1. Introduction
Visual development begins in intrauterine life and continues after birth when both neurological and optical structures are immature [
1]. Normal function depends on two intact visual pathways: the anterior pathways, including eyes, optic nerves, and chiasm; and the posterior or geniculate-striate pathways, including the lateral geniculate nucleus in the thalamus, optic radiations, and visual cortexes (particularly in the occipital lobe of the brain) [
1,
2].
The development of the visual system is immediately initiated after childbirth, through stimuli and environmental interactions, and rapidly improves during the first year [
1,
3]. With this in mind, infants and children are more susceptible to visual development problems during the first few years of life [
1]. Due to the complexity of this system, it can be affected by many pathophysiological mechanisms, including abnormalities in refraction, retinal definition, optic nerve transmission; maturation of the visual cortex and integration of visual data; and external factors, such as nutrition and stimuli/light deprivation [
3].
From this perspective, the current literature has shown that some viral infections, such as those caused by the Zika virus, may be associated with visual impairments in early childhood [
4,
5]. Regarding COVID-19 during pregnancy, recently, it was reported that the coronavirus infection may have a negative impact on maternal and fetal outcomes, including abortions, prematurity, low birth weight, admissions to the neonatal intensive care unit (NICU), fetal distress, and increased maternal and fetal mortality [
6,
7]. Also, a single study showed that the experience of the COVID-19 pandemic might be associated with a higher risk of delay in the development of fine motor and communication for 1-year-old children [
8].
These findings raise concerns about the risks that COVID-19 may impose on healthy vision development in younger children, which can impact their overall development (cognitive, motor, and social). However, these outcomes have been little explored in the current literature so far, leading to difficulty in diagnosing these possible impairments in childhood. In this sense, it is known that new technological resources can be used in order to facilitate the visual tracking of children in different clinical and screening contexts. Thus, it is hypothesized that the use of computational tools can help in the perception and prompt objective diagnosis of visual alterations in children between 0 and 24 months of age.
Therefore, this study aimed to compare functional vision parameters - related to the perception of movement - of children exposed to SARS-CoV-2 with those without contact with the virus in the womb, in postnatal life, or through the family context.
2. Materials and Methods
This is a cross-sectional research conducted with children aged from 0 to 24 months who had contact with the SARS-CoV-2 or were not exposed to it.
2.1. Compliance with Ethical Standards
This study was approved by the Ethics Committee for Research with Human Beings (CAAE: 41500720.0.1001.0121). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. All those responsible for the participants signed a written informed consent form (ICF) before the data collection. The present manuscript was performed according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statements. The study started in March 2021 and ended in December 2022.
2.2. Study Steps
2.2.1. Development of the Computational Method
At first, the technological resource for examining the visual functions of newborns and typical/atypical children was idealised by the researchers. This computational method is based on a set of machine learning tools for face mapping, followed by a tracking procedure applied to the eyes region over time. The elaboration was conducted with the partnership of a team of researchers within the scope of development and evaluation of the visual system and computational methodology from two prestigious public universities in Brazil.
2.2.2. Recruitment of Participants
The participants were recruited from a hospital of the Brazilian public health system and primary health care units in the region of the extreme south of Santa Catarina state - Brazil. They were selected by convenience and followed the eligibility criteria below: (I) children aged from 0 to 24 months of life, (II) of both genders, (III) with gestational ages (GA) included in the prematurity classification or born at term, and (IV) who had contact with coronavirus - during the gestational period, postnatal or through family members residing in the same place - or who were not exposed to it. Participants with neurological diagnoses were excluded, due to the possible negative outcomes on visual development and the creation of selection bias. All the infants recruited for the study were evaluated by a pediatric ophthalmologist according to the protocol of the maternity hospital of origin, which included the red reflex test (RRT) and an evaluation of the eye fundus (fundoscopy). In addition, during the evaluations at the Follow-Up Ambulatory conducted by the research group, the families were instructed to schedule an ophthalmology visit between 6 and 12 months of age, as recommended by the Brazilian Pediatric Ophthalmology Society and the Brazilian Society of Pediatrics [
9].
