1. Introduction
Chinese Baijiu is one of the world’s six renowned distilled Baijiu (Qin et al., 2022). It is produced from starch-rich grains and sorghum(Xing-Lin et al., 2017), and employs Daqu, Xiaoqu, or Fuqu as saccharification and fermentation agents (Tu et al., 2022). Lactic acid, a significant non-volatile organic acid in Baijiu (Wang et al., 2022), is introduced during distillation, contributing to the Baijiu’s sour and fragrant flavor (Fan et al., 2024). Additionally, lactic acid and ethanol are catalyzed by esterifying enzymes to form ethyl lactate, one of the four major esters in Baijiu (Qiao et al., 2023; Wu et al., 2022). Ethyl lactate plays a crucial role in buffering and balancing the Baijiu’s aroma, enhancing its overall mellowness (Jin et al., 2021).
Lactic acid, with its asymmetric carbon atoms, exists as two optical isomers: L(+)-LA and D(-)-LA (Pohanka, 2020). The human body contains only L-lactic dehydrogenase, which means it can metabolize only L(+)-LA (Xu et al., 2022). Excessive intake of D(-)-LA can lead to metabolic disorders (Pohanka, 2020). The World Health Organization strongly advocates for the use of L(+)-LA in the food and pharmaceutical industries due to its safer metabolic profile (Ma et al., 2016; Wang et al., 2010). Consequently, research into the preparation and application of L(+)-LA is attracting widespread attention. Replacing D(-)-LA or mixtures of D(-)/L(+)-LA with L(+)-LA in the food and pharmaceutical sectors is becoming an inevitable trend.
L(+)-LA possesses several advantageous properties: it is easily absorbed (Castillo Martinez et al., 2013), has a mild and stable acidity, and helps preserve the flavor of food (He et al., 2013). As a result, L(+)-LA and its derivatives have gradually replaced other organic acids and are now widely used in the food industry as acidulants, bactericides, emulsifiers, and preservatives (Liu et al., 2022). Additionally, L(+)-LA can be polymerized into linear or cyclic poly-lactic acid, a non-toxic, biocompatible polymer that is broken down into L(+)-LA in the body (Li et al., 2015) and metabolized without causing allergic reactions (Li et al., 2020). This makes it suitable for manufacturing sustained-release capsules, biodegradable fibers (such as surgical sutures) (Baumann et al., 2020), and biological implants (for repairing fractures or other injuries) (Fitzgerald et al., 2018). In agriculture, L(+)-LA is added to animal feed to adjust the pH of the gastrointestinal tract, thereby enhancing the digestion and absorption of nutrients (Santamaría-Fernández et al., 2020).
In this study, two bacterial strains with high selectivity for L(+)-LA production were isolated from the fermented grains of strong-aroma Daqu. These microorganisms were applied to laboratory-scale liquid-state and solid-state fermentation systems with the aim of increasing the proportion of L(+)-EL to total ethyl lactate in Baijiu.
2. Materials and Methods
2.1. Collection of Fermented Grains, Yellow Water, and Liquor Samples
A total of 106 commercially available Baijiu samples from Sichuan, China, were collected and analyzed for this study. Additionally, ten samples of fermented grains, yellow water, and Baijiu were obtained from ten strong-aroma Baijiu factories (Sichuan, China), which were numbered from #1 to #10. From each factory, 1.5 kg of fermented grains, 1.5 L of yellow water, and 1 L of Baijiu were collected from five fermentation pits, with equal amounts taken from each fermentation pit. The fermented grains were stored at -20 °C, the yellow water at 4 °C, and the Baijiu at room temperature. The contents of D(-)-LA, L(+)-LA, D(-)-ethyl lactate (D(-)-EL), and L(+)-EL in these samples were subsequently analyzed.
2.2. Microbial Community Analysis of Fermented Grains
Fermented grains at various stages of fermentation were obtained from a local strong-aroma Baijiu distillery (Yibin, Sichuan, China), and were labeled as Day 0, Day 10, Day 20, Day 30, Day 40, Day 50, and Day 60. Samples were collected from three fermentation pits, with 1 kg of fermented grains taken from each fermentation pit. On the day of sampling, total DNA of microorganisms was extracted for microbial community analysis. The remaining fermented grain samples were stored at -20 °C for subsequent determination of ethanol and acetic acid content.
To collect the microorganisms, 100 g of fermented grains were mixed with 200 mL of sterile saline at 4 °C and shaken at 120 rpm for 30 minutes. After filtration, 100 mL of the filtrate was centrifuged at 12,000 rpm for 10 minutes. The resulting precipitate was used for DNA extraction.
Microbial genomic DNA was extracted from the samples using the Rapid Soil DNA Isolation Kit (Sangon Biotech, Shanghai, China) following the manufacturer’s instructions. DNA concentration and purity were assessed with 0.8% agarose gel electrophoresis and a NanoDrop ND-2000 spectrophotometer (Thermo Fisher Scientific, USA). The V3-V4 regions of the 16S rRNA genes were amplified using specific primers 338F (5'-ACTCCTACGGGAGGCAGCA-3') and 806R (5'-GGACTACHVGGGTWTCTAAT-3') (Xu et al., 2024). PCR parameters were set according to the previously described method (Liu et al., 2021). Next-generation sequencing library preparation and high-throughput sequencing were performed by Shanghai Majorbio Biopharm Technology Co., Ltd (Shanghai, China).
Raw sequencing reads were quality-controlled using Trimmomatic software (
https://cloud.majorbio.com/) and assembled with FLASH software (
https://cloud.majorbio.com/). Operational taxonomic units (OTUs) were clustered at 97% similarity using UPARSE software (version 7.1,
http://drive5.com/uparse/), with single sequences and chimeras removed. Each sequence was taxonomically annotated using the RDP classifier and aligned with the Silva database.
2.3. Detection and Quantification of Lactic Acid and Ethyl Lactate
Sample preparation
The fermentation broth samples were centrifuged, filtered, and diluted before the analysis of lactic acid content. For samples of fermented grains, an equal volume of water was added to the grains and thoroughly homogenized. The mixture was then centrifuged, and the supernatant was collected for analysis.
