1. Introduction
Testicular cancer (TC) is a rare solid organ tumour (1%) which usually occurs in early years of life, most commonly between the age of 18 to 39 years [
1]. Recent studies have reported an increase in incidence with improvement in cure rates of TC in most developed countries including United States and North-Western Europe [
2]. Therefore, we have a large cohort of TC survivors (TCS) increasing in size with each passing year. Although the cure rates are excellent, the treatment involves multiple modalities including high doses of cisplatin based chemotherapy, surgical orchiectomy and radiotherapy. Late side effects of these treatment strategies have been under investigation for a long time [
3]. Subsequently, oncologists have radically modified the treatment plans in most patients. Active surveillance after surgery without any systemic therapy or radiotherapy is becoming more popular in early stage low risk disease. Cisplatin based chemotherapy still remains the mainstay of treatment in advanced and recurrent disease as novel treatments have not proven their efficacy yet [
4]. The excellent cure rates in TC, come at a price of late side effects in survivors [
3,
5]. These side effects include a wide of range of complications causing significant morbidity and early mortality in TCS. On one hand these conditions cause early and premature mortality [
6,
7], while on the other hand they cause significant distress and detrimental effects on physical, social, sexual, economical and psychological health [
8,
9,
10].
Most commonly reported late side effects include second malignant neoplasms, late recurrence of TC, metachronous TC, cardiovascular disease, pulmonary disease, hypogonadism, metabolic syndrome, renal insufficiency, neurotoxicity, ototoxicity and Raynaud’s phenomenon [
5,
6,
7,
8]. Psychosocial sequelae include anxiety, fear of cancer recurrence, risk of suicide, infertility, unemployment and financial toxicity [
11,
12,
13]. In this study we focus on the comorbidities in TCS after 5 years of diagnosis causing mortality and morbidity in this group.
This study is the first attempt to understand TCS cohort in Ireland. Ireland has one of the highest incidence of TC worldwide [
14] with treatment strategies similar to other European countries and United States. We conducted our research in one of the main tertiary care hospitals treating TC. The purpose of this study was to assess point prevalence of late sequelae in TCS and also to provide a model of care. We believe this is the first study of its kind which provides comprehensive data based on clinical assessment of patients and also helps to establish care pathways for TCS. This article is the expanded version of abstract published in American Society of Clinical Oncology Journal as part annual meeting June 2024 abstracts [
15].
2. Methods
2.1. Study Population
We identified all patients who had received treatment at Tallaght University Hospital in Dublin which is one of the tertiary care cancer hospitals in Ireland for testicular cancer. Our records encompassed patients who had received treatment since 2006. A research clinic was set up to perform assessment on these patients. All patients who had crossed 5 years (60 months) mark since diagnosis were invited to become part of the study. Only participants of age 18 and above at diagnosis were included in the study. In addition, the patients who had died after 5 years of diagnosis were also included in the study. No exclusions were made based on treatment modalities, we included all patients, including those who underwent orchiectomy only.
2.2. Ethics and Good Clinical Practice
The study was granted approval in full by JREC (Joint Research and Ethics Committee) of Tallaght University Hospital, Dublin, Republic of Ireland. A written informed consent was obtained from the participants according to the ethics guidelines. Mortality data was collected through retrospective chart review. All staff involved in the study were trained in ethics, data protection and Good Clinical Practice. Data was pseudo-anonymised at the time of collection. Complete anonymity was maintained during data processing and analysis.
2.3. Assessment
A comprehensive assessment was performed in our clinic including symptoms review, systemic inquiry, clinical examination and blood tests. A retrospective review of medical charts, radiology and pathology reports was done to establish initial diagnosis of germ cell tumours, treatments received and previously known co-morbidities.
All second malignant neoplasms (SMNs), late recurrences of TCs and metachronous TCs reported in our study were diagnosed on the basis of pathology and radiology. Diagnosis of cardiovascular complications and hypertension was made after further investigations and assessment by cardiology team. Low testosterone, high FSH (follicle stimulating hormone)/LH (luteinizing hormone), abnormal high glucose, lipids and impaired renal function were based on blood test performed in the clinic. Hearing impairment was confirmed with audiograms in all cases.
HADS (hospital anxiety and depression scale) score was also included in routine assessment to determine the levels of anxiety and depression in our cohort.
2.4. Statistical Analysis
Medical conditions known to the patients before diagnosis of cancer were excluded. All co-morbidities which developed since cancer diagnosis were divided into known or newly diagnosed during the assessment in our clinic. We analysed these comorbidities as percentage of total patients to provide point prevalence in our cohort. Comorbidities were analysed and reported in the groups stratified on the basis of treatment modality for TC, pathology of TC (seminoma Vs Non-seminomatous germ cell tumours), age at diagnosis (below 30 years Vs above 30 years), according to relevance.
