Introduction
Anemia is a global public health concern that affects 36.5% of pregnant women worldwide. This is especially common in sub-Saharan Africa and Southeast Asia [
1]. Similarly, 41% of pregnant women are suffering from anemia in Ethiopia, which ranges from 9% in Addis Ababa to 65.9% in the Somali region [
2]. Anemia during pregnancy is linked to negative health and socioeconomic outcomes. Pregnant women who are anemic frequently experience decreased physical strength and a higher risk of maternal morbidity and mortality. For instance, severe anemia increases the risk of death by 20% [
3]. Anemic pregnant mothers have a higher risk of preterm birth, fetal anemia, low birth weight (LBW), intrauterine fetal growth restriction, decreased gestational weight gain, and perinatal death [
3,
4,
5]. Furthermore, anemia can result in a vicious cycle where a person’s ability to work is diminished, which can have an adverse economic effect on both the individual and society [
6].
The World Health Organization (WHO) designed two broad strategies to address the issues of anemia and undernutrition: nutrition-sensitive and nutrition-specific. Nutrition-specific strategies address the proximal causes of anemia, primarily inadequate dietary intake of hematopoietic nutrients such as iron or vitamin A, supplementation, and access to fortified foods. Dietary diversification is an approach designed to increase the availability, accessibility, and use of locally accessible and acceptable foods that have high micronutrient content and bioavailability across the year [
7]. The Ethiopian government has implemented different strategies to prevent anemia and undernutrition among pregnant women, such as promotion of dietary diversification and iron and folic acid supplementation, malaria prevention and treatment, utilization of bed nets, and deworming pills [
8]. Also, the government has exerted considerable efforts to decrease maternal anemia and undernutrition by adopting and developing a nutrition and food strategy, program, and policy. Furthermore, the government developed the “Seqota Declaration” to guarantee year-round 100% access to sufficient food in the country by 2030 [
9]. Moreover, health extension programs, specifically focusing on community-wide nutrition education, are considered the most significant measures that can help promote adequate nutrition in the first 1000 days [
8].
Despite all national commitments, measures, and efforts, anemia and undernutrition prevalence among pregnant women was high in the country as a whole, particularly in rural areas [
2,
10,
11,
12]. Also, there were considerable regional and urban/rural disparities in the prevalence of anemia and undernutrition at the national level [
2], implying the need for further research into the landscape of anemia and undernutrition prevalence patterns in local settings. However, previous studies on the prevalence of anemia and undernutrition among pregnant women in Ethiopia focused primarily on individual-level factors, with little consideration given to community-level, household-level, and contextual factors. Furthermore, the factors contributing to anemia and undernutrition among pregnant women vary by region in Ethiopia, the prevalence of anemia and undernutrition is highly variable, and existing evidence is insufficient to develop comprehensive prevention strategies.
Undernutrition and anemia are two crucial indicators of maternal undernutrition that can impact the health of both the mother and the fetus. Maternal anemia and undernutrition are linked to an increased risk of preterm birth, stillbirth, LBW, and neonatal deaths. If anemia and undernutrition co-exist at the same time, the risks increase [
13]. There have been several studies conducted to assess anemia [
14,
15,
16,
17] and undernutrition [
18,
19,
20,
21,
22,
23,
24] separately so far. However, to the best of our knowledge, there is no evidence on the coexistence of anemia and undernutrition among pregnant women in developing countries, including Ethiopia, the effect of co-existence on pregnancy outcomes, or whether certain interventions should be targeted toward women who share this dual burden. Therefore, we aimed to assess the prevalence and determinants of co-existing anemia and undernutrition among pregnant women in the Hawela Lida district of Sidama region, Ethiopia.
The result from this study is helpful to inform program managers, policymakers, decision-makers, and implementers in designing effective and efficient prevention strategies to improve maternal nutrition status and achieve the Sustainable Development Goals (SDGs). Furthermore, this study generated results that can inform maternal health champions by providing determinants for co-existing anemia and undernutrition in the context of the Sidama region.
Methods
Study Area
This study was done in the Hawela Lida district of the Sidama region, Ethiopia. Hawela Lida district is one of the 10 districts in the north zone of the Sidama region. It is located 289 kilometers away from Addis Ababa, Ethiopia’s capital city. It has consisted of 11 rural and 2 rural kebeles, which is the least administrative structure in Ethiopia.
Study Design, Period and Population
A community-based cross-sectional study was done among 515 randomly selected pregnant women from June 1–25, 2024. All pregnant women in their first trimester and randomly selected pregnant women residing in the district for at least 6 months were the source and study population for this study, respectively. Pregnant women who had severe illnesses and were temporary residents were excluded from this study.
