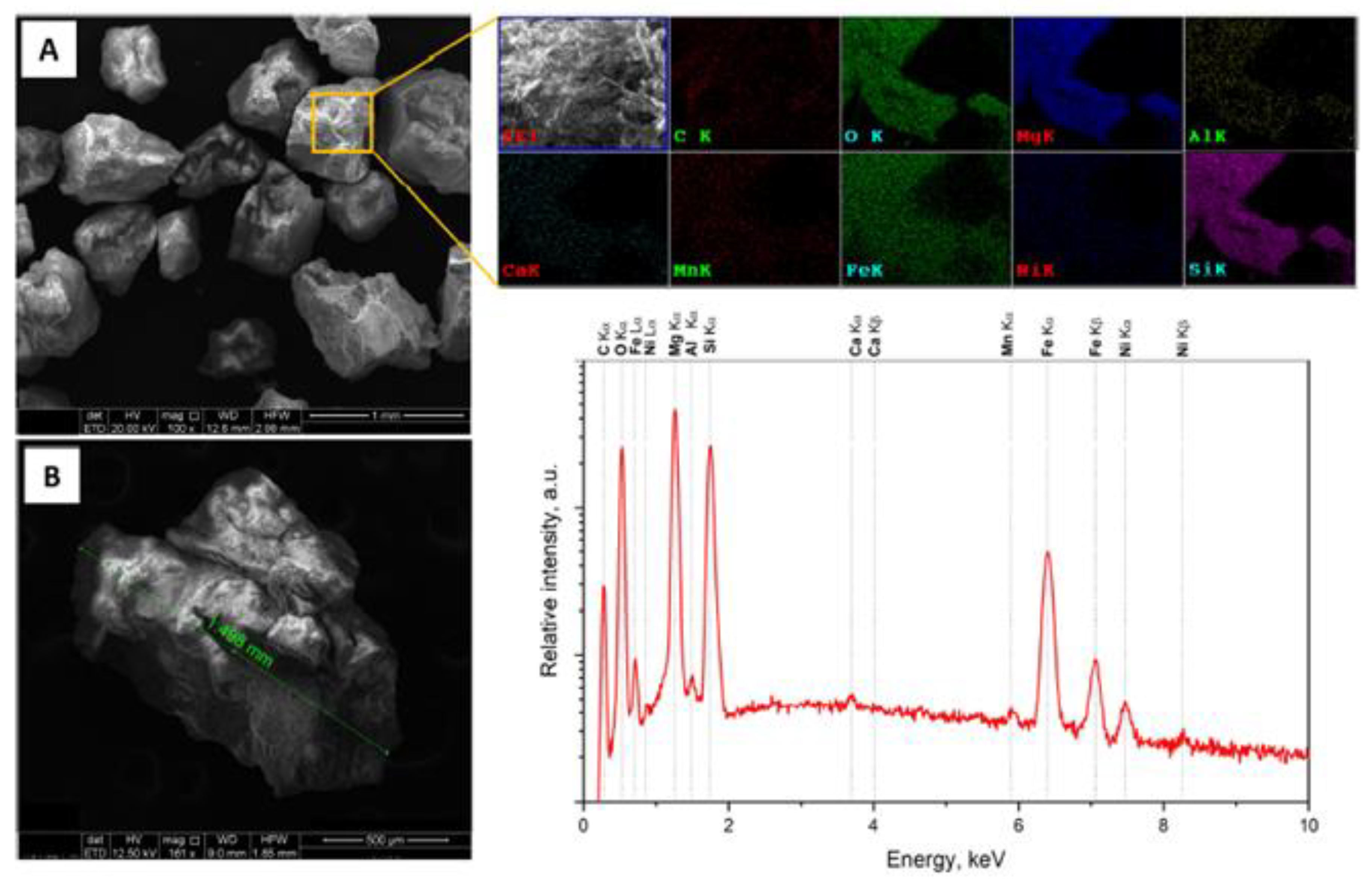
2.1. Synthesis
2.2. Reactant Gas Mixture: Set-Up
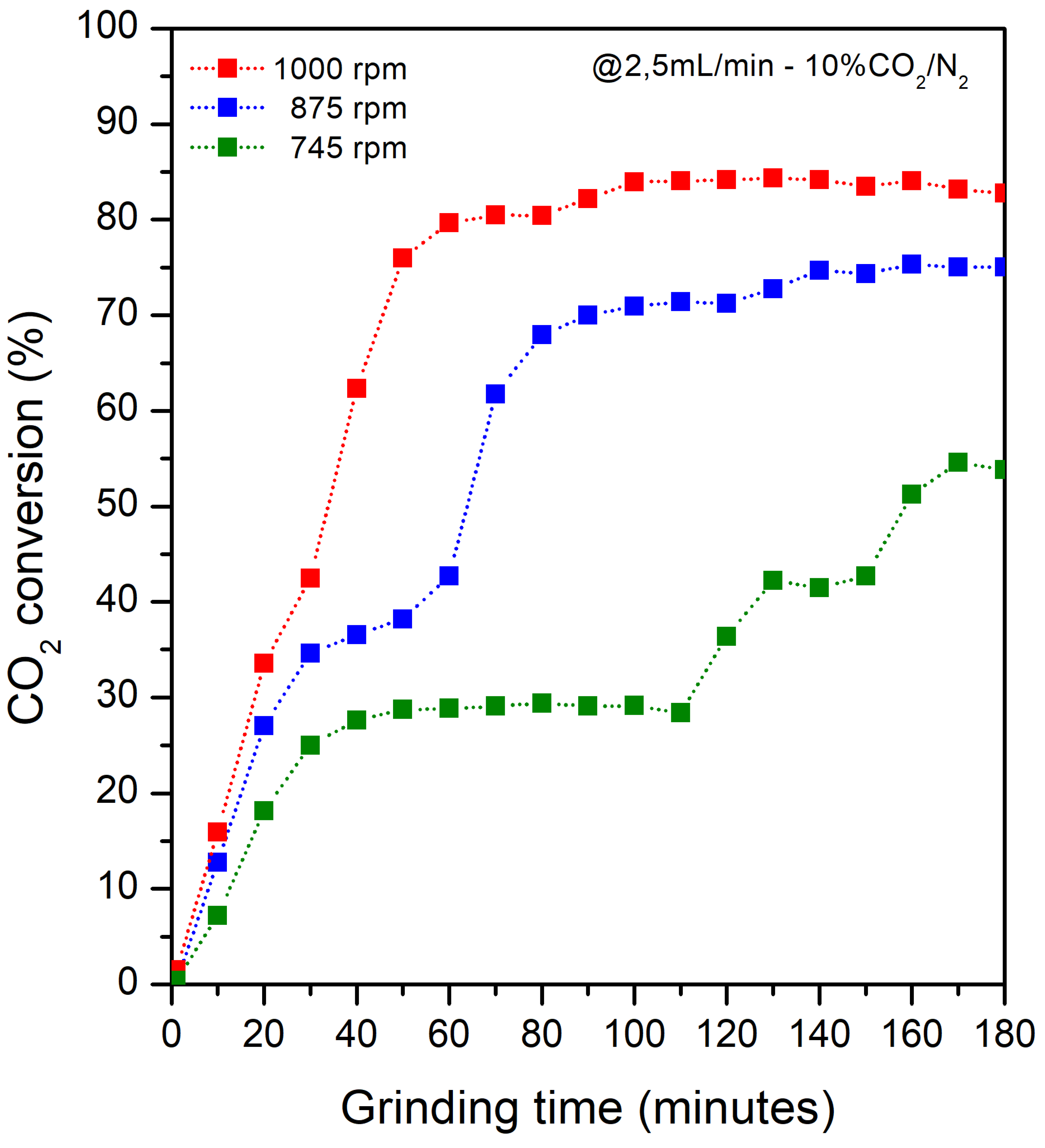
2.3. Percentage Conversion of CO2
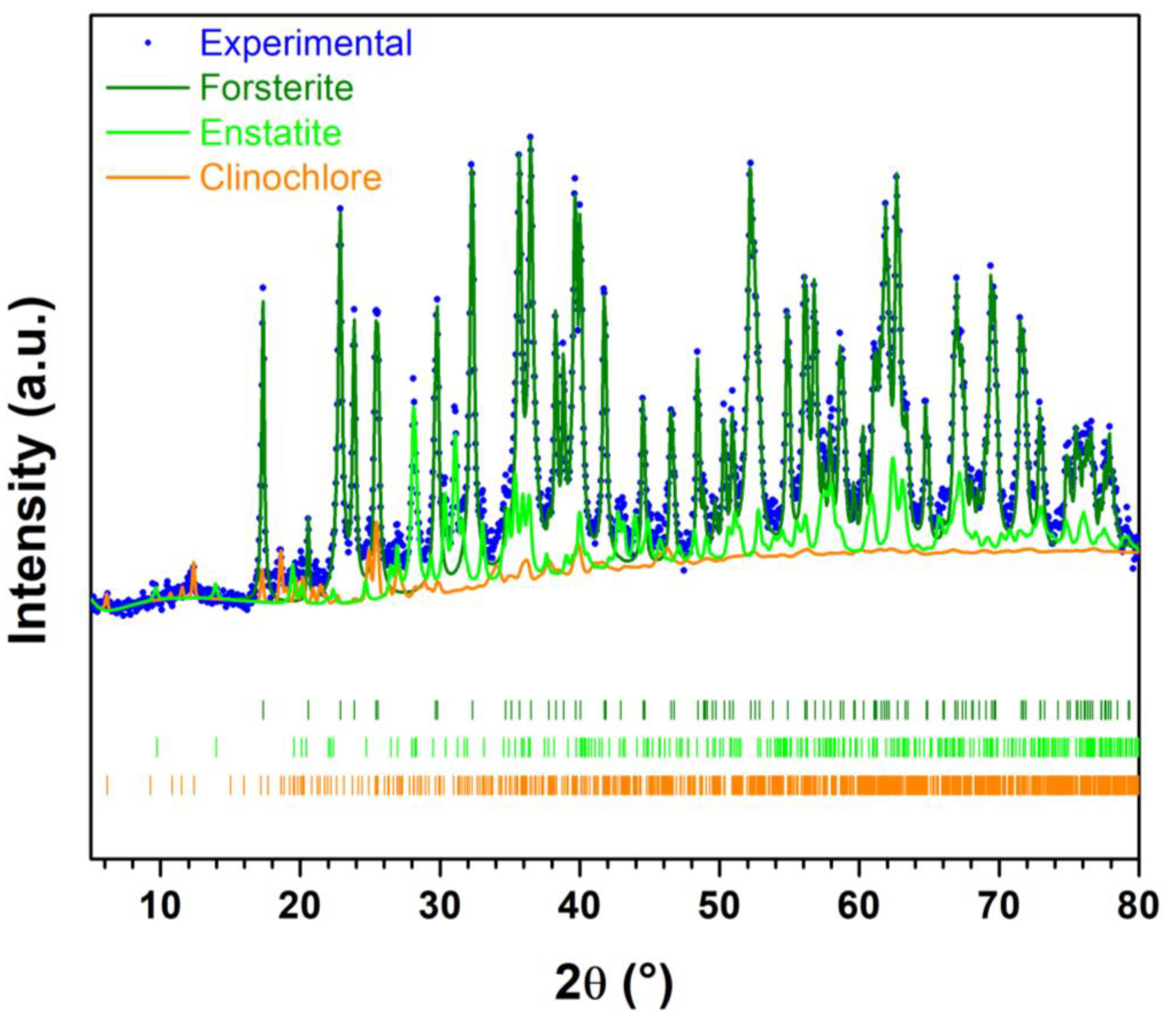
2.4. Assessment of Phase Evolution during Reactive Milling
3. Results and Discussion
4. Conclusions
5. Patents
- Method for Converting Crbon Dioxide into High Added Value Chemical Compounds through a Mechanochemical Process under Continuous Gas Flow Conditions Int. Patent WO/2022200941 A1 2022
- Process for the Conversion of Carbon Dioxide into Value-Added Products by Means of a Process of Mechanochemical Activation of Industrial Processing Scraps Int. Patent WO/2023199254 A9 2023
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busca, G. Critical Aspects of Energetic Transition Technologies and the Roles of Materials Chemistry and Engineering. Energies 2024, 17. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Blunt, M.J.; Anthony, E.J.; Park, A.H.A.; Hughes, R.W.; Webley, P.A.; Yan, J. Advances in Carbon Capture, Utilization and Storage. Appl. Energy 2020, 278, 115627. [Google Scholar] [CrossRef]
- Wei, R.; Alshahrani, T.; Chen, B.; Ibragimov, A.B.; Xu, H.; Gao, J. Advances in Porous Materials for Efficient Separation and Purification of Flue Gas. Sep. Purif. Technol. 2025, 352, 128238. [Google Scholar] [CrossRef]
- Dubey, A.; Arora, A. Advancements in Carbon Capture Technologies: A Review. J. Clean. Prod. 2022, 373, 133932. [Google Scholar] [CrossRef]