2.2.3. Initial Assessment
This step was performed through a caregiver interview before the visual assessment procedures. A trained researcher asked about the participant’s birth data and the mother's vaccination against COVID-19, besides collecting current anthropometric data, such as weight and height. In addition, this researcher asked about the child's contact with the SARS-CoV-2 and the nature of this exposure to classify the participants according to the study groups.
2.2.4. Study Groups
The participants were divided into four groups considering the nature of their contact with COVID-19. These groups were:
Gestational COVID-19 Group (G1), which included children who had their mothers diagnosed with the coronavirus during pregnancy by a positive result for COVID-19 in the RT-PCR test for RNA, Rapid Antigen Search Test or detection of total antibodies and serologies of SARS-CoV-2;
Postnatal COVID-19 group (G2), in which mothers were diagnosed by one of the same aforementioned methods after the child was born;
Familiar COVID-19 group (G3), for children who had contact with a family member living in the same household and who was diagnosed with COVID-19 by the same methods previously considered in the other study groups;
Control group (G4) was composed of participants who were not exposed to COVID-19 previously.
2.3. Visual Assessment
The visual stimulus used to observe and record visual functions was a black and white concentric circles target with a diameter of 10x10cm on a 15x15cm white Polyvinyl Chloride (PVC) card or a black and white figurative face with dimensions of 10x10cm also printed on a 15x15cm card in PVC material according to the study by Ricci et al [
10]. The card was held approximately 30 cm away from the midline of the eyes comprising 19 degrees of visual angle.
During the visual assessment, the brightness of the environment was controlled and the children remained seated with or without support by a researcher. The entire procedure was recorded with a modified HardLine Cutie 6809® to capture the infrared spectrum. An infrared LED illumination system was used to increase the response of the imaging sensor. The complete evaluation was recorded on an Avell C62 MOB Notebook Intel® Core™ i7-11800H and the analysis of the visual parameters was performed by two independent researchers blinded to the classification of the groups through the footage, and in case of disagreement, a third independent evaluator was requested. The functional parameters of vision (slow eye perception movements) recorded using the computational method were divided into primary and secondary variables according to
Table 1.
In horizontal visual tracking, the stimulus initiated in the midline of the eyes on the child's face and was moved in the left and right lateral directions at a speed of approximately 3°/second. In both of the aforementioned primary variables, the secondary variables “Looking continuity” and “Visual alterations” were observed [
11].
Looking continuity was classified as 'Continuous' when the child binocularly followed the stimulus movement in full amplitude of movement (cephalocaudal or lateral-lateral); 'Discontinuous', when the child followed the movement in full amplitude, but with discontinuity of the look at the target; 'Brief', when the child did not follow the full amplitude of movement (angle between 30 and 90 degrees); and 'Does not perform', when the child did not follow the movement of the target. Eye alignment comprised whether the child had any form of visual misalignment or eye alteration during stimulus presentation (strabismus or nystagmus) [
11].
The primary variable 'Fixation Time' was also divided into 'Stable', when the child had the ability to maintain visual attention on the stimulus for a time ≥ 3 seconds, and 'Unstable', for visual attention with a time < 3 seconds [
11,
12].
For the vestibule-ocular reflex, one of the visual targets was kept fixed in the midline of the child's eye, while a researcher performed the passive rotation movement of the participant's neck to the right and left sides three times. In addition to the secondary variables “Looking continuity” and “Eye alignment” (this one classified in the same way as visual alterations), it was observed whether the child kept the eyes fixed on the stimulus during neck rotation for the variables 'Left side' and 'Right side'. The response was 'Present' when the eyes moved in the opposite direction to the head movement and 'Absent' when the eyes did not move [
13].