3. Results and Discussion
3.1. Patient Characteristics
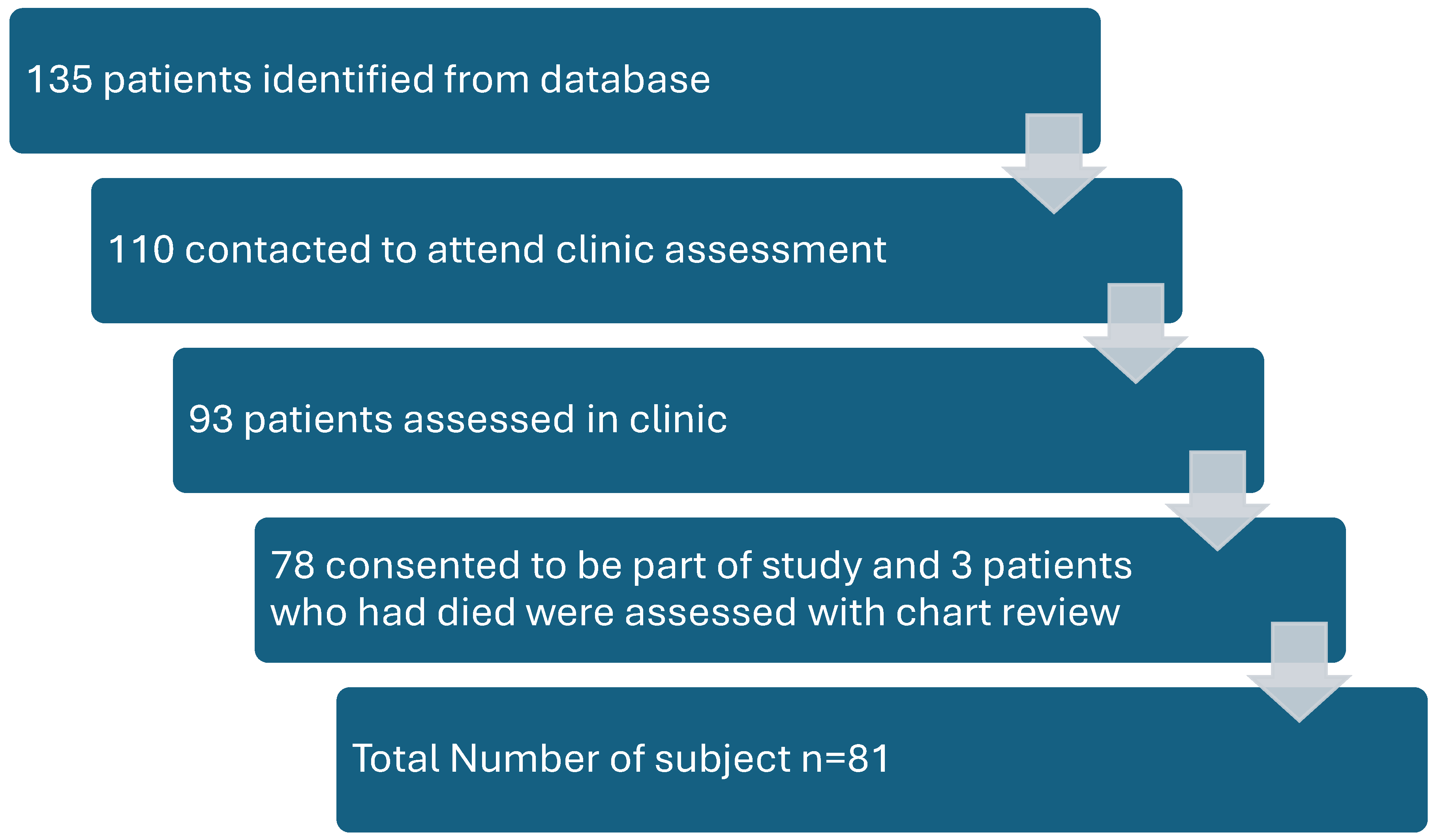
We identified 135 patients fulfilling the inclusion and exclusion criteria of our study, of which we were able to contact only 110 patients. A total of 93 patients agreed to be assessed in our clinic. Out of those, 81 patients consented to be part of the study (
Figure 1). Although, at the time of conception, we intended to include all patients including those who underwent surveillance only after surgery, but unfortunately we noticed an unintentional selection bias in our study. Patients who had received chemotherapy or were experiencing any late side effects were more likely to attend our clinic and participate in the study. This selection bias dramatically affected our study outcomes, exaggerating the prevalence of late side effects and rates of recurrence.
The age at the time of assessment ranged from 25 to 45 years with a median of 34 years and the age at initial diagnosis ranged from 19 to 55 years with a median of 25 years. The median duration from diagnosis to assessment was 129 months (10.7 years) with a range of 60 to 304 months. All of the participants had germ cell tumours, with almost half (57%) classified as non-seminomatous germ cell tumours (NSGCT) and the rest (43%) as seminomas (
Table 1).
On initial presentation, 54% of patients were stage I, 23% stage II and 22% stage III cancer. Due to selection bias mentioned above, these findings are in contrast to the data maintained by National Cancer Registry of Ireland. According to the national database, 69% patients presented with stage I, 16% with stage II and only 10% with stage III. [
16]
Every patient underwent orchiectomy except for one, who presented with extra-testicular germ cell cancer. Active surveillance after orchiectomy was opted in 26 (32%) patients but unfortunately, 22 (85%) of them had recurrence of disease after median duration of 14 months. A significantly higher number (85%) of our study population had received chemotherapy due to unintentional selection bias. Of those 69 patients who received chemotherapy 11 received 3 cycles of BEP (Bleomycin, etoposide and cisplatin), 14 had 4 cycles of BEP, 19 had 4 cycles of EP (etoposide and cisplatin) and 4 had 4 cycles of VIP (etoposide, ifosfamide and cisplatin). In adjuvant setting, 1 cycle of Carboplatin was given to 3 patients while 1 cycle of BEP was given to 4 patients. Details of treatments are mentioned in
Table 1. Radiotherapy was used in 4 (5%) patients including one patient who received whole brain radiotherapy for brain mets. Retroperitoneal lymph node discussion (RPLND) was performed in 14 (17%) patients.
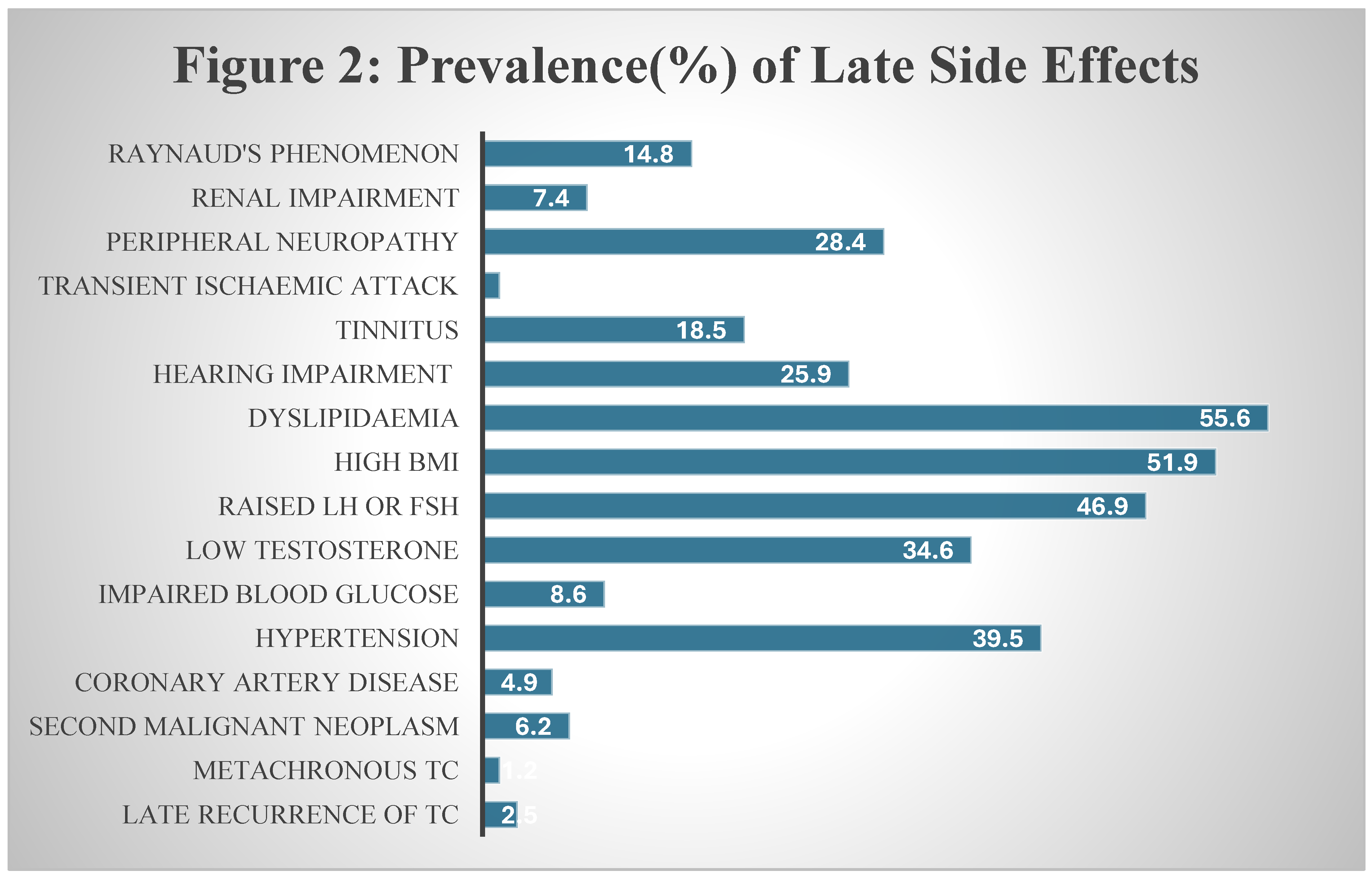
We have summarised the prevalence of these sequelae in
Figure 2 below. The details and discussion on these complications are reported in separate headings.
