Introduction
Pulmonary hypertension (PH) is a major cause of mortality in patients with systemic sclerosis (SSC). However, although SSc-related PH has been classified as WHO Class I, PH in SSc is highly heterogeneous and can be caused by different pathophysiological mechanisms and they may have precapillary PH (PAH), post capillary PH (secondary to left heart diastolic dysfunction), or combined precapillary and post capillary PH [
1,
2,
3,
4].
In addition to various mechanisms leading to pulmonary vascular disease, pulmonary arterial hypertension, and actually pre capillary PH in these patients, primary cardiac involvement is a common complication of SSc. It is related to repeated focal ischemic injury due to microvascular coronary vasospasm (myocardial Raynaud’s phenomenon) and/or autoimmune myocarditis.
The most common feature of myocardial involvement in these patients is heart failure with preserved ejection fraction (HFPEF) leading to left ventricular diastolic dysfunction and increased left ventricular (LV) filling pressure. These patients may have exertional dyspnea and signs of diastolic dysfunction on echocardiography, including PH [
1,
2,
4,
5,
6].
Right heart catheterization (RHC) as the gold standard for definitive diagnosis of PH should be performed in all patients in whom PH is suspected. Once the diagnosis of PH and its hemodynamic classification is confirmed by RHC, further investigations and/or treatment with PH-specific therapies should be initiated as soon as possible [
3].
After documenting the presence of PH in these patients, pulmonary capillary wedge pressure (PCWP)as the surrogate of LV filling pressure, is used to differentiate pre- and post-capillary PH during RHC [
2,
3].
However, since a PCWP less than 15 mmHg can be seen in resting or volume deplete states, PH related to left-sided heart disease cannot be excluded by a normal PCWP. Therefore, to unmask occult post-capillary PH and HFPEF, fluid loading with saline during RHC is recommended [
2,
7,
8].
In the present study, we investigated the response to fluid loading in patients (fluid challenge test) with SSC who were referred for RHC due to their high likelihood of PH on screening echocardiography.
Methods
Patient Selection
Since 2021, following the study by D’Alto et al. [
7]. we have started to perform a
fluid challenge test in some SSC patients referred to RHC by rheumatologists expert in SSC due to the high likelihood of PH in their screening transthoracic echocardiography[
3].
To conduct the current study, the hospital information system (HIS) was queried for all adult patients diagnosed with systemic sclerosis who underwent right heart catheterization and fluid challenge test between January 2021 and January 2024 at Rajaie Cardiovascular Medical and Research Institute, a tertiary center for pulmonary hypertension programs in Tehran, Iran.
Demographic data, clinical characteristics, echocardiographic and RHC data, and cardiac magnetic resonance imaging (CMR) results were obtained from the patients' HIS and hospital records or by contacting their rheumatologist.
The patients with significant systolic (LVEF<45%) and/or diastolic LV dysfunction and left-sided valvular heart disease on transthoracic echocardiography (TTE), significant dyspnea on exertion [New York Heart Association (NYHA) functional class of III and IV] or remarkable extremities’ edema on physical examination, history of uncontrolled systemic hypertension, significant pulmonary involvement and hypoxemia, referred for follow-up RHC and those with missing data were excluded.
All selected patients were followed up until July 2024 to check whether their immunosuppressive therapy had changed in terms of the use of a more effective regimen and/or all-cause mortality by contacting their rheumatologists. In addition, CMR data were requested from patients who had undergone CMR within 4 months of RHC (2 months before to 2 months after RHC) to assess SSc-related myocardial involvement. For our study population, CMR findings of right and left ventricular ejection fraction, the presence of any extent of either late gadolinium enhancement (LGE) and/or myocardial edema(inflammation) were considered.