2.4. Statistical Analysis
For the statistical analysis of this study, the software SPSS® version 20.0 was employed. The data normality was verified by the Shapiro-Wilk test. Descriptive variables were determined by means (± standard deviation) and frequency. In the study, the prevalence and the risk estimate (Odds Ratio) of visual changes according to coronavirus exposure were analysed. All analyses were reproduced considering a p-value < 0.05 (5%).
3. Results
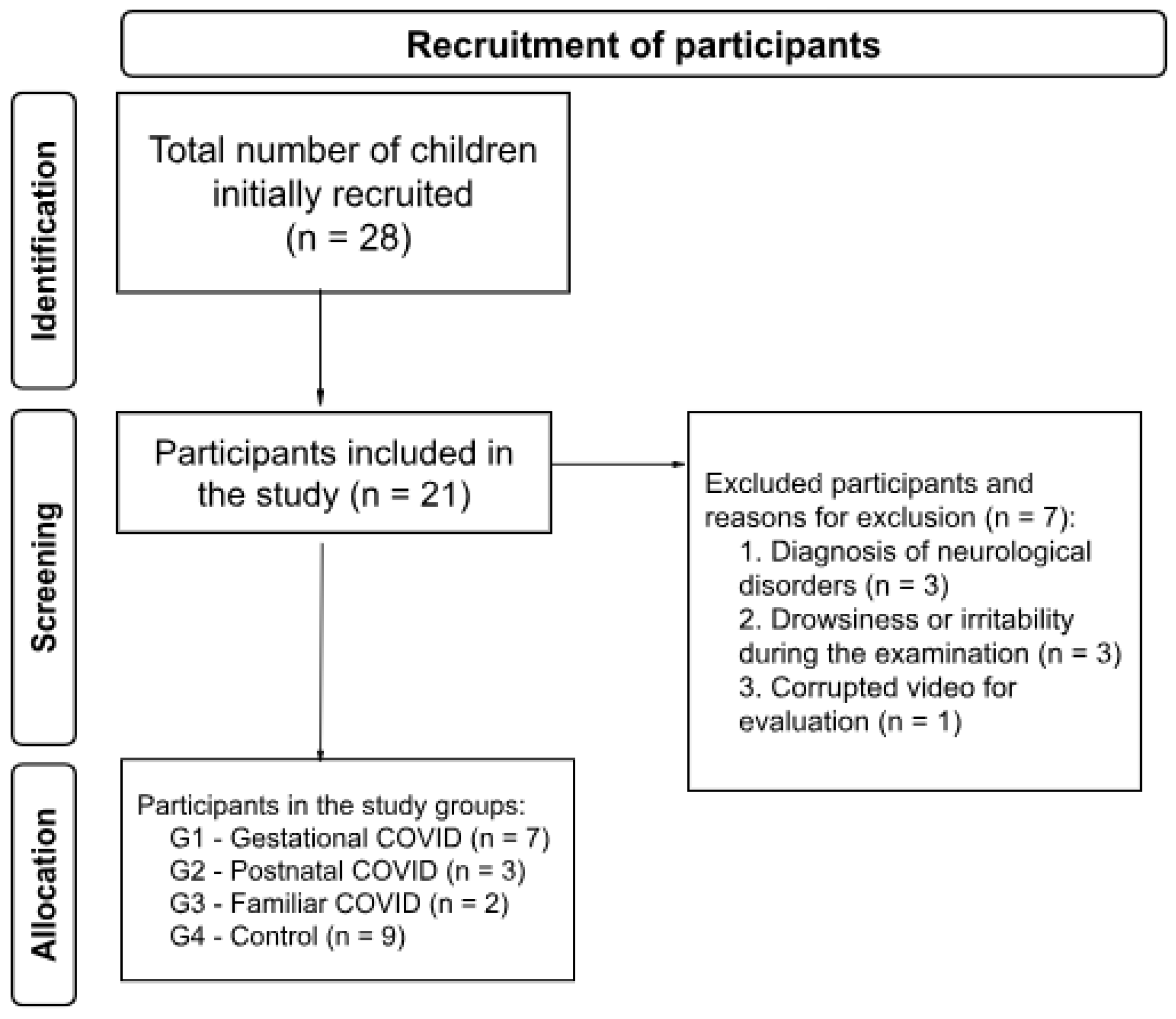
The initial screening performed at the follow-up ambulatory for high-risk newborns was composed of 28 children; however, three of them presented a diagnosis of neurological disorders, three did not complete the evaluation due to drowsiness or irritability during the consultation, and one presented a corrupted video. Thus, a total of 21 children participated in this study. All the infants included in the study showed no alterations in the assessment by the paediatric ophthalmologist, including RRT and fundoscopy, at the time of discharge from the maternity hospital of origin. The participants were divided into four groups, as follows: Gestational COVID Group (G1), Postnatal COVID Group (G2), Familiar COVID Group (G3), and Control Group (G4).
