Submitted:
28 August 2024
Posted:
28 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
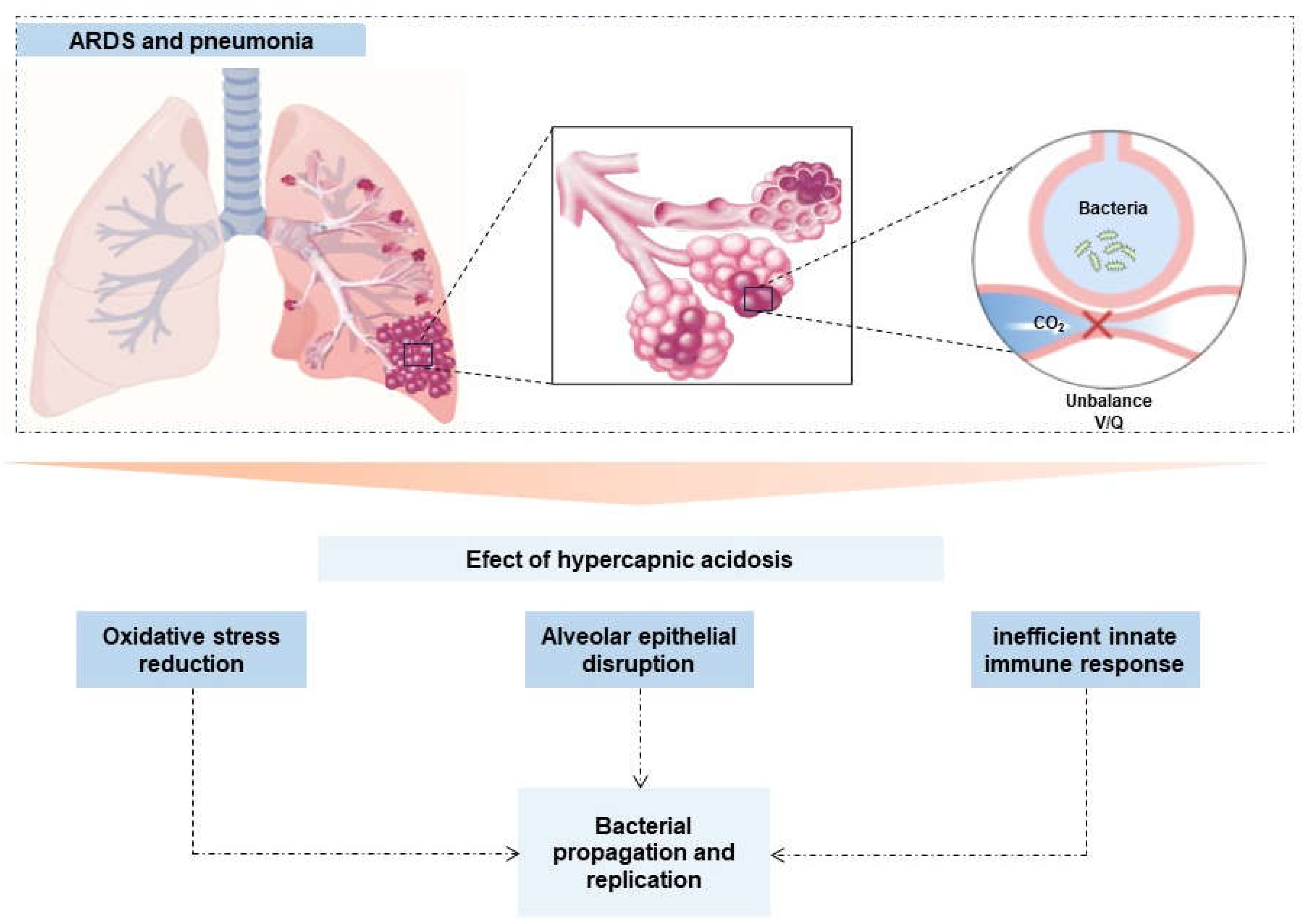
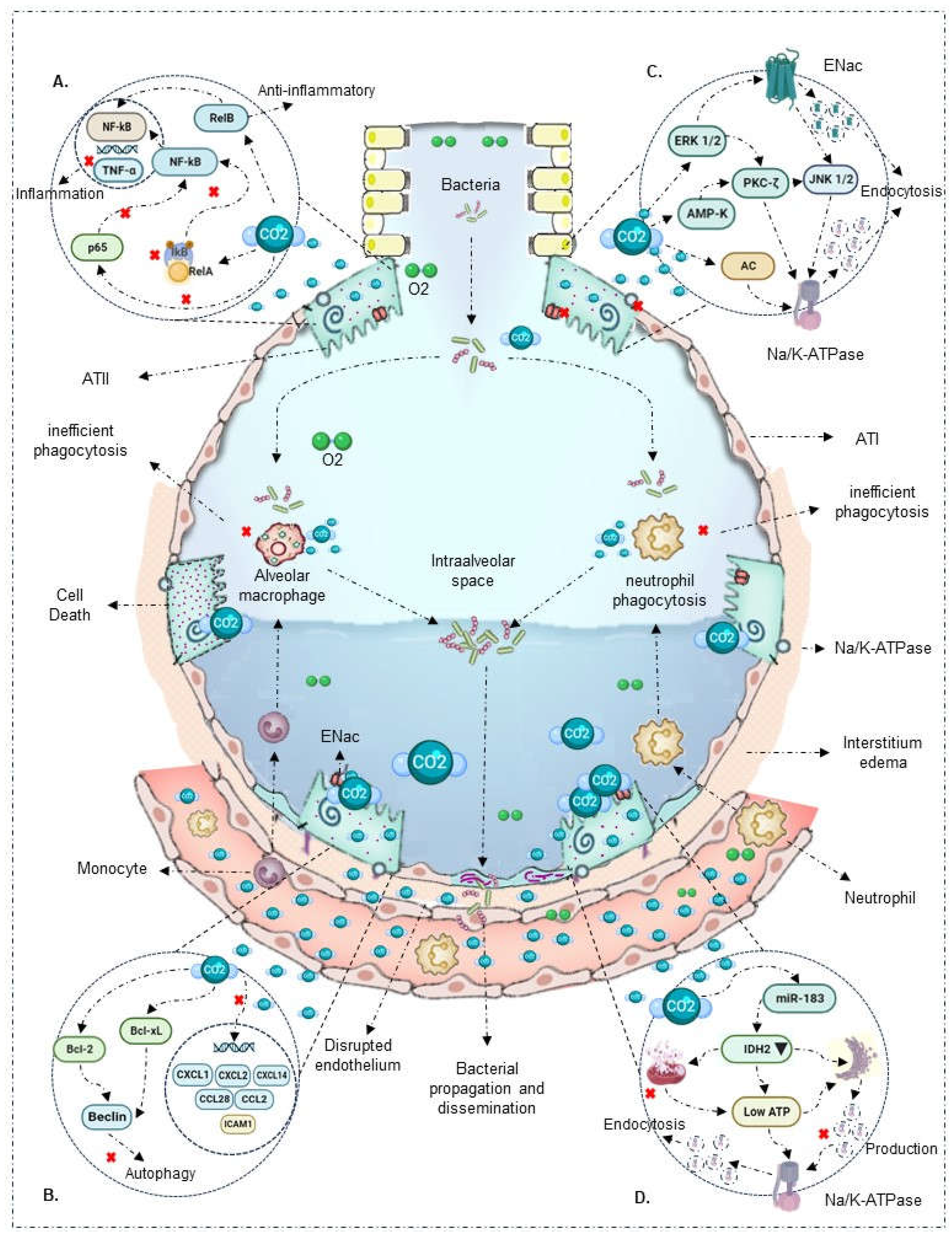
2.1. Innate Immune Response
2.2. Respiratory epithelium
3. Discussion
3.1. Study Limitations
4. Materials and Methods
4.1. Eligibility criteria
4.2. Search strategy
4.3. Information sources
4.4. Data Extraction
4.5. Results mapping
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Section | Item | PRISMA-ScR checklist item | Page # |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a scoping review | 1 |
| Structured summary | 2 | Provide a structured abstract that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives | 1 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach. | 1, 2 |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts, and context) or other relevant key elements used to conceptualize the review questions and/or objectives. | 2 |
| Methods | |||
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration numbe | NA |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language, and publication status), and provide a rationale | 10, 11 |
| Information sources | 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed | 11 |
| Search | 8 | Present the full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 11, Appendix A2 |
| Selection of sources of evidence | 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review | 11 |
| Data charting process | 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that have been tested by the team before their use, and whether data charting was done independently or in duplicate) and any processes for obtaining and confirming data from investigators. | 11, Figure 1. PRISMA-ScR checklist. |
| Data ítems | 11 | List and define all variables for which data were sought and any assumptions and simplifications made | 11 |
| Critical appraisal of individua sources of evidence | 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate) | 11 |
| Synthesis of results | 13 | Describe the methods of handling and summarizing the data that were charted. | 11 |
| Result | |||
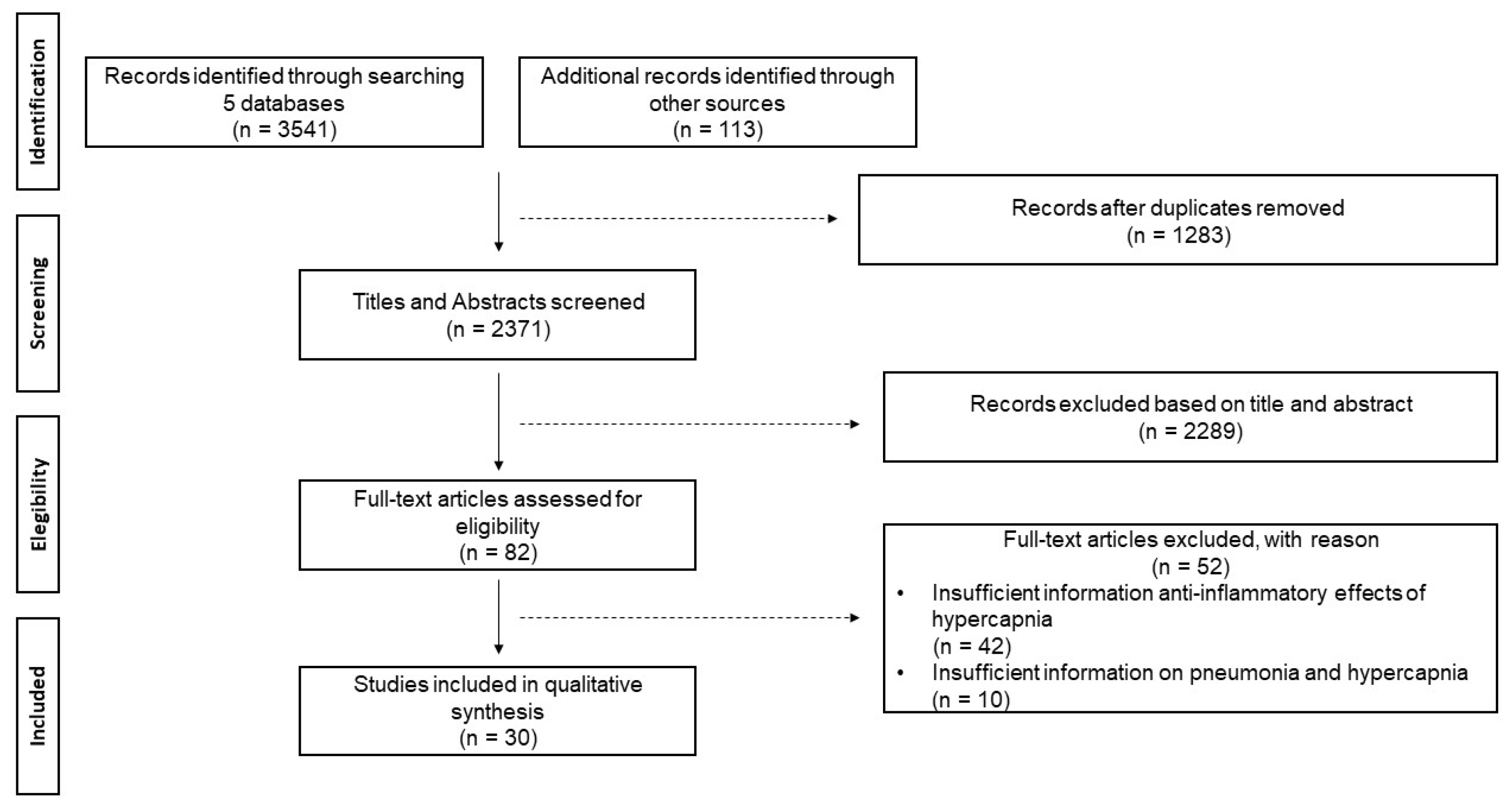
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram | Figure 1. PRISMA-ScR checklist. |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations | Table 2 |
| Critical appraisal within sources of evidence | 16 | If done, present data on critical appraisal of included sources of evidence (see item 12) | |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives. | Table 2, figures 2 and 3 |
| Synthesis of results | 18 | Summarize and/or present the charting results as they relate to the review questions and objectives | Table 2 |
| Discussion | |||
| Summary of evidence | 19 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups | 2 - 10 |
| Limitations | 20 | Discuss the limitations of the scoping review process. | 10 |
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps. | 11 |
| Funding | |||
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review | NA |
| Search number | Search terms |
|---|---|
| 1 | Pneumonia |
| 2 | Hypercapnia |
| 3 | Acute respiratory distress syndrome (ARDS) |
| 4 | Ventilator-Associated Pneumonia (VAP) |
