Introduction
Over the past three decades, there has been a notable increase in the number of patients experiencing infertility and difficulty conceiving naturally [
1]. Given the diverse etiologies of infertility, treatment modalities also vary significantly. In cases where simpler interventions-such as timing of intercourse and intrauterine insemination-prove ineffective, more advanced methods, like in vitro fertilization (IVF), are recommended [
2]. The successful outcome of IVF is contingent upon several factors, including the quality of gametes (sperm and oocytes), their effective interaction, successful fertilization, zygote cleavage, and efficient embryo implantation into the endometrial lining of the uterus [
3]. A primary task for embryologists is to evaluate oocyte quality based on the morphology of both the cumulus complex and the oocyte itself, as well as the morphological characteristics of the oocyte following the removal of cumulus cells. Recently, embryologists have suggested the utilization of follicular fluid (FF) as a means to assess oocyte quality, as this fluid serves as a microenvironment that supports oocyte maturation across various developmental stages [
4,
5].
Follicular fluid is predominantly composed of proteins and steroid hormones, and it also contains cytokines, enzymes, anticoagulants, electrolytes, reactive oxygen species, both enzymatic and non-enzymatic antioxidants, growth factors, and metabolites such as amino acids and lipids that accumulate within the oocyte, assisting in its differentiation [
5,
6,
7,
8,
9,
10,
11,
12].
In addition to hormones and vitamins, which have received extensive research attention, bioelements [
13,
14] may also significantly influence oocyte quality. There is growing interest in understanding the effects of bioelement deficiencies on physiological functions, particularly in reproduction. However, recent literature lacks data on the concentrations of bioelements in human follicular fluid, with research primarily conducted on animal models. Previous studies have made it challenging to identify which specific bioelements should be measured in human follicular fluid.
According to Silberstein et al. (2009), the most frequently identified elements in follicular fluid include calcium (Ca) and magnesium (Mg), followed by copper (Cu), zinc (Zn), iron (Fe), chromium (Cr), and rubidium (Rb). However, it has been concluded that the concentrations of these elements do not significantly impact oocyte maturity [
15,
16].
Sodium and potassium are metals with similar chemical properties. The sodium-potassium pump is in charge of the active transport of ions across the cell membrane, moving ions from regions of lower concentration to areas of higher concentration via carrier molecules and energy expenditure. This mechanism enables sodium ions to actively leave the cell while potassium ions enter, contrary to their concentration gradients (with higher sodium levels outside the cell and higher potassium levels inside). The breakdown of adenosine triphosphate (ATP) into adenosine diphosphate (ADP) and inorganic phosphate releases the energy necessary for this transport, helping to establish the cell membrane potential [
17]. In the female reproductive system, sodium concentration is linked to estrogen synthesis and uterine contractions, along with the roles of potassium and calcium [
14].
It has long been established that calcium is an essential signaling molecule for fertilization in mammals, as it plays a critical role in oocyte activation. Upon the fusion of the sperm and oocyte membranes, the first observable intracellular signaling events involve low-frequency oscillations and a significant increase in free calcium ion concentrations within the cytoplasm. Consequently, it can be postulated that insufficient calcium levels during fertilization may lead to fertilization failure [
14,
18].
Magnesium is crucial for the stabilization of deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and ribosomes, as well as for activating approximately 300 enzymes involved in energy metabolism and reactive oxygen species (ROS) production [
19]. Regardless of its importance, magnesium has not received the same level of emphasis in medical literature or clinical practice as other bioelements such as sodium, potassium, and calcium [
20]. Its relevance is often associated more with male infertility [
21].