This study was approved by the Research and Ethics Committee of the Rajaie Cardiovascular Medical and Research Institute (Ethics code: IR.RHC.REC.1403.032)
Right Heart Catheterization
All patients were studied in the catheterization laboratory at rest in the supine position using a multipurpose A1 catheter while breathing room air. Pressures were all averaged in 3 consecutive heartbeats at the end of expiration. The following variables were measured in each patient: mean right atrial pressure (RAP), right ventricular (RV) systolic and end-diastolic pressure, systolic, diastolic and mean pulmonary artery pressure( PAP), pulmonary capillary wedge pressure (PCWP), systemic arterial and mixed venous oxygen saturation and cardiac output using the Fick method. The cardiac index (CI) was calculated by dividing the CO by the body surface area (BSA).
After obtaining resting data, the fluid challenge test
(FCT) was performed according to D’Alto’s protocol[
7] with 7 ml/kg normal saline over 5-10 minutes and hemodynamic data were measured again.
A positive FCT was defined as an increase in PCWP to more than18 mmHg with saline infusion while in negative fluid challenge PCWP remained ≤18 mmHg.
The clinical update of pulmonary hypertension was taken into account to define the presence of PH and to determine the hemodynamic category (precapillary versus post capillary)[
3]
Statistical Analysis
In a first step, the study population was divided into 2 subgroups, taking into account their baseline hemodynamic data at RHC;
Group 1: patients without pulmonary hypertension, mean PAP<20, PVR<2 Wood unit (no PH)
Group 2: patients with mild isolated precapillary pulmonary hypertension, mean PAP above 20 and below 35 mmHg, PVR>2 wood unit (mPAH)
In addition, each group was divided into two groups with positive FCT and negative FCT based on the results of the fluid challenge test.
IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. was used for all statistical analyzes. Normality of distribution for all variables was tested using the one-sample Kolmogorov-Smirnov test. Categorical variables were expressed as number (percentage) and quantitative variables as mean (standard deviation) or median (interquartile range). Student's t-test and Mann–Whitney, χ2 test, Fisher’s exact tests were used for comparisons and associations as appropriate. P-values <0.05 were considered significant.
Results
Between January 2021 and January 2024, of the total 98 scleroderma patients referred to RHC, 34 patients (29 female) were included. The mean age (SD) was 50.6(8.2) years, ranging from 34 to 69 years.
All patients were referred by rheumatologists experienced in the treatment of SSC from tertiary centers of rheumatology in Tehran/Iran. The median (IQR) duration of SSC was 7.5 (3-12) years.
Regarding symptoms, 4 patients were asymptomatic and were suspected to have PH by screening echocardiography and the rest of the patients (30) had dyspnea on exertion (DOE) in New York Heart Association (NYHA) functional class II.
The median (IQR) of tricuspid regurgitation velocity (TRV) and PAP were 2.89(2.7-2.95) m/s and 38(35-40) mmHg, respectively. The median (IQR) of LVEF was 55(50-55) %. Right ventricular function was within normal limits in 29(85.3%) of patients in echocardiography. No one of patients had pericardial effusion.
In 30 patients with suspected myocardial involvement, a CMR examination was ordered by their rheumatologists before or early after RHC.
Right Heart Catheterization Results
Table 1 shows the baseline characteristics and hemodynamic data.
Baseline RHC data showed that 20 patients had no PH and 14 patients had mPAH . Cardiac index was lower in patients with mPAH, but right atrial pressure (RAP) was not significantly different between the two groups.
Results of the Fluid Challenge Test (FCT)
Ten patients (50%) of the patients who did not have PH had a significant increase in PCWP after the fluid test. Of the patients who had mPAH at baseline, 10 patients (71.4%) had a significant increase in their PCWP.
