Submitted:
28 August 2024
Posted:
28 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Research Subjects and Groups
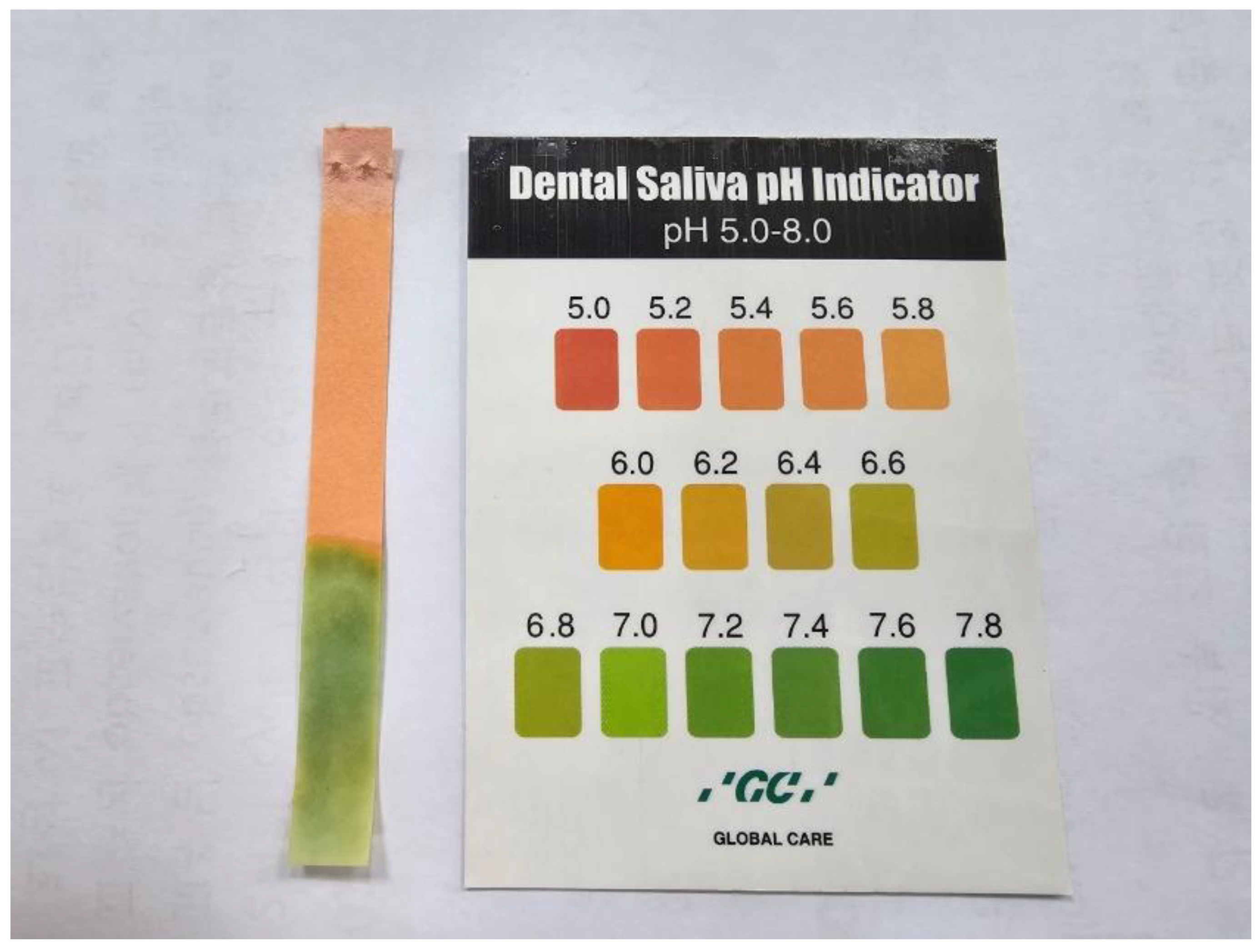
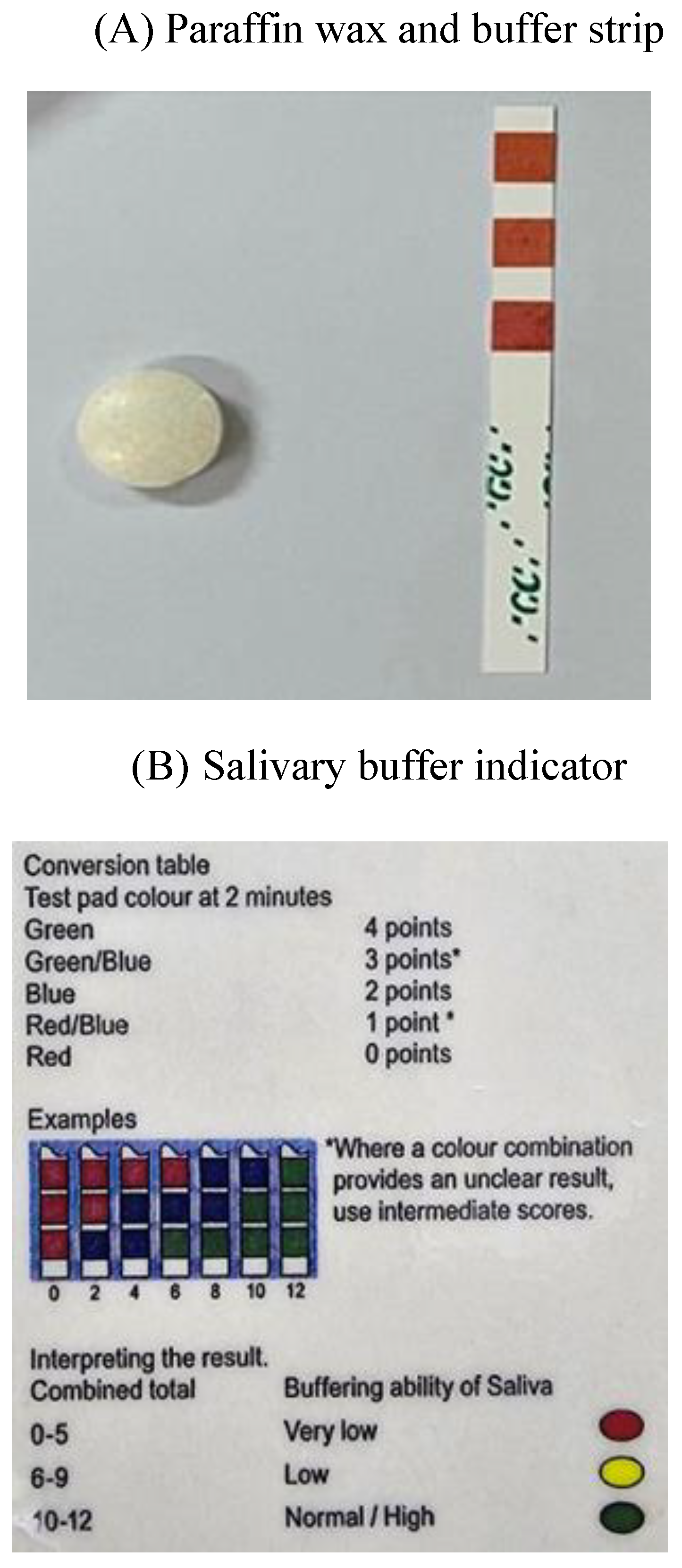
2.2. Evaluation of Saliva
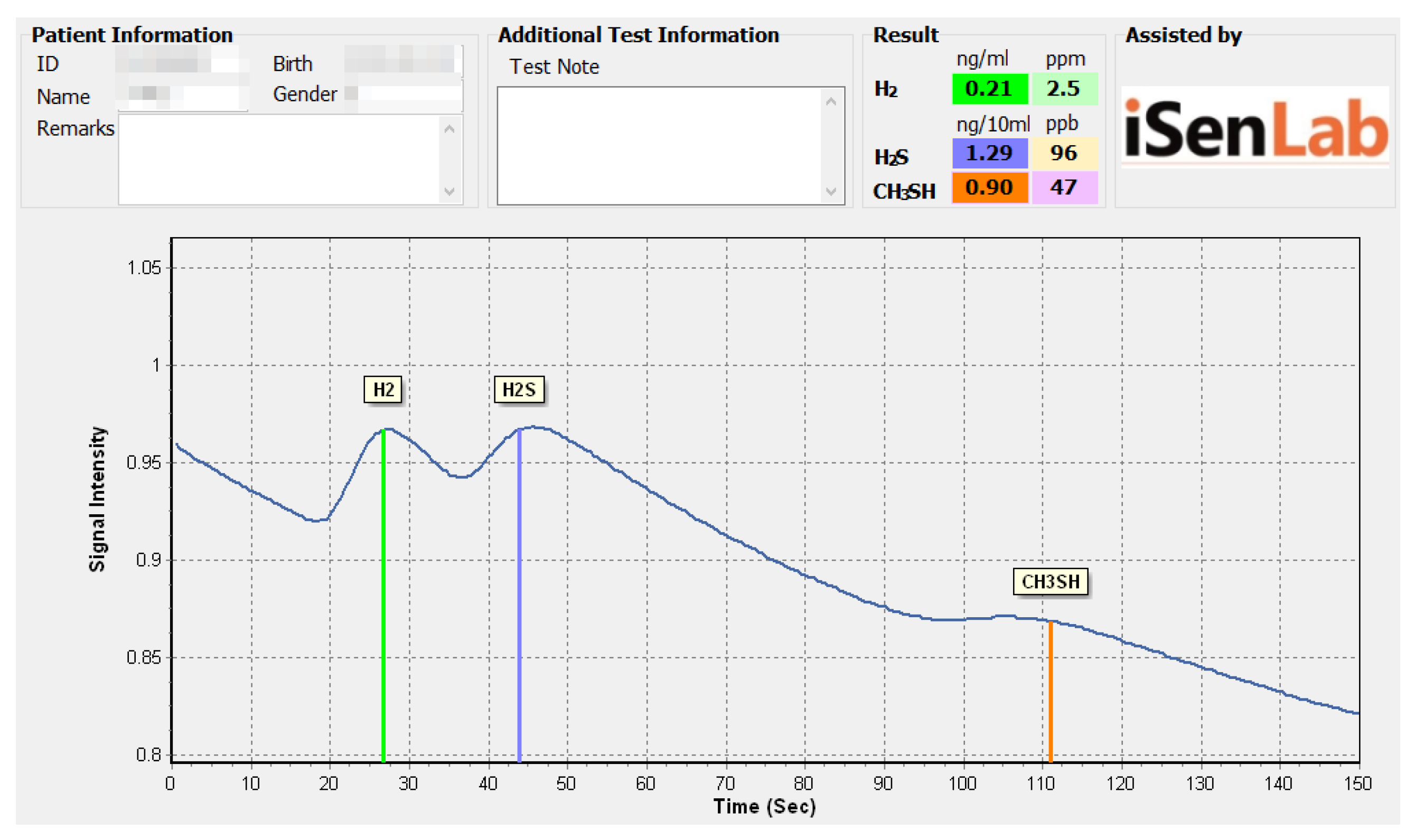
2.3. Evaluation of Halitosis
2.4. Evaluation of Oral Health Condition
2.5. Evaluation of Systemic Disease and Medication
2.6. Statistical Analysis
2.7. Ethics Approval and Consent to Participate
3. Results
3.1. Gender and Age Distribution of Patients
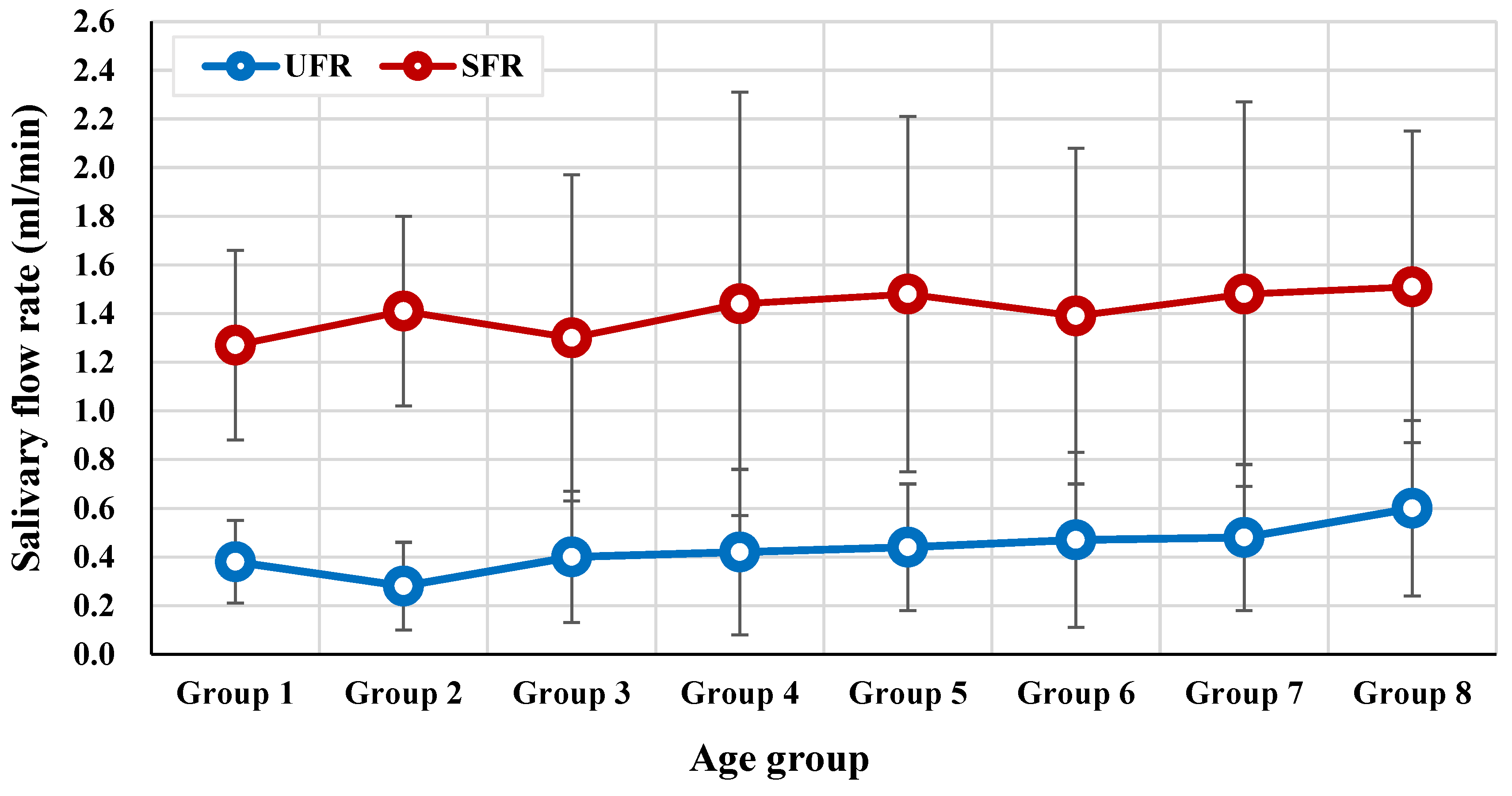
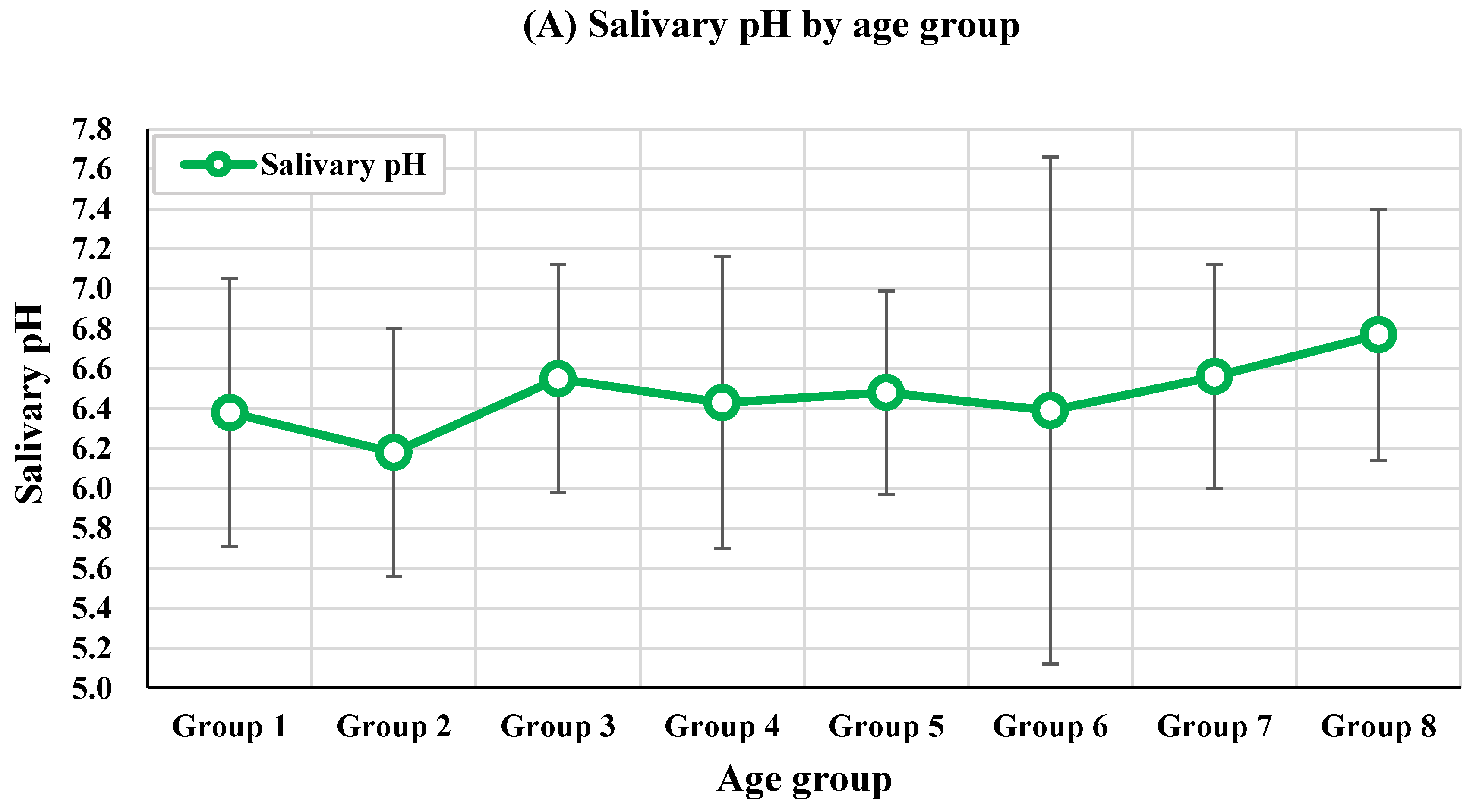
3.2. Salivary Parameters by Age Group
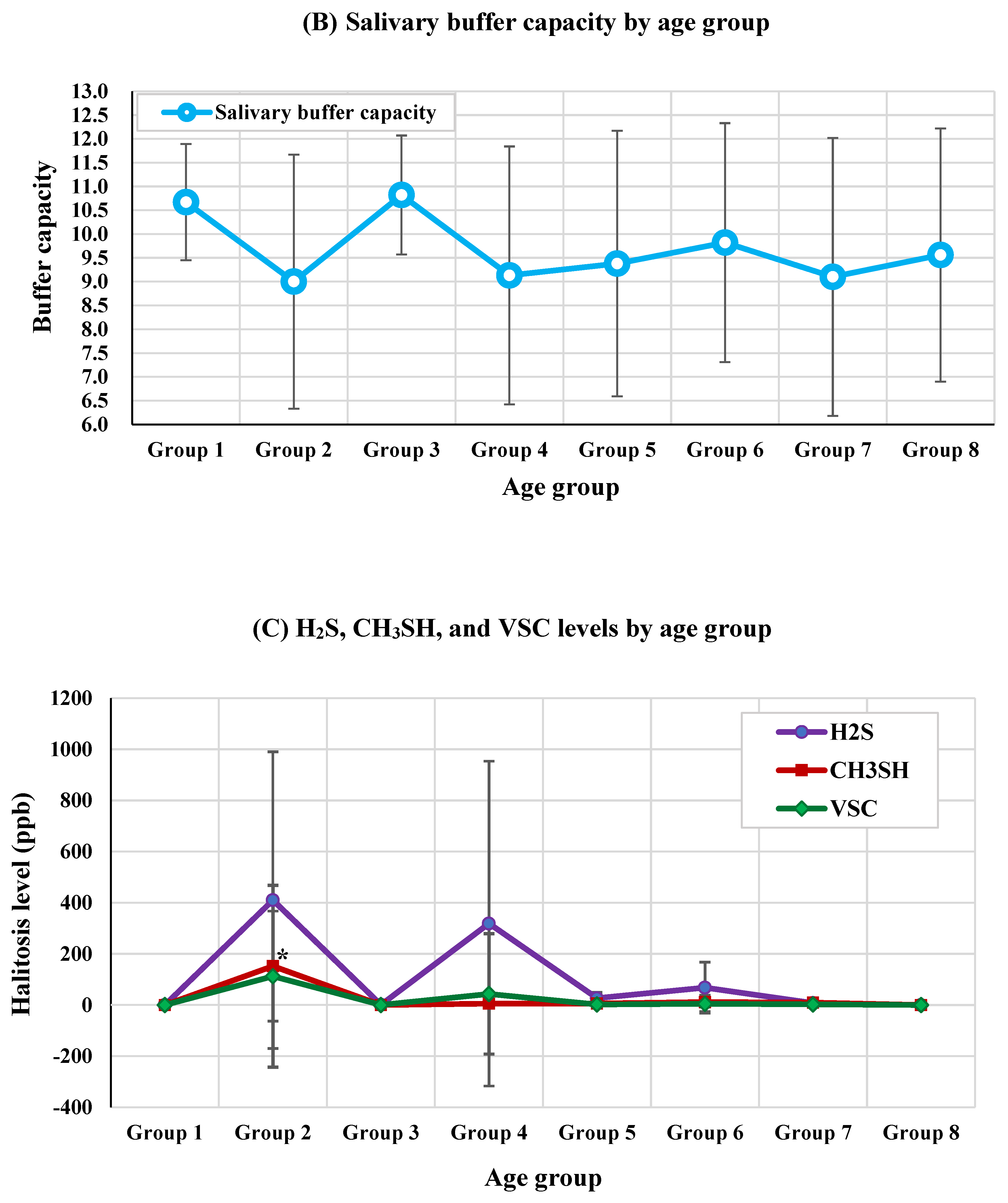
3.3. VSC Levels by Age Group
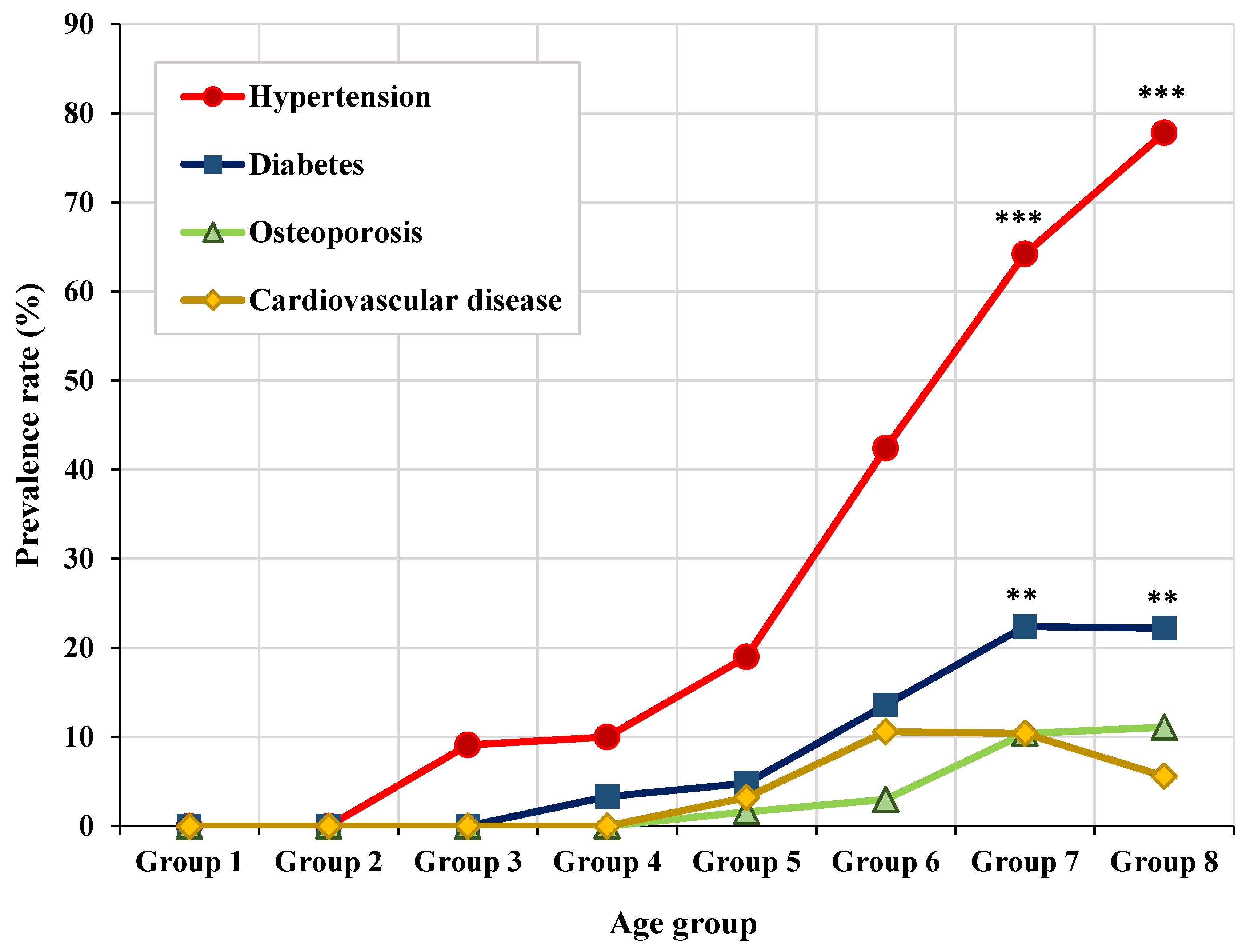
3.4. Distribution of Representative Systemic Diseases by Age Group
3.5. Correlation between Age, Halitosis, and Salivary Flow Rate
3.6. Correlations between oral health, systemic disease, and medication with aging
3.7. Generalized Linear Model of Oral Health, Salivary Parameters and Aging
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed consent statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Consent for Publication
References
- Jang, J.Y.; Lee, D.H. Effects of Oral Health Promotion Program on Oral Function in the Elderly. Korean J Health Serv Manag 2016, 10, 141–151. [Google Scholar] [CrossRef]
- Meng, K.H. Population aging and health promotion activities in Korea. J Korea Assoc Health Promot 2004, 2, 187–197. [Google Scholar]
