Submitted:
28 August 2024
Posted:
29 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
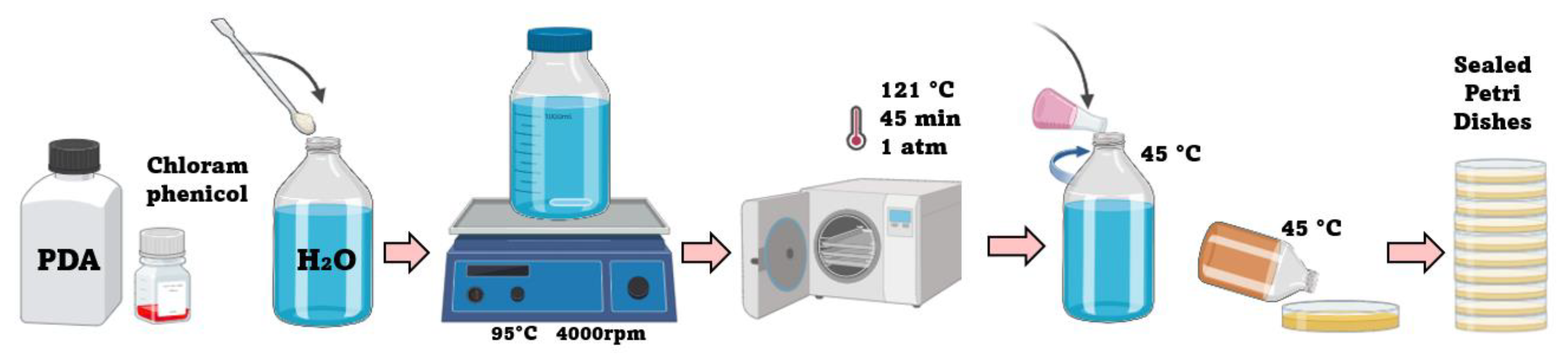
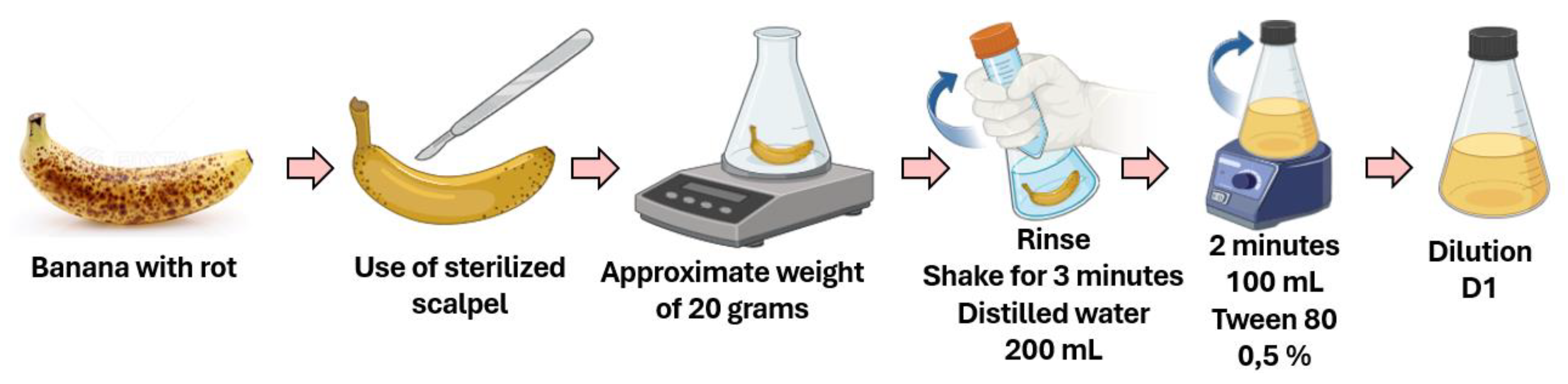
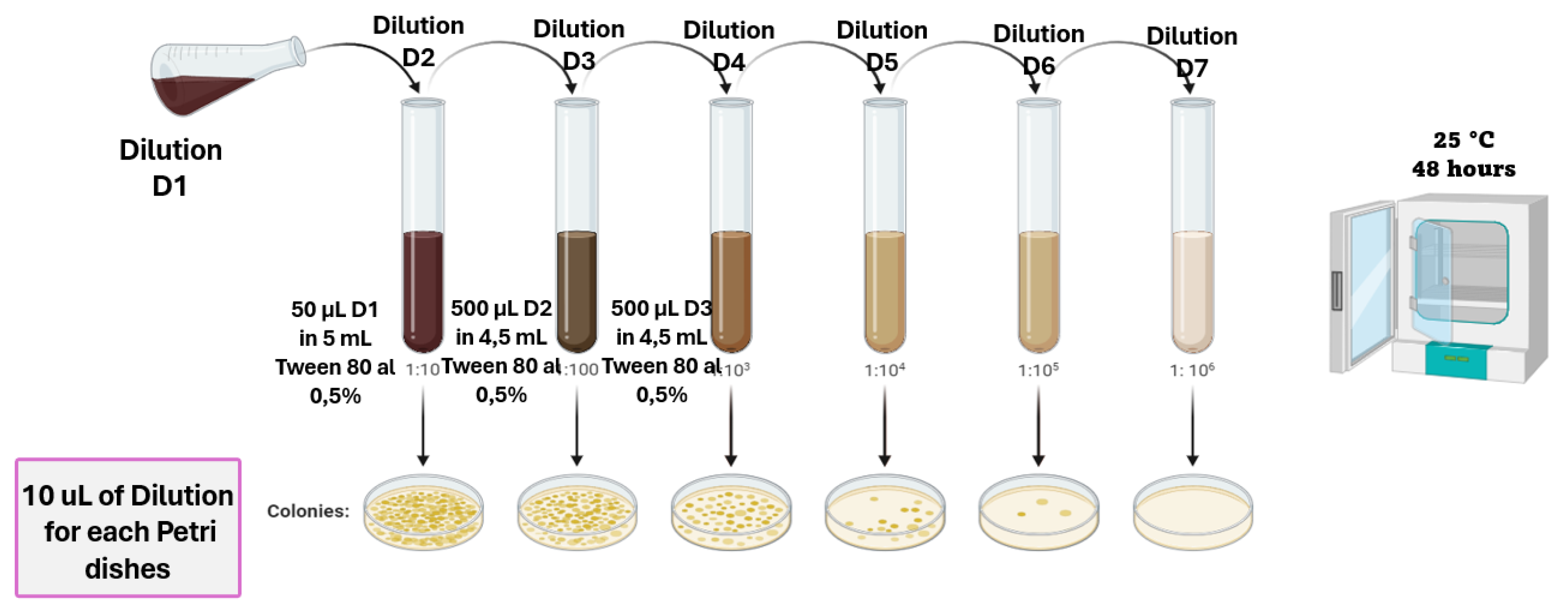
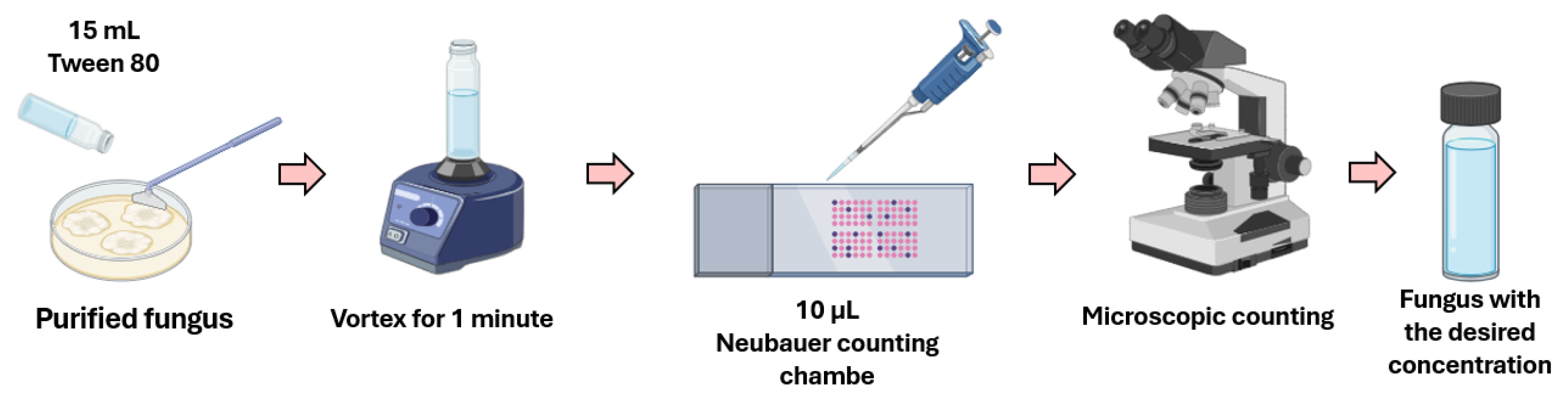