3.2. Second Malignant Neoplasms
We identified 5 (6%) cases of SMNs, occurring at a median of 109 months (9 years) from diagnosis of TC. Chemotherapy had been used in all of these patients, none of them received any radiotherapy. Patients who developed SMNs were all aged 30 years or more at time of TC diagnosis. All 3 deaths in our TC survivors were caused by SMNs. Malignancies included lung cancer, colon cancer, prostate cancer, oesophageal cancer and leiomyosarcoma.
These findings are similar to other studies investigating SMNs in TC survivors. Kvammen et. al. in a wide, registry based analysis also reported SMNs as the commonest cause of death in TCS after 5 years [
7]. The 25-year cumulative incidence of solid SMNs in TCS was reported at 10% by Groot et. al. which is much higher than our observation [
17], but our cohort is relatively much younger with median duration of around 10 years since diagnosis. Similar type of malignancies have been reported by other investigators [
17,
18] and the association with chemotherapy and a higher age at diagnosis is also widely reported in the literature [
7].
3.3. Metachronous Testicular Cancer
Our data showed one case of metachronous TC (1.2%). Patient was initially diagnosed with seminoma, treated with orchiectomy and radiotherapy, subsequently developed NSGCT after 7 years and underwent orchiectomy and chemotherapy. On follow up after 14 years since second TC, no signs of recurrence were found.
Marinakova et. al. performed retrospective study to investigate the incidence second TC in 2124 patients between 1970 and 2020 [
19]. Overall incidence was reported at 4.1% in all cases after a median duration of 6.7 years in seminoma and 9.2 years in NSGCT patients. Similar to SMNs our observed number is lower (1.2%) compared to their findings. Firstly, as mentioned earlier our cohort is still young and this incidence could rise in coming years. Secondly, we observed disproportionately high percentage of patients who had received chemotherapy due to the reasons mentioned above. Chemotherapy has been reported to reduce the incidence of second TC [
19,
20].
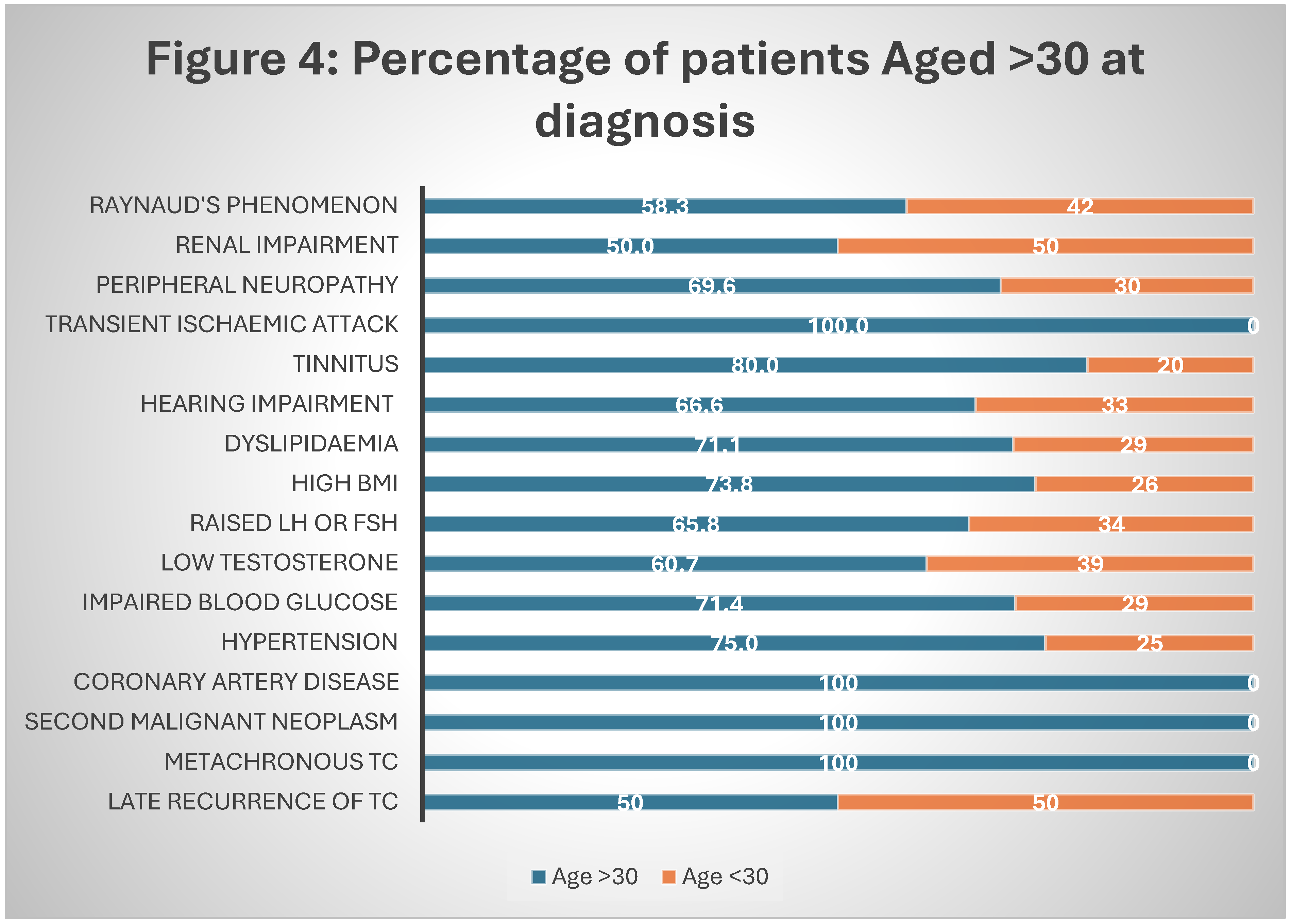
Our patient with metachronous TC had initial cancer at the age of 31, contrary to Hellesnes et. al. finding of higher incidence with age less than 30 [
20] (
Figure 4).