- Gil-Montoya, J.A.; de Mello, A.L.; Barrios, R.; Gonzalez-Moles, M.A.; Bravo, M. Oral health in the elderly patient and its impact on general well-being: A nonsystematic review. Clin Interv Aging 2015, 10, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Richmond, S.; Chestnutt, I.; Shennan, J.; Brown, R. The relationship of medical and dental factors to perceived general and dental health. Community Dent Oral Epidemiol 2007, 35, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic diseases caused by oral infection. Clin Microbiol Rev 2000, 13, 547–558. [Google Scholar] [CrossRef]
- Muller, F. Oral hygiene reduces the mortality from aspiration pneumonia in frail elders. J Dent Res 2015, 94, 14S–16S. [Google Scholar] [CrossRef]
- Lee, Y.H.; Won, J.H.; Auh, Q.S.; Noh, Y.K.; Lee, S.W. Prediction of xerostomia in elderly based on clinical characteristics and salivary flow rate with machine learning. Sci Rep 2024, 14, 3423. [Google Scholar] [CrossRef]
- Fornari, C.B.; Bergonci, D.; Stein, C.B.; Agostini, B.A.; Rigo, L. Prevalence of xerostomia and its association with systemic diseases and medications in the elderly: A cross-sectional study. Sao Paulo Med J 2021, 139, 380–387. [Google Scholar] [CrossRef]
- Jamieson, L.M.; Thomson, W.M. Xerostomia: Its prevalence and associations in the adult Australian population. Aust Dent J 2020, 65 (Suppl. S1), S67–S70. [Google Scholar] [CrossRef]
- Liu, B.; Dion, M.R.; Jurasic, M.M.; Gibson, G.; Jones, J.A. Xerostomia and salivary hypofunction in vulnerable elders: Prevalence and etiology. Oral Surg Oral Med Oral Pathol Oral Radiol 2012, 114, 52–60. [Google Scholar] [CrossRef]
- Yeh, C.K.; Johnson, D.A.; Dodds, M.W. Impact of aging on human salivary gland function: A community-based study. Aging (Milano) 1998, 10, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Dodds, M.W.J.; Haddou, M.B.; Day, J.E.L. The effect of gum chewing on xerostomia and salivary flow rate in elderly and medically compromised subjects: A systematic review and meta-analysis. BMC Oral Health 2023, 23, 406. [Google Scholar] [CrossRef]