2.1. Isolation and Purification of Microorganisms
2.2. Macroscopic Identification
2.3. Microscopic Identification
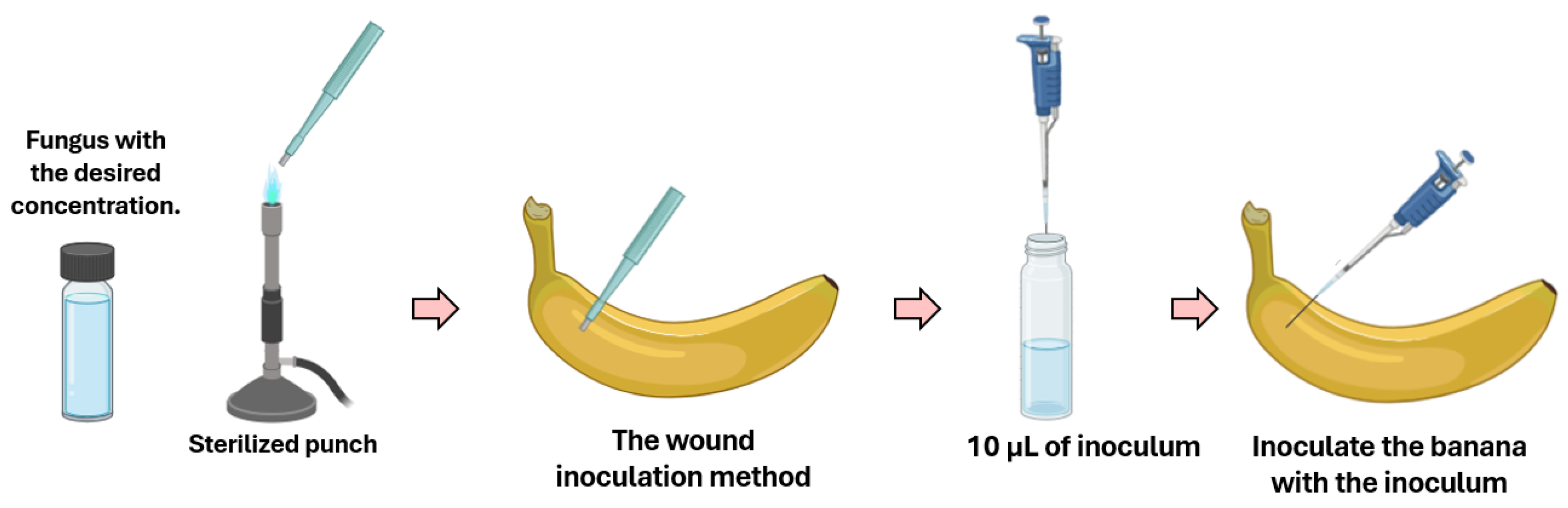
2.4. In Vivo Fungal Activity
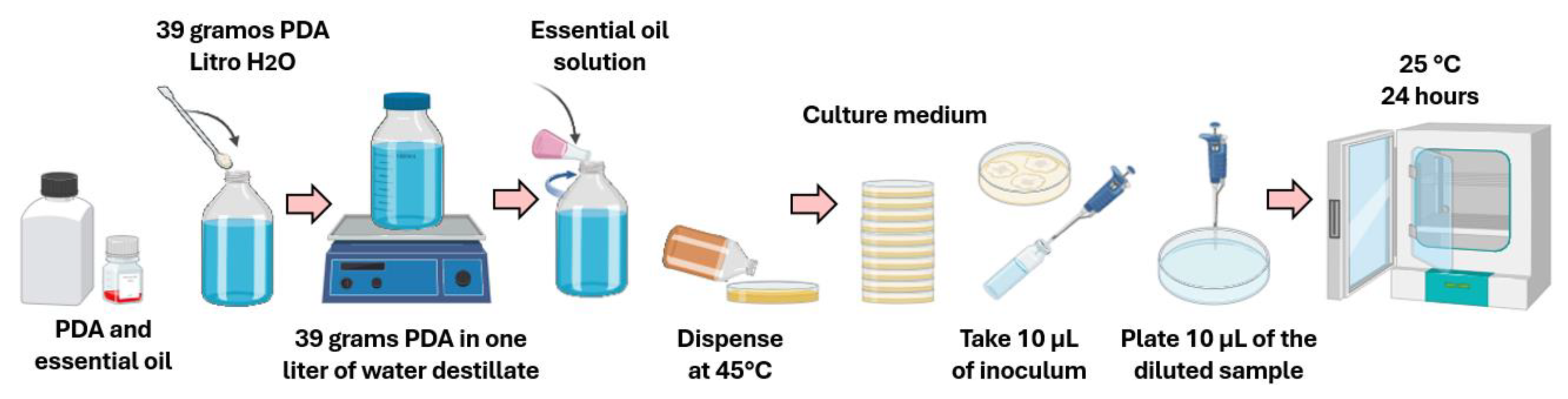