The number of participants in each group is shown in
Figure 1 and birth data is displayed in
Table 2.
Table 3 systematizes the odds ratio data for each one of the functional parameters analyzed in this study.
3.1. Prevalence and Odds Ratio
3.1.1. Gestational COVID Group (G1)
In the prevalence study, 14.3% of the infants who were exposed to COVID-19 during the gestational period had a risk for unstable fixation time, convergent strabismus in the horizontal tracking and vestibule-ocular reflex, and looking discontinuity for the vestibule-ocular reflex. For these same variables, the risk estimate (Odds Ratio) showed that these participants were 3.33 (CI 95%: 0.31 - 36.11) more likely to develop unstable fixation time, 0.50 (CI 95%: 0.26 - 9.46), for convergent strabismus in the horizontal tracking, 0.46 (CI 95%: 0.02 - 8.69) of convergent strabismus and 6.00 (CI 95%: 0.56 - 63.68) of looking discontinuity, both for vestibule-ocular reflex.
The G1 infants did not present higher risks in the vestibule-ocular reflexes (left and right), however, there is a chance that 1.40 of them will not perform these reflexes in the future. Despite this, these children had a prevalence risk of 28.6% for looking discontinuity on horizontal tracking with 2.50 (CI 95%: 0.34-18.33) more chances of developing this change.
3.1.2. Postnatal COVID Group (G2)
In contrast, when the infant is diagnosed with COVID-19 in the postnatal period, the prevalence ratio for visual alterations in horizontal tracking, ocular alignment for vestibule-ocular reflex, and vestibule-ocular reflexes (left and right) does not increase. However, the increased possibility of 1.13 (CI 95%: 0.95-1.35), 1.12 (CI 95%: 0.95-1.32), and 1.29 (CI 95%: 1.00-1.65) for these variables, respectively, may exist for these same children.
Despite this, we found a 66.7% prevalence of looking discontinuity for the vestibule-ocular reflex and 0.25 (CI 95%: 0.02-3.34) odds for developing this abnormal response in these infants, as well as 50% and 0.70 (CI 95%: 0.04-13.18) looking discontinuity on horizontal tracking, and finally 33.3% and 0.77 (CI 95%: 0.06-10.49) for unstable fixation time.
3.1.3. Familiar COVID Group (G3)
For the infants in G3, the only prevalence risk found was for the variable looking continuity in horizontal tracking, and for this, the value was 50% with a risk estimate of 0.70 (CI 95%: 0.04-13.18). However, analyzing the odds ratio of the remaining secondary variables showed increased odds of 1.73 (CI 95%: 1.18-2.53) for looking discontinuity for the vestibule-ocular reflex, 1.46 (CI 95%: 1.08-1.98) for an unstable fixation time, 1.27 (95% CI: 1.00-1.60) for not performing the vestibule-ocular reflexes, as well as 1.12 (95% CI: 0.95-1.32) for the diagnosis of convergent strabismus during horizontal tracking and 1.12 (95% CI: 0.96-1.30) of this same finding during the vestibule-ocular reflex.