- Lee, Y.H.; Hong, J.Y. Oral microbiome as a co-mediator of halitosis and periodontitis: A narrative review. Front Oral Health 2023, 4, 1229145. [Google Scholar] [CrossRef]
- Moreno, L.B.; Colussi, P.R.G.; Marostega, M.G.; Rosalen, N.P.; Rösing, C.K.; Muniz, F.W.M.G. Self-reported halitosis and associated factors among older adults: A cross-sectional study. J Oral Biol Craniofac Res 2022, 12, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, T.; Suzuki, N.; Nakano, Y.; Yasui, M.; Yoneda, M.; Shimazaki, Y.; Hirofuji, T.; Yamashita, Y. Discrimination of the oral microbiota associated with high hydrogen sulfide and methyl mercaptan production. Sci Rep 2012, 2, 215. [Google Scholar] [CrossRef]
- Yaegaki, K.; Sanada, K. Biochemical and clinical factors influencing oral malodor in periodontal patients. J Periodontol 1992, 63, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Agency, K.D.C.a.P. Korea Health Statistics 2022: Korea National Health and nutrition Examination Survey. Available online: https://knhanes.kdca.go.kr/ (accessed on 23 August 2024).
- Cho, H.J.; Chae, J.; Yoon, S.H.; Kim, D.S. Aging and the Prevalence of Polypharmacy and Hyper-Polypharmacy Among Older Adults in South Korea: A National Retrospective Study During 2010-2019. Front Pharmacol. 2022, 13, 866318. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, K.; Shirai, K.; Tang, C.; Hu, Y.; Wang, Y.; Hao, Y.; Dong, J.Y. Prevalence and trends of polypharmacy in U.S. adults, 1999-2018. Glob Health Res Policy 2023, 8, 25. [Google Scholar] [CrossRef]