2.5. In Vitro Antifungal Activity with Essential Oils
3. Results
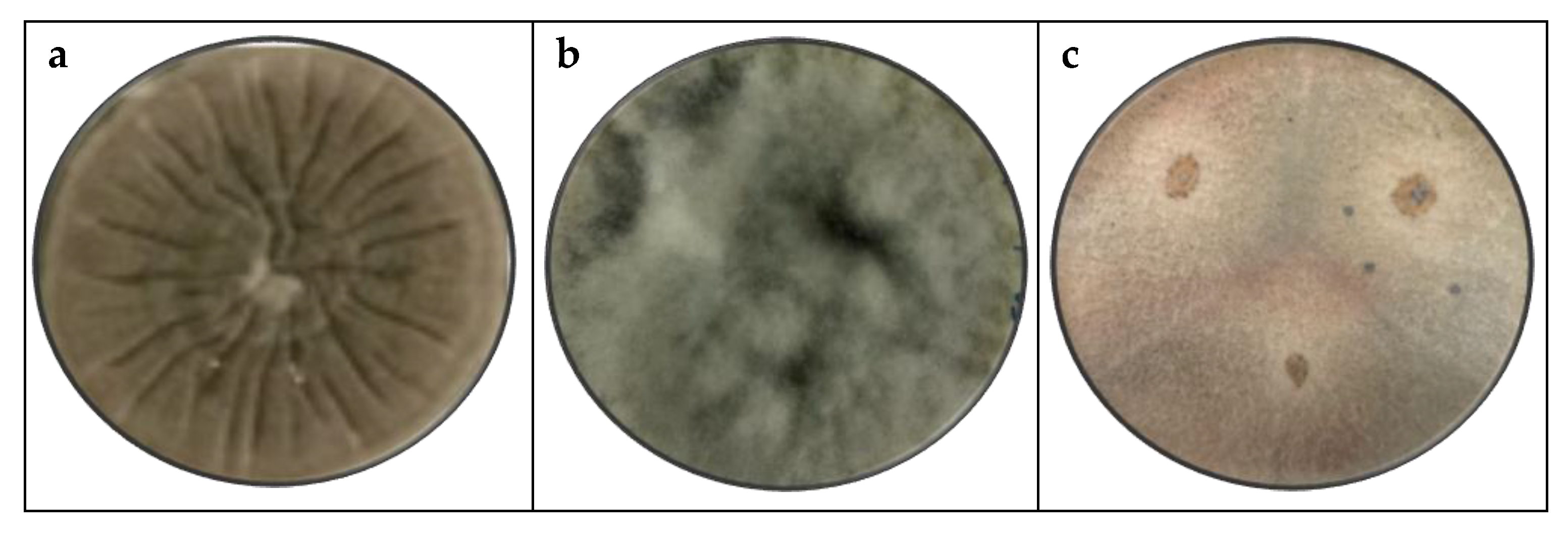
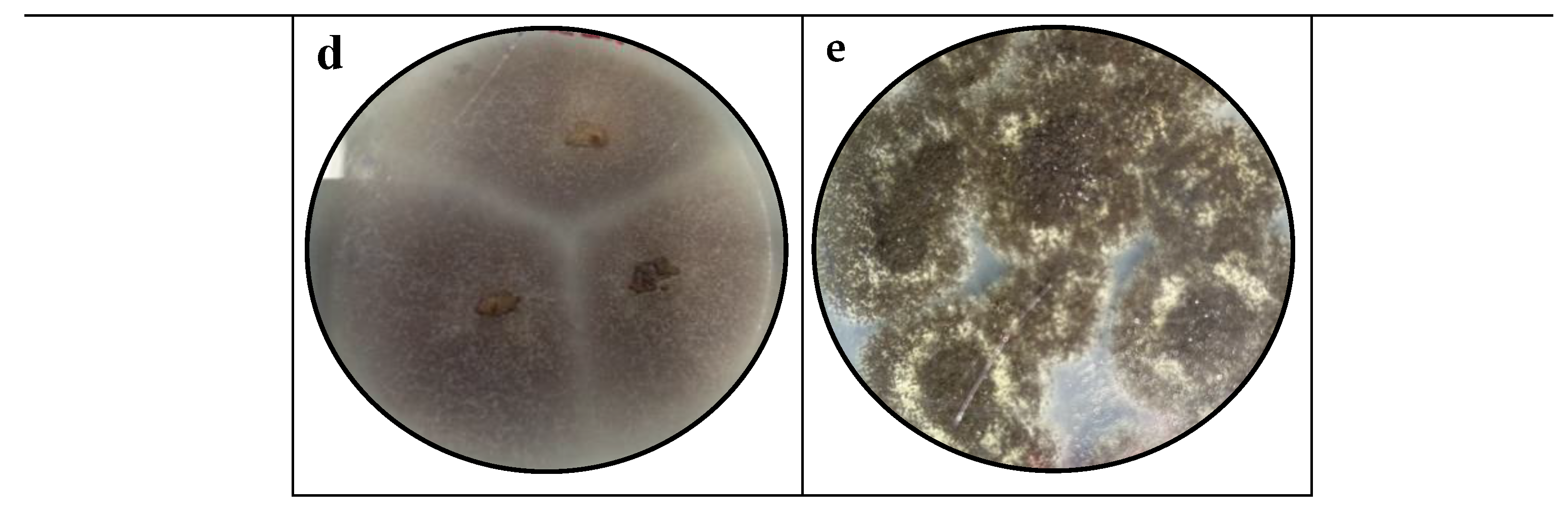
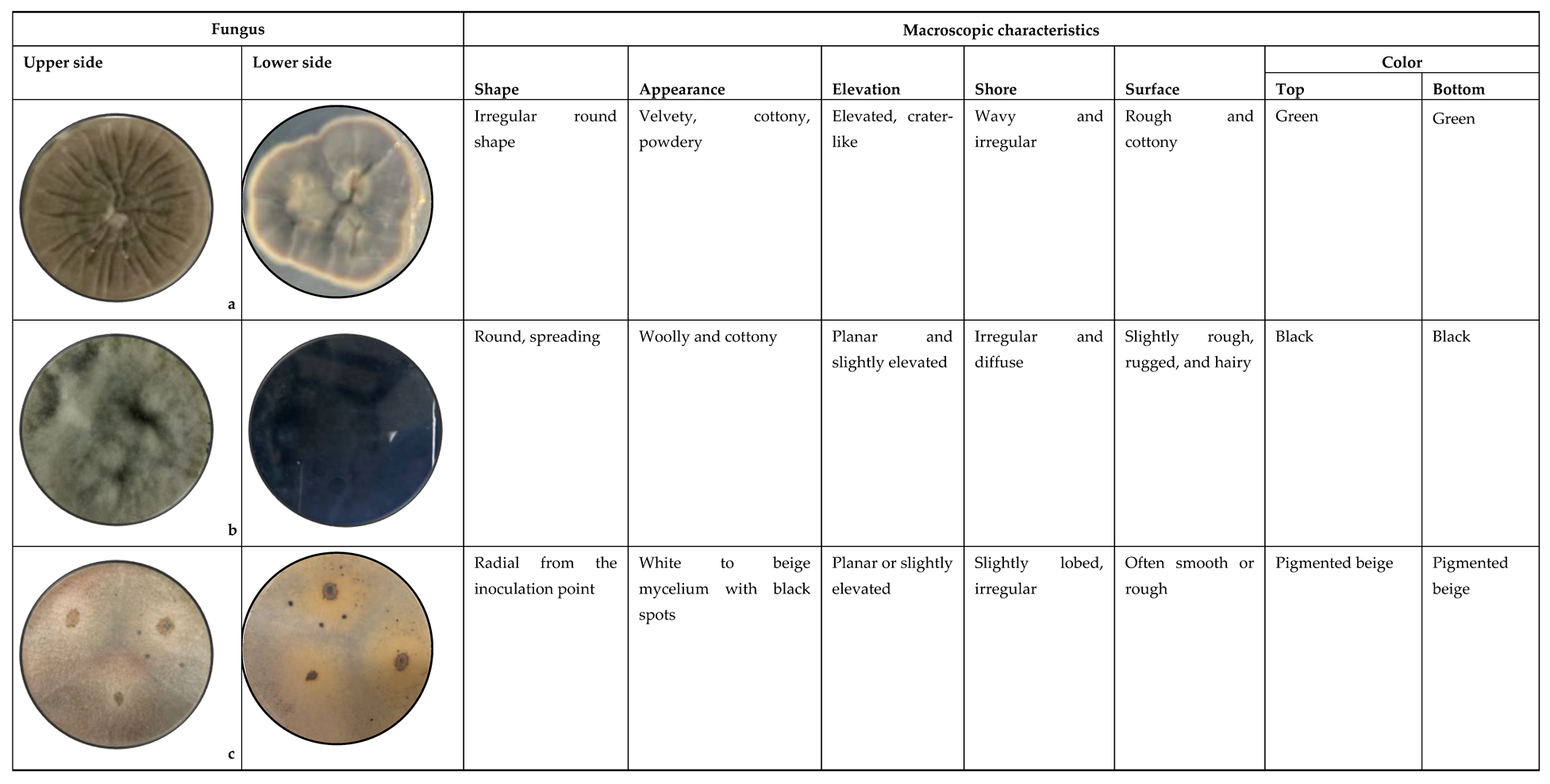
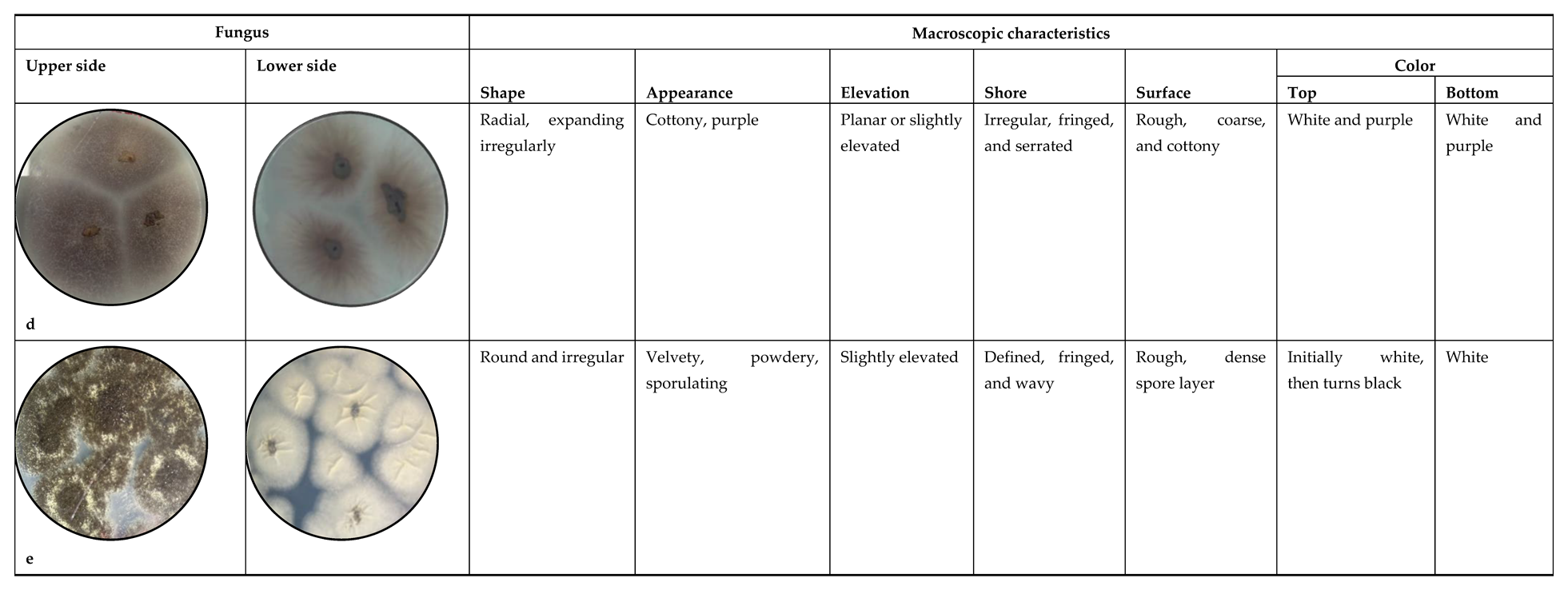