- Liu, W.; Ji, Y.; Huang, Y.; Zhang, X.J.; Wang, T.; Fang, M.X.; Jiang, L. Adsorption-Based Post-Combustion Carbon Capture Assisted by Synergetic Heating and Cooling. Renew. Sustain. Energy Rev. 2024, 191. [Google Scholar] [CrossRef]
- Bukar, A.M.; Asif, M. Technology Readiness Level Assessment of Carbon Capture and Storage Technologies. Renew. Sustain. Energy Rev. 2024, 200, 114578. [Google Scholar] [CrossRef]
- Songolzadeh, M.; Soleimani, M.; Takht Ravanchi, M.; Songolzadeh, R. Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Dziejarski, B.; Krzyżyńska, R.; Andersson, K. Current Status of Carbon Capture, Utilization, and Storage Technologies in the Global Economy: A Survey of Technical Assessment. Fuel 2023, 342. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Structural and Chemical Changes in Mine Waste Mechanically-Activated in Various Milling Environments. Powder Technol. 2017, 308, 13–19. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 Disposal by Means of Silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L.; Sharp, D.H. Carbon Dioxide Disposal in Carbonate Minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- McKelvy, M.J.; Chizmeshya, A.V.G.; Diefenbacher, J.; Béarat, H.; Wolf, G. Exploration of the Role of Heat Activation in Enhancing Serpentine Carbon Sequestration Reactions. Environ. Sci. Technol. 2004, 38, 6897–6903. [Google Scholar] [CrossRef]
- Vega, L.F.; Bahamon, D.; Alkhatib, I.I.I. Perspectives on Advancing Sustainable CO2 Conversion Processes: Trinomial Technology, Environment, and Economy. ACS Sustain. Chem. Eng. 2024, 12, 5357–5382. [Google Scholar] [CrossRef]