- Bozdemir, E.; Yilmaz, H.H.; Orhan, H. Oral mucosal lesions and risk factors in elderly dental patients. J Dent Res Dent Clin Dent Prospects 2019, 13, 24–30. [Google Scholar] [CrossRef]
- Villa, A.; Connell, C.L.; Abati, S. Diagnosis and management of xerostomia and hyposalivation. Ther Clin Risk Manag 2015, 11, 45–51. [Google Scholar] [CrossRef]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J Indian Soc Periodontol 2013, 17, 461–465. [Google Scholar] [CrossRef]
- Tonzetich, J.; Ng, S.K. Reduction of malodor by oral cleansing procedures. Oral Surg Oral Med Oral Pathol 1976, 42, 172–181. [Google Scholar] [CrossRef]
- Mata, A.D.; Marques, D.; Rocha, S.; Francisco, H.; Santos, C.; Mesquita, M.F.; Singh, J. Effects of diabetes mellitus on salivary secretion and its composition in the human. Mol Cell Biochem 2004, 261, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Elmi Rankohi, Z.; Shabanian, M.; Maleki, D. Oral Manifestations of Patients Taking Anti-Hypertensive Medications. Journal of Islamic Dental Association of IRAN 2020, 32, 83–88. [Google Scholar] [CrossRef]
- Ok, S.M.; Ho, D.; Lynd, T.; Ahn, Y.W.; Ju, H.M.; Jeong, S.H.; Cheon, K. Candida Infection Associated with Salivary Gland-A Narrative Review. J Clin Med 2020, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Beetz, I.; Schilstra, C.; Visink, A.; van der Schaaf, A.; Bijl, H.P.; van der Laan, B.F.; Steenbakkers, R.J.; Langendijk, J.A. Role of minor salivary glands in developing patient-rated xerostomia and sticky saliva during day and night. Radiother Oncol 2013, 109, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Vissink, A.; Spijkervet, F.K.; Van Nieuw Amerongen, A. Aging and saliva: A review of the literature. Spec Care Dent 1996, 16, 95–103. [Google Scholar] [CrossRef]