3.1. Macroscopic Identification
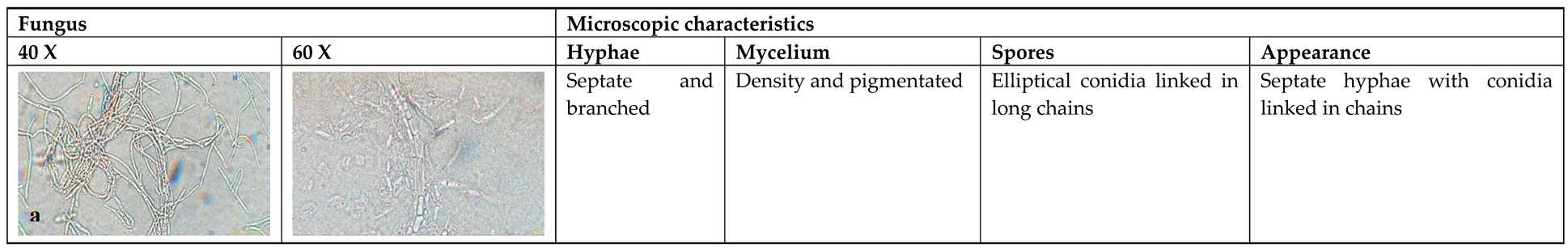
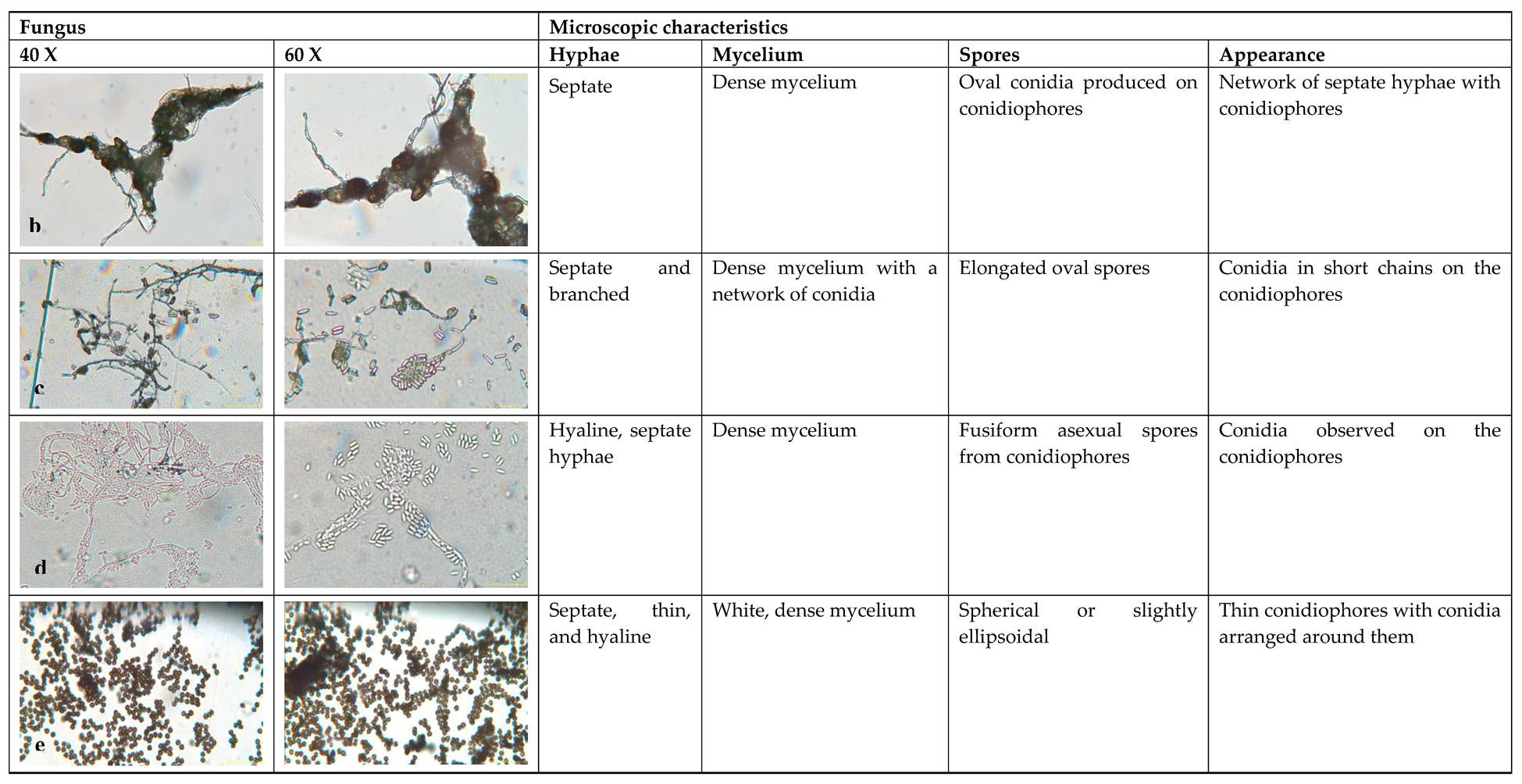
3.2. Microscopic Identification
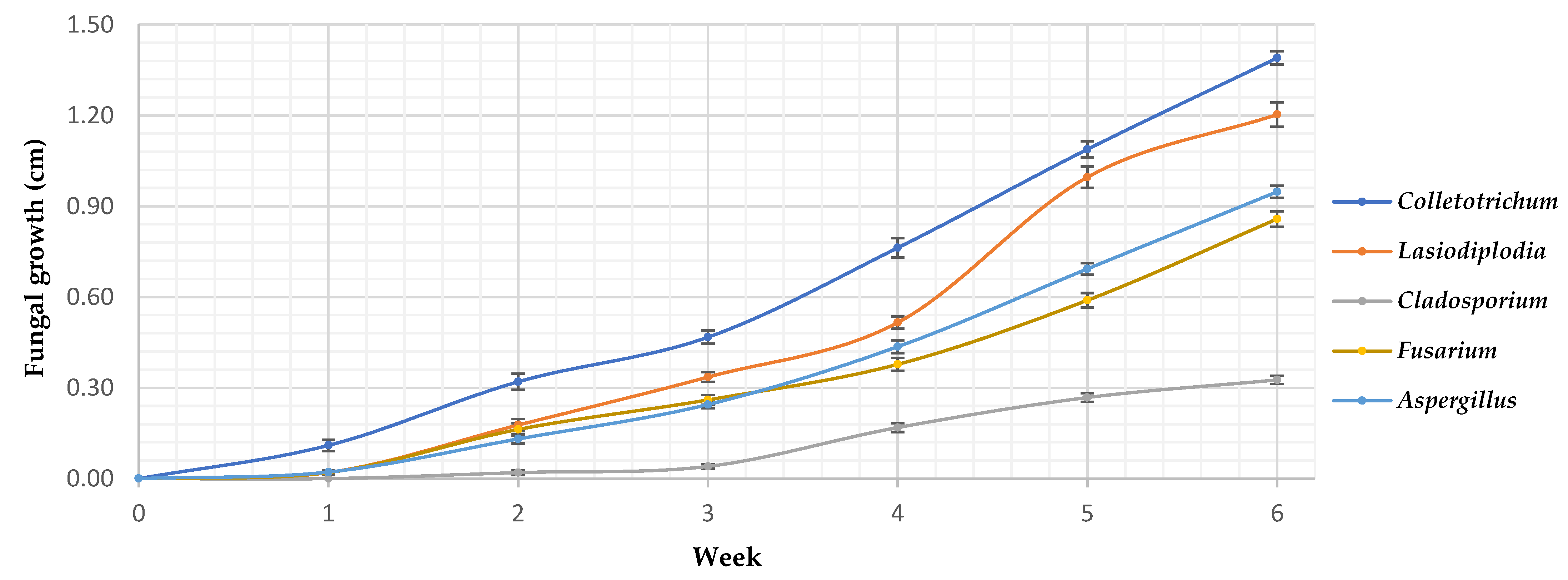
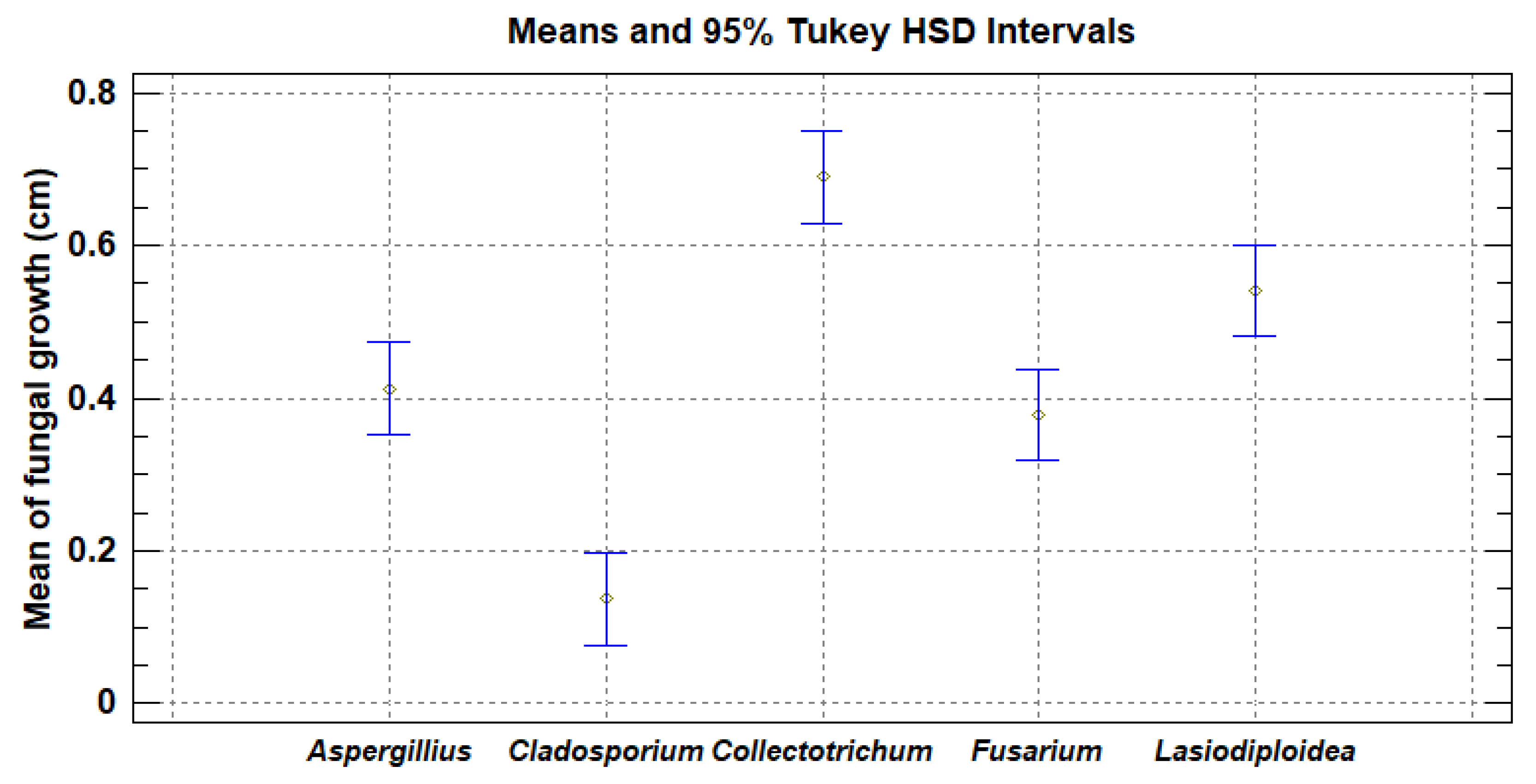
3.3. In vivo fungal activity