3.4. Late Recurrence of Testicular Cancer
Two (2.4%) cases of late recurrences of TC were noted with mean duration of 72 months (6 years) since the initial diagnosis. One of them had NSGCT treated with surgery and chemotherapy while the other one had seminoma initially diagnosed at stage IB and treated with surgery only. NSGCT recurred as a teratoma which was surgically resected and seminoma was treated with chemotherapy. Both patients were disease free at the time of follow up which was more than 5 years since recurrence.
Ondrusova et. al also reported 2.6% relapse rate in their retrospective analysis [
21]. Late relapses have been reported with both seminoma and NSGCT [
22] with the interval of 6-7 years from initial diagnosis [
21,
23]. Late recurrence of teratoma in second patient after chemotherapy has also been widely reported in literature [
21,
23,
24].
3.5. Cardiovascular Disease
In this study, four (5%) patients were found to have coronary artery disease. Half of them were already diagnosed but the other half was only diagnosed during their assessment in clinic. All of these patients (100%) had received chemotherapy and were aged more than 30 years at the time of TC diagnosis (
Figure 4). No cases of cardiomyopathy were found in our study.
Lubberts et. al. assessed the incidence of cardiovascular disease in a multicentre cohort including 4748 TCS. They reported 5.7% incidence at a median of 16 years of follow up [
25] very similar to our finding. Cisplatin based chemotherapy and an older age has been repeatedly reported in studies as major risk factors [
25,
26,
27].
Interestingly, 2 of these 4 patients were not aware of their underlying coronary artery disease. Systemic enquiry by our researchers led to further investigations by our cardiology team, which in turn diagnosed and managed their condition. This finding emphasises the need for screening clinics to reduce the mortality associated with cardiovascular complications in TCS.
3.6. Hypertension
Hypertension was found to be second most common underlying condition in our study with almost 40% patients affected. The median duration to develop hypertension was 109 months (9 years) but only one third of these patients were diagnosed in primary healthcare and the rest were diagnosed in our clinic. Chemotherapy was used in 81% of the cases and 75% were aged more than 30 years at the time of diagnosis.
As mentioned above, Lubberts et al. conducted a comprehensive study to evaluate cardiovascular risk factors in TC population of Netherlands [
25]. They reported hypertension in 50% of 4787 TC survivors compared to 39.5% in our study. This difference can be explained with two principal differences in characteristics of cohorts. Firstly, median follow up in this Dutch study was 22 years compared to 10 years in our study. Secondly, only 54% patients in their study received chemotherapy in contrast to 85% in our study. Cisplatin based chemotherapy has been reported in other studies to increase the risk of developing hypertension [
5,
28]. This finding is also reflected in our study where more than 80% of patients who developed hypertension had been given chemotherapy (
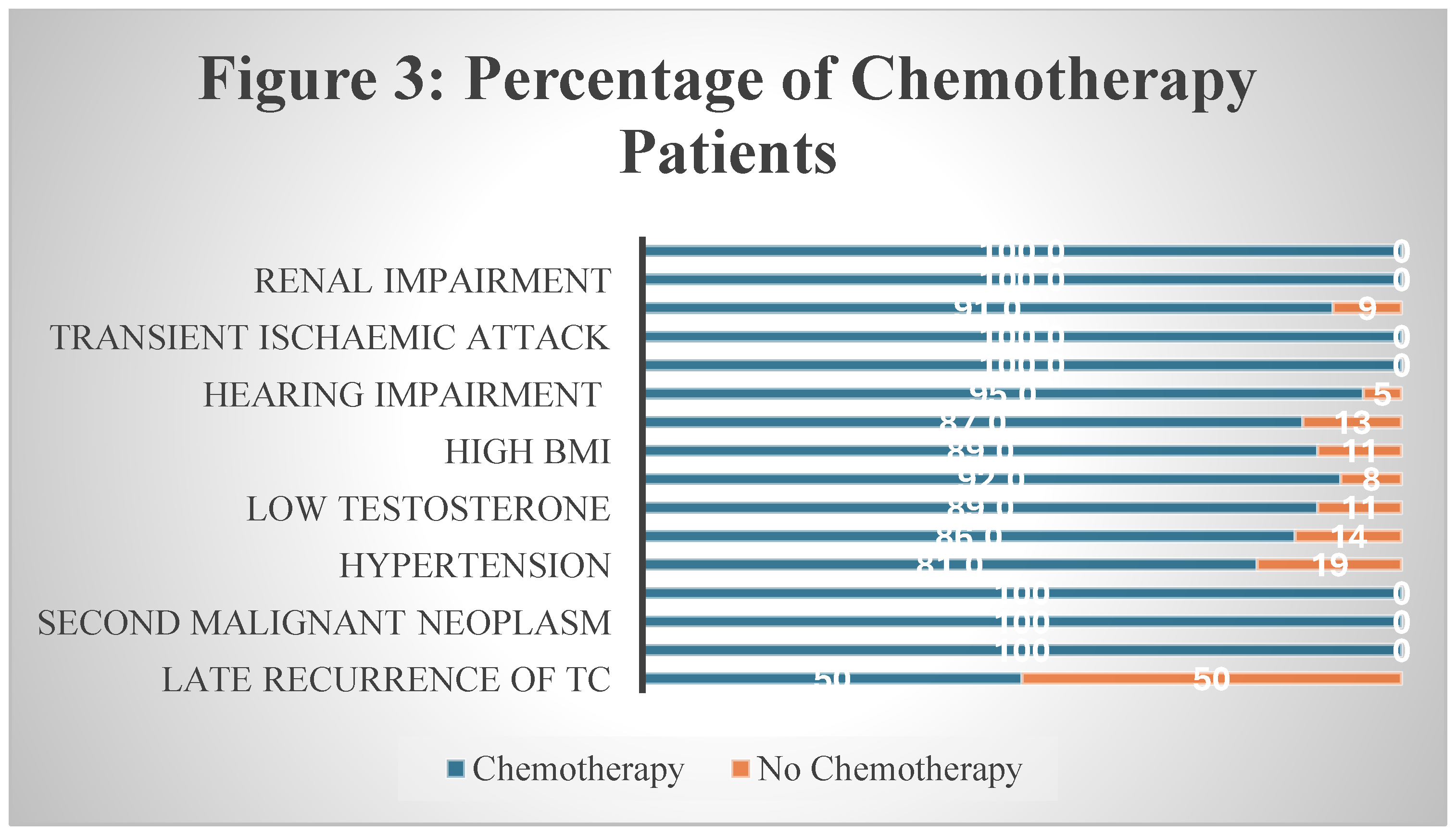
Figure 3).