4. Discussion
This cross-sectional study aimed mainly to compare functional vision parameters - related to movement perception - of children who were exposed to SARS-CoV-2 (G1, G2, and G3) with those who had no contact with the virus (Control Group). To our knowledge, this is a pioneering study on the subject, conducted with exposure and control groups composed of infants born during the pandemic, generating similar chances of exposure to the virus.
Although current evidence suggests that children are mostly asymptomatic or have mild disease, much remains to be explored regarding the age groups at the highest risk for developing sequelae [
14,
15]. The lower number of infections or diseases in children has been explained by differences in the pediatric immune response or differences in their susceptibility to infection [
16,
17]. In parallel, the immune system of children is believed to respond less aggressively to SARS-CoV-2 infection, producing less extensive lung damage and other extrapulmonary manifestations than cases in adults [
16].
However, it is important to consider that children have a mosaic of disease presentations, ranging from asymptomatic to pediatric inflammatory syndrome [
16,
18]. In this context, current evidence suggests several ocular manifestations of COVID-19 in the pediatric population, including episcleritis, conjunctivitis, optic neuritis, cranial nerve palsies, retinal vein occlusion, retinal vasculitis, retinal changes, orbital myositis, and orbital cellulitis [
19]. With this in mind, this is the first cross-sectional study to evaluate the impact of COVID-19 on visual outcomes of infants related to movement perception, also called functional vision parameters.
The present study demonstrated an increased risk for some of the variables being studied in the exposure groups. The G1 presented an increased risk for unstable fixation time, convergent strabismus in the horizontal tracking and vestibule-ocular reflex, looking discontinuity on horizontal tracking and looking discontinuity for the vestibule-ocular reflex, but the odds ratio for these variables presented a large confidence interval, not allowing establishing a risk relation for intrauterine exposure to the coronavirus. The G2, in parallel, showed no increased risk by means of the odds ratios, with no possibility of inferring a risk relationship between postnatal exposure to SARS-CoV-2 and functional vision impairment in infants.
Finally, the G3 was more inclined to look discontinuity on horizontal tracking, as well as had increased odds ratios with shorter confidence intervals for not performing the vestibule-ocular reflexes, looking discontinuity for the vestibule-ocular reflex, and unstable fixation time. While these findings suggest a higher risk for functional vision impairments in this group of infants, it is essential to remember that none of the prevalence analyses demonstrated statistically significant values to draw more robust inferences about the exposure to coronavirus as a risk factor for the development of these visual function complications.
These findings are consistent with current evidence showing that, despite the immense range of possible ocular manifestations in the pediatric population affected by COVID-19, the visual prognosis observed has been of minimal or no deficits in most of the cases, possibly not leading to such dramatic changes in the visual function of infants exposed to the virus [
19]. However, these findings are based on small case studies and case series published during the pandemic, and there are limited studies that evaluate these sequelae in the long term and with considerable sample size. This may also be associated with the fact that children are more often asymptomatic and underrepresented in cohort epidemiologic studies involving the disease [
16,
18].
Other variables, such as gestational age and birth weight, have been shown to have a considerable influence on the risk of developing a range of sequelae in the development of the visual pathway. These sequelae already established in the scientific literature include strabismus, refractive errors, low visual acuity, color and contrast sensitivity deficits, visual field defects, and other clinical conditions such as optic atrophy, congenital cataract, and retinopathy of prematurity [
20,
21,
22,
23].
Thus, prematurity and low birth weight may be more important risk factors for changes in functional parameters of vision in child development than exposure to SARS-CoV-2 itself - even in intrauterine life - since the last trimester of pregnancy is extremely important for the development of the visual system, because at this stage the retina and visual cortex are undergoing maturation, differentiation, and cell remodeling, and the first visual functions appear [
24].