HPLC and GC-MS procedures
Lactic acid was detected and quantified using high-performance liquid chromatography (Shimadzu Prominence-i LC-2030C). A 10 µL sample was injected into a Shim-pack GIST C18 reversed-phase chromatography column (4.6m × 250mm × 5µm). The mobile phase solutions contain 10 mM potassium dihydrogen phosphate and 5% methanol. The flow rate was maintained at 1 mL/min, and the column temperature was set to 25 °C. Detection occurred at a wavelength of 210 nm (Ren et al., 2024a). To determine the proportions of D(-)-LA and L(+)-LA in total lactic acid, a 10 µL sample was injected into a reversed-phase chromatography column Chiral AAOA 3u 50*4.6mm (FMB-AAOA3-EONU). The mobile phase solutions contain 2 mM copper sulfate and 5% isopropanol. The flow rate was maintained at 1 mL/min, and the column temperature was set to 25 °C. Detection occurred at a wavelength of 254 nm. The representative HPLC spectra for lactic acid, D(-)-lactic acid, and L(+)-lactic acid were shown in the supplementary materials (Figures S1A and S1B), demonstrating the distinct separation of each lactic acid isomer.
Ethyl lactate was detected and quantified using gas chromatography-mass spectrometry (GC-MS, Agilent 7890A-5975B) equipped with an FBX-DEX DAC Beta chromatographic column (25m × 0.25mm × 0.25μm) and a Mass Selective Detector. The injection volume is 0.4 μL. The GC-MS system operated under specified conditions: helium served as the carrier gas, maintained at a consistent flow rate of 1 mL/min, with the oven temperature initially sustained at 50 °C for 3 minutes, then escalated to 120 °C at a rate of 3 °C/min, and subsequently held at 120 °C for 1 minute, then increased to 200 °C at a rate of 8 °C/min. The mass spectrometer operated in electron ionization mode at 70 eV, with the ion source temperature set at 230 °C. The concentrations of lactic acid and ethyl lactate were determined by comparing the chromatographic peak areas to those of internal standards. The GC spectrum of D(-)-ethyl lactate and L(+)-ethyl lactate was shown in the supplementary materials (Figure S1C).
2.4. Isolation and Screening of Lactic Acid-Producing Strains
Add 100 mL of sterile saline to 100 g of Day 0 fermented grains and homogenize thoroughly. Perform a 10-fold serial dilution of the mixture, then spread the diluted samples onto MRS medium and incubate at 37 °C for 24 hours. Isolate single colonies by streaking. Transfer the selected single colonies to bromocresol purple-CaCO3 medium plates and identify colonies with larger transparent and color-changing circles. Culture these selected single colonies in MRS medium (without agar) at 37 °C for 48 hours and measure the pH of the culture medium. Cultures with a pH < 4.0 were further analyzed by HPLC to determine the lactic acid content and proportions of D(-)-LA and L(+)-LA in total lactic acid.
To prepare 1 liter of LA bacteria selective medium (MRS medium), use the following components: peptone 10g, beef extract 10g, yeast extract 5g, K2HPO4 2g, diammonium citrate 2g, sodium acetate 5g, glucose 20g, Tween-80 1mL, MgSO4·7H2O 0.58g, MnSO4·7H2O 0.25g, agar 15g (Vinderola & Reinheimer, 1999). Adjust the pH to 6.2-6.4 and autoclave at 115 °C for 30 minutes. To prepare the LA bacteria isolation medium (bromocresol purple-CaCO3 medium): adjust the pH of the MRS medium to 5.8-6.0, then add bromocresol purple to 0.0224 g/L and CaCO3 to 5 g/L.
2.5. Identification of A6 and B12 Strains
Culture the A6 and B12 strains on MRS solid medium at 37 °C for 24 hours and observe the colony morphology. Perform Gram staining and spore staining on the bacteria. Examine the bacterial morphology of the A6 and B12 strains under a microscope using an oil immersion lens. Physiological and biochemical identification was carried out according to the methods outlined in Bergey's Manual of Determinative Bacteriology(Rehman et al., 2015).
The genomes of strains A6 and B12 were extracted using the Rapid Bacterial Genomic DNA Isolation Kit. The 16S rRNA gene sequences were amplified using the following primers: forward primer 27F (5'-TACGGYTACCTTGTTACGACTT-3') and reverse primer 1492R (5'-AGAGTTTGATCMTGGCTCAG-3'). The PCR products were A-tailed at the 3' end using the dA-Tailing Kit and then ligated to the T vector with the pUCm-T Vector Rapid Cloning Kit. All kits were purchased from Sangon Biotech (Shanghai, China), and all procedures were carried out according to the manufacturer's instructions. Nucleotide sequences were analyzed using DNAman software (Liu et al., 2014). Species assignment for the A6 and B12 strains was performed using the National Center for Biotechnology Information (NCBI) BLAST platform, based on the BLAST results yielding the highest total score (Zhao et al., 2024). Phylogenetic trees for the A6 and B12 strains, along with similar Bacillus strains, were constructed using Molecular Evolutionary Genetics Analysis (MEGA 11.0).
2.6. Characterization and Optimization of L(+)-LA Fermentation Characteristics of A6 and B12
Strains A6 and B12, preserved on slants, were streaked onto M6 solid medium and incubated at 37 °C for 48 hours. A single colony from the M6 solid medium was then inoculated into 25 mL of seed culture medium and cultured at 37 °C for 14 hours. The seed culture was used to inoculate the fermentation medium at a 5% inoculum size. Fermentation was carried out at 37 °C with continuous shaking at 120 rpm for 60 hours. During fermentation, the pH was maintained at 5.5-6.0 by adjusting with ammonia water.
To eliminate interference from the initial culture medium, the optical density (OD) of the bacteria was measured as follows: 5 mL of the fermentation broth was centrifuged at 8,000 rpm for 10 minutes. The supernatant was discarded, and the pellet was washed three times with sterile saline. The pellet was then resuspended in 5 mL of sterile saline (Tang et al., 2014). The OD value was measured at 600 nm using a UV-1800 spectrophotometer (MAPADA, Shanghai).
To investigate the effect of seed age on the fermentation process, equal volumes of seed cultures of different ages were added to the fermentation medium. To evaluate the effect of bacterial inoculation size, varying amounts of seed cultures were inoculated into the fermentation medium, and the L(+)-LA content was measured as described in section 2.3. Samples of the fermentation broth were taken from the fermentation tank and centrifuged at 12,000 rpm. The pH of the supernatant was measured using an ST10-OHAUS pH meter. The residual sugar content of the fermentation broth was determined using the Fehling reagent substitution method (Jayaraman et al., 2017).