Sample Size Calculation and Sampling Procedures
We calculated the minimum required sample size utilizing OpenEpi version 3 for this study. The sample size required to estimate the prevalence of anemia is computed by considering the anticipated prevalence of anemia (19.3%), according to the report of a previous study [
10], a margin of error of 5%, a 5% level of confidence, and a design effect (DEF) of 2. The DE is calculated using the formula DEFF = 1+ (m-1)* infraclass correlation coefficient (ICC), while’m’ is the average number of clusters. The required number of clusters was computed by multiplying the initial calculated sample size by the ICC value. However, previously conducted studies didn’t report the ICC value to facilitate sample size replication. We received an ICC value of 0.01 to facilitate our sample size calculation from a range value based on the existing recommendation.
[
25]. Accordingly, the minimum needed number of clusters is 240 * 0.01 = 2.4. However, we included 10
kebeles (clusters) to maintain the adequacy of power for this study. The sample size was corrected for the non-response rate by adding 10% of the anticipated non-response rate. Based on the above information, the final estimated sample size was 528. We used a multi-stage sampling method to recruit the study participants for this study. The primary sampling unit was the district, and Hawela Lida district was chosen purposefully from the Sidama region using the purposive sampling method. We selected purposively because, immediately following this baseline study, the interventional study was implemented to investigate the effect of amaranth grain flat bread on anemia for six months. This district will provide the geographic access to carefully implement, coordinate, supervise, and correct any unexpected problems because it is near Hawassa City. The secondary sampling unit was
kebeles, and 10
kebeles were selected using a simple random sampling procedure. The third sampling unit was households containing pregnant women, identified by conducting a house-to-house census in the selected
kebeles. Pregnant women who were not available after three consecutive visits were considered non-respondents for this study. One mother was included by using a lottery sampling procedure when two or more pregnant mothers occur in the chosen households.
Study Variables
The outcome variable was co-existing anemia and undernutrition. In pregnancy, anemia is defined as hemoglobin <11 g/dl and is classified into three categories based on WHO criteria: mild (10.0–10.9 g/dl), moderate (7.0–9.9 g/dl), and severe (less than 7.0 g/dl) [
26]. Hemoglobin analysis was performed by laboratory technologists in all health posts. Each pregnant woman’s hemoglobin concentration was determined by taking a finger-prick blood sample with a HemoCue Hb 301 (HemoCue AB, Angelholm, Sweden). The site was disinfected, and a prick was conducted on the tip of the middle finger. The device used to measure hemoglobin concentration will eliminate the first drop of blood and collect the second drop to fill the microcuvette. The cuvette holder held the microcuvette. To improve test reliability, the meter’s performance was examined daily using control standards. While the meter was prepared to utilize capillary blood, the microcuvette owner was first pulled to the loading position, and then the sample was filled continuously for the examination. Within 10 minutes of filling, it was placed in the holder and pushed into its measuring position. Finally, using WHO’s field survey recommendation techniques, the result was recorded and displayed after a duration of 15–60 seconds [
27,
28]. Before the data was entered, the hemoglobin level was corrected for altitude using the formula [
28]. Serum ferritin and C-reactive protein were tested further in all samples with hemoglobin levels less than 11 g/dl. The Cobas 6000 e601 module from Roche (Germany) was used to analyze serum ferritin, and the Cobas 6000 c501 module was used to analyze CRP. All laboratory procedures were carried out in accordance with standard operating procedures (SOPs). In this study, pregnant women with mid-upper arm circumference (MUAC) measurements less than 23 cm were classed as undernourished, whereas those with values greater than 23 cm were classified as normal.
The independent variables are socio-economic and demographic, such as women age, education status, husband education and occupation status, wealth index, family size, and mass media utilization; reproductive characteristics like planned pregnancy, previous history of stillbirth, women age at first marriage, and previous history of stillbirth; knowledge and practice of dietary diversity; and household food security. Every detail of individual and community-level variable measurements is provided in supplementary file 1.
Blood Collection and Serum Preparation Procedures
Standard procedures were followed for blood sample collection and laboratory analyses. Phlebotomists prepared the necessary materials (alcohol swabs, sterile gloves, a tourniquet, a vacutainer needle, a vacutainer tube, and a syringe) and collected blood from each participant. They were to clean and dry the collection site, apply a tourniquet, locate the vein, and prepare the needle and tube. Participants were instructed to make a fist, and the phlebotomist inserted the needle to collect 5 ml of blood. Afterward, the needle was withdrawn, pressure was applied to the puncture site, and the needle was safely disposed of. The phlebotomist expressed gratitude to the participants for their cooperation.
After blood collection, the hemoglobin concentration was measured in the field using a hemocue photometer (Hb 301+ System) with collected blood drops. An altitude adjustment for hemoglobin was made based on WHO standards. To prevent hemolysis and contamination, serum extraction occurred within 45 minutes of sample collection at the nearby health institution. The extracted serum samples were then frozen at -20 °C and transported on dry ice to the Hawassa Referral Comprehensive Specialized Hospital for further analysis.