- Affoo, R.H.; Foley, N.; Garrick, R.; Siqueira, W.L.; Martin, R.E. Meta-Analysis of Salivary Flow Rates in Young and Older Adults. J Am Geriatr Soc 2015, 63, 2142–2151. [Google Scholar] [CrossRef]
- Beetz, I.; Schilstra, C.; van der Schaaf, A.; van den Heuvel, E.R.; Doornaert, P.; van Luijk, P.; Vissink, A.; van der Laan, B.F.; Leemans, C.R.; Bijl, H.P.; et al. NTCP models for patient-rated xerostomia and sticky saliva after treatment with intensity modulated radiotherapy for head and neck cancer: The role of dosimetric and clinical factors. Radiother Oncol 2012, 105, 101–106. [Google Scholar] [CrossRef]
- Seerangaiyan, K.; Juch, F.; Winkel, E.G. Tongue coating: Its characteristics and role in intra-oral halitosis and general health-a review. J Breath Res 2018, 12, 034001. [Google Scholar] [CrossRef]
- Li, Y.; Cui, J.; Liu, Y.; Chen, K.; Huang, L.; Liu, Y. Oral, Tongue-Coating Microbiota, and Metabolic Disorders: A Novel Area of Interactive Research. Front Cardiovasc Med 2021, 8, 730203. [Google Scholar] [CrossRef] [PubMed]
- Ogami, K.; Ueda, T.; Ryu, M.; Tajima, S.; Sakurai, K. Evaluation of Factors Associated with Tongue Coating Status in Elderly with Care Needs. Bull Tokyo Dent Coll 2018, 59, 163–169. [Google Scholar] [CrossRef]
- Kikutani, T.; Tamura, F.; Nishiwaki, K.; Suda, M.; Kayanaka, H.; Machida, R.; Yoshida, M.; Akagawa, Y. The degree of tongue-coating reflects lingual motor function in the elderly. Gerodontology 2009, 26, 291–296. [Google Scholar] [CrossRef]