Prepare the culture media (1 L) according to the following recipe. M6 medium: glucose 5.5g, beef extract 10g, yeast extract 5g, K2HPO4 2g, sodium acetate 5g, Tween-80 1mL, MgSO4·7H2O 0.2g, MnSO4·7H2O 0.05g, agar 15g. Seed medium: glucose 20g, beef extract 10g, yeast extract 10g, CaCl2 0.111g, MnSO4·7H2O 0.042g, Tween-80 0.5mL. Fermentation medium: beef extract 6g, yeast extract 5g, glucose 40g, K2HPO4 2g, diammonium citrate 2g, Tween-80 1mL, MgSO4·7H2O 0.1g, MnSO4·7H2O 0.25g, agar 15g. Adjust the pH value of media to 6.8 and autoclave at 115 °C for 30 minutes.
2.7. Addition of A6 and B12 Bacteria to the Liquid-State Fermentation System
Daqu was obtained from a local Baijiu factory (Yibin, Sichuan), and Xiaoqu was purchased from the market. To prepare yeast cultures, 1 g of Daqu or 0.1 g of Xiaoqu was added to 100 mL of YPD medium and incubated at 30 °C with shaking at 120 rpm for 24 hours . Subsequently, 5 mL of this culture was used to inoculate 100 mL of fresh YPD medium, which was then incubated under the same conditions. This process was repeated twice to further enrich the yeast cultures. The yeast cells were collected by centrifugation at 8000 rpm for 10 minutes and labeled as Yeast-Daqu and Yeast-Xiaoqu, respectively. For bacterial preparation, A6 and B12 strains were transferred to seed culture medium, incubated at 37 °C for 14 hours, and then centrifuged at 8000 rpm for 10 minutes to collect the bacteria.
A total of 36 fermentation containers were prepared for four fermentation models: Daqu, Xiaoqu, Yeast-Daqu, and Yeast-Xiaoqu. Each fermentation model included three groups: control, A6 added, and B12 added, with each group replicated three times. In each 10 L fermentation container, 5 L of malt extract medium was added. For the Daqu model, 50 g of Daqu was added, and for the Xiaoqu model, 5 g of Xiaoqu was included. For the Yeast-Daqu model, the yeast pellet derived from 250 mL of Daqu yeast culture was added, and for the Yeast-Xiaoqu model, the yeast pellet from 250 mL of Xiaoqu yeast culture was used. A6 or B12 bacteria (from 250 mL of bacterial culture) were introduced into the experimental groups, whereas the control groups received no additional bacteria. Fermentation was carried out for 7 days at a constant room temperature and weight loss was measured daily throughout the fermentation period.
To determine ethanol yield, 100 mL of fermentation broth was transferred to a 500 mL distillation flask, to which 100 mL of water was added. The mixture was distilled until 100 mL of distillate was collected, and the alcohol content was measured using the density method. Additionally, 4 L of fermentation broth was distilled, and 500 mL of Baijiu samples were collected to measure the content of D(-)-EL and L(+)-EL.
Prepare the media according to the following recipe. YPD medium (1 L): yeast extract 10 g, peptone 20 g, glucose 20 g, chloramphenicol 0.05 g (added after sterilization). Malt extract medium (1 L): malt extract 130 g, glucose 65 g.
2.8. Addition of A6 and B12 Bacteria to the Solid-State Fermentation System
Commercially available glucoamylase, active dry yeast, sorghum, and rice husk were used to prepare the starting fermented grains. Sorghum (80 kg) was crushed and mixed with 50 L of boiling water, then incubated for 2 hours. Next, 8 kg of rice husk was added, thoroughly mixed, and steamed for 90 minutes. Hot water at 90°C was added until the total mass reached 195 kg. The mixture of hot water and steamed grains was continuously mixed for 10 minutes to ensure even water absorption and then rapidly cooled to 22 °C using cold air.
A total of 27 fermentation containers were prepared for three fermentation models: Glucoamylase+yeast, Daqu, and Xiaoqu. Each fermentation model included three groups: control, A6 added, and B12 added, with each group replicated three times. In each 10 L fermentation container, 7 kg of the prepared mixture was added. For the Glucoamylase+yeast model, 5 g of active dry yeast and 20 g of glucoamylase were included. For the Daqu model, 500 g of Daqu was added, and for the Xiaoqu model, 25 g of Xiaoqu was included. In the experimental groups, A6 or B12 bacteria (from 350 mL of bacterial culture) were added, while the control groups received no additional bacteria. Fermentation was carried out for 30 days at a constant room temperature.
To assess acidity, 100 mL of water was added to 100 g of fermented grains. After homogenization, 20 g of the mixture was added to 80 mL of water and incubated for 30 minutes with constant mixing every 10 minutes. The resulting solution was subjected to acid-base titration. Total acid production was evaluated by measuring the amount of NaOH (mmol) consumed during titration.
For ethanol yield measurement, 100 g of fermented grains was transferred to a 500 mL distillation flask, and 200 mL of water was added. The mixture was distilled to obtain 100 mL of distillate, and the alcohol content was determined using the density method. Additionally, 5 kg of the fermentation mixture was distilled, and 500 mL of the Baijiu sample was analyzed for D(-)-EL and L(+)-EL content.