Serum ferritin levels were analyzed using the Elecsys 2010 analyzer, an automated clinical analyzer manufactured by Elecsys in Mannheim, Germany. The analysis utilized the enzyme-linked immunosorbent assay (ELISA) method. Additionally, a serum C-rreactive protein (CPR) level, used to assess inflammation, was measured using the Cobas 6000 system, a clinical chemistry analyzer manufactured by Roche Diagnostic GmbH.
Data Quality Assurance
The PI trained the data collectors, field assistants, and field supervisors on the study tool for two days, emphasizing the significance of the research, the data collection procedure, objectives, sampling procedures, blood sample collection procedures, and ethical considerations. The data was collected using a well-designed, standardized, pretested, structured, face-to-face interviewer-administered. Following the pre-test, necessary adjustments were made before beginning the main data collection procedure on the tool. The data collection procedure was strictly monitored. The completeness, consistency, and accuracy of data were reviewed on a daily basis during data collection. The data was cleaned, coded, and then exported to Stata 17 for further processing and analysis. To reduce the likelihood of reporting bias, data collectors, field assistants, and supervisors were blinded to the exposure and outcome variables. Furthermore, to reduce the risk of bias, maximum efforts were taken by carefully selecting subjects who represent the source population, increasing response, and training data collectors, field assistants, and supervisors.
Ethics Statement
This study was ethically approved by the Hawassa University College of Medicine and Health Sciences’ institutional review board (IRB) with reference number of IRB/027/16. A letter of support was received from Hawassa University School of Public Health, Sidama region, Hawela Lida district, and kebeles leaders. Pregnant women provided written consent before to data collection and after receiving information about the study. All data collection techniques were carried out in strict confidentially. No specific personal identifiers were collected, and only researchers had access to data that may identify individual respondents during or after data collection. Furthermore, pregnant women who shown severe anemia and undernutrition during the survey were transferred to a neighboring health care facility for additional assessment and treatment.
Data Analysis Techniques
Prior to the main analyses, quantitative variables were processed through recoding, computations, and categorization. Descriptive analyses were performed to obtain descriptive measures for key variables of interest, such as frequency, percentage, mean, and standard deviation (SD). For this research, the wealth index was computed using Principal Component analytic (PCA) [
32], and the S1 file contains information of variable preparation and analytic processes.
We estimated the intra-class correlation coefficient (ICC) utilizing a multi-level logistic regression intercept only model [
33,
34]. The computed ICC value was larger than 5%, which is one reason to use the multi-level regression analysis for this research. To control the impacts of clusters and known confounders, bivariable and multivariable analyses were performed using multilevel mixed effects modified Poisson regression models with robust standard error. Variables having p-values < 0.25 on the bivariable analysis and practical significance backed by relevant literature were built-in in a multivariable regression model to identify determinants independently related with CAU, controlling for other variables in the model [
35].
We constructed multi-level models to account for the effects of clustering and the hierarchical structure of our data. Thus, four models were evaluated for this research. Model 1 was an empty or intercept-only model, whereas Model 2 had only individual-level determinants, Model 3 contained only community-level determinants, and Model 4 consisted of both individual and community-level determinants. The ICC value and median prevalence ratio (MPR) were used to evaluate the random model data [
36]. The MPR is a predictor of the unexplained
kebeles-level heterogeneity, while the ICC value was utilized to characterize the percentage of variability in the prevalence of undernutrition that is attributable to the clustering variable
(kebele). The MPR predicts unexplained
kebeles-level heterogeneity, whereas the ICC value characterizes the fraction of variability in the prevalence of CAU due to the clustering variable (
kebele). The MPR was calculated using the formula MPR= ~
and is defined as the mean of the prevalence ratio between the areas with the highest and lowest risk of CAU prevalence when two areas are randomly selected [
37,
38].
This study examined effect modification and multicollinearity. The variance inflation factor (VIF) < 5 indicated that multicollinearity had a little impact on the study’s results [
39]. The S1 file contains detailed information about the effect modification findings.
The strength and presence of a statistically significant association between CAU and the independent factors were determined using APRs with a 95% confidence interval and a 5% level of significance. A statistically significant association between CAU and the independent factors was confirmed when the 95% confidence intervals of the APRs did not contain 1 or had a P-value less than 0.05.
Results
Socio-Demographic Characteristics of Research Subject
We properly interviewed 515 pregnant women out of 528, resulting in a response rate of 97.53% for this study. The mean (+ SD) age of the study participants was 25.89 (+ 4.53) years. Almost all 486 (94.4%) study participants were of Sidama ethnicity. The majority of research participants, 438 (85.2%), identified as Protestant Christians. Nearly all pregnant women were married, with 511 (99.2%) and 468 (90.9%) being housewives, respectively.
