Submitted:
28 August 2024
Posted:
29 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Selection
2.2. Morphologic Analysis and Tissue Microarray (TMA)
2.3. Immunohistochemical Assay for SWI/SNF-Subunits Complex Evaluation
2.4. Follow up and Survival Analysis of SWI/SNF-Partial Inactivation in Sinonasal Squamous Cell Carcinoma
3. Results
3.1. Squamous Cell Carcinoma with High-Grade Morphology in the Paranasal Sinuses and Nasal Cavity
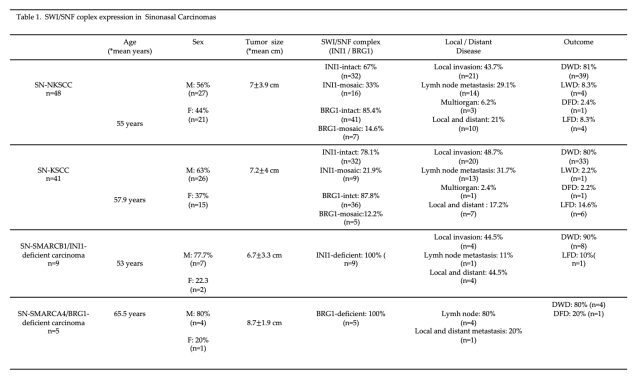
3.2. Partial Loss (Mosaic) of INI1/SMARCB1 in Sinonasal Non-Keratinizing Squamous Cell Carcinoma
3.3. Partial Loss (Mosaic) of BRG1/SMARCA4 in Sinonasal Non-Keratinizing Squamous Cell Carcinoma
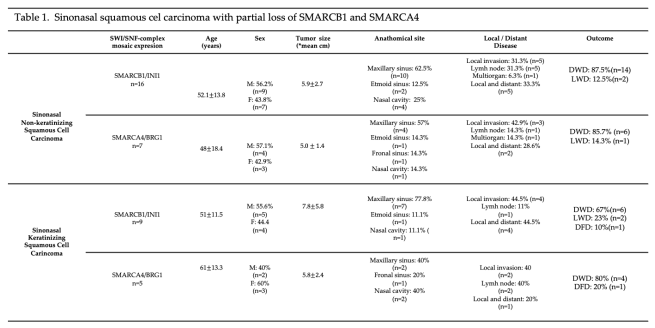
3.4. Partial Loss (Mosaic) of INI1/SMARCB1 in Sinonasal Keratinizing Squamous Cell Carcinoma
3.5. Partial Loss (Mosaic) of BRG1/SMARCA4 in Sinonasal Keratinizing Squamous Cell Carcinoma
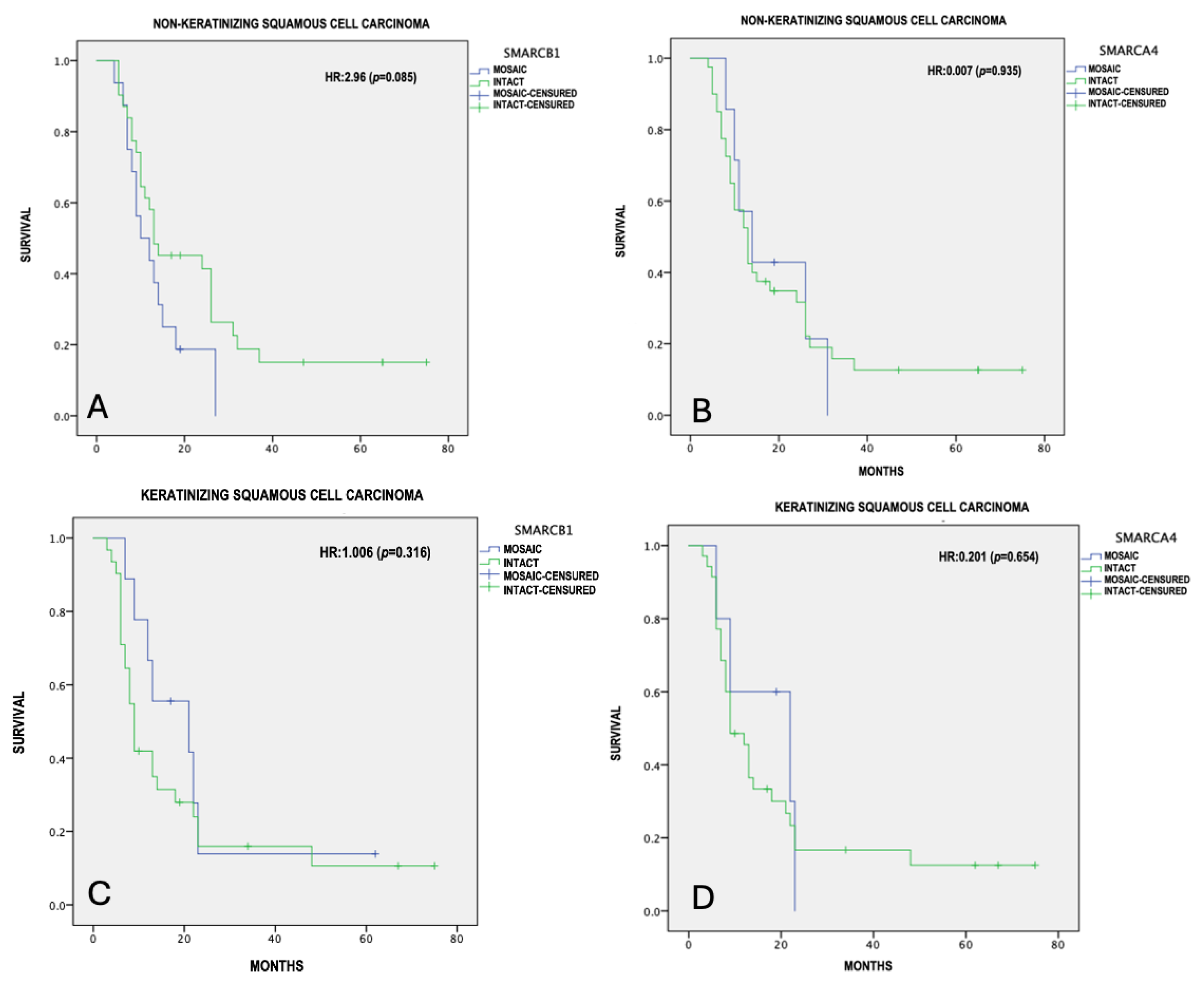
3.6. Survival Analysis of SNSCC with Partial Inactivation of SMARCB1 and SMARCA4
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mittal, P.; Roberts, C.W.M. The SWI/SNF complex in cancer — biology, biomarkers and therapy. Nat. Rev. Clin. Oncol. 2020, 17, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, I.-M.; Hornick, J.L. SWI/SNF complex-deficient soft tissue neoplasms: An update. Semin. Diagn. Pathol. 2020, 38, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Foulkes, W.D. Hereditary SWI/SNF complex deficiency syndromes. Semin. Diagn. Pathol. 2018, 35, 193–198. [Google Scholar] [CrossRef]
- Tessier-Cloutier, B.; Kleinman, C.L.; Foulkes, W.D. SWI/SNF-deficient undifferentiated malignancies: where to draw the line†. J. Pathol. 2021, 256, 139–142. [Google Scholar] [CrossRef]
- Thompson, L.D.R.; Bishop, J.A. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Nasal Cavity, Paranasal Sinuses and Skull Base. Head Neck Pathol. 2022, 16, 1–18. [Google Scholar] [CrossRef]
- Agaimy, A.; Bishop, J.A. SWI/SNF-deficient head and neck neoplasms: An overview. Semin. Diagn. Pathol. 2021, 38, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.A. OSPs and ESPs and ISPs, Oh My! An Update on Sinonasal (Schneiderian) Papillomas. Head Neck Pathol. 2017, 11, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.H.; Reh, D.D. Incidence and survival in patients with sinonasal cancer: A historical analysis of population-based data. Head Neck 2011, 34, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.B.; Hansen, E.E.M.; Lewis, J.S.J.; Faden, D.L.M. Is it Time for a Molecular-based Classification System for Sinonasal Squamous Cell Carcinoma? Am. J. Surg. Pathol. 2022, 46, 873–877. [Google Scholar] [CrossRef]