| 5 | Anti-Inflammatories |
| 6 | Anti-Inflammatories |
| 7 | 1 AND 2 AND 3 |
| 8 | 4 AND 2 |
| 9 | 5 AND 2 AND 1 |
References
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA [Internet]. 2016 Feb 23;315(8):788. [CrossRef]
- Máca J, Jor O, Holub M, Sklienka P, Burša F, Burda M, et al. Past and Present ARDS Mortality Rates: A Systematic Review. Respir Care [Internet]. 2017 Jan;62(1):113–22. [CrossRef]
- Wu N, Hanrahan J, Bornstein J, Chen S-Y. Healthcare costs utilization and costs of patients hospitalized with acute respiratory distress syndrome (ARDS) in US commercially-insured individuals and Medicare beneficiaries. In: 21 Acute Critical Care [Internet]. European Respiratory Society; 2015. p. PA2139. [CrossRef]
- Amato MBP, Barbas CSV, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, et al. Effect of a Protective-Ventilation Strategy on Mortality in the Acute Respiratory Distress Syndrome. N Engl J Med [Internet]. 1998 Feb 5;338(6):347–54. [CrossRef]
- The Acute Respiratory Distress Syndrome Network. Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. N Engl J Med [Internet]. 2000 May 4;342(18):1301–8. [CrossRef]
- Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev [Internet]. 2013 Feb 28; [CrossRef]
- Tuxen D V., Williams TJ, Scheinkestel CD, Czarny D, Bowes G. Use of a Measurement of Pulmonary Hyperinflation to Control the Level of Mechanical Ventilation in Patients with Acute Severe Asthma. Am Rev Respir Dis [Internet]. 1992 Nov;146(5_pt_1):1136–42. [CrossRef]
- Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med [Internet]. 1990 Sep;16(6):372–7. [CrossRef]
- Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: A prospective study. Crit Care Med [Internet]. 1994 Oct;22(10):1568–78. [CrossRef]
- Shigemura M, Lecuona E, Sznajder JI. Effects of hypercapnia on the lung. J Physiol [Internet]. 2017 Apr 15;595(8):2431–7. [CrossRef]
- Nin N, Muriel A, Peñuelas O, Brochard L, Lorente JA, Ferguson ND, et al. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med [Internet]. 2017 Feb 20;43(2):200–8. [CrossRef]
- Chonghaile MN, Higgins BD, Costello J, Laffey JG. Hypercapnic Acidosis Attenuates Lung Injury Induced by Established Bacterial Pneumonia. Anesthesiology [Internet]. 2008 Nov 1;109(5):837–48. [CrossRef]
- Madotto F, Rezoagli E, McNicholas BA, Pham T, Slutsky AS, Bellani G, et al. Patterns and Impact of Arterial CO2 Management in Patients With Acute Respiratory Distress Syndrome. Chest [Internet]. 2020 Nov;158(5):1967–82. [CrossRef]
- Gendreau S, Geri G, Pham T, Vieillard-Baron A, Mekontso Dessap A. The role of acute hypercapnia on mortality and short-term physiology in patients mechanically ventilated for ARDS: a systematic review and meta-analysis. Intensive Care Med [Internet]. 2022 May 16;48(5):517–34. [CrossRef]
- Masterson C, Horie S, McCarthy SD, Gonzalez H, Byrnes D, Brady J, et al. Hypercapnia in the critically ill: insights from the bench to the bedside. Interface Focus [Internet]. 2021 Apr 6;11(2):20200032. [CrossRef]
- Beitler JR, Malhotra A, Thompson BT. Ventilator-induced Lung Injury. Clin Chest Med [Internet]. 2016 Dec;37(4):633–46. [CrossRef]