Study Participants’ Reproductive Health Characteristics
The mean age of first marriage (+ SD) among study participants was 21.01 + 3.10 years. 81 (15.7%) women have previously had an abortion, and 93 (18.1%) have experienced an infection during their present pregnancy. Similarly, 49 (9.5%) and 28 (5.4%) women have had past history of stillbirths and neonatal deaths, respectively.
Prevalence of Co-Existing Anemia and Undernutrition
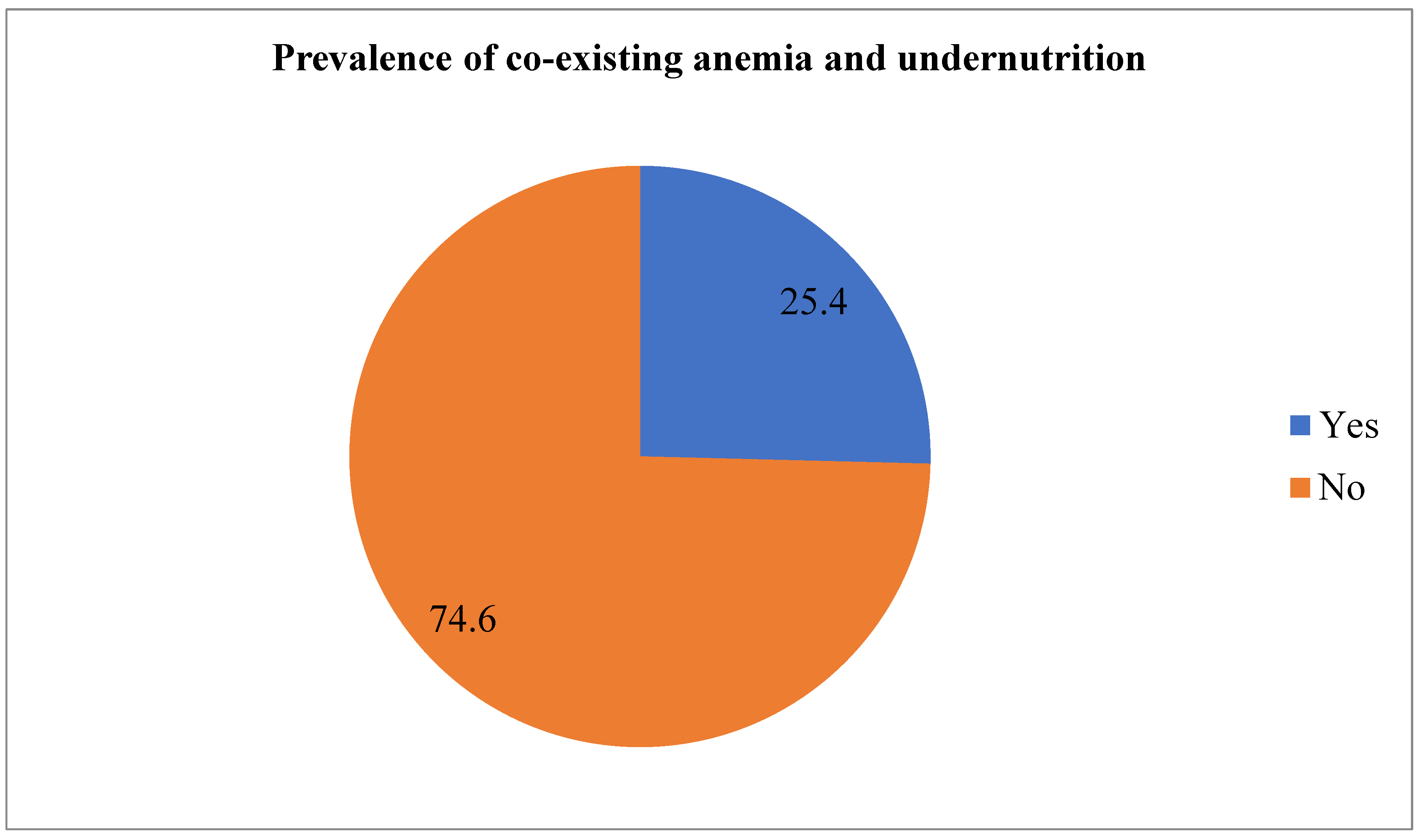
The overall prevalence of CAU was 25.4% (95% CI: 21.9-28.9) (
Figure 1). The overall prevalence of anemia among pregnant women was 42.9% (95% CI: 38.4-47.6), while the prevalence of undernutrition was 41.7% (95% CI: 37.3-45.6). Similarly, 15.5, 19.2, and 8.2% of study participants had mild, moderate, and severe anemia, respectively (Figure 2).