- Agaimy, A. The Expanding Family of SMARCB1(INI1)-deficient Neoplasia. Adv. Anat. Pathol. 2014, 21, 394–410. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Lund, V.J. Novel Biomarkers in Sinonasal Cancers: from Bench to Bedside. Curr. Oncol. Rep. 2020, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A. Proceedings of the North American Society of Head and Neck Pathology, Los Angeles, CA, March 20, 2022: SWI/SNF-deficient Sinonasal Neoplasms: An Overview. Head Neck Pathol. 2022, 16, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, Q. Constructing Tissue Microarrays Without Prefabricating Recipient Blocks. Am. J. Clin. Pathol. 2005, 124, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Allred, D.C.; Harvey, J.M.; Berardo, M.; Clark, G. Prognostic and predictive factors in breast cancer by inmunohistochemical analysis. Mod. Pathol. 1998, 11, 159. [Google Scholar]
- de Matos, L.L.; Stabenow, E.; Tavares, M.R.; Ferraz, A.R.; Capelozzi, V.L.; Pinhal, M.A.d.S. IMMUNOHISTOCHEMISTRY QUANTIFICATION BY A DIGITAL COMPUTER-ASSISTED METHOD COMPARED TO SEMIQUANTITATIVE ANALYSIS. Clinics 2006, 61, 417–424. [Google Scholar] [CrossRef]
- Hermsen, M.A.; Bossi, P.; Franchi, A.; Lechner, M. Sinonasal Cancer: Improving Classification, Stratification and Therapeutic Options. Cancers 2023, 15, 1675. [Google Scholar] [CrossRef]
- Agarwal, A.; Bhatt, A.; Bathla, G.; Kanekar, S.; Soni, N.; Murray, J.; Vijay, K.; Vibhute, P.; Rhyner, P. Update from the 5th Edition of the WHO Classification of Nasal, Paranasal, and Skull Base Tumors: Imaging Overview with Histopathologic and Genetic Correlation. Am. J. Neuroradiol. 2023, 44, 1116–1125. [Google Scholar] [CrossRef]
- Carrillo, J.; Güemes, A.; Ramírez-Ortega, M.; Oñate-Ocaña, L. Prognostic factors in maxillary sinus and nasal cavity carcinoma. Eur. J. Surg. Oncol. (EJSO) 2005, 31, 1206–1212. [Google Scholar] [CrossRef]
- López, F.; Inclán, C.G.; Pérez-Escuredo, J.; Marcos, C.; Scola, B.; Suárez, C.; Llorente, J.L.; Hermsen, M.A. KRAS and BRAF mutations in sinonasal cancer. Oral Oncol. 2012, 48, 692–697. [Google Scholar] [CrossRef]
- Al-Qurayshi, Z.; Smith, R.; Walsh, J.E. Sinonasal Squamous Cell Carcinoma Presentation and Outcome: A National Perspective. Ann. Otol. Rhinol. Laryngol. 2020, 129, 1049–1055. [Google Scholar] [CrossRef]