- O’Croinin DF, Nichol AD, Hopkins N, Boylan J, O’Brien S, O’Connor C, et al. Sustained hypercapnic acidosis during pulmonary infection increases bacterial load and worsens lung injury*. Crit Care Med [Internet]. 2008 Jul;36(7):2128–35. [CrossRef]
- Wang N, Gates KL, Trejo H, Favoreto S, Schleimer RP, Sznajder JI, et al. Elevated CO 2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. FASEB J [Internet]. 2010 Jul 24;24(7):2178–90. [CrossRef]
- Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Prim [Internet]. 2019 Mar 14;5(1):18. [CrossRef]
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med [Internet]. 2018 Oct 2;169(7):467–73. [CrossRef]
- Reyes Martín E, Prieto Martín A, Díaz Martín D, Álvarez-Mon Soto M. Inmunidad innata e inmunidad adaptativa. Med - Programa Form Médica Contin Acreditado [Internet]. 2013 Mar;11(28):1760–7. [CrossRef]
- O’Toole D, Hassett P, Contreras M, Higgins BD, McKeown STW, McAuley DF, et al. Hypercapnic acidosis attenuates pulmonary epithelial wound repair by an NF- B dependent mechanism. Thorax [Internet]. 2009 Nov 1;64(11):976–82. [CrossRef]
- Cummins EP, Oliver KM, Lenihan CR, Fitzpatrick SF, Bruning U, Scholz CC, et al. NF-κB Links CO2 Sensing to Innate Immunity and Inflammation in Mammalian Cells. J Immunol [Internet]. 2010 Oct 1;185(7):4439–45. [CrossRef]
- Oliver KM, Lenihan CR, Bruning U, Cheong A, Laffey JG, McLoughlin P, et al. Hypercapnia Induces Cleavage and Nuclear Localization of RelB Protein, Giving Insight into CO2 Sensing and Signaling. J Biol Chem [Internet]. 2012 Apr;287(17):14004–11. [CrossRef]
- Contreras M, Ansari B, Curley G, Higgins BD, Hassett P, O’Toole D, et al. Hypercapnic acidosis attenuates ventilation-induced lung injury by a nuclear factor-κB–dependent mechanism. Crit Care Med [Internet]. 2012 Sep;40(9):2622–30. [CrossRef]
- Yang W, Yue Z, Cui X, Guo Y, Zhang L, Zhou H, et al. Comparison of the effects of moderate and severe hypercapnic acidosis on ventilation-induced lung injury. BMC Anesthesiol [Internet]. 2015 Dec 30;15(1):67. [CrossRef]
- Masterson C, O’Toole D, Leo A, McHale P, Horie S, Devaney J, et al. Effects and Mechanisms by Which Hypercapnic Acidosis Inhibits Sepsis-Induced Canonical Nuclear Factor-κB Signaling in the Lung. Crit Care Med [Internet]. 2016 Apr;44(4):e207–17. [CrossRef]
- Horie S, Ansari B, Masterson C, Devaney J, Scully M, O’Toole D, et al. Hypercapnic acidosis attenuates pulmonary epithelial stretch-induced injury via inhibition of the canonical NF-κB pathway. Intensive Care Med Exp [Internet]. 2016 Dec 22;4(1):8. [CrossRef]
- Keogh CE, Scholz CC, Rodriguez J, Selfridge AC, von Kriegsheim A, Cummins EP. Carbon dioxide-dependent regulation of NF-κB family members RelB and p100 gives molecular insight into CO2-dependent immune regulation. J Biol Chem [Internet]. 2017 Jul;292(27):11561–71. [CrossRef]
- Liu Y, Chacko BK, Ricksecker A, Shingarev R, Andrews E, Patel RP, et al. Modulatory effects of hypercapnia on in vitro and in vivo pulmonary endothelial–neutrophil adhesive responses during inflammation. Cytokine [Internet]. 2008 Oct;44(1):108–17. [CrossRef]
- Ni Chonghaile M, Higgins BD, Costello JF, Laffey JG. Hypercapnic acidosis attenuates severe acute bacterial pneumonia-induced lung injury by a neutrophil-independent mechanism*. Crit Care Med [Internet]. 2008 Dec;36(12):3135–44. [CrossRef]