3. Results and Discussion
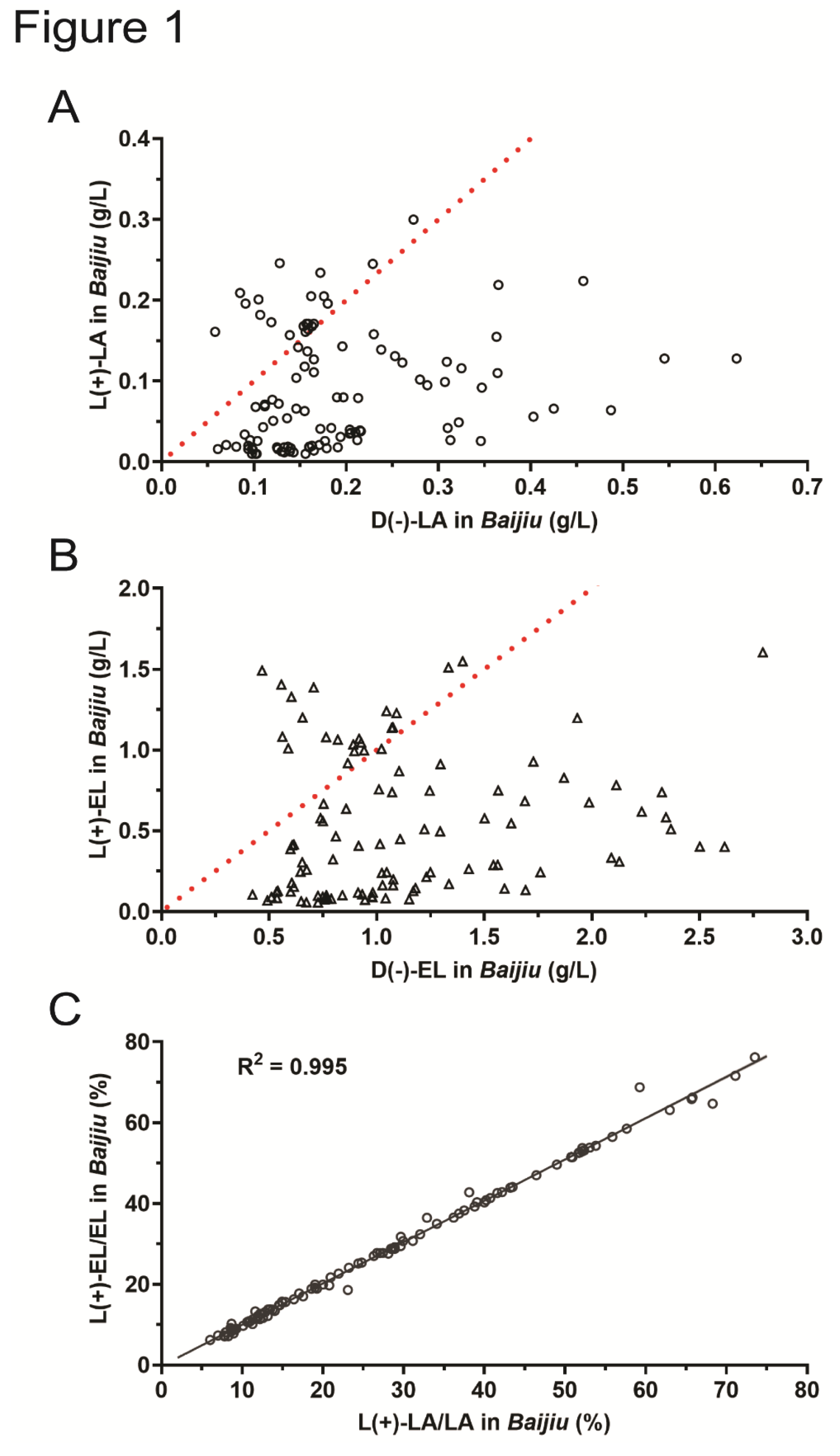
3.1. The Strong-Aroma Baijiu Contains Both Isomers of Lactic Acid and Ethyl Lactate
The strong-aroma Baijiu contains a variety of organic acids and esters, among which the most abundant acids are lactic acid, acetic acid, butyric acid, and caproic acid; the most abundant esters are ethyl caproate, ethyl lactate, ethyl acetate, and ethyl butyrate. Among these four acids, lactic acid is the only one that has a chiral carbon atom and shows two chiral isomers, L(+)-LA and D(-)-LA. Therefore, ethyl lactate also has two optically active isomers, L(+)-EL and D(-)-EL.
To investigate the isomeric content of lactic acid and ethyl lactate in strong-aroma
Baijiu, 106 commercially available samples were collected. Our results revealed that all
Baijiu samples contained two isomers of lactic acid, with each isomer displaying significant variation in content (
Figure 1A). A putative red dotted line indicating an equal distribution of the two isomers (50% L(+)-LA and 50% D(-)-LA) was drawn. This line divided the 106
Baijiu samples into two groups: 21 samples above the line, characterized by a higher content of L(+)-LA than D(-)-LA, and 85 samples below the line, characterized by a higher content of D(-)-LA than L(+)-LA (
Figure 1A), which suggested that the D(-)-LA prevails in 106 commercially available
Baijiu samples.
Moreover, both L(+)-EL and D(-)-EL were detected in all 106
Baijiu samples. Consistent with the content distribution of the two lactic acid isomers, the content of D(-)-EL in 85 samples was greater than that of L(+)-EL (
Figure 1B). This leads us to speculate that the chirality of lactic acid and ethyl lactate in
Baijiu is strongly correlated. To verify this speculation, we conducted a correlation analysis on the content of L(+)-LA and L(+)-EL in 106
Baijiu samples. The results showed that the contents of L(+)-LA and L(+)-EL were highly correlated (R
2 = 0.995) (
Figure 1C). A possible explanation is that L(+)-LA and L(+)-EL in
Baijiu are in a dynamic equilibrium of continuous esterification and hydrolysis.