Determinants of Co-Existing Anemia and Undernutrition
Pregnant women who had obtained model family training from health extension workers had a 34% higher likelihood of CAU than their counterparts (APR = 0.66; 95% CI: 0.45-0.96). Pregnant women’s inadequate dietary diversity increased the likelihood of CAU prevalence (APR = 2.17; 95% CI: 1.43-3.28) as compared to women who have adequate dietary diversity. Pregnant women in the food-insecure household had 51% more CAU prevalence as compared to the food-secure household (APR = 1.51; 95% CI: 1.18-1.95). Women who have poor knowledge of nutrition had a higher prevalence of CAU than their counterparts (APR = 1.55; 95% CI: 1.06-2.26). While the community-level road access decreased the likelihood of CAU prevalence (APR = 0.65; 95% CI: 0.43–0.98) as compared to the community-level road inaccessibility, the likelihood of CAU prevalence was 6.19 times higher for pregnant women who lived in high community-level women autonomy (APR = 6.19; 95% CI: 3.42–11.22) as compared to women who lived in low community-level women autonomy (
Table 1).
Discussion
The prevalence of CAU among pregnant women was 25.4%. Inadequate dietary diversity, poor knowledge of nutrition, food-insecure status, training on model families, community-level women’s autonomy, and road inaccessibility were determinants of CAU among pregnant women.
The prevalence of undernutrition among pregnant women was 25.4%. There has been no previous publication on the prevalence of CAU and its determinants in Ethiopia or other African nations, limiting the comparability of our findings.
Pregnant mothers’ inadequate dietary diversity was significantly associated with CAU prevalence. This could be because women who practice food diversity obtain a variety of nutrients from different diets, which could make them more nutrient-dense than mothers with lower dietary diversity scores. Furthermore, this could be because women do not consume extra meals during pregnancy, and maternal dietary habits, socio-cultural beliefs, and food taboos can all affect nutrition during pregnancy. The prevention of malnutrition in all of its forms, both before and during pregnancy, is dependent on appropriate nutrition practices, essential nutrition services, and healthy diets. Thus, it is critical for all prenatal care to equip pregnant women with dietary teaching and counseling, which should be expanded. Furthermore, the researchers contended that this could be expressed as follows: Eating a diverse diet is vital for obtaining all of the nutrients required to prevent undernutrition caused by nutritional deficiencies. [
40].
This research found that the training on model family decreased the prevalence of CAU. The possible rationale is as follows: women who have received model family training from health extension workers tend to have good knowledge of nutrition during pregnancy and lactation or nutrition benefits during the first 1000 days, a positive attitude toward nutritious diets and avoiding food taboos, health-seeking behavior, and information on the benefits of dietary diversity practices.
This study indicated that household food-insecure status was positively related with CAU prevalence. This could be because a lack of food in the household leads to insufficient daily nutritional needs and poor dietary intake, resulting in undernutrition in pregnant women. Researchers also claimed that one of the primary underlying causes of undernutrition is household food insecurity, which occurs when a household does not always have physical, social, or financial access to enough food to meet their nutritional needs for a healthy life. Women tend to consume less than men, which could be attributed to the time of limited food availability. Furthermore, the women used coping methods to reduce their food intake while providing nourishment for their small children and newborns during a food shortage. As a result, improving community food security for homes is critical to avoiding and eliminating acute undernutrition and its negative long-term consequences [
41].
Pregnant women’s poor knowledge of nutrition increased the prevalence of CAU. This could be because a lack of nutritional comprehension usually leads to an insufficient dietary intake, resulting in undernutrition. Furthermore, women who have knowledge of nutrition may be better able to perceive the benefits of eating a healthy and appropriate diet during their pregnancy, as well as be prepared to adopt such a diet.
This study documented that high community-level women autonomy decreased the prevalence of CAU. Communities with significant women’s autonomy had higher levels of knowledge about nutrition and health-seeking behavior. Autonomous mothers are more educated and financially independent, have more job opportunities, and understand the importance of adequate dietary diversity. Another factor might be that literate populations utilize more community-level mass media, which may increase community discussion about maternal health issues. Mothers who reside in affluent communities may have been exposed to more mass media, which has increased their awareness and knowledge of nutrition and appropriate dietary diversification practices, according to the WHO report [
42]. The results of research carried out in low-income nations provide further support to this idea [
43,
44].
The prevalence of CAU increased for pregnant women who lived in poor road accessible communities. One possible rationale for this is because community-level road inaccessibility reduces access to fundamental services such as health and education. Researchers also highlighted that geographic difference in health services in Ethiopia [
45,
46,
47]. Another reason might be that health care providers give less attention to screening and linking undernutrition pregnant women from road inaccessible places, which contributed to the high prevalence of CAU in settings with low road accessibility.