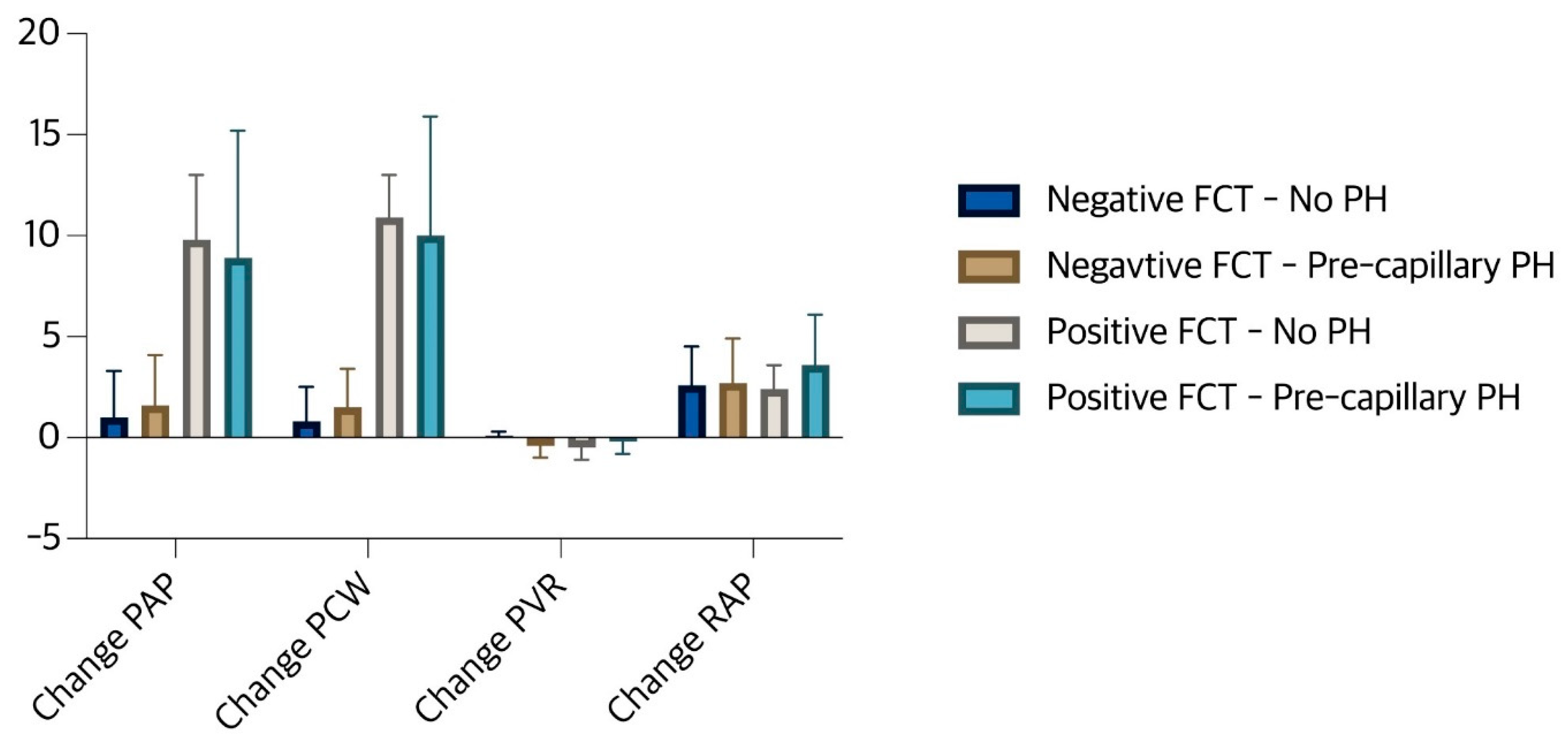
Table 2 shows the comparison of baseline hemodynamic values between the 4 subgroups of the study population and
Figure 1 shows the magnitude of changes in RAP, PCWP, mean PAP and PVR after fluid testing in these subgroups. With the exception of RAP, there was no significant difference between the 4 subgroups in terms of baseline hemodynamic status. However, as shown in
Figure 1, there was no significant change in RAP in all 4 groups after fluid loading. There was no significant change in cardiac output/index after FCT. Except in group no PH, an increase in PCWP, albeit insignificant, led to a decrease in the calculated PVR in the other sub groups.
CMR Findings
Table 3 shows the comparison of CMR findings between 4 subgroups of the study population. CMR was performed in 30 patients with suspected myocardial involvement. There was no difference in left and right ventricular function between the groups. Eighteen of 30 (60%) patients had LGE and/or myocardial edema (inflammation) on their CMR examination. There was no statistically significant difference between the patients with positive and negative FCT in terms of the presence of LGE/inflammation on CMR examination, but the presence of LGE/inflammation was significantly more common in the patients with mPAH.