Figure 3.
Percentage of Chemotherapy patients.
Figure 3.
Percentage of Chemotherapy patients.
3.7. Impaired High Glucose
Our assessment revealed abnormally high glucose levels in 7 (22%) patients, based on fasting glucose and HbA1c readings. Of those 7 patients, 6 had received chemotherapy for treatment of TC and 5 were aged more than 30 years at diagnosis (
Figure 3 and
Figure 4). A strong correlation of impaired glucose levels with chemotherapy and older age (>30) at TC diagnosis is reported by other investigators as well. Lubberts et al found a staggering 40% of TCS with abnormally high glucose levels [
25]. This discrepancy between the two studies could be due to longer duration of follow up (10 Vs 22 years). Both studies followed similar methods of assessment and cut offs for normal levels. Pre-diabetic levels of impaired glucose are more prevalent in this cohort compared to diabetes [
8]. Due to study limitations, we are not able to report the proportion of diabetics in this cohort.
3.8. High Body Mass Index
Body mass index (BMI) was calculated based on weight and height measurements in clinic. We found high BMI in 42 (52%) patients with the majority (81%) belonging to the chemotherapy group. Internationally recognised range of normal BMI was utilised for assessment using height and weight measured in the clinic [
29]. Many studies have reported higher incidence of obesity and pre-obese high BMI after chemotherapy in TCS [
5,
8,
25]. Kerns et al. assessed 1214 TCS who had received chemotherapy, in a multi-institute study. The study revealed 71.5% of TCS with high BMI with a median of 4.2 years since diagnosis [
8]. Our study showed similar results with a high prevalence mainly in chemotherapy group. Although a similar percentage (77%) was reported in all TCS by Agrawal et al. based on questionnaire based study conducted in US, chemotherapy cohort still had much higher incidence [
5].
3.9. Dyslipidaemia
More than half (51%) of our patients had abnormal high levels of cholesterol or triglycerides on blood tests. Only a small minority (13%) were aware of this underlying condition as most of them were diagnosed on screening. Dyslipidaemia was clearly predominant in chemotherapy group with more than 85% patients. Also more common in TCS diagnosed at >30 years of age.
While evaluating CVD risk factors in TCS, Lubberts et al showed 85% patient with dyslipidaemia [
25]. Similar to differences in other results, a higher incidence in their study could be due to older cohort of TCS (10 years Vs 22 years of follow up duration). As reported in our results, only 15% of TCS in this Dutch study were aware of their condition. In both cohorts, around 85% of patients were not conscious of their underlying disease.
3.10. Hypogonadism
We assessed serum testosterone and FSH/LH levels in all of our patients. An Abnormal low serum testosterone was subsequently confirmed with early morning fasting levels. We found low levels of testosterone in 28 (34.5%) and an abnormal high FSH or LH in 38 (47%) patients. Patients who had received chemotherapy accounted for almost all cases of gonadal dysfunction representing almost 90% of this cohort. Even with high prevalence of symptoms, only 17% of patients were aware of their underlying condition.
Our stud findings are comparable to the study on hypogonadism conducted in United Kingdom by R. A. Huddart et. al. [
30]. They evaluated hormone levels 640 patients in survivorship phase of TC. Low levels of testosterone were found in 38% patients of chemotherapy and radiotherapy group compared to 11% in surveillance group. High FSH in surveillance and chemotherapy/radiotherapy group was 42% and 70% respectively. Similar trend was seen with raised LH. We reported similar results in our cohort. R. A. Huddart also highlighted issues of infertility and sexual dysfunction associated with these hormonal abnormalities, we have reported these previously in a questionnaire based survey [
31].
3.11. Ototoxicity
In this cohort, the prevalence of hearing impairment was 29% almost exclusively associated with chemotherapy (95% cases). Like in all other co-morbidities, majority of cases (76%) were diagnosed during screening in our clinic. Similarly, tinnitus was reported by 15 (18.5%) patients and all of them belonged to chemotherapy group.
Many studies have investigated the ototoxicity related with cisplatin exposure during treatment of TC. Most commonly reported incidence is around 30-40% in TCS from chemotherapy group [
5,
8,
32]. A rise in incidence with higher at age at diagnosis was also reported in a US study [
5].
3.12. Neurotoxicity
Only one of our patients was diagnosed with transient ischaemic attacks after detailed investigations of symptoms. No strokes were detected or reported in our study.
On the contrary, 23 (28%) patients reported residual peripheral neuropathy after a median follow up of 8.5 years. This assessment is solely based on patient reported questionnaires since we couldn’t perform any further investigations like nerve conduction studies to confirm the diagnosis. Agrawal et. al. reported similar proportion (26.5%) in US population of TCS [
5].
3.13. Pulmonary Disease
In this study, we didn’t find any cases of pulmonary disease diagnosed after TC diagnosis or treatment. Even though 42 (52%) of our patients had received one or more cycles of bleomycin.
The most notable study on this subject was performed by H. S. Haugnes et. al. in Norway. Around 16-17% long term survivors were found to have compromised pulmonary function after chemotherapy treatment. The results from our study are possibly a consequence of more cautious use of bleomycin in TC patients.
3.14. Renal Impairment
We found 6 (7.4%) patients with persistent renal impairment after a median follow up of more than 10 years. Of note, only 1 out of these 6 patients had an established diagnosis of renal compromise. The rest of 5 patients were diagnosed during screening in our clinic and later confirmed by nephrology team. Similar to ototoxicity, nephrotoxicity was only found in patients who received chemotherapy.
In majority of studies evaluating renal impairment in TCS, the incidence is reported around 3% [
5,
8]. A much higher incidence (23%) of reduced glomerular filtration rate has been mentioned after 1 year of cisplatin based treatment [
33].