This study has some limitations, which should be mentioned. The first is related to the methodology of the cross-sectional descriptive study itself, which does not allow us to make causal inferences, since this methodology cannot adequately assess an outcome after an exposure. In addition, some variables of interest may not have presented statistically significant values and with narrower confidence intervals due to the sample size - more limited precisely because of the difficulty of diagnosis in children, who are often asymptomatic [
16,
18].
On the other hand, this work is innovative in using a new computational resource to assess the visual functional parameters of infants, fostering an advance for scientific research in the field of Pediatrics. In addition, for visual assessment, it was used the tool already established in literature by Ricci et al [
10], with the evaluators blinded to the exposure category of the participants.
5. Conclusions
This work revealed that there were no statistically significant differences in the prevalence and odds ratios between the exposure and control groups. Consequently, exposure of infants to SARS-CoV-2 in the womb, in postnatal life, or through the family context could not be proven to be a risk factor for alterations in functional vision parameters in these groups.
Author Contributions
Conceptualization, G.R., V.C., M.C., A.S. and C.M..; methodology, G.R., V.C., A.S. and C.M.; software, G.R., A.S. and C.M.; validation, G.R., V.C., M.C., A.S. and C.M; formal analysis, G.R., V.C., and C.M.; investigation, G.R., V.C., M.C., A.S. and C.M.; resources, G.R. and C.M.; data curation, G.R., V.C., M.C., A.S. and C.M.; writing—original draft preparation, G.R., V.C. and C.M.; writing—review and editing, G.R., V.C., M.C., A.S. and C.M.; visualization, G.R., V.C., M.C., A.S. and C.M.; supervision, C.M.; project administration, G.R. and C.M.; funding acquisition, M.C. and C.M.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) - Brazil, grant number 2021TR000344. MFC is a CNPq Researcher Fellowship.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee for Research with Human Beings of UNIVERSIDADE FEDERAL DE SANTA CATARINA (protocol code CAAE: 41500720.0.1001.0121; date of approval: 22/06/2021).
Informed Consent Statement
Informed consent was obtained from the parents of all subjects involved in the study.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the Federal University of Santa Catarina for the infrastructure for the follow-up ambulatory and data collection.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brémond-Gignac, D.; Copin, H.; Lapillonne, A.; Milazzo, S. European Network of Study and Research in Eye Development. Visual development in infants: physiological and pathological mechanisms. Curr Opin Ophthalmol 2011, 22, S1–S8. [Google Scholar] [CrossRef]
- Goodale, M.A.; Meenan, J.P.; Bülthoff, H.H.; Nicolle, D.A.; Murphy, K.J.; Racicot, C.I. Separate neural pathways for the visual analysis of object shape in perception and prehension. Curr Biol 1994, 4, 604–610. [Google Scholar] [CrossRef]
- Zimmermann, A.; Carvalho, K.M.M.; Atihe, C.; Zimmermann, S.M.V.; Ribeiro, V.L.M. Visual development in children aged 0 to 6 years. Arq Bras Oftalmol 2019, 82, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Baran, L.C.P.; Lima, D.S.; Silva, L.A.; Tabares, H.S.; Dias, S.L.; Zin, A.A.; et al. Visual Acuity alterations in heavily impaired Congenital Zika Syndrome (CZS) children. Front. Ophthalmol 2022, 2, 948409. [Google Scholar] [CrossRef]
- Freitas, D.A.; Souza-Santos, R.; Carvalho, L.M.A.; Barros, W.B.; Neves, L.M.; Brasil, P.; et al. Congenital Zika syndrome: A systematic review. PLoS One 2020, 15, e0242367. [Google Scholar] [CrossRef]