Iron plays a vital role in the synthesis of nucleic acids and proteins, as well as in cellular respiration, development, and differentiation. It is involved in numerous redox reactions catalyzed by cytochromes, influencing energy production, drug and hormone metabolism, and the activation of defense systems via nicotinamide adenine dinucleotide phosphate (NADP) oxidase [
13]. Elevated iron concentrations in follicular fluid can lead to granulosa cell death, negatively impacting oocyte maturation and quality, and may contribute to the development of endometriosis [
22,
23,
24]. Recent studies have highlighted the implications of altered iron metabolism in the onset of endometriosis [
25,
26,
27]. Increased iron concentration may result from the heightened influx of degraded erythrocytes due to significant menstrual reflux or bleeding [
27,
28]. According to Ito et al., the accumulation of iron and hemoglobin leads to oxidative stress, which can induce DNA hypermethylation and histone modification. Such hypermethylation is associated with defective endometrial development in patients with endometriosis [
27,
29].
This study aimed to assess the levels of bioelements: sodium (Na), potassium (K), calcium (Ca), magnesium (Mg), and iron (Fe) in follicular fluid derived from stimulated cycles. The primary objective was to determine whether the concentrations of named bioelements influence oocyte and embryo quality, thereby impacting the overall outcomes of the IVF process. Additionally, the research sought to explore whether any of these bioelements could serve as predictive markers for IVF success.
Methods
Study Population:
The research obtained approval from the Ethics Committee of the Clinical Center Kragujevac under decision number 01-20-582 dated 25th June 2020. This study is experimental in nature and involves the use of human-origin materials in vitro. A total of 120 patients were enrolled in the study.
Prior to their participation, all 120 individuals were fully briefed on the research objectives. Subsequently, they provided their informed consent by signing the necessary documentation to partake in the study.
Prior to the commencement of the study, all patients underwent a series of analyses and diagnostic procedures, including microbiological examinations such as vaginal and cervical swabs for bacteria and fungi, Chlamydia trachomatis testing, bacterial vaginosis assessment, Toxoplasma gondii screening, Venereal Disease Research Laboratory Test (VDRL), as well as virological examinations for Hepatitis B surface antigen (HbsAg), Hepatitis C virus (HCV), Human immunodeficiency virus (HIV), and Rubella. Furthermore, hormonal status evaluations were conducted during the 2-3 days of the menstrual cycle, encompassing tests for follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, progesterone, testosterone, prolactin, thyroid-stimulating hormone (TSH), triiodothyronine (T3), thyroxine (T4), and anti-Müllerian hormone (AMH). Cervical screening procedures, including Papanicolaou smears and colposcopy, were also performed, in addition to ultrasonographic examinations with a vaginal probe and assessment of tubal patency through hysterosalpingography.
Inclusion Criteria for the Study: Couples who have exhausted other infertility treatment options, couples who have one child conceived through in vitro fertilization within the existing community, women up to age 43, preserved ovarian function, a normal body mass index (BMI less than 30) for women, and a partner’s spermogram within normal limits (if there is a confirmed issue with the female partner, such as blocked fallopian tubes) or if the male partner has diagnosed issues like decreased sperm count, reduced sperm motility, or a lower percentage of morphologically normal sperm (if no issue is confirmed in the female partner).
Exclusion Criteria from the Study: Couples who have not exhausted other infertility treatment options, women without preserved ovarian reserve, women with a BMI over 30 kg/m², any anomalies or benign tumors of the uterus, fallopian tubes, or ovaries that interfere with the IVF process, the occurrence or development of pregnancy, the presence of malignant or suspicious tumors in the uterus, fallopian tubes, or ovaries, any diseases (internal, immunological, infectious, neurological, psychiatric) if assisted reproductive technology procedures are conducted without specialist approval, diseases where anesthesia or pregnancy could possibly represent a threat to the patient’s life. Patients with other endocrine disorders affecting fertility and those whose partners have been diagnosed with azoospermia are also excluded from the study.
Patients undergoing examination were separated into two groups based on the outcome of in vitro fertilization: the first group included the patients who did not achieve pregnancy, while the second group included those who did. They were also separated into two age groups: those aged 35 and younger, and those older than 35.