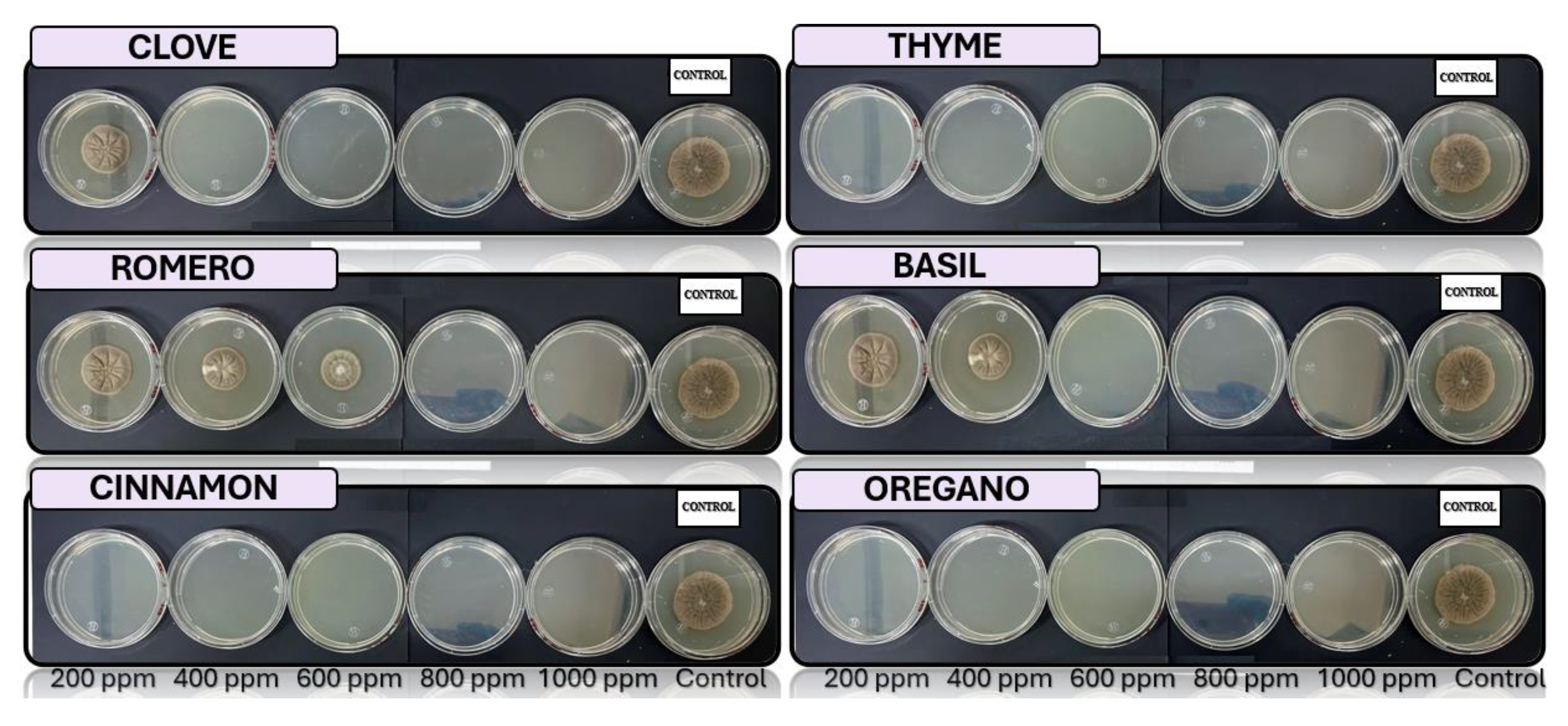
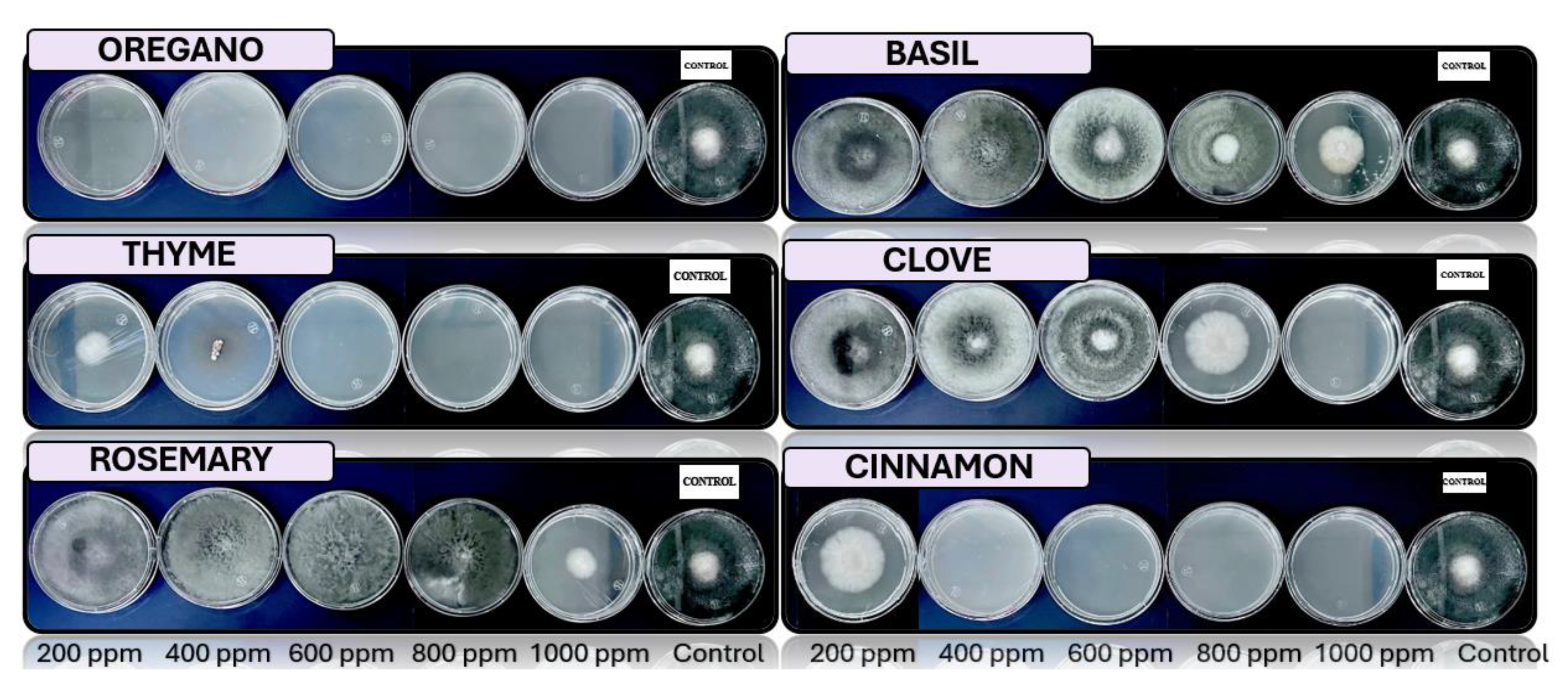
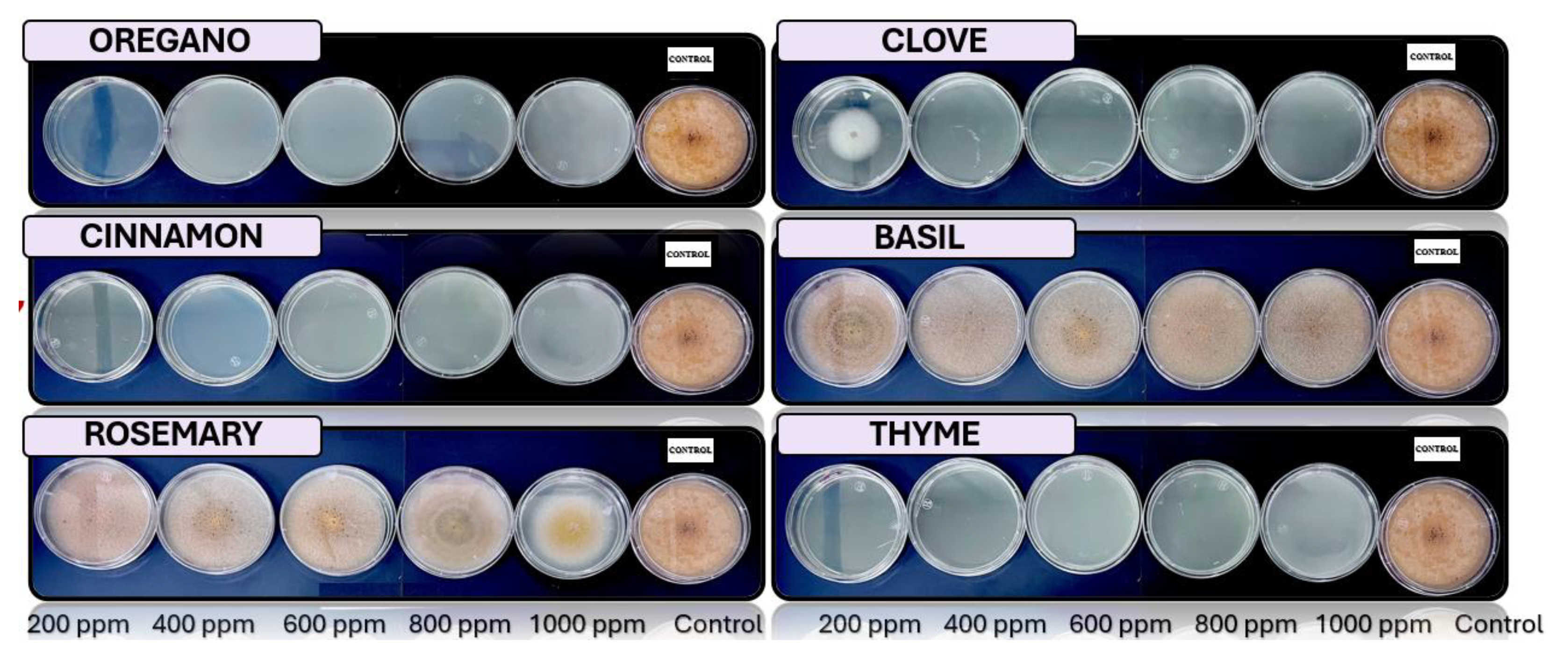
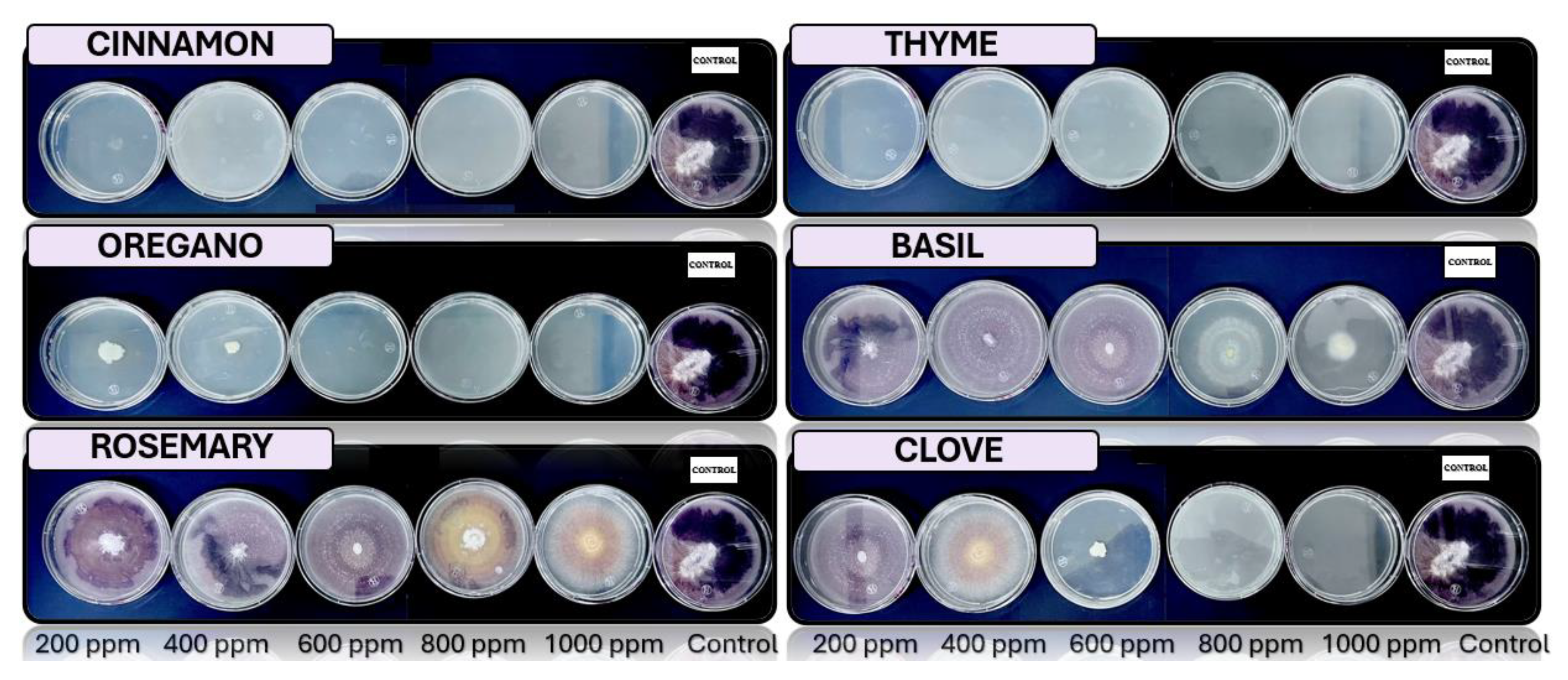
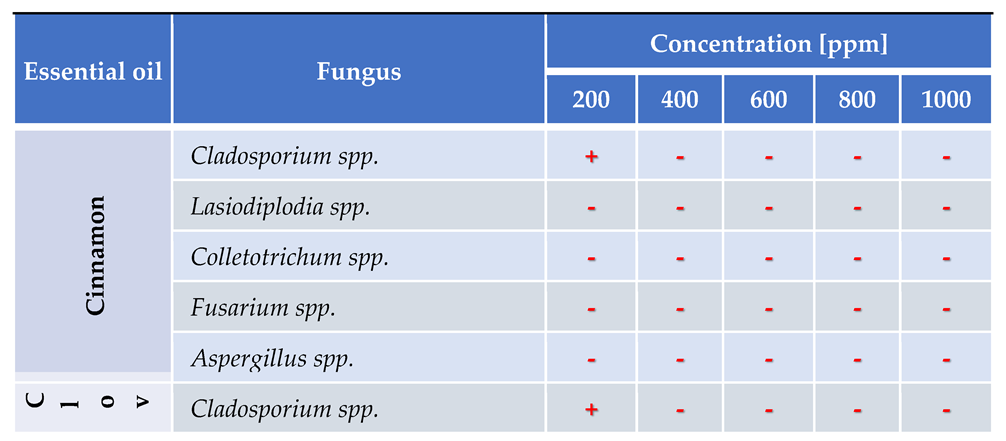
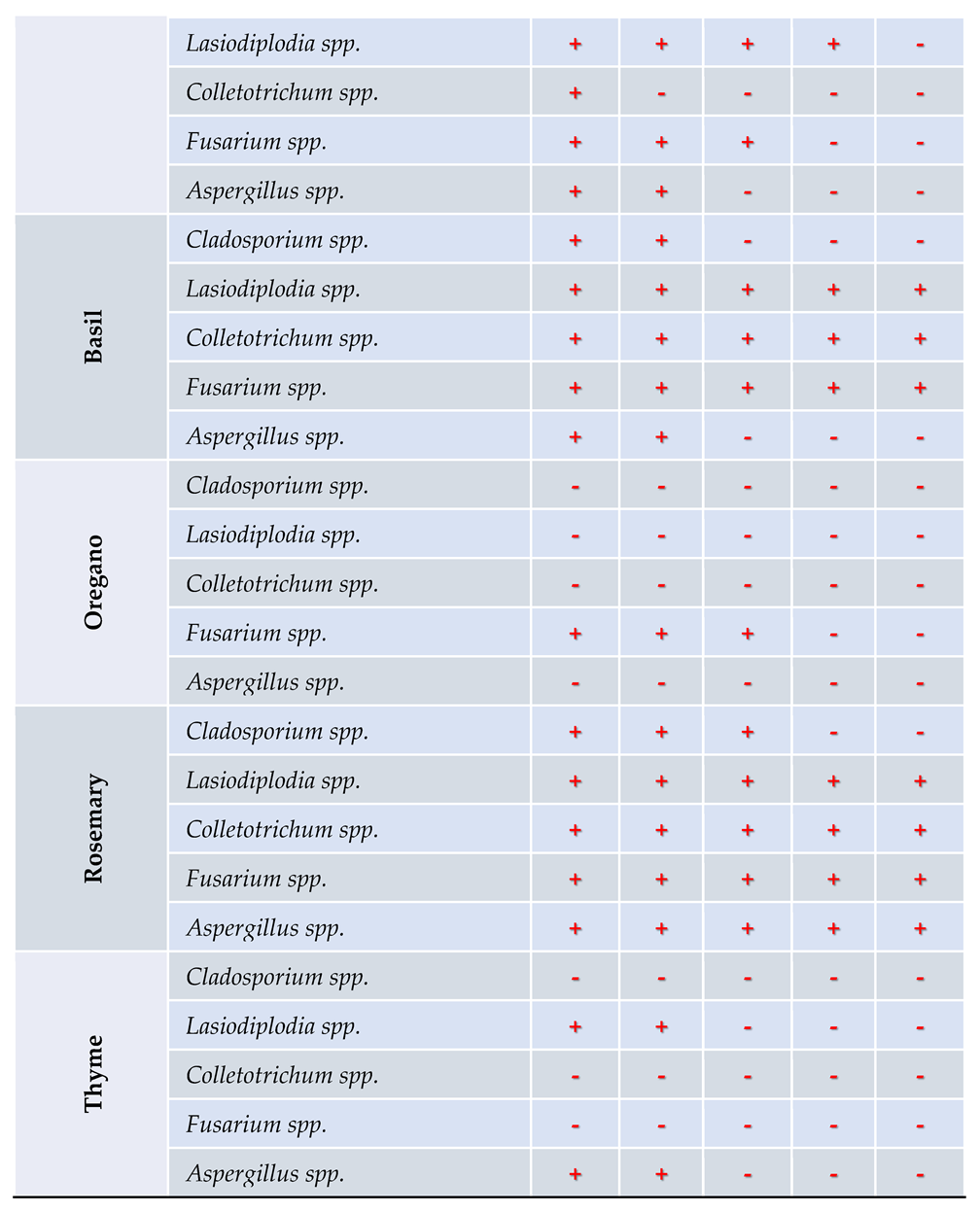
3.4. In Vitro Antifungal Activity with Essential Oils
4. Discussion
4.1. Macroscopic Identification
4.2. Microscopic Identification
4.3. In Vivo Fungal Activity
4.4. In Vitro Antifungal Activity with Essential Oils
5. Conclusions
Contributions
Acknowledgments
Conflicts of Interest
References
- Mata Anchundia D, Suatunce Cunuhay P, Poveda Morán R. Análisis económico del banano orgánico y convencional en la provincia Los Ríos, Ecuador. Avances 2021;23:419–30.