- O’Connor, W.K.; Dahlin, D.C.; Rush, G.E.; Dahlin, C.L.; Collins, W.K. Carbon Dioxide Sequestration by Direct Mineral Carbonation: Process Mineralogy of Feed and Products. Miner. Metall. Process. 2002, 19, 95–101. [Google Scholar] [CrossRef]
- Haug, T.A.; Kleiv, R.A.; Munz, I.A. Investigating Dissolution of Mechanically Activated Olivine for Carbonation Purposes. Appl. Geochemistry 2010, 25, 1547–1563. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Hamidi, H.; Junin, R.; Karaei, M.A. A Review on Carbon Dioxide Mineral Carbonation through PH-Swing Process. Chem. Eng. J. 2015, 279, 615–630. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Mechanical Activation of Magnesium Silicates for Mineral Carbonation, a Review. Miner. Eng. 2018, 128, 69–83. [Google Scholar] [CrossRef]
- Baláž, P.; Turianicová, E.; Fabián, M.; Kleiv, R.A.; Briančin, J.; Obut, A. Structural Changes in Olivine (Mg, Fe)2SiO4 Mechanically Activated in High-Energy Mills. Int. J. Miner. Process. 2008, 88, 1–6. [Google Scholar] [CrossRef]
- Zhang, Q.; Sugiyama, K.; Saito, F. Enhancement of Acid Extraction of Magnesium and Silicon from Serpentine by Mechanochemical Treatment. Hydrometallurgy 1997, 45, 323–331. [Google Scholar] [CrossRef]
- Kalinkina, E. V.; Kalinkin, A.M.; Forsling, W.; Makarov, V.N. Sorption of Atmospheric Carbon Dioxide and Structural Changes of Ca and Mg Silicate Minerals during Grinding I. Diopside. Int. J. Miner. Process. 2001, 61, 273–288. [Google Scholar] [CrossRef]
- Kalinkin, A.M.; Kalinkina, E. V.; Politov, A.A.; Makarov, V.N.; Boldyrev, V. V. Mechanochemical Interaction of Ca Silicate and Aluminosilicate Minerals with Carbon Dioxide. J. Mater. Sci. 2004, 39, 5393–5398. [Google Scholar] [CrossRef]