Follow Up
Patients were followed for a median (IQR) of 15 (7-20) months. During follow-up, 3 patients died. Mortality was due to SSc-related complications in two patients. These two patients (both female) had positive FCT and were categorized as combined pre- and post-capillary PH. Both had both edema and LGE in their CMR. The third patient was a 57-year-old male who had had SSC for 3 years. He had no PH on hemodynamic testing, his fluid challenge test was negative, and he did not perform CMR. The reason for his mortality was unclear to us.
Of 34 patients, the immunosuppressive therapies were changed in 22 patients according to the opinion of the rheumatologist. Eleven patients received specific therapy for myocardial involvement (SSc related myocarditis) after a positive FCT and myocardial edema/LGE in their CMR examination were detected. Specific pulmonary vasodilators were started for all patients with precapillary PH.
Discussion
Our study examines the utility of fluid challenge testing in patients with systemic sclerosis (SSc) suspected of having pulmonary hypertension (PH). The results highlight several important points. Fluid challenge testing may identify a subset of SSc patients with latent post-capillary PH. While right heart catheterization (RHC) did not reveal significant pulmonary hypertension in a proportion of patients, the FCT showed a positive response in a substantial proportion of patients (58%), indicating latent or masked post-capillary PH. In addition, a positive FCT in patients with mild precapillary PH suggests that the fluid challenge test may identify patients with more advanced disease characterized by structural changes in the heart and lungs.
In patients with systemic sclerosis (or scleroderma), the primary form of pulmonary hypertension is typically class I or precapillary PH, characterized by increased pulmonary arterial pressure due to the underlying vasculopathy associated with the disease. This form of PH is relatively well studied and understood in the context of systemic sclerosis. However, there is less evidence and understanding of class II or post-capillary PH in these patients, which occurs secondary to left heart problems such as left ventricular dysfunction or valvular disease. The prevalence of class II PH in patients with systemic sclerosis is not well understood as it is often overlooked or underdiagnosed [
1,
2,
7]. In systemic sclerosis, the underlying vasculopathy and fibrotic changes may contribute to left ventricular dysfunction leading to increased left-sided filling pressure and subsequent post-capillary PH. These patients may have a combination of precapillary (Class I) and post-capillary (Class II) components. The fluid challenge test can help distinguish between these two subtypes, which have different pathophysiology and require different treatment approaches [
1,
2,
5,
7].
However, in addition to the FCT, other diagnostic tests should be considered to complement the assessment of cardiac involvement in scleroderma patients, including electrocardiography, echocardiography, CMR, biomarkers and cardiopulmonary exercise testing. By combining the information from the FCT with these other diagnostic modalities, clinicians can obtain a more comprehensive assessment of cardiac involvement in scleroderma. This multifaceted approach can help detect early subclinical changes, monitor disease progression and provide appropriate therapeutic interventions [
1,
2,
9,
10,
11,
12,
13].
CMR can offer detailed information about myocardial tissue composition, including the presence and extent of fibrosis, inflammation, and edema. The fluid challenge test can complement the structural and functional information obtained from CMR by assessing the heart's dynamic response to volume loading. This can help differentiate between fixed structural changes and more functional or reversible cardiac impairment [
11,
12,
14,
15].
As far as we have researched, the current study is the first study to examine the association between CMR findings and hemodynamics in SSc patients. However, in this study, despite an increased frequency of LGE/inflammation in the patients’ CMR, we found no correlation between the CMR findings and a positive FCT. Although this finding may indicate that FCT may not be a reliable indicator of myocardial involvement in SSc, several explanations should be considered. As previous studies have shown, the presence of LGE/inflammation in the CMR of SSc patients is a very common finding, and SSc patients may have myocardial abnormalities in their CMR even in the absence of overt cardiac dysfunction. For example, half of the SSc patients in the Poindron et al. study had diffuse myocardial fibrosis on CMR, but there was no difference in right or left ventricular volume or ejection fraction between the patients with and without LGE [
14]. Ntusi et al. also showed increased LGE/inflammation in SSc patients despite preserved ventricular size and function. On the other hand, several other studies have demonstrated LV diastolic dysfunction and increased filling pressures in a remarkable number of SSc patients by hemodynamic studies including FCT [
6,
15]. But in none of these studies were the hemodynamic findings collected together with the CMR findings.