- Peltekova V, Engelberts D, Otulakowski G, Uematsu S, Post M, Kavanagh BP. Hypercapnic acidosis in ventilator-induced lung injury. Intensive Care Med [Internet]. 2010 May 6;36(5):869–78. [CrossRef]
- Gates KL, Howell HA, Nair A, Vohwinkel CU, Welch LC, Beitel GJ, et al. Hypercapnia Impairs Lung Neutrophil Function and Increases Mortality in Murine Pseudomonas Pneumonia. Am J Respir Cell Mol Biol [Internet]. 2013 Nov;49(5):821–8. [CrossRef]
- Nardelli LM, Rzezinski A, Silva JD, Maron-Gutierrez T, Ornellas DS, Henriques I, et al. Effects of acute hypercapnia with and without acidosis on lung inflammation and apoptosis in experimental acute lung injury. Respir Physiol Neurobiol [Internet]. 2015 Jan;205:1–6. [CrossRef]
- Casalino-Matsuda SM, Nair A, Beitel GJ, Gates KL, Sporn PHS. Hypercapnia Inhibits Autophagy and Bacterial Killing in Human Macrophages by Increasing Expression of Bcl-2 and Bcl-xL. J Immunol [Internet]. 2015 Jun 1;194(11):5388–96. [CrossRef]
- Casalino-Matsuda SM, Wang N, Ruhoff PT, Matsuda H, Nlend MC, Nair A, et al. Hypercapnia Alters Expression of Immune Response, Nucleosome Assembly and Lipid Metabolism Genes in Differentiated Human Bronchial Epithelial Cells. Sci Rep [Internet]. 2018 Sep 10;8(1):13508. [CrossRef]
- Casalino-Matsuda SM, Berdnikovs S, Wang N, Nair A, Gates KL, Beitel GJ, et al. Hypercapnia selectively modulates LPS-induced changes in innate immune and DNA replication-related gene transcription in the macrophage. Interface Focus [Internet]. 2021 Apr 6;11(2):20200039. [CrossRef]
- Vadász I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J Clin Invest [Internet]. 2008 Jan 10; [CrossRef]
- Nichol AD, OʼCronin DF, Howell K, Naughton F, OʼBrien S, Boylan J, et al. Infection-induced lung injury is worsened after renal buffering of hypercapnic acidosis. Crit Care Med [Internet]. 2009 Nov;37(11):2953–61. [CrossRef]
- Welch LC, Lecuona E, Briva A, Trejo HE, Dada LA, Sznajder JI. Extracellular signal-regulated kinase (ERK) participates in the hypercapnia-induced Na,K-ATPase downregulation. FEBS Lett [Internet]. 2010 Sep 24;584(18):3985–9. [CrossRef]
- Vohwinkel CU, Lecuona E, Sun H, Sommer N, Vadász I, Chandel NS, et al. Elevated CO2 Levels Cause Mitochondrial Dysfunction and Impair Cell Proliferation. J Biol Chem [Internet]. 2011 Oct;286(43):37067–76. [CrossRef]
- Vadász I, Dada LA, Briva A, Helenius IT, Sharabi K, Welch LC, et al. Evolutionary Conserved Role of c-Jun-N-Terminal Kinase in CO2-Induced Epithelial Dysfunction. Königshoff M, editor. PLoS One [Internet]. 2012 Oct 8;7(10):e46696. [CrossRef]
- Lecuona E, Sun H, Chen J, Trejo HE, Baker MA, Sznajder JI. Protein Kinase A-Iα Regulates Na,K-ATPase Endocytosis in Alveolar Epithelial Cells Exposed to High CO 2 Concentrations. Am J Respir Cell Mol Biol [Internet]. 2013 May;48(5):626–34. [CrossRef]
- Gwoździńska P, Buchbinder BA, Mayer K, Herold S, Morty RE, Seeger W, et al. Hypercapnia Impairs ENaC Cell Surface Stability by Promoting Phosphorylation, Polyubiquitination and Endocytosis of β-ENaC in a Human Alveolar Epithelial Cell Line. Front Immunol [Internet]. 2017 May 23;8. [CrossRef]
- Cortes-Puentes GA, Westerly B, Schiavo D, Wang S, Stroetz R, Walters B, et al. Hypercapnia Alters Alveolar Epithelial Repair by a pH-Dependent and Adenylate Cyclase-Mediated Mechanism. Sci Rep [Internet]. 2019 Jan 23;9(1):349. [CrossRef]