- Fabian, M.; Shopska, M.; Paneva, D.; Kadinov, G.; Kostova, N.; Turianicová, E.; Briančin, J.; Mitov, I.; Kleiv, R.A.; Baláž, P. The Influence of Attrition Milling on Carbon Dioxide Sequestration on Magnesium-Iron Silicate. Miner. Eng. 2010, 23, 616–620. [Google Scholar] [CrossRef]
- Bolm, C.; Hernández, J.G. Mechanochemistry of Gaseous Reactants. Angew. Chemie - Int. Ed. 2019, 58, 3285–3299. [Google Scholar] [CrossRef] [PubMed]
- Farina, V.; Gamba, N.S.; Gennari, F.; Garroni, S.; Torre, F.; Taras, A.; Enzo, S.; Mulas, G. CO2 Hydrogenation Induced by Mechanochemical Activation of Olivine With Water Under CO2 Atmosphere. Front. Energy Res. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Torre, F.; Farina, V.; Taras, A.; Pistidda, C.; Santoru, A.; Bednarcik, J.; Mulas, G.; Enzo, S.; Garroni, S. Room Temperature Hydrocarbon Generation in Olivine Powders: Effect of Mechanical Processing under CO2 Atmosphere. Powder Technol. 2020, 364, 915–923. [Google Scholar] [CrossRef]
- Taras, A.; Farina, V.; Cappai, L.; Enzo, S.; Garroni, S.; Mulas, G. Method for Converting Crbon Dioxide into High Added Value Chemical Compounds through a Mechanochemical Process under Continuous Gas Flow Conditions Int. Patent WO/2022200941 A1 2022.
- Simula, M.D.; Taras, A.; Pinna L.; Piu S.; Enzo, S.; Garroni, S.; Mulas, G.. Process for the Conversion of Carbon Dioxide into Value-Added Products by Means of a Process of Mechanochemical Activation of Industrial Processing Scraps Int. Patent WO/2023199254 A9 2023.
- Graulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database - An Open-Access Collection of Crystal Structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Lutterotti, L. Total Pattern Fitting for the Combined Size-Strain-Stress-Texture Determination in Thin Film Diffraction. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. with Mater. Atoms 2010, 268, 334–340. [Google Scholar] [CrossRef]
- Yang, M.; Ye, M.; Han, H.; Ren, G.; Han, L.; Zhang, Z. Near-Infrared Spectroscopic Study of Chlorite Minerals. J. Spectrosc. 2018, 2018. [Google Scholar] [CrossRef]
- Callister, William, D.; Rethwisch, D.G. Materials Science and Engineering An Introduction; Eight Edit.; Wiley, 2010; ISBN 0470419970.
- Delogu, F.; Monagheddu, M.; Mulas, G.; Schiffini, L.; Cocco, G. Impact Characteristics and Mechanical Alloying Processes by Ball Milling: Experimental Evaluation and Modelling Outcomes. Int. J. non-equilibrium Process. 2000, 11, 235–269. [Google Scholar]
- Napolitano, E.; Mulas, G.; Enzo, S.; Delogu, F. Kinetics of Mechanically Induced Anatase-to-Rutile Phase Transformations under Inelastic Impact Conditions. Acta Mater. 2010, 58, 3798–3804. [Google Scholar] [CrossRef]
- Ugapeva, S.S.; Oleinikov, O.B.; Zayakina, N. V. Rare Hydrated Magnesium Carbonate Minerals Nesquehonite and Dypingite of the Obnazhennaya Kimberlite Pipe, in the Yakutian Kimberlite Province. Minerals 2023, 13. [Google Scholar] [CrossRef]