3.2. The L(+)-Isomer Proportion of Ethyl Lactate and Lactic Acid in Zaopei, Huangshui, and Baijiu Shows a Strong Correlation
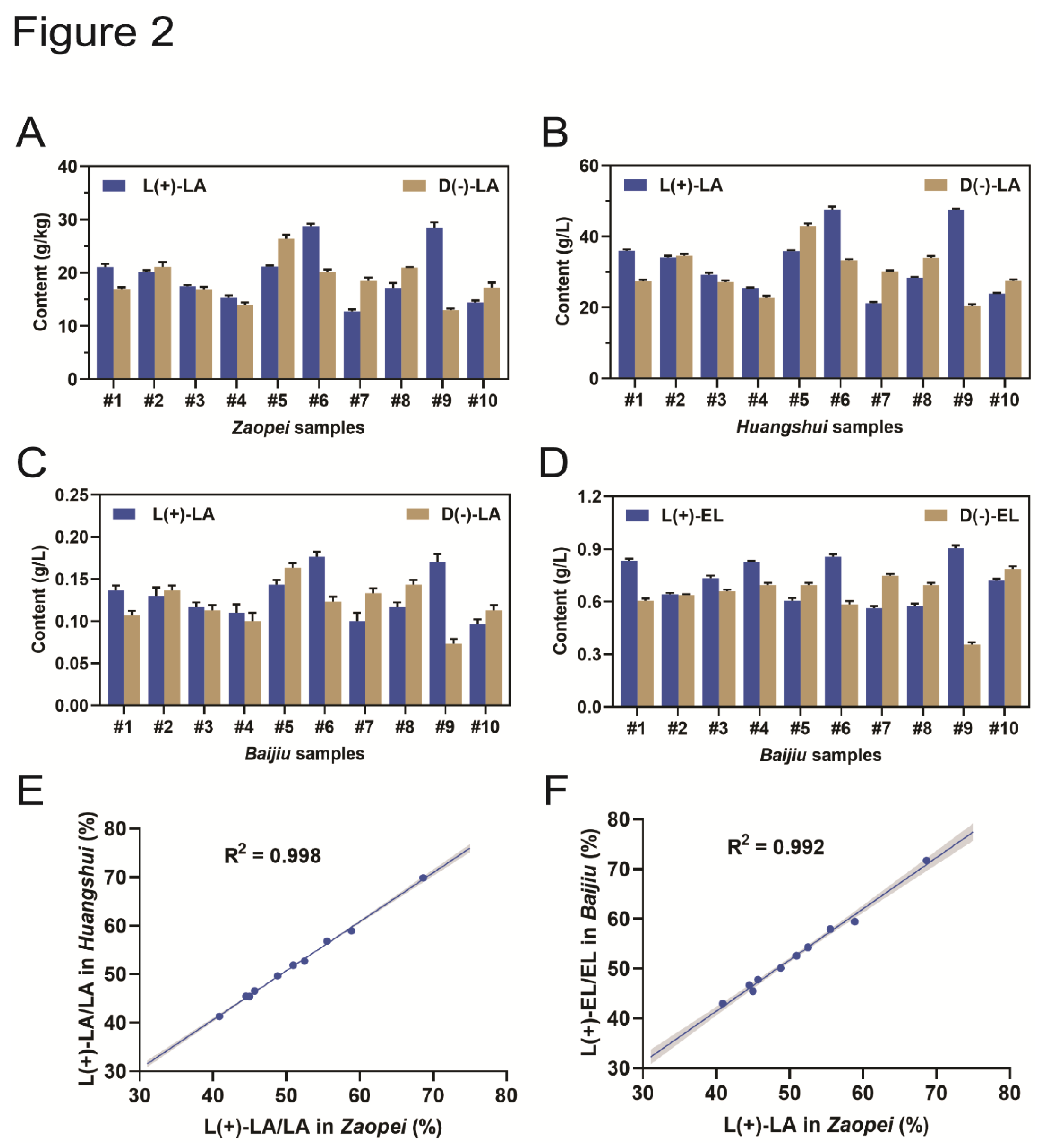
During the Baijiu production process, grains are fermented by microorganisms to form Zaopei, also called fermented grains, and Huangshui, the percolate after solid-state fermentation (Ren et al., 2024b). Lactic acid and ethyl lactate in Zaopei and Huangshui are transferred to Baijiu during the distillation process. Therefore, the lactic acid contained in Baijiu comes from the microbial fermentation. We hypothesized that the lactic acid components in Zaopei, Huangshui, and Baijiu are correlated.
To study this,
Zaopei,
Huangshui, and
Baijiu samples were collected from 10 strong-aroma
Baijiu factories and their lactic acid content was measured. The results showed that all
Zaopei and
Huangshui samples contained L(+)-LA and D(-)-LA (
Figure 2A,B). Moreover, the L(+)-LA content in
Zaopei and
Huangshui was strongly correlated (R
2 = 0.998) (
Figure 2E). The
Baijiu samples from 10 distilleries all contained L(+)-LA and D(-)-LA, as well as L(+)-EL and D(-)-EL (
Figure 2C,D). The L(+)-LA content in
Zaopei and
Baijiu was strongly correlated (R
2 = 0.992) (
Figure 2F). which is in good agreement with the results obtained in 106 commercially available strong-aroma
Baijiu samples (
Figure 1C).
In conclusion, we found that the chiral composition of lactic acid produced by fermentation and lactic acid in Baijiu shows a strong correlation. In strong-aroma Baijiu, lactic acid is in free and esterified forms. Therefore, we can adjust the chiral composition of lactic acid and ethyl lactate in Baijiu by improving the microbial composition of the fermentation process.
3.3. Lactic Acid-Producing Microorganisms Are Abundant in Fermented Grains
Microbial samples were collected at different time points and analyzed to evaluate the abundance of lactic acid-producing microorganisms and the changes in microbial communities during fermentation. The dominant bacterial communities in the fermented grains included
Firmicutes,
Proteobacteria, and
Actinobacteria (
Figure 3A).
Firmicutes were the predominant microbes, constituting 73.85% to 98.53% of the microbial community. Their proportion decreased from 82.13% to 73.85% during the first 20 days of fermentation and then increased to 98.53% after Day 20. This microbial community comprised 13 dominant genera, including
Lactobacillus,
Bacillus,
Staphylococcus,
Clostridium,
Caproiciproducens,
Weissella,
Pediococcus,
Enterococcus,
Enterobacter,
Pseudomonas,
Kroppenstedtia,
Acinetobacter, and
Thermoactinomyces. Among these, seven genera are known to produce lactic acid:
Lactobacillus,
Bacillus,
Staphylococcus,
Weissella,
Pediococcus,
Enterococcus, and
Enterobacter (
Figure 3A). The relative abundance of bacterial communities exhibited distinct trends throughout the fermentation process. The proportion of
Lactobacillus increased significantly from 26.71% on Day 0 to 89.83% on Day 60. Conversely, the relative abundance of
Bacillus decreased markedly from 41.01% on Day 0 to 0.25% on Day 60. Similarly, the abundance of
Staphylococcus decreased from 11.40% on Day 0 to 0.19% on Day 60.
Lactic acid production through microbial metabolism primarily follows three pathways: homolactic fermentation, heterolactic fermentation, and bifidobacterial lactic acid fermentation. Key enzymes for these pathways are illustrated in
Figure 3B. Using PICRUSt2 (Gao et al., 2023), we analyzed the abundance of genes encoding key enzymes for lactic acid metabolism at various time points (
Figure 3C). Enzymes associated with homolactic fermentation, such as EC2.7.1.11 and EC4.1.2.13, were found to be highly abundant, indicating that homolactic fermentation predominates in lactic acid production.
Ethanol and lactic acid contents in the fermented grains were monitored throughout the fermentation process. The results revealed that ethanol and lactic acid fermentations occurred with different time dynamics. The ethanol content increased rapidly during the first 20 days and then stabilized. In contrast, the lactic acid content increased gradually until Day 20 and then rose sharply from Day 20 to Day 50 (
Figure 3D). During this period, the abundance of key enzymes EC2.7.1.40 and EC1.1.1.27 remained high (
Figure 3C).