Material:
During the ovarian stimulation procedure, a short protocol [
30] was employed. Oocyte maturation was induced using either 5000 IU of chorionic gonadotropin or 250 mcg of alpha chorionic gonadotropin. For luteal phase support, 125 mg of depot progesterone was administered.
Oocyte aspiration was carried out under ultrasound guidance with a double-lumen aspiration needle. Both follicular fluid and oocytes were collected for analysis. The follicular fluid, which contained the oocytes, was isolated and then centrifuged at 3000 rpm for 10 minutes to obtain pure follicular fluid devoid of cellular elements. A 5 ml aliquot of the total follicular fluid volume was used for analysis.
All disposable plastic materials and media were sterile, IVF-tested, and approved. Media were utilized directly from their original, ready-to-use packaging.
Cultivation from gametes to blastocysts was conducted in a K-MINC incubator set to 37°C, with an atmosphere comprising 6% CO2, 5% O2, and the remainder N2, and maintained at 95% humidity.
Methods:
Follicular fluid was extracted by puncturing follicles larger than 18 mm following controlled ovarian stimulation, performed in the intervention room at the Department of Assisted Reproduction Technologies. The examination of the follicular fluid and the collection of oocytes were conducted under a stereomicroscope in the embryology laboratory at the same department, ensuring controlled conditions.
The embryological procedures involved in the ART process include several key steps: oocyte collection, in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), fertilization assessment, embryo evaluation, and embryo transfer. The concentration of the analyzed enzymes in the follicular fluid was measured by the Laboratory Diagnostic Service at the Clinical Center Kragujevac.
Ovulation Stimulation:
Ovarian response to stimulation was monitored through ultrasound examinations and laboratory hormone analyses, including estradiol, progesterone, LH, and FSH levels. Medication dosages for stimulation were adjusted daily based on these results.
Stimulation continued until the leading follicle reached a diameter of 20 mm or until at least two follicles each reached a diameter of 18 mm. When serum estradiol levels indicated the existance of two or more follicles with a diameter greater than 18 mm, the final trigger injection was administered. Follicle aspiration was then performed 34-36 hours later.
Follicle Aspiration:
Follicle aspiration is a surgical procedure conducted under ultrasound guidance while the patient is under general anesthesia. The duration of the procedure typically ranges from 20 to 30 minutes, depending on the number and availability of follicles in the ovaries. Upon completion of the aspiration, the collected follicular fluid was examined microscopically in the embryology laboratory. The number of oocytes and their quality were assessed based on the appearance of the cumulus-oocyte complex.
Embryological Procedures Troughout the IVF Process:
Following follicle aspiration, oocytes are counted and their maturity is assessed based on the appearance of the cumulus-corona-oocyte complex. The oocytes are then placed in an equilibrated medium for fertilization and stored in an incubator until fertilization occurs. Oocyte quality is evaluated based on nuclear maturity after the removal of cumulus cells. The oocytes may be classified as mature MII (indicated by the presence of the first polar body in the perivitelline space), immature MI (absence of the first polar body), or immature GV (characterized by vesicles in the ooplasm and absence of the first polar body).
Fertilization is carried out using either in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI). Fertilization is checked 16-20 hours after the procedure, with successful fertilization indicated by the presence of two pronuclei and two polar bodies.
All viable zygotes obtained through IVF or ICSI are stored in the incubator for further development. The resulting embryos are assessed every 24 hours until the embryo transfer, which occurs on the 3rd or 5th day after fertilization.
Table 1.
outlines the specific criteria used for evaluating embryos on days 3 and 5.