- Kryvenko V, Wessendorf M, Morty RE, Herold S, Seeger W, Vagin O, et al. Hypercapnia Impairs Na,K-ATPase Function by Inducing Endoplasmic Reticulum Retention of the β-Subunit of the Enzyme in Alveolar Epithelial Cells. Int J Mol Sci [Internet]. 2020 Feb 21;21(4):1467. [CrossRef]
- Gabrielli NM, Mazzocchi LC, Kryvenko V, Tello K, Herold S, Morty RE, et al. TRAF2 Is a Novel Ubiquitin E3 Ligase for the Na,K-ATPase β-Subunit That Drives Alveolar Epithelial Dysfunction in Hypercapnia. Front Cell Dev Biol [Internet]. 2021 Jul 2;9. [CrossRef]
- Kryvenko V, Wessendorf M, Tello K, Herold S, Morty RE, Seeger W, et al. Hypercapnia Induces Inositol-Requiring Enzyme 1α–Driven Endoplasmic Reticulum–associated Degradation of the Na,K-ATPase β-Subunit. Am J Respir Cell Mol Biol [Internet]. 2021 Dec;65(6):615–29. [CrossRef]
- Curley G, Laffey JG, Kavanagh BP. Bench-to-bedside review: Carbon dioxide. Crit Care [Internet]. 2010;14(2):220. [CrossRef]
- Morales Quinteros L, Bringué Roque J, Kaufman D, Artigas Raventós A. Importance of carbon dioxide in the critical patient: Implications at the cellular and clinical levels. Med Intensiva (English Ed [Internet]. 2019 May;43(4):234–42. [CrossRef]
- Vadász I, Hubmayr RD, Nin N, Sporn PHS, Sznajder JI. Hypercapnia: A Nonpermissive Environment for the Lung. Am J Respir Cell Mol Biol [Internet]. 2012 Apr;46(4):417–21. [CrossRef]
- Curley GF, Kavanagh BP, Laffey JG. Hypocapnia and Hypercapnia. In: Murray and Nadel’s Textbook of Respiratory Medicine [Internet]. Elsevier; 2016. p. 1527–1546.e8. [CrossRef]
- Curley G, Hayes M, Laffey JG. Can “permissive” hypercapnia modulate the severity of sepsis-induced ALI/ARDS? Crit Care [Internet]. 2011;15(2):212. [CrossRef]
- Silvestre C, Vyas H. Is permissive hypercapnia helpful or harmful? Paediatr Child Health (Oxford) [Internet]. 2015 Apr;25(4):192–5. [CrossRef]
- Hamacher J, Hadizamani Y, Borgmann M, Mohaupt M, Männel DN, Moehrlen U, et al. Cytokine–Ion Channel Interactions in Pulmonary Inflammation. Front Immunol [Internet]. 2018 Jan 4;8. [CrossRef]
- Baloğlu E, Mairbäurl H. In Search of a Sensor: How Does CO 2 Regulate Alveolar Ion Transport? Am J Respir Cell Mol Biol [Internet]. 2021 Dec;65(6):571–2. [CrossRef]
- Vadász I, Sznajder JI. Gas Exchange Disturbances Regulate Alveolar Fluid Clearance during Acute Lung Injury. Front Immunol [Internet]. 2017 Jul 4;8. [CrossRef]
| Citacion | Year | Country | Type of study |
|---|---|---|---|
| Vadász I | 2008 Jan | USA | Experimental |
| O’Croinin DF | 2008 Jul | Ireland | Experimental |
| Liu Y | 2008 Aug | USA | Experimental |
| Chonghaile MN | 2008 Nov | Ireland | Experimental |
| Ni Chonghaile M | 2008 Dec | Ireland | Experimental |
| Nichol AD | 2009 Nov | Ireland | Experimental |
| O'Toole D | 2009 Nov | Ireland | Experimental |
| Wang N | 2010 Feb | USA | Experimental |
| Welch LC | 2010 Sep | USA | Experimental |
| Peltekova V | 2010 Oct | Canada | Experimental |
| Cummins EP | 2010 Oct | Ireland | Experimental |
| Vohwinkel CU | 2011 Oct | USA | Experimental |
| Oliver K | 2012 Apr | Ireland | Experimental |
| Contreras M | 2012 Sep | Ireland | Experimental |
| Vadász I | 2012 Oct | USA | Experimental |