3.15. Raynaud’s Phenomenon
At the median follow up of 12 years, Raynaud’s phenomenon was reported by 12(15%) patients, all of them received chemotherapy for treatment of TC. Other studies have reported Raynaud’s in 18% of all TCS and 33% in chemotherapy group [
5,
8]. Both patients diagnosed with coronary artery disease in our clinic had Raynaud’s phenomenon as well, which underscores the close association between the two complications.
Figure 4.
Percentage of patients in each co-morbidity aged more than 30 years at diagnosis.
Figure 4.
Percentage of patients in each co-morbidity aged more than 30 years at diagnosis.
3.16. Anxiety and Depression
Out of 78 patients we assessed in our clinic, 76 filled out HADS (Hospital Anxiety and Depression Score) proforma. Internationally accepted scale was used to interpret HADS results [
34].
We found 17% patients with mild anxiety and an additional 14.5% with moderate and severe anxiety. This result goes in line with 20% prevalence of anxiety in TCS after 5 years reported by AB Smith et. al. [
35]. The systemic review by AB Smith et. al. reported no increased risk of depression in TCS compared to general population. In our study only 8% patients reported mild depression and one respondent reported moderate levels.
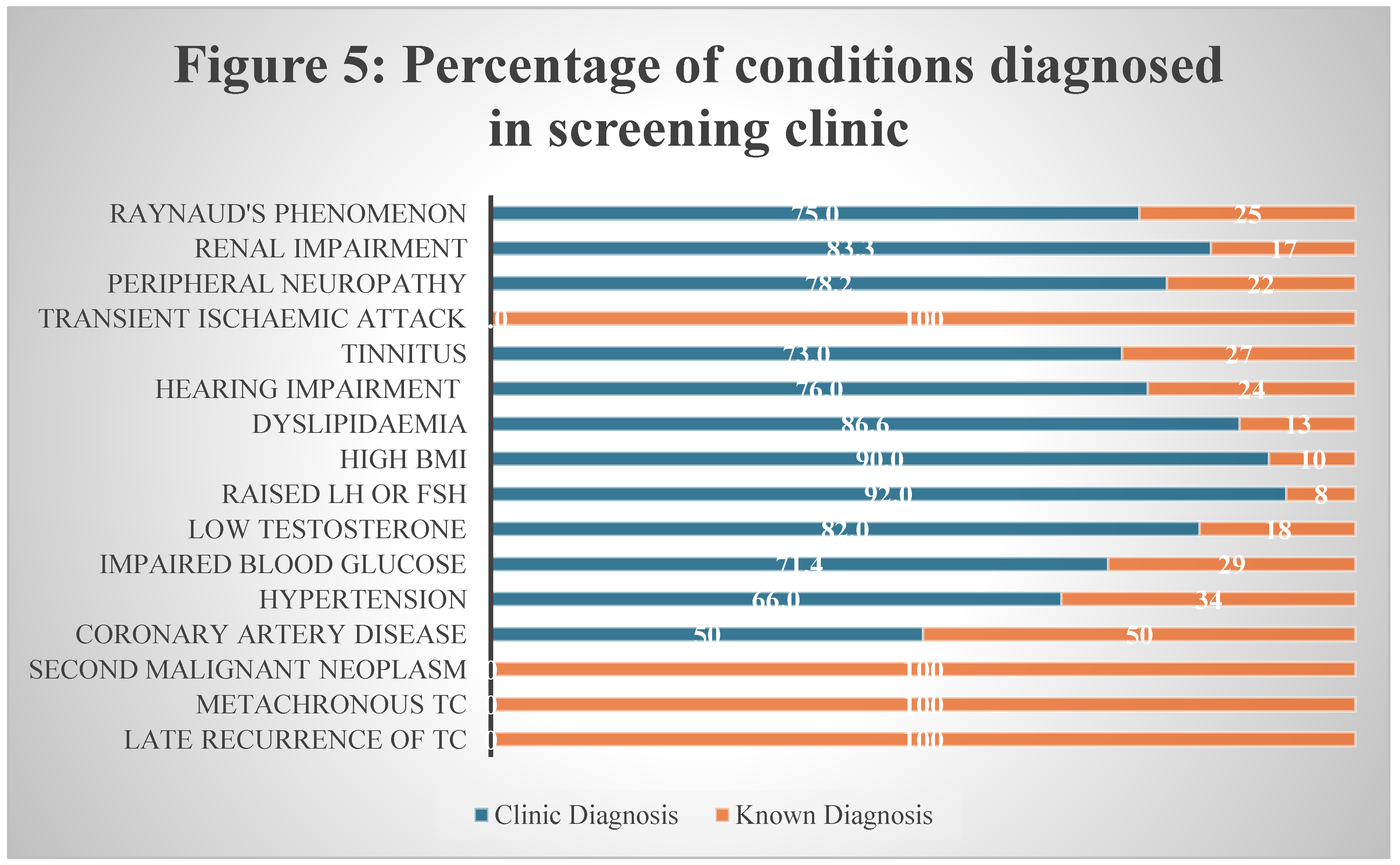
3.17. Efficacy of Screening Clinic in TCS
We have attached our screening proformas in supplementary material. Our nurse-led research clinic can serve as a model for survivorship care in TCS. As shown in
Figure 5 below, major proportion of the late complications were diagnosed in clinic during assessment. Further investigations under specialist team such as cardiology and endocrinology were performed for abnormal findings during screening.
Kvammen et. al. have featured the causes of inferior survivor in TCS with their registry based study [
7]. SMNs followed by cardiovascular complications were identified as the most common cause of early death in TCS. In our study we have established that most of the cardiac risk factors including hypertension, obesity, diabetes and hypogonadism can be detected and corrected with regular assessment of TCS. In this way, early deaths due to cardiac complications can be prevented. Lubberts et. al. also noticed that when cardiac risk factors were treated, no excess risk of cardiac complications was found [
25]. In the same way, regular symptoms check and clinical examination might be able to identify early signs of malignancy and reduce the mortality related to SMNs. We do believe that a more comprehensive strategy is needed for early detection of solid tumours. A good example is early screening for breast and prostate cancer in patients with BRCA mutations.
4. Strengths and Limitations
To our knowledge this is the first research to provide a comprehensive care plan and a snapshot of every late side effect in all TCS. Previous studies providing prevalence and incidence in all TCS were mainly based on registry analysis or questionnaire based surveys [
5,
7]. Other studies evaluating patients with clinical assessment either focused on a group of patients [
8] or a group of disease [
25].
In this study, we also propose a working model to improve the morbidity and mortality in TCS through nurse-led, purpose-built clinics with referral pathways in place. During our study we noticed a great amount of interest from the survivors and the patient communities.