- Tomooka, K.; Saito, I.; Furukawa, S.; Maruyama, K.; Eguchi, E.; Iso, H.; Tanigawa, T. Yellow Tongue Coating is Associated With Diabetes Mellitus Among Japanese Non-smoking Men and Women: The Toon Health. J Epidemiol 2018, 28, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Furuya, J.; Beniya, A.; Suzuki, H.; Hidaka, R.; Matsubara, C.; Obana, M.; Yoshimi, K.; Yamaguchi, K.; Hara, K.; Nakagawa, K.; et al. Factors associated with the number of microorganisms on the tongue surface in patients following acute stroke. J Oral Rehabil 2020, 47, 1403–1410. [Google Scholar] [CrossRef]
- Chalmers, J.M.; King, P.L.; Spencer, A.J.; Wright, F.A.; Carter, K.D. The oral health assessment tool--validity and reliability. Aust Dent J 2005, 50, 191–199. [Google Scholar] [CrossRef]
- Arai, K.; Sumi, Y.; Uematsu, H.; Miura, H. Association between dental health behaviours, mental/physical function and self-feeding ability among the elderly: A cross-sectional survey. Gerodontology 2003, 20, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Girestam Croonquist, C.; Dalum, J.; Skott, P.; Sjögren, P.; Wårdh, I.; Morén, E. Effects of Domiciliary Professional Oral Care for Care-Dependent Elderly in Nursing Homes - Oral Hygiene, Gingival Bleeding, Root Caries and Nursing Staff's Oral Health Knowledge and Attitudes. Clin Interv Aging 2020, 15, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Patterson Norrie, T.; Villarosa, A.R.; Kong, A.C.; Clark, S.; Macdonald, S.; Srinivas, R.; Anlezark, J.; George, A. Oral health in residential aged care: Perceptions of nurses and management staff. Nurs Open 2019, 7, 536–546. [Google Scholar] [CrossRef]
- Sterer, N.; Rosenberg, M. Streptococcus salivarius promotes mucin putrefaction and malodor production by Porphyromonas gingivalis. J Dent Res 2006, 85, 910–914. [Google Scholar] [CrossRef]