Table 1.
outlines the specific criteria used for evaluating embryos on days 3 and 5.
| Embryo quality on day 3 post-fertilization |
| Class I |
Excellent, without or with 1-10% fragmentation, perfect symmetry |
| Class II |
Medium, with 11-25% of fragmentation, moderate asymmetry |
| Class III |
Poor, > 25% of fragmentation, expressed asymmetry |
| Blastocyst quality on day 5 day post-fertilization |
| Based on the appearance of ICM |
I |
A high number of closely packed cells |
| II |
A high number of cells that are not closely packed |
| III |
Very small number of cells |
| Based on the appearance of the trophectoderm |
I |
Many cells form a cohesive epithelium |
| II |
A small number of cells that form a loose epithelium |
| III |
A very small number of cells |
On the third or fifth day following oocyte fertilization, up to three embryos were transferred under transabdominal ultrasound guidance. From the day of oocyte aspiration, patients received daily intramuscular injections of 250 mg of depot progesterone as luteal phase support. Pregnancy was confirmed by a positive serum β-hCG level 14 days after embryo transfer. Clinical pregnancies were verified by transvaginal ultrasound, which identified a gestational sac with a viable embryo at the sixth week of gestation.
Parameters Assessed in the Study:
The concentrations of Na (measured by selective electrode), K (measured by selective electrode), Ca (measured by ion-selective electrode), Mg (measured by spectrophotometry), and Fe (measured by colorimetry) in the follicular fluid were determined using the AU 680 instrument from Beckman Coulter.
Statistical Evaluation and Results Interpretation:
Prior to statistical data analysis, the normality of the data distribution was assessed using the Kolmogorov-Smirnov test. Based on the p-value from this test, the appropriate statistical test was selected: parametric ANOVA for p > 0.05, indicating a normal distribution, or non-parametric Mann-Whitney and Kruskal-Wallis tests for p < 0.05, indicating a non-normal distribution.
The Spearman rank correlation coefficient was employed to evaluate the direction and strength of the relationship between two variables. This coefficient (rho) ranges from -1 to +1, where the sign denotes the nature of the correlation- positive (both variables increase or decrease together) or negative (one variable decreases as the other increases, and vice versa). The magnitude of the coefficient reflects the strength of the correlation: 0.10 to 0.29 indicates a small effect, 0.30 to 0.49 a moderate effect, and 0.50 to 1.00 a large effect.
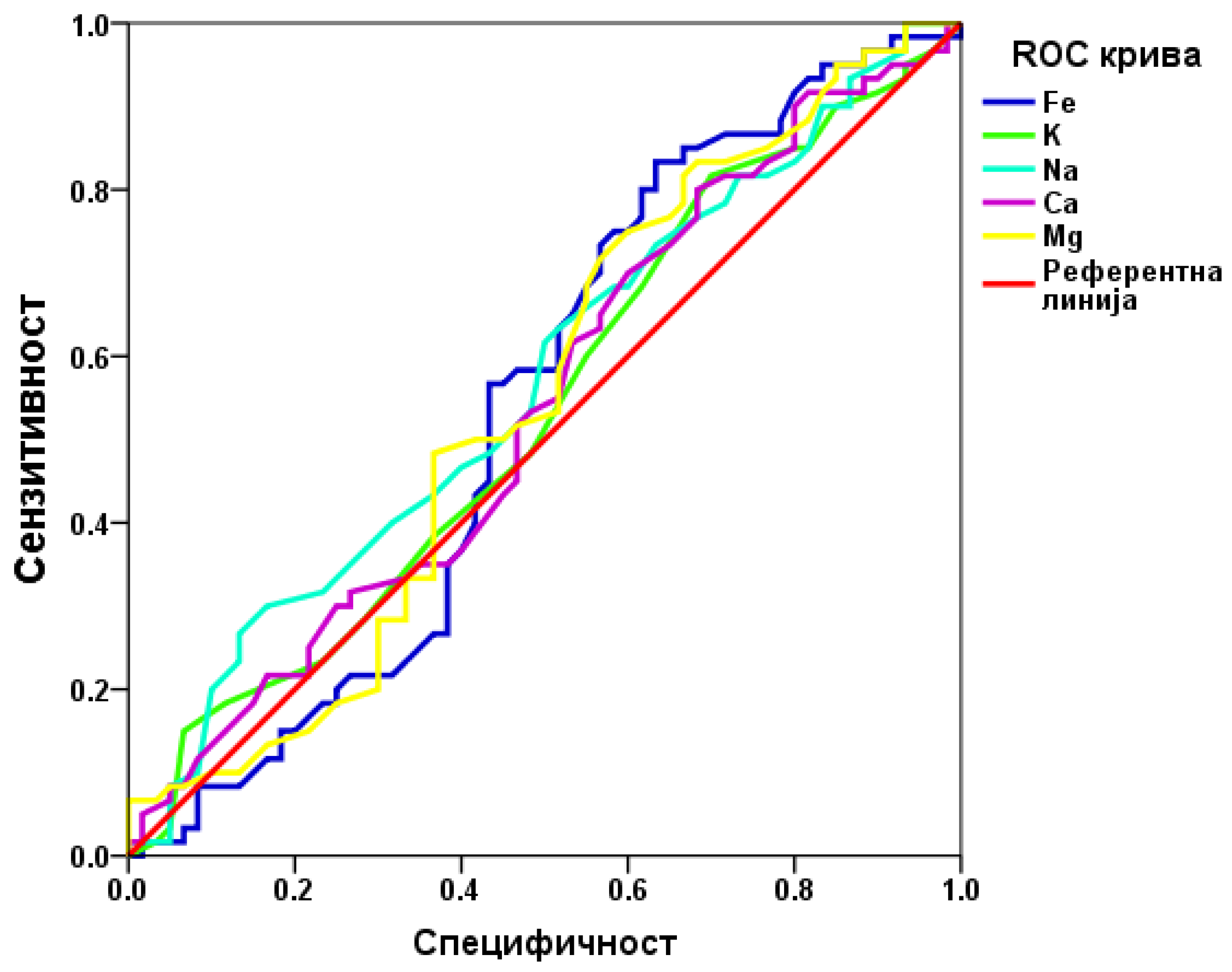