- Ballirano, P.; De Vito, C.; Mignardi, S.; Ferrini, V. Phase Transitions in the MgCO2H2O System and the Thermal Decomposition of Dypingite, Mg5(CO3)4(OH)25H2O: Implications for Geosequestration of Carbon Dioxide. Chem. Geol. 2013, 340, 59–67. [Google Scholar] [CrossRef]
- Enzo, S.; Mulas, G.; Frattini, R. The Structure of Mechanically Alloyed AlxFe(1-x) End-Products after Annealing. Mater. Sci. Forum 1998, 269–272. [Google Scholar] [CrossRef]
- Farina, V.; Simula, M.D.; Taras, A.; Cappai, L.; Sougrati, M.T.; Mulas, G.; Garroni, S.; Enzo, S.; Stievano, L. Unveiling Redox Mechanism at the Iron Centers in the Mechanochemically Activated Conversion of CO2 in the Presence of Olivine. J. Mater. Sci. 2022, 57, 10017–10027. [Google Scholar] [CrossRef]
- Masci, L.; Dubacq, B.; Verlaguet, A.; Chopin, C.; Andrade, V. De; Herviou, C. A XANES and EPMA Study of Fe3+ in Chlorite: Importance of Oxychlorite and Implications for Cation Site Distribution and Thermobarometry. Am. Mineral. 2019, 104, 403–417. [Google Scholar] [CrossRef]
- Toby, B.H. R Factors in Rietveld Analysis: How Good Is Good Enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Turianicová, E.; Baláž, P.; Tuček, Ľ.; Zorkovská, A.; Zeleňák, V.; Németh, Z.; Šatka, A.; Kováč, J. A Comparison of the Reactivity of Activated and Non-Activated Olivine with CO2. Int. J. Miner. Process. 2013, 123, 73–77. [Google Scholar] [CrossRef]
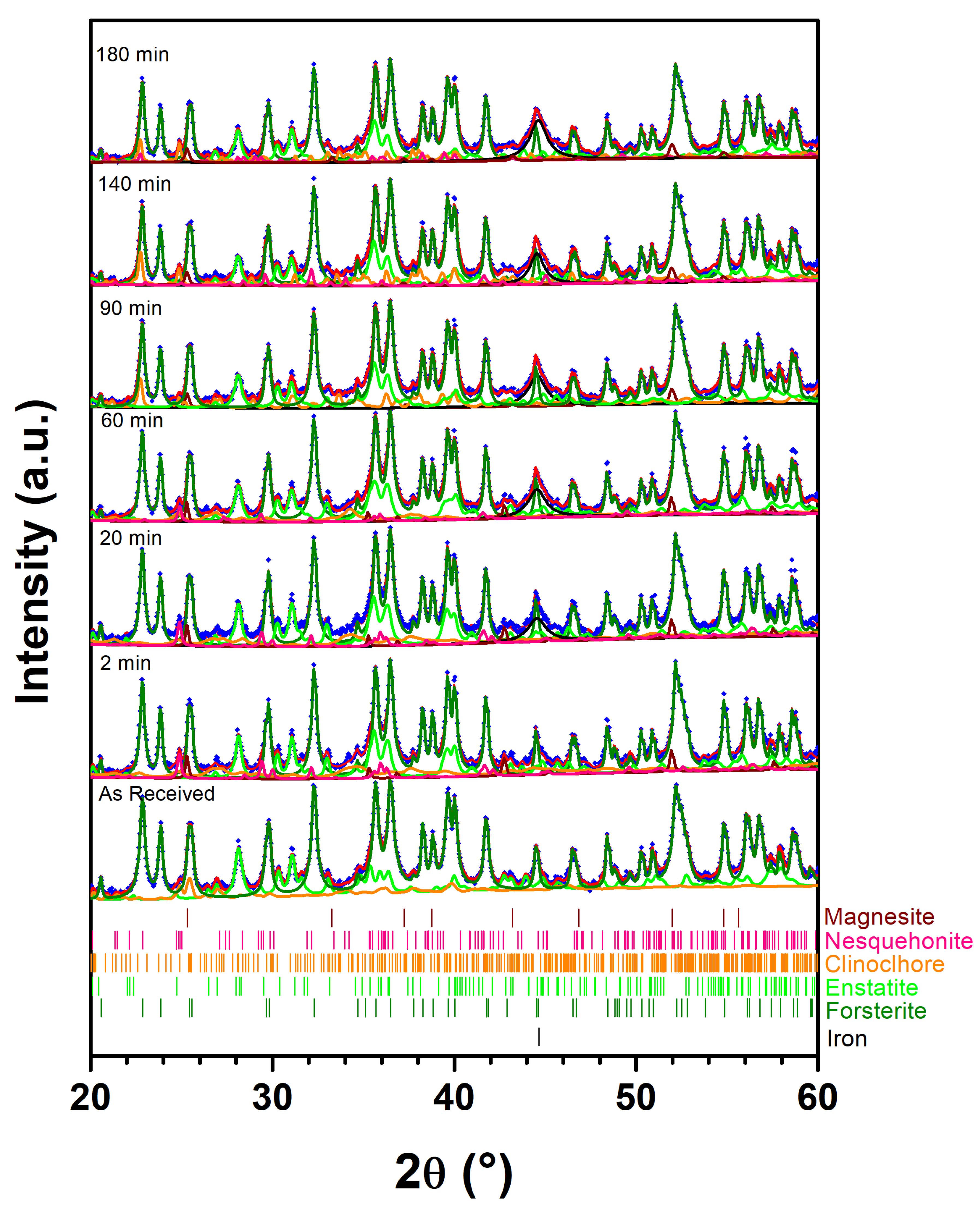

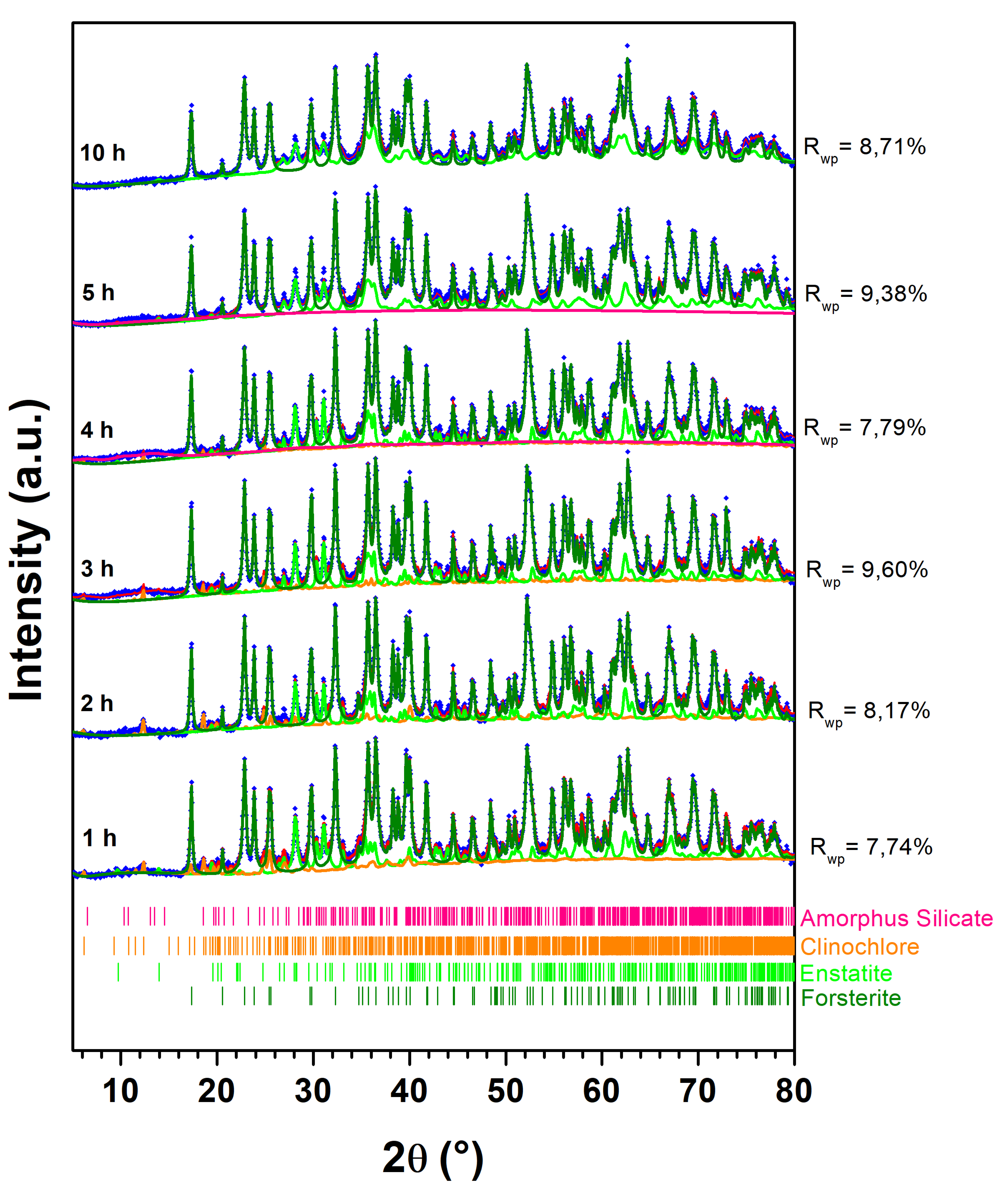
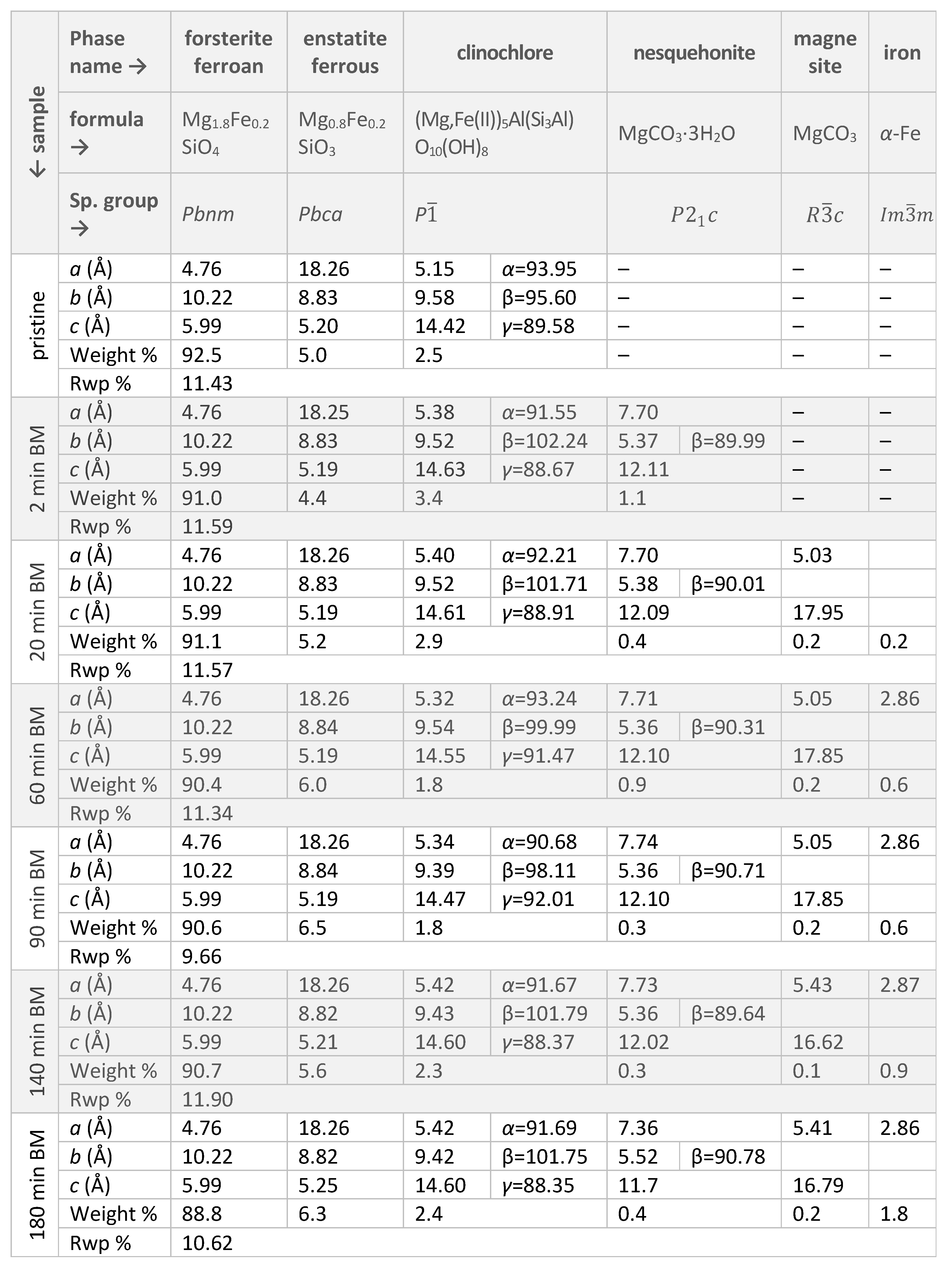 |
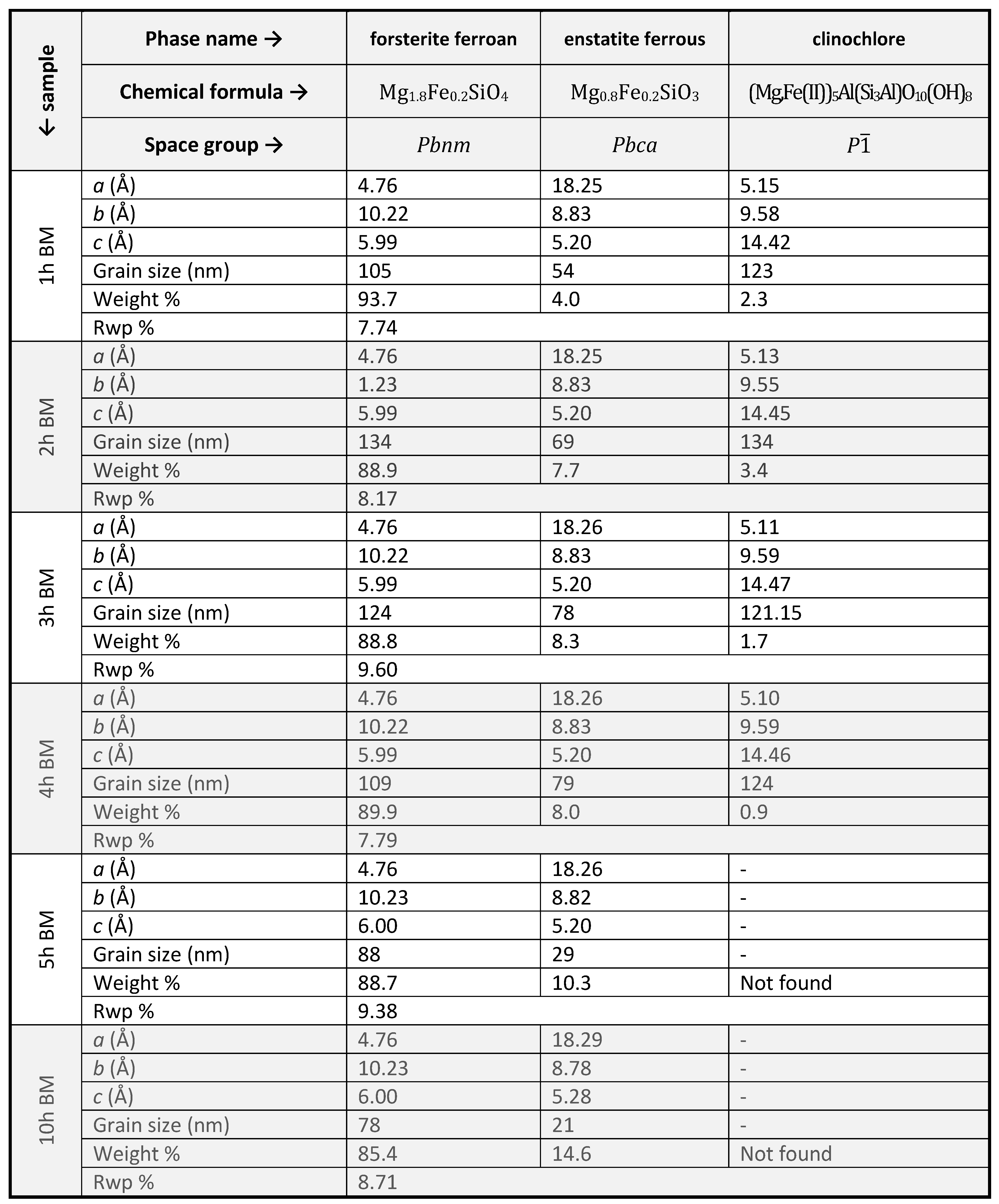 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