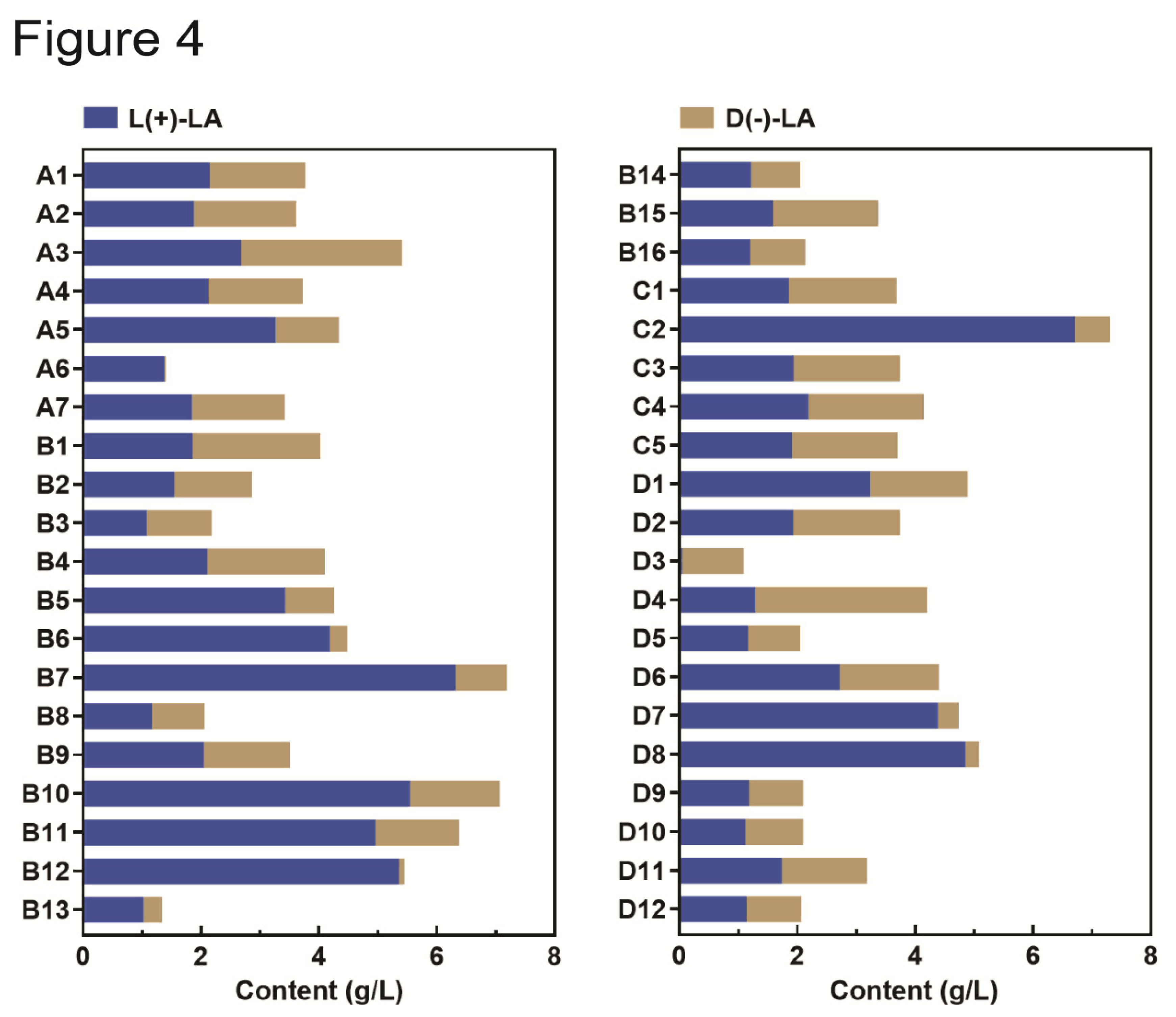
3.4. Isolation of Two L(+)-LA-Producing Strains from Initial Fermented Grains
Microorganisms present in fermented grains play various roles in the fermentation process and experience community succession. Bacterial species are the most abundant in initial fermented grains before fermentation begins (Day 0) (
Figure 3A). To screen lactic acid-producing bacteria, we used initial fermented grains from four
Baijiu distilleries (A, B, C, and D), isolating a total of 40 lactic acid-producing strains. Each strain was assigned a number corresponding to its distillery of origin.
The lactic acid production of these strains was tested in the MRS medium over 48 hours. The results revealed significant variability in lactic acid yield among the strains (
Figure 4). Strains B7 and C2 produced high yields of 7.13 g/L and 7.30 g/L, respectively, while strains B13 and D3 produced lower yields of 1.34 g/L and 1.09 g/L. Additionally, the configuration of lactic acid produced by these strains varied. Strains A6 and B12 strongly preferred to produce L(+)-LA, with L(+)-LA comprising 98.57% and 98.53% of their total lactic acid production, respectively. In contrast, the L(+)-LA produced by strain D3 accounted for 4.59% of its total lactic acid (
Figure 4).
Baijiu fermentation is driven by multiple microbial species. The presence of D(-)-LA-producing bacteria, L(+)-LA-producing bacteria, and bacteria capable of producing both isomers in fermented grains may contribute to the mixed presence of D(-)-LA and L(+)-LA in the final product. D(-)-LA-producing bacteria and L(+)-LA-producing bacteria occupy similar ecological niches and exhibit competitive interactions. To reduce the concentration of D(-)-LA in Baijiu, increasing the abundance of L(+)-LA-producing bacteria could be a viable strategy.