- Sarafidou, K.; Alexakou, E.; Talioti, E.; Bakopoulou, A.; Anastassiadou, V. The oral microbiome in older adults –a state-of-the-art review. Archives of Gerontology and Geriatrics Plus 2024, 1. [Google Scholar] [CrossRef]
- Rizzardi, K.F.; Indiani, C.; Mattos-Graner, R.O.; de Sousa, E.T.; Nobre-Dos-Santos, M.; Parisotto, T.M. Firmicutes Levels in the Mouth Reflect the Gut Condition With Respect to Obesity and Early Childhood Caries. Front Cell Infect Microbiol 2021, 11, 593734. [Google Scholar] [CrossRef] [PubMed]
- Percival, R.S.; Challacombe, S.J.; Marsh, P.D. Flow rates of resting whole and stimulated parotid saliva in relation to age and gender. J Dent Res 1994, 73, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Al Kibria, G.M.; Nemirovsky, A.; Sharmeen, A.; Day, B. Age-stratified prevalence, treatment status, and associated factors of hypertension among US adults following application of the 2017 ACC/AHA guideline. Hypertens Res 2019, 42, 1631–1643. [Google Scholar] [CrossRef]
- Sinclair, A.; Saeedi, P.; Kaundal, A.; Karuranga, S.; Malanda, B.; Williams, R. Diabetes and global ageing among 65-99-year-old adults: Findings from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract 2020, 162, 108078. [Google Scholar] [CrossRef]
- Kim, H.C.; Lee, H.; Lee, H.H.; Son, D.; Cho, M.; Shin, S.; Seo, Y.; Kim, E.J.; Korean Society of Hypertension -Hypertension Epidemiology Research Working Group. Korea Hypertension Fact Sheet 2023: Analysis of nationwide population-based data with a particular focus on hypertension in special populations. Clin Hypertens 2024, 30, 7. [Google Scholar] [CrossRef] [PubMed]
- Won, K.C.; Kwon, H.S.; Ko, S.H.; Bae, J.H.; Yang, Y.S.; Choi, J.H.; K., H. Diabetes fact sheet in Korea 2022. 2023.05.02 ed.; Won, K.C.: Korean Diabetes Association, 2022.