The predictive efficiency of the examined parameter was assessed using ROC analysis. Statistical significance was defined as p < 0.05. All statistical analyses were conducted using SPSS 20 software, with results presented as mean values in both tabular and graphical formats.
Discussion
The primary aim of this study was to identify new, easily analyzable parameters in follicular fluid that could predict the outcome of in vitro fertilization (IVF) and potentially enhance therapy during ovarian stimulation. The study explored whether various elements of follicular fluid could serve as predictors for IVF outcomes and oocyte quality, which are critical for successful embryo formation and pregnancy [
30,
31].
In addition to the composition of follicular fluid, several other factors can influence oocyte quality and IVF outcomes, including patient age, serum anti-Müllerian hormone (AMH) levels, body mass index (BMI), the number of antral follicles, stimulation protocol, endometrial thickness, sperm selection and quality, and the number of high-quality oocytes. These variables can significantly impact IVF results [
12,
30,
31,
32,
33,
34,
35,
36,
37]. The primary goal of IVF is to achieve optimal follicle development (around 17 mm in diameter), obtain a higher number of mature oocytes, and develop embryos of high quality for transfer, which should ideally result in a normal pregnancy and the birth of a healthy child [
12,
30,
38,
39,
40].
Our study included 120 participants with an average age of 36.74 years. The body mass index of participants was within normal ranges (<30 kg/m²), and the average serum AMH level was near the lower end of normal (2.56 ng/ml). The average number of retrieved oocytes per patient was 8.22, with 6.7 mature oocytes capable of fertilization, indicating adequate stimulation. The highest number of Class I embryos obtained was 2.98, positively influencing the overall outcome.
This study aimed to assess the concentrations of selected bioelements (Na, K, Ca, Mg, and Fe) in follicular fluid, their distribution across different age groups, and their impact on oocyte quality and IVF outcomes.
Previous research suggests a near-linear relationship between follicle size, bioelement concentrations in follicular fluid, and their levels in blood, indicating that follicular fluid may originate from blood transudate [
15]. Our results showed no statistically significant differences in bioelement concentrations between age groups (≤35 years and >35 years) or between patients with positive and negative IVF outcomes. Iron and magnesium concentrations were higher in older patients, while other bioelements (potassium, sodium, calcium) were slightly higher in younger women. Notably, all bioelements were found in slightly higher concentrations in follicular fluid from patients with negative IVF outcomes.