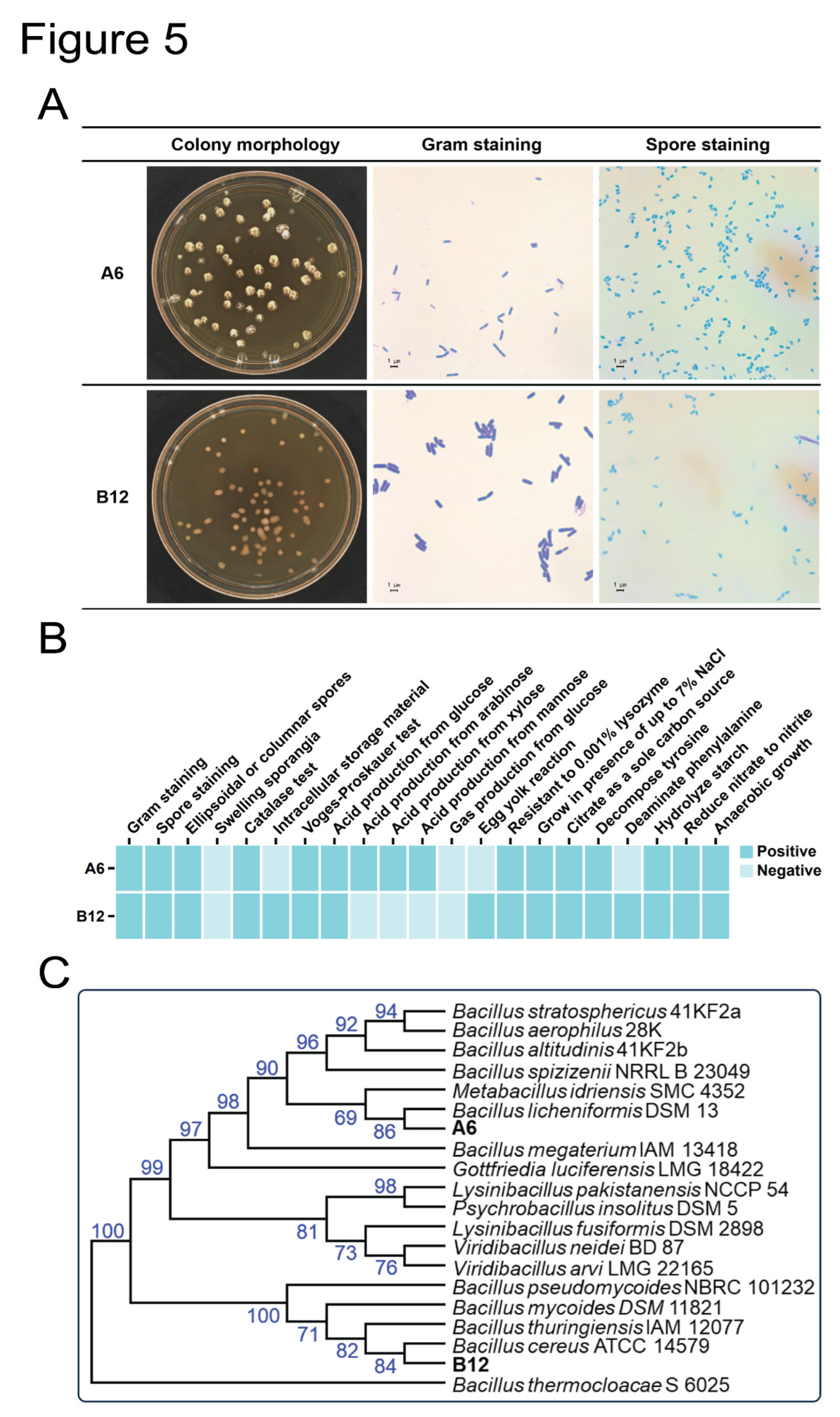
3.5. Identification of Strains A6 and B12
To determine the taxonomic status of the two high L(+)-LA-producing strains, A6 and B12, we analyzed their morphology, physiological and biochemical characteristics, and 16S rRNA gene sequences. The morphology of A6 colonies varied with growth. Initially, A6 colonies had a mucous or membranous surface with a mucous matrix underneath. As they matured, the colonies became rough and crusty, forming “licheniform” colonies that adhered to the agar (
Figure 5A). After 24 hours of growth on agar plates, A6 colonies appeared whitish, opaque, and irregularly shaped with a wrinkled surface. In contrast, B12 colonies were round with complete edges and a smooth appearance. Additionally, B12 bacteria (0.5-0.8 µm × 1.5-2.5 µm) were larger than A6 bacteria (0.3-0.4 µm × 1.0-1.6 µm). Both A6 and B12 strains are Gram-positive, rod-shaped bacteria, occurring singly or in pairs, and form ellipsoidal to cylindrical spores in unswollen sporangia (
Figure 5A). Based on these morphological characteristics(Lu et al., 2018), A6 and B12 were preliminarily identified as
Bacillus species.
To further identify strains A6 and B12, several physiological and biochemical tests were performed. Both strains are facultative anaerobes and tested positive for the Voges-Proskauer test. According to Bergey’s Manual of Determinative Bacteriology, strain A6 displayed characteristics consistent with
Bacillus licheniformis, including growth on MRS agar plates, growth in the presence of up to 7% NaCl, starch hydrolysis, resistance to 0.001% lysozyme, and reduction of nitrate to nitrite (
Figure 5B). The strain B12 was identified as
Bacillus cereus, based on its production of a large amount of storage material with a vacuolar or foamy appearance on MRS agar plates (
Figure 5B).
A phylogenetic tree based on the 16S rRNA gene sequences of strains A6 and B12 was constructed to confirm the classical taxonomic results (Fig. 5C). The phylogenetic analysis revealed that strain A6 is closely related to Bacillus licheniformis DSM 13(Veith et al., 2004), while strain B12 is closely related to Bacillus cereus ATCC14579(Mirończuk et al., 2008). These phylogenetic results align with the morphological, physiological, and biochemical identification results. Consequently, strains A6 and B12 were identified as Bacillus licheniformis and Bacillus cereus, respectively.