| Lecuona E | 2013 May | USA | Experimental |
| Gates KL | 2013 Nov | USA | Experimental |
| Nardelli LM | 2015 Jan | Brazil | Experimental |
| Casalino-Matsuda SM | 2015 Apr | USA | Experimental |
| Yang W | 2015 Dec | China | Experimental |
| Masterson C | 2016 Apr | Ireland | Experimental |
| Horie S | 2016 Dec | Ireland | Experimental |
| Gwoździńska P | 2017 May | Germany | Experimental |
| Keogh C | 2017 Jul | Ireland | Experimental |
| Casalino-Matsuda SM | 2018 Sep | USA | Experimental |
| Cortes-Puentes GA | 2019 Jan | USA | Experimental |
| Kryvenko V | 2020 Feb | Germany | Experimental |
| Casalino-Matsuda SM | 2021 Apr | USA | Experimental |
| Gabrielli NM | 2021 Jul | Germany | Experimental |
| Kryvenko V | 2021 Dec | Germany | Experimental |
| Author | Experimental Model | Secondary Injury | CO2 Concentration | Immunomodulatory Effect |
|---|---|---|---|---|
| Alteration in transcription of innate response | ||||
| O'Toole et al., 2009 [22] |
In vitro: Confluent bronchial, respiratory, and alveolar A549 type II epithelial cells | No | 10%, 15% | Hypercapnia directly inhibits NF-κB activation |
| Liu et al., 2008 [30] |
In vivo (rats) and In vitro: Human pulmonary microvascular endothelial cells | LPS and bacterial TNF (24 hours) | 5%, 10% | In vitro model shows that 4 hours of hypercapnia and metabolic acidosis increase NF-κB expression |
| Wang N et al., 2010 [18] |
In vitro: THP-1 cells, human alveolar macrophages, RAW 264.7 mouse macrophages | Bacterial LPS and TLR | 5%, 9%, 12.5%, 20% | Hypercapnia attenuates TNF and IL-6 mRNA induction independently of extracellular metabolic acidosis. Also, it does not affect IkBα and RelA/p65 phosphorylation |
| Cummins et al., 2010 [23] |
In vitro: Respiratory epithelial cells | Endotoxin | 5%, 10% | Hypercapnia blocks IκBα phosphorylation, degradation, and p65 translocation, inactivating NF-κB |
| Oliver et al., 2012 [24] |
In vivo (rats) and In vitro: Alveolar epithelial A549 cells | No | 5%, 10% | Hypercapnia promotes RelB cleavage and localization during the non-canonical signaling pathway, exerting an anti-inflammatory and immunosuppressive effect |
| Contreras et al., 2012 [25] |
In vivo (rats) and In vitro: Alveolar epithelial A549 cells | No | 5% | Hypercapnia inactivates NF-κB in vivo and in vitro, maintaining cytoplasmic IκBα concentrations |
| Yang et al., 2015 [26] |
In vivo (rats) | None | CO2 ventilation: 35 - 150 mmHg | Hypercapnia reduced IkBα expression and NF-κB activity |
| Masterson et al., 2016 [27] |
In vivo (rats) and In vitro: Alveolar epithelial cells | Escherichia coli (4 hours) | Inspired CO2 ventilation: 5% | Hypercapnia inhibits p65 subunit translocation, reduces IκBβ intrinsic phosphorylation, IκBα concentrations, and maintains NF-κB inactivation |
| Horie et al., 2016 [28] |
In vitro: Bronchial and alveolar A549 cells | Lung stretch (24 - 120 hours) | 5%, 10% | Hypercapnia blocks IκBα phosphorylation, degrades proteins, inactivating NF-κB |
| Keogh C et al., 2017 [29] |
In vitro: Alveolar epithelial A549 cells | No | 5%, 10% | Hypercapnia promotes non-canonical NF-κB RelB/p100 activation, exerting an anti-inflammatory and immunosuppressive effect |
| Decease in innate immune response capacity | ||||