- Taverna, C.; Agaimy, A.; Franchi, A. Towards a Molecular Classification of Sinonasal Carcinomas: Clinical Implications and Opportunities. Cancers 2022, 14, 1463. [Google Scholar] [CrossRef] [PubMed]
- Pacini, L.; Cabal, V.N.; Hermsen, M.A.; Huang, P.H. EGFR Exon 20 Insertion Mutations in Sinonasal Squamous Cell Carcinoma. Cancers 2022, 14, 394. [Google Scholar] [CrossRef] [PubMed]
- Rooper, L.M.; Agaimy, A.; Dickson, B.C.M.; Dueber, J.C.; Eberhart, C.G.; Gagan, J.; Hartmann, A.; Khararjian, A.M.; London, N.R.; MacMillan, C.M.; et al. DEK-AFF2 Carcinoma of the Sinonasal Region and Skull Base. Am. J. Surg. Pathol. 2021, 45, 1682–1693. [Google Scholar] [CrossRef]
- Turri-Zanoni, M.; Gravante, G.; Castelnuovo, P. Molecular Biomarkers in Sinonasal Cancers: New Frontiers in Diagnosis and Treatment. Curr. Oncol. Rep. 2022, 24, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Sheng, W.; Wang, L.; Zhu, X.; Tan, C.; Ni, S.; Weng, W.; Huang, D.; Wang, J. SWI/SNF Complex-deficient Undifferentiated Carcinoma of the Gastrointestinal Tract. Am. J. Surg. Pathol. 2021, 46, 889–906. [Google Scholar] [CrossRef]
- Agaimy, A. Abbas SWI/SNF-deficient Sinonasal Carcinomas. Adv. Anat. Pathol. 2022, 30, 95–103. [Google Scholar] [CrossRef]
- Skálová, A.; Taheri, T.; Bradová, M.; Vaněček, T.; Franchi, A.; Slouka, D.; Kostlivý, T.; de Rezende, G.; Michálek, J.; Klubíčková, N.; et al. SMARCB1-deficient sinonasal adenocarcinoma: a rare variant of SWI/SNF-deficient malignancy often misclassified as high-grade non-intestinal-type sinonasal adenocarcinoma or myoepithelial carcinoma. Virchows Arch. 2023, 485, 245–256. [Google Scholar] [CrossRef]
- Agaimy, A.; Hartmann, A.; Antonescu, C.R.; Chiosea, S.I.; El-Mofty, S.K.; Geddert, H.; Iro, H.; Lewis, J.S.J.; Märkl, B.; Mills, S.E.; et al. SMARCB1 (INI-1)-deficient Sinonasal Carcinoma. Am. J. Surg. Pathol. 2017, 41, 458–471. [Google Scholar] [CrossRef]
- Parker, N.A.; Al-Obaidi, A.; Deutsch, J.M. SMARCB1/INI1-deficient tumors of adulthood. F1000Research 2020, 9, 662. [Google Scholar] [CrossRef]
- Caltabiano, R.; Magro, G.; Polizzi, A.; Praticò, A.D.; Ortensi, A.; D’orazi, V.; Panunzi, A.; Milone, P.; Maiolino, L.; Nicita, F.; et al. A mosaic pattern of INI1/SMARCB1 protein expression distinguishes Schwannomatosis and NF2-associated peripheral schwannomas from solitary peripheral schwannomas and NF2-associated vestibular schwannomas. Child's Nerv. Syst. 2017, 33, 933–940. [Google Scholar] [CrossRef]