In addition, the fluid challenge test in scleroderma patients may reveal various types of cardiac dysfunction that are not necessarily correlated with cardiac imaging results. The fluid challenge test can provide information about right ventricular function, which may be impaired in scleroderma patients with or without overt pulmonary hypertension. A positive fluid challenge test may indicate an impaired ability of the right ventricle to adapt to increased preload, even in the absence of significant pulmonary vascular disease [
16]. In the present study, although RAP did not change significantly after FCT, subtle RV abnormalities and abnormal septal movements after volume loading as a reason for the increase in PCWP and a positive FCT cannot be excluded. Furthermore, the FCT may reveal underlying autonomic dysregulation, which may be a feature of scleroderma and contribute to cardiovascular instability. The ability of the heart to maintain adequate hemodynamic responses to volume changes may be impaired in the setting of autonomic dysfunction [
17].
Regarding mortality risk in our study population, mortality was observed in three patients, two of whom had positive FCT, combined pre-post capillary pH and LGE/inflammation in the CMR. Thus, although mortality in SSc patients with PH is very high, the result of the current study is not conclusive enough to investigate the mortality risk. The small sample size and the relatively short follow-up period may explain this result. However, we excluded the patients who had significant SSc-related interstitial lung disease (SSc-ILD), and the survival rate is worse in patients with SSc-ILD[
1,
18], and it was expected that our study group would have a better clinical outcome in terms of survival.
Study Limitations
The careful selection of the study population and the availability of CMR data are the strengths of the study. However, the relatively small sample size may limit the generalizability of the results. The 20-month follow-up period may not be sufficient to fully assess long-term outcomes. In addition, the lack of a control group and the potential for selection bias could be considered limitations. It would have been better if we could have also determined the level of natriuretic peptide in this study, but as this was not checked in half of the patients due to the limited availability of this biomarker, we did not include it.
Recommendations for Future Research
Based on our findings, some specific areas are recommended for future research;
1. Optimization of fluid testing: Investigate the optimal time to perform fluid challenge testing in relation to baseline RHC. Explore different fluid loading protocols (volume, rate, duration) to determine the most effective and reliable method to identify latent PH. Develop standardized criteria for interpreting response to fluid loading, including hemodynamic parameters and other clinical indicators, to improve consistency and reliability in identifying patients with latent PH, including postcapillary PH.Conduct studies to determine the predictive value of fluid testing for long-term outcomes such as disease progression, mortality and response to treatment.
2. Understanding the mechanism of latent PH: Investigate the role of microvascular dysfunction and changes in vascular reactivity in the development of latent PH in SSc patients. Explore the possible contribution of inflammation, fibrosis and other factors to these microvascular changes. Evaluate the impact of latent PH on cardiac function, including ventricular hypertrophy, diastolic dysfunction and arrhythmias. Determine whether early detection and treatment of latent PH can improve cardiac function and reduce the risk of complications.
3. Impact of early intervention: Conduct clinical trials to evaluate the effectiveness of different treatment strategies for patients with latent PH identified by fluid loading tests. These could include medications targeting pulmonary vascular resistance, anti-inflammatory therapies, or other approaches. Assess the long-term impact of early intervention on disease progression, survival, and quality of life in patients with latent PH. Determine whether early treatment can delay or prevent the development of overt PH and its associated complications.
4. Relationship to SSc subtypes: Explore the prevalence of latent PH in different SSc subtypes (e.g., limited cutaneous, diffuse cutaneous) and investigate whether the fluid test is more sensitive in certain subtypes. Examine the relationship between fluid test response and disease severity in SSc, including extent of skin involvement, internal organ damage, and functional limitations.
5. Integration into clinical practice: Develop evidence-based guidelines and recommendations for the use of fluid testing in SSc patients, taking into account the results of future research.