| O’Croinin et al., 2008 [17] |
In vivo (rats) | Escherichia coli (48 hours) | Inspired CO2 ventilation: 5% | Prolonged hypercapnia affects neutrophil phagocytic activity, worsening bacterial infection-induced lung injury |
| Liu et al., 2008 [30] |
In vivo (rats) and In vitro: Human pulmonary microvascular endothelial cells | LPS and bacterial TNF (24 hours) | 5%, 10% | 4 hours of hypercapnia increased neutrophil adhesion and expression of ICAM1, VCAM1, E-selectin, and IL-8, exhibiting a proinflammatory effect |
| Chonghaile et al., 2008 [12] |
In vivo (rats): Established pneumonia | Escherichia coli (6 hours) | Inspired CO2 ventilation: 5% | In a model of established pneumonia, hypercapnia increased TNF-α and IL-6 levels, improved airway pressures. In the presence of antibiotic therapy, it reduced bacterial count and pneumonia-induced histological injury |
| Ni Chonghaile et al., 2008 [31] |
In vivo (rats) | Escherichia coli (6 hours) | Inspired CO2 ventilation: 5% | During pulmonary sepsis, hypercapnia affects neutrophil phagocytic activity, reducing bacterial death and worsening lung injury |
| Nichol et al., 2009 [39] |
In vivo (rats) and In vitro: Bronchial epithelial cells | Endotoxin and Escherichia coli | Inspired CO2 ventilation: 5% | Buffered hypercapnia in the absence of metabolic acidosis does not alter phagocytic capacity and neutrophil concentration. However, it increases maximum airway pressure, exacerbating lung injury |
| Wang N et al., 2010 [18] |
In vitro: THP-1 cells, human alveolar macrophages, RAW 264.7 mouse macrophages | Lipopolysaccharides and bacterial TLR | 5%, 9%, 12.5%, 20% | Hypercapnia inhibits macrophage phagocytosis |
| Peltekova et al., 2010 [32] |
In vivo (rats) | Harmful ventilation | Inspired CO2 ventilation: 0%, 5%, 12%, 25% | Hypercapnia decreases TNFα levels and increases nitrotyrosine formation, enhancing lung injury |
| Cummins et al., 2010 [23] |
In vitro: Respiratory epithelial cells | Endotoxin | 5%, 10% | Hypercapnia reduces proinflammatory response gene expression (CCL2, ICAM1, and TNF-α) |
| Oliver et al., 2012 [24] |
In vivo (rats) and In vitro: Alveolar epithelial A549 cells | No | 5%, 10% | Hypercapnia suppresses TNF-α expression independent of pH |
| Gates et al., 2013 [33] |
In vivo (rats) | Pseudomonas aeruginosa (96 hours) | Inspired CO2 ventilation: 10% | In a model of established pneumonia, hypercapnia alters neutrophil phagocytic capacity, increases bacterial load and dissemination to other organs, and reduces early cytokine response (IL-6, TNF) |
| Nardelli et al., 2015 [34] |
In vivo (rats) | Paraquat | PaCO2 ventilation: 35 - 80 mmHg | Hypercapnia, independent of acidosis, reduces IL-6, IL-1β, and type III pro-collagen expression. It also decreases neutrophil count and apoptosis processes |
| Casalino-Matsuda et al., 2015 [35] |
In vitro: Human alveolar macrophages | No | 5%, 15% | Hypercapnia increases anti-apoptotic factors Bcl-2, Bcl-xL, inhibiting Beclin 1 and autophagy and bacterial death |
| Yang et al., 2015 [26] |
In vivo (rats) | None | CO2 ventilation: 35 - 150 mmHg | Hypercapnia attenuates TNF-α levels independent of pH |
| Casalino-Matsuda et al., 2018 [36] |