- Ruiz Medina MD, Ruales J. Postharvest Alternatives in Banana Cultivation. 2024. [CrossRef]
- Vilaplana R, Pazmiño L, Valencia-Chamorro S. Control of anthracnose, caused by Colletotrichum musae, on postharvest organic banana by thyme oil. Postharvest Biology and Technology 2018;138:56–63. [CrossRef]
- Arcila-Lozano CC, Loarca-Piña G, Lecona-Uribe S, González de Mejía E. El orégano: propiedades, composición y actividad biológica de sus componentes. Archivos Latinoamericanos de Nutrición 2004;54:100–11.
- Villanueva C, Vilca R, Ítalo A, Cotrina G. Aplicación de Aceite Esencial de Canela (cinnamomum verum) y Clavo de Olor (syzygium aromaticum) en la cobertura comestible y tiempo de vida útil de la Fresa (fragaria ananassa). Ciencia Latina Revista Científica Multidisciplinar, vol. 5, Perú: 2021, p. 1504–26. [CrossRef]
- Ruiz M, Ávila J, Ruales J. Diseño de un recubrimiento comestible bioactivo para aplicarlo en la frutilla (Fragaria vesca) como proceso de postcosecha 2016;17:276–87.
- López Luengo MT. El romero. Planta aromática con efectos antioxidantes. Offarm 2008;27:60–3.
- Farías C, Cisternas C, Morales G, Muñoz L, Valenzuela Rodrigo. Albahaca: Composición química y sus beneficios en salud. Revista Chilena de nutrición 2022;49. [CrossRef]
- Alzate D, Mier G, Afanador L, Durango D, García C. Evaluación de la fitotoxicidad y la actividad antifúngica contra Colletotrichum acutatum de los aceites esenciales de tomillo (Thymus vulgaris), limoncillo (Cymbopogon citratus), y sus componentes mayoritarios 2009;16.
- [10] Bensch K, Braun U, Groenewald JZ, Crous PW. The genus Cladosporium. Studies in Mycology 2012;72:1–401. [CrossRef]
- Crous PW, Braun U, Schubert K, Groenewald JZ. Delimiting Cladosporium from morphologically similar genera. Studies in Mycology 2007;58:33–56. [CrossRef]
- Picos-Muñoz PA, García-Estrada RS, León-Félix J, Sañudo-Barajas A, Allende-Molar R, Picos-Muñoz PA, et al. Lasiodiplodia theobromae en Cultivos Agrícolas de México: Taxonomía, Hospedantes, Diversidad y Control. Revista mexicana de fitopatología 2015;33:54–74.
- Sangeetha G, Anandan A, Rani SU. Morphological and molecular characterisation of Lasiodiplodia theobromae from various banana cultivars causing crown rot disease in fruits. Archives Of Phytopathology And Plant Protection 2012. [CrossRef]
- Villacís-Aldaz LA, León-Gordon O, Santana-Mayorga R, Mangui-Tobar J, Carranza G, Pazmiño-Miranda P. Actividad anti fúngica (in vitro) de extractos vegetales para el control de antracnosis (Colletotrichum acutatum). Journal of the Selva Andina Biosphere 2017;5:59–64. [CrossRef]
- Randy P. Marchites por fusarium del plátano 2015. [CrossRef]
- Ortiz E, Riascos D, Angarita M, Castro O, Rivera C, Romero D, et al. Tópicos taxonómicos para el estudio del género Fusarium 2020;33:61–6.
- Chang PK, Horn BW, Abe K, Gomi K. Aspergillus: Introduction. Encyclopedia of Food Microbiology: Second Edition, Elsevier Inc.; 2014, p. 77–82. [CrossRef]
- Rokas A. Aspergillus. Current Biology 2013;23:R187–8. [CrossRef]
- Suárez L, Rangel A. Aislamiento de microorganismos para control biológico de Moniliophthora roreri 2013;62.
- Ogórek R, Pusz W, Lejman A. Characteristic and taxonomy of Cladosporium fungi. Prace Pogladowe 2012;19:80–5.
- Santos JEA, Silva DEM, Vieira RFBS, Cordeiro MVM, Almeida MMM, Lima M a. S, et al. First Report of Lasiodiplodia brasiliensis Causing Crown Rot on Banana in Brazil. Plant Disease 2023;107:2538. [CrossRef]
- Camargo Piñeres Y, Zambrano Montenegro G, Ortega-Cuadros M, Gutierrez Montero DJ, Yepes JA, Camargo Piñeres Y, et al. Actividad antifúngica in vitro del aceite esencial de Swinglea glutinosa Merr sobre Colletotrichum sp. patógeno de mango (Mangifera indica L.). Revista Colombiana de Biotecnología 2021;23:62–71. [CrossRef]
- Jiménez M, Logrieco A, Bottalico A. Occurrence and Pathogenicity of Fusarium Species in Banana Fruits. Journal of Phytopathology 1993;137:214–20. [CrossRef]
- Pascal A, Simon T. Aspergillus fumigatus in Poultry 2011;20.
- Aguilar-Anccota R, Apaza-Apaza S, Maldonado E, Calle-Cheje Y, Rafael-Rutte R, Montalvo K, et al. Control in vitro e in vivo de Thielavipsis paradoxa y Collettrichum musae cn biofungicidas en frutos de banano orgánico. Manglar 2024;21:57–63. [CrossRef]
- Angarita Merchán M, Torres Caicedo MI, Díaz Torres AK. Técnicas de Biología Molecular en el desarrollo de la investigación. Revisión de la literatura. Revista Habanera de Ciencias Médicas 2017;16:796–807.
- Pérez-Rodríguez LR, Pérez-Moreno L, Guzmán-Mendoza R, Sanzón-Gómez D, Belmonte-Vargas JR, Pérez-Rodríguez LR, et al. Sensibilidad in vitro de hongos fitopatógenos causantes de enfermedades en fresa a controladores biológicos y fungicidas, en el estado de Guanajuato, México. Acta universitaria 2019;29. [CrossRef]

|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