The purpose of this research was to include all TCS who had completed the surveillance period. Unfortunately, patients who had received chemotherapy or having any symptoms were more likely to attend clinic and participate in the study. Therefore, there was an unintentional selection bias, exaggerating the incidence of chemotherapy related co-morbidities.
A more accurate estimate could be possible with certain conditions like Raynaud’s phenomenon and peripheral neuropathy by using specialised investigations. Due to limitations in resources, we couldn’t perform these investigations.
5. Conclusion
The cure rates in TC have improved with multimodal treatment strategies but the care for survivors remains a challenge. TCS are facing a spectrum of physical, psychological and social challenges after the cure of cancer. Serious deficiencies can be seen in the survivorship care as most cancer centres don’t offer any follow up at the end of surveillance. Our study provides the magnitude of difficulties and a practical solution. We are hoping that further studies will build up on this knowledge and improve the survivorship care as well as raise awareness among healthcare providers and survivors. Five decades ago testicular cancer patients emerged as pioneers in solid organ cure, now they can become the pioneers in survivorship care.
Author Contributions
M Raheel Khan: Idea, literature search, data analysis, writing – draft, writing – review and editing Patrice Kearney Sheehan: literature search, writing – review and editing Ashley Bazin: idea, writing – review and editing Abdur Rehman Farooq: idea, writing – review and editing Christine Leonard: idea, writing – review and editing Umair Aleem: idea, writing – review and editing Lynda Corrigan: critical analysis, writing – review and editing Ray Mc Dermott: idea, data analysis, critical analysis, writing – review and editing.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
References
- Kusler, K.A.; Poynter, J.N. International testicular cancer incidence rates in children, adolescents and young adults. Cancer Epidemiology 2018, 56, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chan, S.C.; Tin, M.S.; Liu, X.; Lok, V.T.-T.; Ngai, C.H.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.-J.; et al. Worldwide Distribution, Risk Factors, and Temporal Trends of Testicular Cancer Incidence and Mortality: A Global Analysis. Eur. Urol. Oncol. 2022, 5, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.; Fossa, S.D.; Williams, A.; Travis, L.B. Long-term Morbidity of Testicular Cancer Treatment. Urol. Clin. North Am. 2015, 42, 393–408. [Google Scholar] [CrossRef]
- Oing, C.; Kollmannsberger, C.; Oechsle, K.; Bokemeyer, C. Investigational targeted therapies for the treatment of testicular germ cell tumors. Expert Opin. Investig. Drugs 2016, 25, 1033–1043. [Google Scholar] [CrossRef]
- Agrawal, V.; Dinh, P.C.; Fung, C.; O Monahan, P.; Althouse, S.K.; Norton, K.; Cary, C.; Einhorn, L.; Fossa, S.D.; Adra, N.; et al. Adverse Health Outcomes Among US Testicular Cancer Survivors After Cisplatin-Based Chemotherapy vs Surgical Management. JNCI Cancer Spectr. 2019, 4, pkz079. [Google Scholar] [CrossRef] [PubMed]
- Kvammen, Ø.; Myklebust, T.; Solberg, A.; Møller, B.; Klepp, O.H.; Fosså, S.D.; Tandstad, T. Long-term Relative Survival after Diagnosis of Testicular Germ Cell Tumor. Cancer Epidemiology Biomarkers Prev. 2016, 25, 773–779. [Google Scholar] [CrossRef]
- Kvammen, Ø.; Myklebust, T.; Solberg, A.; Møller, B.; Klepp, O.H.; Fosså, S.D.; Tandstad, T. Causes of inferior relative survival after testicular germ cell tumor diagnosed 1953–2015: A population-based prospective cohort study. PLOS ONE 2019, 14, e0225942. [Google Scholar] [CrossRef]
- Kerns, S.L.; Fung, C.; Monahan, P.O.; Ardeshir-Rouhani-Fard, S.; Abu Zaid, M.I.; Williams, A.M.; Stump, T.E.; Sesso, H.D.; Feldman, D.R.; Hamilton, R.J.; et al. Cumulative Burden of Morbidity Among Testicular Cancer Survivors After Standard Cisplatin-Based Chemotherapy: A Multi-Institutional Study. J. Clin. Oncol. 2018, 36, 1505–1512. [Google Scholar] [CrossRef]
- Kerns, S.L.; Fung, C.; Fossa, S.D.; Dinh, P.C.; Monahan, P.; Sesso, H.D.; Frisina, R.D.; Feldman, D.R.; Hamilton, R.J.; Vaughn, D.; et al. Relationship of Cisplatin-Related Adverse Health Outcomes with Disability and Unemployment Among Testicular Cancer Survivors. JNCI Cancer Spectr. 2020, 4, pkaa022. [Google Scholar] [CrossRef]
- Hellesnes, R.; Myklebust, T. .; Fosså, S.D.; Bremnes, R.M.; Karlsdottir, Á.; Kvammen, Ø.; Tandstad, T.; Wilsgaard, T.; Negaard, H.F.S.; Haugnes, H.S. Testicular Cancer in the Cisplatin Era: Causes of Death and Mortality Rates in a Population-Based Cohort. J. Clin. Oncol. 2021, 39, 3561–3573. [Google Scholar] [CrossRef]
- Gunnes, M.W.; Lie, R.T.; Bjørge, T.; Ghaderi, S.; Syse, A.; Ruud, E.; Wesenberg, F.; Moster, D. Suicide and violent deaths in survivors of cancer in childhood, adolescence and young adulthood-A national cohort study. Int. J. Cancer 2016, 140, 575–580. [Google Scholar] [CrossRef]
- Kreiberg, M.; Bandak, M.; Lauritsen, J.; Andersen, K.K.; Skøtt, J.W.; Johansen, C.; Agerbaek, M.; Holm, N.V.; Lau, C.J.; Daugaard, G. Psychological stress in long-term testicular cancer survivors: a Danish nationwide cohort study. J. Cancer Surviv. 2020, 14, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Raphael, M.J.; Gupta, S.; Wei, X.; Peng, Y.; Soares, C.N.; Bedard, P.L.; Siemens, D.R.; Robinson, A.G.; Booth, C.M. Long-Term Mental Health Service Utilization Among Survivors of Testicular Cancer: A Population-Based Cohort Study. J. Clin. Oncol. 2021, 39, 779. [Google Scholar] [CrossRef] [PubMed]
- Deady, D.S. and D.H. Comber. Cancer Trends - Cancer of Testis. 2012 [cited 2023; Available from: https://www.ncri.ie/publications/cancer-trends-and-projections/cancer-trends-cancers-testis.