Conclusions
One in four pregnant women was CAU in the study setting. Inadequate dietary diversity, poor knowledge of nutrition, food-insecure status, training on model families, community-level women’s autonomy, and road inaccessibility were determinants of CAU among pregnant women. Thus, any programs related to maternal nutritional improvement strategies should address these determinants to decrease the high prevalence of CAU. Likewise, particularly intervention approaches should be considered for pregnant women with poor knowledge of nutrition, pregnant women who have not obtained training in a model family, and pregnant mothers who have inadequate dietary diversity. Moreover, there is a significant level of food insecurity in the research area, which adds to the high rate of CAU among pregnant women. Designing and enhancing the promotion of food security policies based on Ethiopian national directives and WHO guidelines is therefore a thoughtful demand. Furthermore, there is an urgent need for nutritional screening for women living in poor, road-accessible areas to circumvent the high prevalence of CAU. Finally, creating women’s autonomy-raising reforms for communities with low women’s autonomy with the help of rural health extension workers must be considered.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Supporting Information: S2 file: Detail information of some methods and result section (DOCX).
S1 file: English version study questionnaire (DOCX).
S3 file: Stata data set.
Authors’ contributions
Conceptualization: Amanuel Yoseph. Data curation: Amanuel Yoseph. Formal analysis: Amanuel Yoseph. Investigation: Amanuel Yoseph, Lakew Mussie, Mehretu Belayineh. Methodology: Amanuel Yoseph, Lakew Mussie, Mehretu Belayineh. Project administration: Amanuel Yoseph, Lakew Mussie, Mehretu Belayineh. Resources: Amanuel Yoseph. Software: Amanuel Yoseph, Lakew Mussie, Mehretu Belayineh. Supervision: Amanuel Yoseph, Lakew Mussie, Mehretu Belayineh. Validation: Amanuel Yoseph, Lakew Mussie, Mehretu Belayineh. Visualization: Amanuel Yoseph, Lakew Mussie, Mehretu Belayineh. Writing – original draft: Amanuel Yoseph, Lakew Mussie, Mehretu Belayineh. Writing – review & editing: Amanuel Yoseph, Lakew Mussie, Mehretu Belayineh.
Acknowledgments
We’d like to thank the Nestle Foundation for their financial support. This study would not be possible without their financial assistance. We are also thankful to the Sidama region, Hawela Lida district health office, study subjects, data collectors, field assistants, and supervisors for their direct contributions to the study’s successful completion. Finally, we would like to thank the team at Hawassa University School of Public Health for their invaluable help during the study’s design and data analysis.
List of Abbreviations
AIC: Akaike information criteria; APR: Adjusted prevalence ratio; BIC: Bayesian information criteria; CI: confidence interval; CPR: Crude prevalence ration; EDHS: Ethiopian Demographic and Health Survey; FANTA: Food and nutrition technical assistance; FAO: Food and agriculture organization; HCPs: HEW: Health extension worker; MPR: Median prevalence ratio; NGO: Non-governmental organization; PI: Principal Investigator; ICC: Intra-class correlation coefficient; IUGR: Intrauterine growth retardation; IRB: Institutional review board; SD: Standard deviation: WHO: World Health Organization; WRA: Women of reproductive age; VIF: Variance inflation factor.
References
- World Health Organization. Anemia in women and children. 2021. Available online from https://www.who.int/data/gho/data/themes/topics/anaemia_in_women_and_children.
- “ CSAC (2017) [Ethiopia] and ICF,” Ethiopia Minin Demographic and Health Survey: Key Indicators Report: Addis Ababa, Ethiopia, and Rockville, CSA and ICF, Maryland, USA.
- Derso, T.; Abera, Z.; Tariku, A. Magnitude and associated factors of anemia among pregnant women in Dera District: A cross-sectional study in northwest Ethiopia. BMC Res. Notes 2017, 10, 359. [Google Scholar] [CrossRef]
- Haggaz, A.D.; Radi, E.A.; Adam, I. Anaemia and low birthweight in western Sudan. Transactions of the Royal Society of Tropical Medicine and Hygiene 2010, 104, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.; Mogren, I.; Lindmark, G.; Massawe, S.; Nystrom, L. The risks for pre-term delivery and low birth weight are independently increased by the severity of maternal anaemia. South African Medical Journal 2009, 99, 98–102. [Google Scholar]
- Moench-Pfanner, R.; Silo, S.; Laillou, A.; Wieringa, F.; Hong, R.; et al. The economic burden of malnutrition in pregnant women and children under 5 years of age in Cambodia. Nutrients 2016, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Nutritional anaemias: Tools for effective prevention and control; World Health Organization: Geneva, 2017. [Google Scholar]
- MoFED Health sector growth and Transformation Plan (GTP) 2010/11-2014/15. The Federal Democratic Republic of Ethiopia, 2010.
- Federal Democratic Republic of Ethiopia National Nutrition Program Multi-sectoral Implementation Guide. Addis Ababa: 2016.