- de Medeiros, K.S.; Sarmento, A.C.A.; Costa, A.P.F.; Macêdo, L.T.A.; da Silva, L.A.S.; de Freitas, C.L.; et al. Consequences and implications of the coronavirus disease (COVID-19) on pregnancy and newborns: A comprehensive systematic review and meta-analysis. Int J Gynaecol Obstet 2022, 156, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, M.; Zhu, Z.; Liu, Y. Coronavirus disease 2019 (COVID-19) and pregnancy: a systematic review. J Matern Fetal Neonatal Med 2022, 35, 1619–1622. [Google Scholar] [CrossRef]
- Huang, P.; Zhou, F.; Guo, Y.; Yuan, S.; Lin, S.; Lu, J.; et al. Association Between the COVID-19 Pandemic and Infant Neurodevelopment: A Comparison Before and During COVID-19. Front Pediatr 2021, 9, 662165. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, J.D.; Hopker, L.M.; De Carvalho, L.E.M.R.; Vadas, M.G.; Zin, A.A.; Mendonça, T.S.; et al. Diretrizes brasileiras sobre avaliação oftalmológica de crianças saudáveis menores de 5 anos: exames recomendados e frequência. Arq Bras Oftalmol 2021, 84, 561–568. [Google Scholar] [CrossRef]
- Ricci, D.; Cesarini, L.; Groppo, M.; De Carli, A.; Gallini, F.; Serrao, F.; et al. Early assessment of visual function in full term newborns. Early Hum Dev 2008, 84, 107–113. [Google Scholar] [CrossRef]
- Gallego, M.L. Detección de respuestas visuales en recién nacidos pretérmino: resultados preliminares de un estudio piloto con batería de optotipos. Integración 2007, 51, 7–19. [Google Scholar]
- Ricci, D.; Romeo, D.M.; Gallini, F.; Groppo, M.; Cesarini, L.; Pisoni, S.; et al. Early visual assessment in preterm infants with and without brain lesions: correlation with visual and neurodevelopmental outcome at 12 months. Early Hum Dev 2011, 87, 177–182. [Google Scholar] [CrossRef]
- Costa, M.F. Movimentos oculares no bebê: O que eles nos indicam sobre o status oftalmológico e neurológico. Psicologia USP 2007, 18, 47–61. [Google Scholar] [CrossRef]
- Götzinger, F.; Santiago-García, B.; Noguera-Julián, A.; Lanaspa, M.; Lancella, L.; Calò Carducci, F.I.; et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 2020, 4, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020, 109, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, P.; Valdés, M.; González, R.A. Molecular biology of coronaviruses: an overview of virus-host interactions and pathogenesis. Biología molecular de los coronavirus: una visión panorámica de las interacciones virus-hospedero y de la patogénesis. Bol Med Hosp Infant Mex 2021, 78, 41–58. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, X.; Chen, L. Possible causes for decreased susceptibility of children to coronavirus. Pediatr Res 2020, 88, 342. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.W.; Zachariah, P.; Gorelik, M.; Boneparth, A.; Kernie, S.G.; Orange, J.S.; et al. Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City. JAMA 2020, 324, 294–296. [Google Scholar] [CrossRef]
- Alnahdi, M.A.; Alkharashi, M. Ocular manifestations of COVID-19 in the pediatric age group. Eur J Ophthalmol 2023, 33, 21–28. [Google Scholar] [CrossRef]
- Burgess, P.; Johnson, A. Ocular defects in infants of extremely low birth weight and low gestational age. Br J Ophthalmol 1991, 75, 84–87. [Google Scholar] [CrossRef]
- Dowdeswell, H.J.; Slater, A.M.; Broomhall, J.; Tripp, J. Visual deficits in children born at less than 32 weeks' gestation with and without major ocular pathology and cerebral damage. Br J Ophthalmol 1995, 79, 447–452. [Google Scholar] [CrossRef]
- Fielder, A.R. The impact of low birth weight on the visual pathway. Br J Ophthalmol 1998, 82, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Page, J.M.; Schneeweiss, S.; Whyte, H.E.; Harvey, P. Ocular sequelae in premature infants. Pediatrics 1993, 92, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Glass, P. Development of the Visual System and Implications for Early Intervention. Infants & Young Children 2002, 15, 1–10. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).