| Features | n (%) | Male n (%) | Female n (%) | p-value | |
|---|---|---|---|---|---|
| Sex | Male | 66 (24.1) | - | - | - |
| Female | 208 (75.9) | ||||
| Age | Group 1 (age 10s) | 9 (3.3) | 5(55.6%) | 4(44.4%) | 0.028* |
| Group 2 (age 20s) | 10 (3.6) | 6(60.0%) | 4(40.0%) | ||
| Group 3 (age 30s) | 11 (4.0) | 3(27.3%) | 8(72.7%) | ||
| Group 4 (age 40s) | 30 (10.9) | 6(20.0%) | 24(80.0%) | ||
| Group 5 (age 50s) | 63 (23.0) | 11(17.5%) | 52(82.5%) | ||
| Group 6 (age 60s) | 66 (24.1) | 13(19.7%) | 53(80.3%) | ||
| Group 7 (age 70s) | 67 (24.5) | 19(28.4%) | 48(71.6%) | ||
| Group 8 (age 80s) | 18 (6.6) | 3(16.7%) | 15(83.3%) | ||
| Total | 274 (100.0) | 66(24.1%) | 208(75.9%) | ||
| Features | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | Group 7 | Group 8 | p-value | Post-hoc |
| Age 10s (n = 9) |
Age 20s (n = 10) |
Age 30s (n = 11) |
Age 40s (n = 30) |
Age 50s (n = 63) |
Age 60s (n = 66) |
Age 70s (n = 67) |
Age 80s (n = 18) |
|||
| Mean±SD or n (%) | Mean±SD or n (%) | Mean±SD or n (%) | Mean±SD or n (%) | Mean±SD or n (%) | Mean±SD or n (%) | Mean±SD or n (%) | Mean±SD or n (%) | |||
| Age (year) | 15.89±3.26 | 24.20±1.74 | 36.27±3.04 | 45.33±3.09 | 54.57±3.07 | 64.26±2.88 | 74.04±2.91 | 82.72±2.22 | <0.001*** | Age group 1<2<3<4<5<6<7<8 |
| H2S (ppb) | 0.00±0.00 | 410.00±579.82 | 1.00±1.00 | 318.50±635.00 | 26.50±22.81 | 68.00±99.58 | 7.89±12.34 | 0.00±0.00 | 0.393 | - |
| CH3SH (ppb) | 0.00±0.00 | 152.00±214.96 | 0.00±0.00 | 5.25±9.18 | 6.00±6.93 | 11.50±20.37 | 9.22±16.86 | 0.00±0.00 | 0.049* | Age group 2 > Others |
| VSC (ppb) | 0.00±0.00 | 112.40±355.44 | 0.273±0.65 | 43.17±235.49 | 2.06±9.74 | 4.82±32.00 | 2.30±10.50 | 0.00±0.00 | 0.051 | - |
| Halitosis | 0 (0.0%) | 1 (10.0%) | 0 (0.0%) | 1 (3.3%) | 2 (3.2%) | 2 (3.0%) | 1 (1.5%) | 0 (0.0%) | 0.807 |
| Correlation | VSC (ppb) |
Halitosis | UFR (mL/min) |
SFR (mL/min) |
|
|---|---|---|---|---|---|
| Age (years) | Correlation Coefficient | 0.052 | 0.054 | −0.024 | 0.082 |
| p-value | 0.392 | 0.371 | 0.690 | 0.178 | |
|
VSC (ppb) |
Correlation Coefficient | 1.000 | 0.621 | 0.038 | 0.083 |
| p-value | <0.001*** | 0.533 | 0.171 | ||
| Halitosis | Correlation Coefficient | 1.000 | −0.010 | 0.074 | |
| p-value | 0.871 | 0.221 | |||
|
UFR (mL/min) |
Correlation Coefficient | 1.000 | 0.513 | ||
| p-value | <0.001*** | ||||
| SFR (mL/min) |
Correlation Coefficient | 1.000 | |||
| p-value | |||||
| Features | Aging | ||
|---|---|---|---|
| Correlation coefficient | p-value | ||
| Oral health | Sticky saliva | 0.022 | 0.721 |
| Oral hygiene | 0.087 | 0.152 | |
| Calculus deposition | 0.005 | 0.930 | |
| Tongue coating | 0.205 | 0.001*** | |
| Ulcer | 0.023 | 0.701 | |
| Oral candidiasis | 0.063 | 0.299 | |
| Systemic medication | Amlodipine | 0.249 | 0.001*** |
| Metformin | 0.031 | 0.614 | |
| Alendronate | 0.118 | 0.051 | |
| Furosemide | 0.068 | 0.278 | |
| Systemic disease | Hypertension | 0.495 | <0.001*** |
| Diabetes mellitus | 0.255 | <0.001*** | |
| Osteoporosis | 0.195 | 0.001** | |
| Cardiovascular diseases | 0.150 | 0.013* | |
| Parameter | B | SE | 95% Wald CI | p-value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Sticky saliva [ref.=none] | 6.039 | 2.383 | 1.368 | 10.709 | 0.011* |
| Oral hygiene_poor [ref.=good] | 11.752 | 4.864 | 2.219 | 21.286 | 0.016* |
| Oral hygiene_moderate [ref.=good] | 6.903 | 2.563 | 1.880 | 11.926 | 0.007** |
| Calculus deposition [ref.=none] | 2.533 | 5.711 | -8.660 | 13.725 | 0.657 |
| Oral ulcer [ref.=none] | -5.036 | 3.5272 | -11.949 | 1.877 | 0.153 |
| Tongue coating [ref.=none] | 4.615 | 2.007 | 0.681 | 8.549 | 0.021* |
| Halitosis [ref.=none] | 0.774 | 0.307 | 0.216 | 1.332 | 0.043* |
| Oral candidiasis [ref.=none] | -0.348 | 1.207 | -2.714 | 2.018 | 0.773 |
| Salivary pH | 0.308 | 1.239 | -2.120 | 2.735 | 0.804 |
| Saliva buffer capacity | -0.132 | 0.372 | -0.861 | 0.598 | 0.724 |
| UFR | 6.332 | 3.649 | -0.821 | 13.484 | 0.083 |
| SFR | -0.439 | 1.539 | -3.455 | 2.578 | 0.776 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