- Dessalegn, F.N.; Wanamo, T.E.; Wordofa, D. Prevalence of Iron Deficiency Anemia and Associated Factors among Pregnant Women Attending Antenatal Care Follow up at Dodola General Hospital West Arsi Zone Oromia Region South East Ethiopia. Arch Med 2021, 13, 40. [Google Scholar]
- Laelago, F.; Paulos, W.; Halala Handiso, Y. Prevalence and predictors of iron deficiency anemia among pregnant women in Bolosso Bomibe district, Wolaita Zone, Southern Ethiopia Community-based cross-sectional study. Cogent Public Health 2023, 10, 2183562. [Google Scholar] [CrossRef]
- Woldegebriel, A.G.; Gebrehiwot, G.G.; Desta, A.A.; Ajemu, K.F.; Berhe, A.A.; Woldearegay, T.W.; Bezabih, N.M. Determinants of Anemia in Pregnancy: Findings from the Ethiopian Health and Demographic Survey. Anemia 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Patel, A.; Prakash, A.A.; Das, P.K.; Gupta, S.; Pusdekar, Y.V.; Hibberd, P.L. Maternal anemia and underweight as determinants of pregnancy outcomes: Cohort study in eastern rural Maharashtra, India. BMJ Open 2018, 8, e021623. [Google Scholar] [CrossRef]
- Deriba, B.S.; Bala, E.T.; Bulto, G.A.; Geleta, T.A.; Ayalew, A.F.; et al. Determinants of Anemia among Pregnant Women at Public Hospitals in West Shewa, Central Ethiopia: A Case-Control Study. Anemia 2020, 2020, 2865734. [Google Scholar] [CrossRef]
- Addis Alene, K.; Mohamed Dohe, A. Prevalence of anemia and associated factors among pregnant women in an urban area of Eastern Ethiopia. Anemia 2014, 2014, 561567. [Google Scholar] [CrossRef]
- Bekele, A.; Tilahun, M.; Mekuria, A. Prevalence of anemia and its associated factors among pregnant women attending antenatal care in health institutions of Arba Minch town, Gamo Gofa Zone, Ethiopia: A cross-sectional study. Anemia 2016, 2016, 1073192. [Google Scholar] [CrossRef] [PubMed]
- Teshome, M.S.; Meskel, D.H.; Wondafrash, B. Determinants of anemia among pregnant women attending antenatal care clinic at public health facilities in Kacha Birra District, Southern Ethiopia. Journal of Multidisciplinary Healthcare 2020, 13, 1007–1015. [Google Scholar] [CrossRef]
- Lesage, J.; Hahn, D.; Leonhardt, M.; Blondeau, B.; Breant, B.; et al. Maternal undernutrition during late gestation-induced intrauterine growth restriction in the rat is associated with impaired placental GLUT3 expression, but does not correlate with endogenous corticosterone levels. Journal of endocrinology 2022, 174, 37–44. [Google Scholar] [CrossRef]
- Muze, M.; Yesse, M.; Kedir, S.; Mustefa, A. Prevalence and associated factors of undernutrition among pregnant women visiting ANC clinics in Silte zone, Southern Ethiopia. BMC Pregnancy and Childbirth 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nigatu, M.; Gebrehiwot, T.T.; Gemeda, D.H. Household food insecurity, low dietary diversity, and early marriage were predictors for Undernutrition among pregnant women residing in Gambella, Ethiopia. Advances in Public Health 2018, 2018, 1350195. [Google Scholar] [CrossRef]
- Shiferaw, A.; Husein, G. Acute under nutrition and associated factors among pregnant women in Gumay District, Jimma Zone, South West Ethiopia. J Women’s Health Care 2019, 8, 2167-0420.1000459. [Google Scholar]
- Tadesse, A.; Hailu, D.; Bosha, T. Nutritional status and associated factors among pastoralist children aged 6–23 months in Benna Tsemay Woreda, South Omo zone, Southern Ethiopia. Int J Nutr Food Sci 2018, 7, 11–23. [Google Scholar] [CrossRef]
- Tikuye, H.H.; Gebremedhin, S.; Mesfin, A.; Whiting, S. Prevalence and factors associated with undernutrition among exclusively breastfeeding women in Arba Minch Zuria District, Southern Ethiopia: A cross-sectional community-based study. Ethiopian journal of health sciences 2019, 29. [Google Scholar] [CrossRef]
- Zewdie, S.; Fage, S.G.; Tura, A.K.; Weldegebreal, F. Undernutrition among pregnant women in rural communities in southern Ethiopia. International Journal of Women’s Health 2021, 13, 73–79. [Google Scholar] [CrossRef]
- Donner, A.; Birkett, N.; Buck, C. Randomization by cluster. Sample size requirements and analysis. Am J Epidemiol 1981, 114, 906–914. [Google Scholar] [CrossRef]
- Obai, G.; Odongo, P.; Wanyama, R. Prevalence of anaemia and associated risk factors among pregnant women attending antenatal care in Gulu and Hoima Regional Hospitals in Uganda: A cross sectional study. BMC Pregnancy Childbirth 2016, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Villanova, P.A. National committee for clinical laboratory standards: Reference and selected procedures for the quantitative determination of haemoglobin in blood. 2nd ed. Approved standards; 1994.