In our study, higher potassium levels correlated with a greater number of follicles and oocytes, while higher sodium levels were associated with higher estradiol levels at trigger and a greater number of follicles and oocytes. These findings are consistent with earlier studies indicating that Na and K ratios do not affect oocyte quality or IVF outcomes [
41,
42].
Higher calcium levels were linked to younger patients, a greater number of follicles and oocytes, and more Class I embryos. Magnesium levels did not correlate with any monitored parameters. Literature presents varied conclusions about factors influencing oocyte and embryo quality, with some studies showing that low calcium and magnesium concentrations do not affect oocyte quality, while others suggest high magnesium concentrations improve embryonic development by regulating calcium homeostasis. On the other hand, high magnesium concentration and a high magnesium-to-calcium ratio (2:1) improve early embryonic development because magnesium regulates calcium homeostasis in the zygote [
42]. Low concentrations of magnesium and calcium may inhibit meiosis continuation, with the inhibitory effect of low magnesium concentration being greater than that of low calcium concentration [
42].
Iron concentration in follicular fluid was the only parameter correlating with BMI (-0.182; p = 0.046), with higher iron levels associated with lower BMI. This finding aligns with previous research, which also found no association between iron levels in follicular fluid and embryo quality [
43].
ROC analysis aimed to determine if any bioelements in follicular fluid could predict IVF outcomes. The results indicated that none of the examined bioelements are useful predictive markers for IVF outcomes.
Both our study and previous research have not found a correlation between bioelement concentrations in follicular fluid and IVF outcomes or oocyte quality. Since follicular fluid serves as the microenvironment for the in vivo development of oocytes, it is essential that it contains all necessary components for follicle development and oocyte maturation. Although numerous targeted analyses [
12,
44] have been conducted, identifying a clinically useful biomarker has proven challenging due to existing limitations, and this issue has yet to be resolved [
45].
These findings highlight the need for further research to improve IVF outcomes. Future studies should also consider assessing bioelement concentrations in blood during ovulation stimulation in addition to follicular fluid.
Table 2.
Average Values of Monitored Parameters During the In Vitro Fertilization Process.
Table 2.
Average Values of Monitored Parameters During the In Vitro Fertilization Process.
| |
Mean |
SD |
| Age |
36,74 |
4,74 |
| BMI (kg/m2) |
24,89 |
2,67 |
| Serum AMH (ng/ml) |
2,56 |
2,89 |
| Estradiol on the day of final injection (pg/ml) |
5618,02 |
3182,48 |
| Number of follicles on the day of final injection |
12,61 |
9,95 |
| Number of oocytes |
8,22 |
5,77 |
| Number of MII oocytes |
6,70 |
4,83 |
| Fertilized oocytes |
4,96 |
3,60 |
| Number of embryos |
5,33 |
3,35 |
| Number of Class 1 embryos |
2,98 |
2,66 |
Table 3.
Concentrations of Parameters in Follicular Fluid Across 120 Patients.
Table 3.
Concentrations of Parameters in Follicular Fluid Across 120 Patients.
| |
Mean |
Median |
Min |
Max |
SD |
| Sodium (mmol/l) |
152,54 |
151,00 |
135 |
186 |
10,08 |
| Potassium (mmol/l) |
4,88 |
4,90 |
4,10 |
5,90 |
0,41 |
| Calcium (mmol/l) |
2,30 |
2,29 |
1,96 |
2,77 |
0,16 |
| Magnesium (mmol/l) |
0,55 |
0,56 |
0,21 |
0,94 |
0,14 |
| Iron (µmol/l) |
6,98 |
6,05 |
0,50 |
17,30 |
3,60 |
Table 4.