In vitro: Bronchial epithelial cells | Lung injury (24 hours) | 20% | Sustained hypercapnia for 24 hours alters the regulation of immunoregulatory genes such as CXCL1, CXCL2, CXCL14, CCL28, IL-6R, and TLR4 |
| Casalino-Matsuda et al., 2021 [37] |
In vitro: Human alveolar macrophages, RAW 264.7 mouse macrophages | No | 20% | Hypercapnia downregulates NF-κB pathway genes, type I interferon and antiviral signaling genes, cytokines, and other associated genes |
| Disruption and resealing of alveolar epithelial cells | ||||
| Vadász et al., 2008 [38] |
In vitro: Alveolar epithelial cells | No | PaCO2: 60 - 120 mmHg | Hypercapnia increases AMPK overexpression, inducing PKC-ζ activation, promoting Na/K-ATPase endocytosis, and inhibiting alveolar fluid reabsorption |
| Nichol et al., 2009 [39] |
In vivo (rats) and In vitro: Bronchial epithelial cells | Endotoxin and Escherichia coli | Inspired CO2 ventilation: 5% | Buffered hypercapnia, without metabolic acidosis, decreases lung cell wound repair rate |
| O'Toole et al., 2009 [22] |
In vitro: Confluent bronchial, respiratory, and alveolar A549 type II epithelial cells | No | 10%, 15% | Hypercapnia decreases lung cell wound healing through a mechanism associated with direct NF-Κb activation inhibition |
| Welchl et al., 2010 [40] |
In vitro: Alveolar epithelial cells | No | 5% | Hypercapnia activates ERK 1/2, promoting Na/K-ATPase endocytosis and inhibiting alveolar fluid reabsorption |
| Vohwinkel et al., 2011 [41] |
In vitro: Alveolar epithelial A549 cells and fibroblasts | No | 5% | Hypercapnia increases microRNA-183 expression, downregulating isocitrate dehydrogenase 2 expression, affecting mitochondrial function and cell proliferation |
| Vadász et al., 2012 [42] |
In vitro: Alveolar epithelial cells | No | PaCO2: 60 - 120 mmHg | Hypercapnia induces JNK activation, leading to Na/K-ATPase downregulation and alveolar epithelial dysfunction |
| Lecuona et al., 2013 [43] |
In vitro: Alveolar epithelial cells | No | PaCO2: 60 - 120 mmHg | Hypercapnia activates soluble adenylate cyclase CO2/HCO3-sensitive, stimulating cAMP production and PKA activity, favoring Na/K-ATPase endocytosis and alveolar epithelial dysfunction |
| Gwoździńska et al., 2017 [44] |
In vitro: Alveolar epithelial A549 cells | No | 5% | Hypercapnia promotes ERK/AMPK/JNK axis activation, affecting ENaC cellular activity via polyubiquitination mechanism |
| Cortes-Puentes et al., 2019 [45] |
In vitro: Alveolar epithelial cells | No | 80 Torr | Hypercapnia activates soluble adenylate cyclase, delaying lung membrane resealing and alveolar epithelial cell repair |
| Kryvenko et al., 2020 [46] |
In vitro: Alveolar epithelial A549 and rat type II cells | No | 5%, 15% | Hypercapnia causes Na/K-ATPase-β retention in the endoplasmic reticulum and significant reduction in the alveolar membrane |
| Gabrielli et al., 2021 [47] |
In vitro: Alveolar epithelial A549 cells | No | 5% | Hypercapnia promotes ubiquitination of E3 ligase, favoring Na/K-ATPase β subunit endocytosis and degradation mediated by PKC-ζ |
| Kryvenko et al., 2021 [48] |
In vitro: Alveolar epithelial A549 cells | No | 5% | Hypercapnia affects Na/K-ATPase β subunit in the endoplasmic reticulum and basal membrane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