- Esposito, A.; Stucchi, E.; Baronchelli, M.; Di Mauro, P.; Ferrari, M.; Lorini, L.; Gurizzan, C.; London, N.R.J.; Hermsen, M.; Lechner, M.; et al. Molecular Basis and Rationale for the Use of Targeted Agents and Immunotherapy in Sinonasal Cancers. J. Clin. Med. 2022, 11, 6787. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Taboni, S.; Carobbio, A.L.C.; Emanuelli, E.; Maroldi, R.; Bossi, P.; Nicolai, P. Sinonasal Squamous Cell Carcinoma, a Narrative Reappraisal of the Current Evidence. Cancers 2021, 13, 2835. [Google Scholar] [CrossRef]
- Ayyanar, P.; Mishra, P.; Preetam, C.; Adhya, A.K. SMARCB1/INI1 Deficient Sino-Nasal Carcinoma: Extending the Histomorphological Features. Head Neck Pathol. 2021, 15, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Weichert, W. SMARCA4-deficient Sinonasal Carcinoma. Head Neck Pathol. 2017, 11, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Agaimy, A.; Weichert, W. SMARCA4-deficient Sinonasal Carcinoma. Head Neck Pathol. 2017, 11, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Kohashi, K.; Oda, Y. Oncogenic roles of SMARCB1/INI1 and its deficient tumors. Cancer Sci. 2017, 108, 547–552. [Google Scholar] [CrossRef]
- Agaimy, A.; Jain, D.; Uddin, N.; Rooper, L.M.; Bishop, J.A. SMARCA4-deficient Sinonasal Carcinoma. Am. J. Surg. Pathol. 2020, 44, 703–710. [Google Scholar] [CrossRef]
- Agaimy, A.; Franchi, A.; Lund, V.J.; Skálová, A.; Bishop, J.A.; Triantafyllou, A.; Andreasen, S.; Gnepp, D.R.; Hellquist, H.; Thompson, L.D.; et al. Sinonasal Undifferentiated Carcinoma (SNUC): From an Entity to Morphologic Pattern and Back Again-A Historical Perspective. Adv. Anat. Pathol. 2020, 27, 51–60. [Google Scholar] [CrossRef]
- Alzumaili, B.; Sadow, P.M. IDH2-Mutated Sinonasal Tumors: A Review. Adv. Anat. Pathol. 2022, 30, 104–111. [Google Scholar] [CrossRef]
- Turri-Zanoni, M.; Gravante, G.; Castelnuovo, P. Molecular Biomarkers in Sinonasal Cancers: New Frontiers in Diagnosis and Treatment. Curr. Oncol. Rep. 2022, 24, 55–67. [Google Scholar] [CrossRef]
- Hongo, T.; Yamamoto, H.; Jiromaru, R.; Yasumatsu, R.; Kuga, R.; Nozaki, Y.; Hashimoto, K.; Matsuo, M.; Wakasaki, T.; Tamae, A.; et al. PD-L1 expression, tumor-infiltrating lymphocytes, mismatch repair deficiency, EGFR alteration and HPV infection in sinonasal squamous cell carcinoma. Mod. Pathol. 2021, 34, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