Distribution of Determined Parameter Concentrations in Follicular Fluid by Patient Age.
Table 4.
Distribution of Determined Parameter Concentrations in Follicular Fluid by Patient Age.
| |
≤ 35 years |
> 35 years |
| N=46 |
N=74 |
| Sodium (mmol/l) |
153,04±11,31 |
152,23±9,31 |
| Potassium (mmol/l) |
4,91±0,45 |
4,86±0,38 |
| Calcium (mmol/l) |
2,31±0,19 |
2,29±0,15 |
| Magnesium (mmol/l) |
0,54±0,15 |
0,56±0,14 |
| Iron (mmol/l) |
6,71±3,38 |
7,00±3,74 |
Table 5.
Concentrations of Determined Parameters in Follicular Fluid Relative to In Vitro Fertilization Outcomes.
Table 5.
Concentrations of Determined Parameters in Follicular Fluid Relative to In Vitro Fertilization Outcomes.
| |
Negative |
Positive |
| N=60 |
N=60 |
| Sodium (mmol/l) |
153,88±10,66 |
151,20±9,37 |
| Potassium (mmol/l) |
4,91±0,41 |
4,86±0,41 |
| Calcium (mmol/l) |
2,31±0,17 |
2,28±0,16 |
| Magnesium (mmol/l) |
0,57±0,15 |
0,54±0,13 |
| Iron (mmol/l) |
7,30±4,04 |
6,48±3,07 |
Table 6.
Correlation of Parameters in Follicular Fluid with Age, BMI, and Serum AMH.
Table 6.
Correlation of Parameters in Follicular Fluid with Age, BMI, and Serum AMH.
| |
Age |
BMI |
Serum AMH |
| r/rho |
P |
r/rho |
p |
r/rho |
p |
| Sodium (mmol/l) |
-0,1311
|
0,154 |
-0,0291
|
0,754 |
0,1312
|
0,153 |
| Potassium (mmol/l) |
-0,0941
|
0,308 |
-0,0191
|
0,839 |
0,1642
|
0,073 |
| Calcium (mmol/l) |
-0,2051
|
0,024 |
-0,0371
|
0,668 |
0,1632
|
0,076 |
| Magnesium (mmol/l) |
0,0221
|
0,816 |
-0,0991
|
0,283 |
-0,0982
|
0,288 |
| Iron (mmol/l) |
-0,0101
|
0,917 |
-0,1821
|
0,046 |
-0,0482
|
0,602 |
Table 8.
Correlation Analysis of Follicular Fluid Parameters with Oocyte Quality, Fertilization Success, and Embryo Quality.
Table 8.
Correlation Analysis of Follicular Fluid Parameters with Oocyte Quality, Fertilization Success, and Embryo Quality.
| |
Number of MII oocytes |
Number of fertilized oocytes |
Number of embryos |
Class I embryos |
Positive outcome |
| rho |
p |
r/rho |
r/rho |
r/rho |
p |
p |
p |
rho |
p |
| Sodium (mmol/l) |
0,1092
|
0,238 |
0,0712
|
0,440 |
0,1481
|
0,106 |
0,1732
|
0,058 |
-0,1202
|
0,190 |
| Potassium (mmol/l) |
0,1492
|
0,104 |
0,1162
|
0,207 |
0,1771
|
0,052 |
0,1582
|
0,085 |
-0,0612
|
0,508 |
| Calcium (mmol/l) |
0,1562
|
0,090 |
0,0982
|
0,287 |
0,1831
|
0,046 |
0,2002
|
0,028 |
-0,0702
|
0,446 |
| Magnesium (mmol/l) |
-0,1212
|
0,188 |
-0,1152
|
0,210 |
-0,0711
|
0,439 |
-0,0682
|
0,459 |
-0,0792
|
0,390 |
| Iron (µmol/l) |
-0,2062
|
0,024 |
0,1412
|
0,125 |
-0,1411
|
0,125 |
-0,1622
|
0,077 |
-0,0712
|
0,443 |