- World health organization. Hemoglobin concentrations for the diagnosis of anemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization (WHO/NMH/NHD/ MNM/11.1). 2011.
- World health organization. Improving nutrition outcomes with better water, sanitation and hygiene: Practical solutions for policies and programmes. 2015.
- Alem, M.; Enawgaw, B.; Gelaw, A.; Kenaw, T.; Seid, M.; Olkeba, Y. Prevalence of Anemia and Associated Risk Factors among Pregnant Women Attending Antenatal Care in Azezo Health Center Gondar Town, Northwest Ethiopia. J. Interdiscip. Histopathol. 2013, 1, 137–144. [Google Scholar] [CrossRef]
- Getachew, M.; Yewhalaw, D.; Tafess, K.; Getachew, Y.; Zeynudin, A. Anaemia and associated risk factors among pregnant women in Gilgel Gibe dam area, Southwest Ethiopia. Parasites & vectors 2012, 5, 1–8. [Google Scholar]
- Gwatkin, D.R. Health inequalities and the health of the poor: What do we know? What can we do? Bull World Health Organ. 2000, 78, 3–18. [Google Scholar]
- Tabachnick, B.G.; Fidell, L.S.; Ullman, J.B. Using multivariate statistics; Pearson: Boston, MA, 2007. [Google Scholar]
- Kleiman, E. Understanding and analyzing multilevel data from real-time monitoring studies: An easily-accessible tutorial using R. 2017.
- Hosmer, D.W.; Le Cessie, S. Applied Logistic Regression; Wiley: New York, 2000. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Gebeyehu, F.G.; Geremew, B.M.; Belew, A.K.; Zemene, M.A. Number of antenatal care visits and associated factors among reproductive age women in Sub-Saharan Africa using recent demographic and health survey data from 2008–2019: A multilevel negative binomial regression model. PLOS Glob. Public Heal. 2022, 2, e0001180. [Google Scholar] [CrossRef]
- Merlo, J.; Chaix, B.; Ohlsson, H.; Beckman, A.; Johnell, K.; Hjerpe, P.; Råstam, L.; Larsen, K. A brief conceptual tutorial of multilevel analysis in social epidemiology: Using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J. Epidemiology Community Heal. 2006, 60, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Senaviratna, N.A.M.R.; Cooray, T.M.J.A. Diagnosing Multicollinearity of Logistic Regression Model. Asian J. Probab. Stat. 2019, 1–9. [Google Scholar] [CrossRef]
- Lee, S.E.; A Talegawkar, S.; Merialdi, M.; E Caulfield, L. Dietary intakes of women during pregnancy in low- and middle-income countries. Public Health Nutr 2012, 16, 1340–1353. [Google Scholar] [CrossRef]
- Adem, H.A.; Usso, A.A.; Hebo, H.J.; Workicho, A.; Ahmed, F. Determinants of acute undernutrition among pregnant women attending primary healthcare unit in Chinaksen District, Eastern Ethiopia: A case-control study. PeerJ 2023, 11, e15416. [Google Scholar] [CrossRef]
- World Health Organization. Nutrition in adolescence –Issues and Challenges for the Health Sector. Available online: https://iris.who.int/bitstream/handle/10665/43342/92;jsessionid=268FA3727B4187B66A6232E6FC093BD5?sequence=1 (accessed on 20 August 2024).
- Sserwanja, Q.; Mutisya, L.M.; Musaba, M.W. Exposure to different types of mass media and timing of antenatal care initiation: Insights from the 2016 Uganda Demographic and Health Survey. BMC Women's Health 2022, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Zaheer, S.; Safdar, N.F.; Turk, T.; Hashmi, S. Women’s awareness, knowledge, attitudes, and behaviours towards nutrition and health in Pakistan: Evaluation of kitchen gardens nutrition program. PLoS ONE 2023, 18, e0291245. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, K.; Cordaro, J.; Fanzo, J.; Gibney, M.; Kennedy, E.; et al. The economic causes of malnutrition. Good nutrition: Perspectives for the 21st century: Karger Publishers. 2016; pp. 92–104.
- Ver Ploeg, M.; Breneman, V.; Dutko, P.; Williams, R.; Snyder, S.; et al. Access to affordable and nutritious food: Updated estimates of distance to supermarkets using 2010 data. 2012.
- French, S.A.; Tangney, C.C.; Crane, M.M.; Wang, Y.; Appelhans, B.M. Nutrition quality of food purchases varies by household income: The SHoPPER study. BMC Public Health 2019, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).