- Khan, M.R.; Sheehan, P.K.; Bazin, A.; Leonard, C.; Aleem, U.; Trainor, S.; Corrigan, L.; McDermott, R.S. Long term sequelae of testicular cancer and treatment: A point prevalence observational study. J. Clin. Oncol. 2024, 42, e17015–e17015. [Google Scholar] [CrossRef]
- Ireland, N.C.R., Cancer Factsheet: Testis. 2020.
- Groot, H.J.; Lubberts, S.; De Wit, R.; Witjes, J.A.; Kerst, J.M.; De Jong, I.J.; Groenewegen, G.; van den Eertwegh, A.J.; Poortmans, P.M.; Klümpen, H.-J.; et al. Risk of Solid Cancer After Treatment of Testicular Germ Cell Cancer in the Platinum Era. J. Clin. Oncol. 2018, 36, 2504–2513. [Google Scholar] [CrossRef] [PubMed]
- Ondrus, D.; Ondrusova, M.; Friedova, L. Second malignancies in long-term testicular cancer survivors. Int. Urol. Nephrol. 2013, 46, 749–756. [Google Scholar] [CrossRef]
- Mrinakova, B.; Trebaticky, B.; Kajo, K.; Ondrusova, M.; Lehotska, V.; Waczulikova, I.; Ondrus, D. Bilateral testicular germ cell tumors – 50 years experience. Bratisl. Med J. 2021, 122, 449–453. [Google Scholar] [CrossRef]
- Hellesnes, R.; Myklebust, T.; Bremnes, R.M.; Karlsdottir, Á.; Kvammen, Ø.; Negaard, H.F.S.; Tandstad, T.; Wilsgaard, T.; Fosså, S.D.; Haugnes, H.S. Metachronous Contralateral Testicular Cancer in the Cisplatin Era: A Population-Based Cohort Study. J. Clin. Oncol. 2021, 39, 308–318. [Google Scholar] [CrossRef]
- Ondrusova, M.; Suchansky, M.; Psota, M.; Zeleny, T.; Ondrus, D. Late relapse in stage I of nonseminomatous germ cell testicular cancer on surveillance. Bratisl. Med J. 2018, 119, 3–5. [Google Scholar] [CrossRef]
- George, D.W.; Foster, R.S.; Hromas, R.A.; Robertson, K.A.; Vance, G.H.; Ulbright, T.M.; Gobbett, T.A.; Heiber, D.J.; Heerema, N.A.; Ramsey, H.C.; et al. Update on Late Relapse of Germ Cell Tumor: A Clinical and Molecular Analysis. J. Clin. Oncol. 2003, 21, 113–122. [Google Scholar] [CrossRef]
- Dieckmann, K.-P.; Albers, P.; Classen, J.; DE Wit, M.; Pichlmeier, U.; Rick, O.; Müllerleile, U.; Kuczyk, M. LATE RELAPSE OF TESTICULAR GERM CELL NEOPLASMS: A DESCRIPTIVE ANALYSIS OF 122 CASES. J. Urol. 2005, 173, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Baniel, J.; Foster, R.S.; Gonin, R.; E Messemer, J.; Donohue, J.P.; Einhorn, L.H. Late relapse of testicular cancer. J. Clin. Oncol. 1995, 13, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Lubberts, S.; Groot, H.J.; de Wit, R.; Mulder, S.; Witjes, J.A.; Kerst, J.M.; Groenewegen, G.; Lefrandt, J.D.; van Leeuwen, F.E.; Nuver, J.; et al. Cardiovascular Disease in Testicular Cancer Survivors: Identification of Risk Factors and Impact on Quality of Life. J. Clin. Oncol. 2023, 41, 3512–3522. [Google Scholar] [CrossRef]
- Bjerring, A.W.; Fosså, S.D.; Haugnes, H.S.; Nome, R.; Stokke, T.M.; Haugaa, K.H.; E Kiserud, C.; Edvardsen, T.; I Sarvari, S. The cardiac impact of cisplatin-based chemotherapy in survivors of testicular cancer: a 30-year follow-up. Eur. Hear. J. - Cardiovasc. Imaging 2020, 22, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Fosså, S.D.; Gilbert, E.; Dores, G.M.; Chen, J.; McGlynn, K.A.; Schonfeld, S.; Storm, H.; Hall, P.; Holowaty, E.; Andersen, A.; et al. Noncancer Causes of Death in Survivors of Testicular Cancer. JNCI J. Natl. Cancer Inst. 2007, 99, 533–544. [Google Scholar] [CrossRef]
- Lauritsen, J.; Hansen, M.K.; Bandak, M.; Kreiberg, M.B.; Skøtt, J.W.; Wagner, T.; Kier, M.G.G.; Holm, N.V.; Agerbæk, M.; Gupta, R.; et al. Cardiovascular Risk Factors and Disease After Male Germ Cell Cancer. J. Clin. Oncol. 2020, 38, 584–592. [Google Scholar] [CrossRef]
- Zierle-Ghosh, A. and A. Jan, Physiology, Body Mass Index, in StatPearls. 2024, StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.: Treasure Island (FL).
- A Huddart, R.; Norman, A.; Moynihan, C.; Horwich, A.; Parker, C.; Nicholls, E.; Dearnaley, D.P. Fertility, gonadal and sexual function in survivors of testicular cancer. Br. J. Cancer 2005, 93, 200–207. [Google Scholar] [CrossRef]
- Khan, M.; Sheehan, P.K.; Bazin, A.; Leonard, C.; Aleem, U.; Corrigan, L.; McDermott, R. 11P Impact of testicular cancer on socio-economic and sexual health of survivors: A questionnaire-based survey. ESMO Open 2024, 9. [Google Scholar] [CrossRef]
- Brydøy, M.; Oldenburg, J.; Klepp, O.; Bremnes, R.M.; Wist, E.A.; Wentzel-Larsen, T.; Hauge, E.R.; Dahl, O.; Fosså, S.D. Observational Study of Prevalence of Long-term Raynaud-Like Phenomena and Neurological Side Effects in Testicular Cancer Survivors. JNCI J. Natl. Cancer Inst. 2009, 101, 1682–1695. [Google Scholar] [CrossRef]
- Inai, H.; Kawai, K.; Ikeda, A.; Ando, S.; Kimura, T.; Oikawa, T.; Onozawa, M.; Miyazaki, J.; Uchida, K.; Nishiyama, H. Risk factors for chronic kidney disease after chemotherapy for testicular cancer. Int. J. Urol. 2012, 20, 716–722. [Google Scholar] [CrossRef]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Smith, A.; Rutherford, C.; Butow, P.; Olver, I.; Luckett, T.; Grimison, P.; Toner, G.; Stockler, M.; King, M. A systematic review of quantitative observational studies investigating psychological distress in testicular cancer survivors. Psycho-Oncology 2017, 27, 1129–1137. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).