Conclusions
In conclusion, this study provides preliminary evidence that fluid challenge testing may be a useful adjunct to baseline RHC testing to identify SSc patients with latent postcapillary PH. Further studies with larger sample sizes and longer follow-up periods are needed to confirm these results and determine the optimal role of the fluid challenge test in the clinical management of SSc patients with suspected PH.
References
- Haque, A. , et al., Pulmonary hypertension phenotypes in patients with systemic sclerosis. European Respiratory Review, 2021. 30(161).
- Hsu, V.M. , et al., Assessment of pulmonary arterial hypertension in patients with systemic sclerosis: comparison of noninvasive tests with results of right-heart catheterization. The Journal of rheumatology, 2008. 35(3): p. 458-465.
- Vazquez, Z.G. and J.R. Klinger, Guidelines for the treatment of pulmonary arterial hypertension. Lung, 2020. 198(4): p. 581-596.
- Khangoora, V. , et al., Connective tissue disease-associated pulmonary hypertension: A comprehensive review. Pulmonary Circulation, 2023. 13(4): p. e12276.
- Parks, J.L. , et al., Systemic sclerosis and the heart. Rheumatic Disease Clinics, 2014. 40(1): p. 87-102.
- Bourji, K.I. , et al., Poor survival in patients with scleroderma and pulmonary hypertension due to heart failure with preserved ejection fraction. Pulmonary Circulation, 2017. 7(2): p. 409-420.
- D’Alto, M. , et al., Hemodynamic changes after acute fluid loading in patients with systemic sclerosis without pulmonary hypertension. Pulmonary circulation, 2018. 9(1): p. 2045894018816089.
- D'Alto, M. , et al., Clinical relevance of fluid challenge in patients evaluated for pulmonary hypertension. Chest, 2017. 151(1): p. 119-126.
- Chung, L. , et al., Survival and predictors of mortality in systemic sclerosis-associated pulmonary arterial hypertension: outcomes from the pulmonary hypertension assessment and recognition of outcomes in scleroderma registry. 2014, Wiley Online Library.
- Dumitru, R.B. , et al., Cardiovascular outcomes in systemic sclerosis with abnormal cardiovascular MRI and serum cardiac biomarkers. RMD open, 2021. 7(3): p. e001689.
- Meduri, A. , et al., Cardiac magnetic resonance in systemic sclerosis patients with cardiac symptoms. European Review for Medical & Pharmacological Sciences, 2017. 21(21).
- Agoston-Coldea, L. , et al., Current advances in cardiac magnetic resonance imaging in systemic sclerosis. European Review for Medical & Pharmacological Sciences, 2021. 25(10).
- Panopoulos, S. , et al., Cardiac magnetic resonance imaging before and after therapeutic interventions for systemic sclerosis-associated myocarditis. Rheumatology, 2023. 62(4): p. 1535-1542.
- Poindron, V. , et al. T1 mapping cardiac magnetic resonance imaging frequently detects subclinical diffuse myocardial fibrosis in systemic sclerosis patients. in Seminars in arthritis and rheumatism. 2020. Elsevier.
- Ntusi, N.A. , et al., Subclinical myocardial inflammation and diffuse fibrosis are common in systemic sclerosis–a clinical study using myocardial T1-mapping and extracellular volume quantification. Journal of Cardiovascular Magnetic Resonance, 2014. 16(1): p. 21.
- Tedford, R.J. , et al., Right ventricular dysfunction in systemic sclerosis–associated pulmonary arterial hypertension. Circulation: Heart Failure, 2013. 6(5): p. 953-963.
- Di Battista, M. , et al., Autonomic dysfunction in systemic sclerosis: A scoping review. Seminars in Arthritis and Rheumatism, 2023. 63: p. 152268.
- Chauvelot, L. , et al., Hemodynamic response to treatment and outcomes in pulmonary hypertension associated with interstitial lung disease versus pulmonary arterial hypertension in systemic sclerosis: data from a study identifying prognostic factors in pulmonary hypertension associated with interstitial lung disease. Arthritis & Rheumatology, 2021. 73(2): p. 295-304.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).