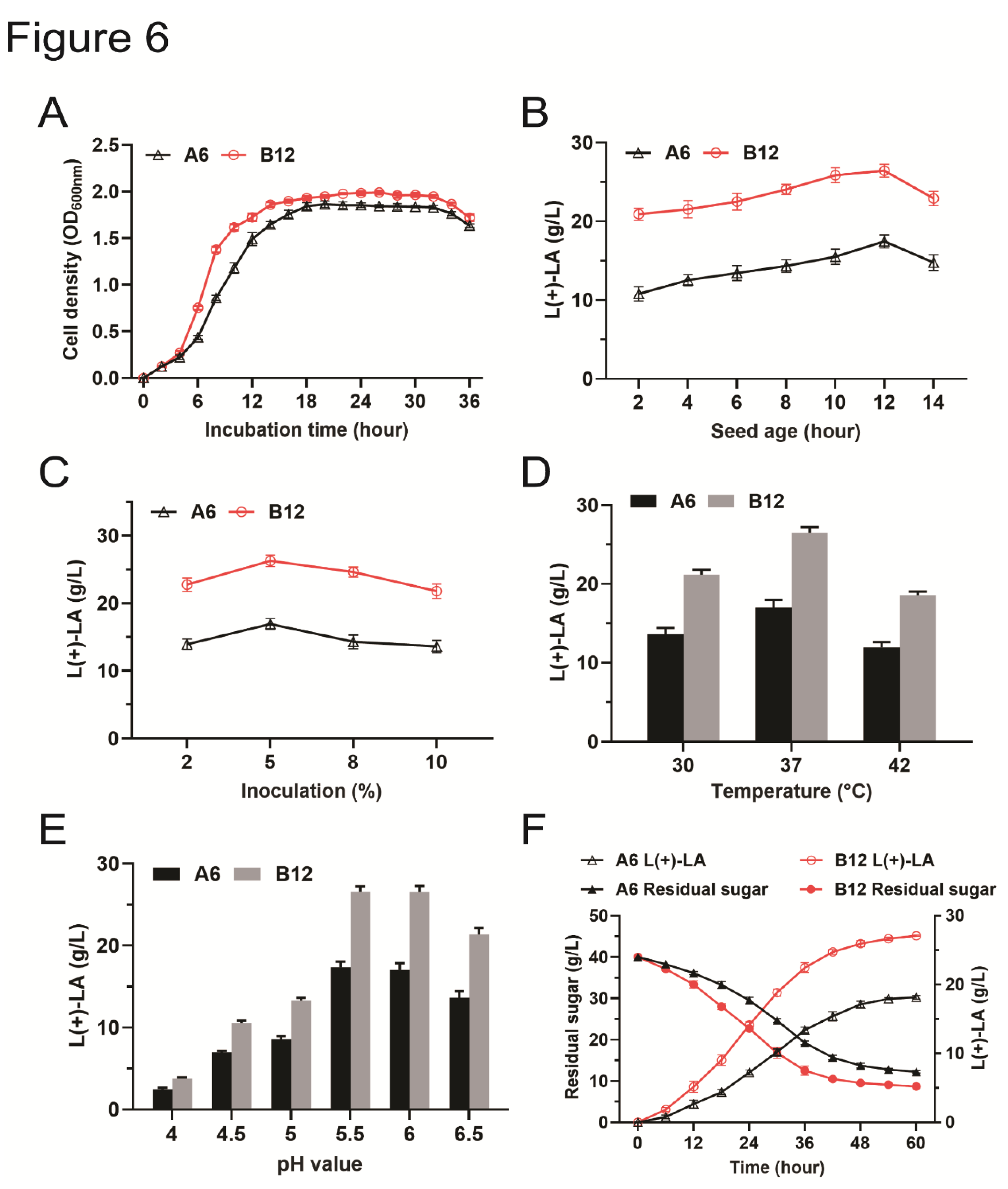
3.6. Optimization of Culture Conditions and Fermentation Performance of Strains A6 and B12
The quality of the seed culture has a significant impact on bacterial growth rate and L(+)-LA production during fermentation. To determine the optimal seed culture duration, we characterized the growth properties of strains A6 and B12. Both strains were inoculated into MRS medium, with samples taken every 2 hours to measure the OD
600 value. The growth curves for strains A6 and B12 were similar. The logarithmic growth phase for both strains commenced approximately 4 hours after inoculation and continued until around 12-14 hours (
Figure 6A).
Bacterial cultures of strains A6 and B12, at the logarithmic growth phase, were inoculated into MRS medium to assess the impact of seed age on L(+)-LA fermentation. As illustrated in
Figure 6B, L(+)-LA production increased with seed age up to 12 hours. This improvement can be attributed to the slower growth of younger seed cultures in the early stages post-inoculation, which delays the fermentation cycle and results in lower L(+)-LA yields. The highest L(+)-LA production was observed with a seed age of 12 hours. However, extending the seed age to 14 hours led to a decline in L(+)-LA production, likely due to premature bacterial decay after inoculation (
Figure 6B). Thus, a seed culture age of 12 hours is optimal for maximizing L(+)-LA production.
Inoculum size is a critical factor influencing the fermentation process and efficiency (Abbasiliasi et al., 2017). We evaluated the impact of different inoculum sizes on L(+)-LA production for these two strains. The highest L(+)-LA production was achieved with an inoculum size of 5%. In contrast, production decreased when the inoculum size was either reduced to 2% or increased to 8% or 10% (
Figure 6C). Therefore, an inoculum size of 5% was selected for use in subsequent studies.
To further optimize fermentation conditions, we investigated the production of L(+)-LA by strains A6 and B12 under varying temperature and pH conditions. At 30 °C, the L(+)-LA yields were 13.6 g/L for A6 and 21.2 g/L for B12. Increasing the temperature to 37 °C resulted in the highest L(+)-LA yields, with A6 and B12 producing 16.9 g/L and 26.5 g/L, respectively. However, at a higher temperature of 42 °C, L(+)-LA production decreased to 11.9 g/L for A6 and 18.5 g/L for B12 (
Figure 6D). Therefore, 37 °C is the optimal temperature for L(+)-LA fermentation with strains A6 and B12.
At pH 4.0, L(+)-LA production was low for strains A6 and B12, with yields of 2.4 g/L and 3.7 g/L, respectively. As the pH increased, L(+)-LA production improved, suggesting that bacterial growth was inhibited at lower pH levels (
Figure 6E). L(+)-LA yields for strains A6 and B12 peaked at pH 5.5, with respective yields of 17.3 g/L and 26.5 g/L. L(+)-LA yields at pH 6.0 were similar to those observed at pH 5.5. Further increasing the pH to 6.5 did not enhance L(+)-LA production.
Strains A6 and B12 were subjected to L(+)-LA fermentation under the optimized conditions. The yields reached 18.1 g/L for A6 and 27.1 g/L for B12 (
Figure 6F). At the end of fermentation, the residual sugar content was 12.2 g/L for A6 and 8.7 g/L for B12. The conversion rate of glucose to lactic acid for strain B12 was 86.5%, indicating that B12 predominantly exhibits homolactic fermentation. In contrast, the glucose-to-lactic acid conversion rate for strain A6 was 65.1%, which is below the threshold for homolactic fermentation (>80%)(Subramanian et al., 2015) but above the threshold for heterolactic fermentation (<50%) (Arriola et al., 2011). Thus, strain A6 may exhibit both homolactic and heterolactic fermentation. Overall, both strains demonstrated strong L(+)-LA production capabilities under the tested conditions.
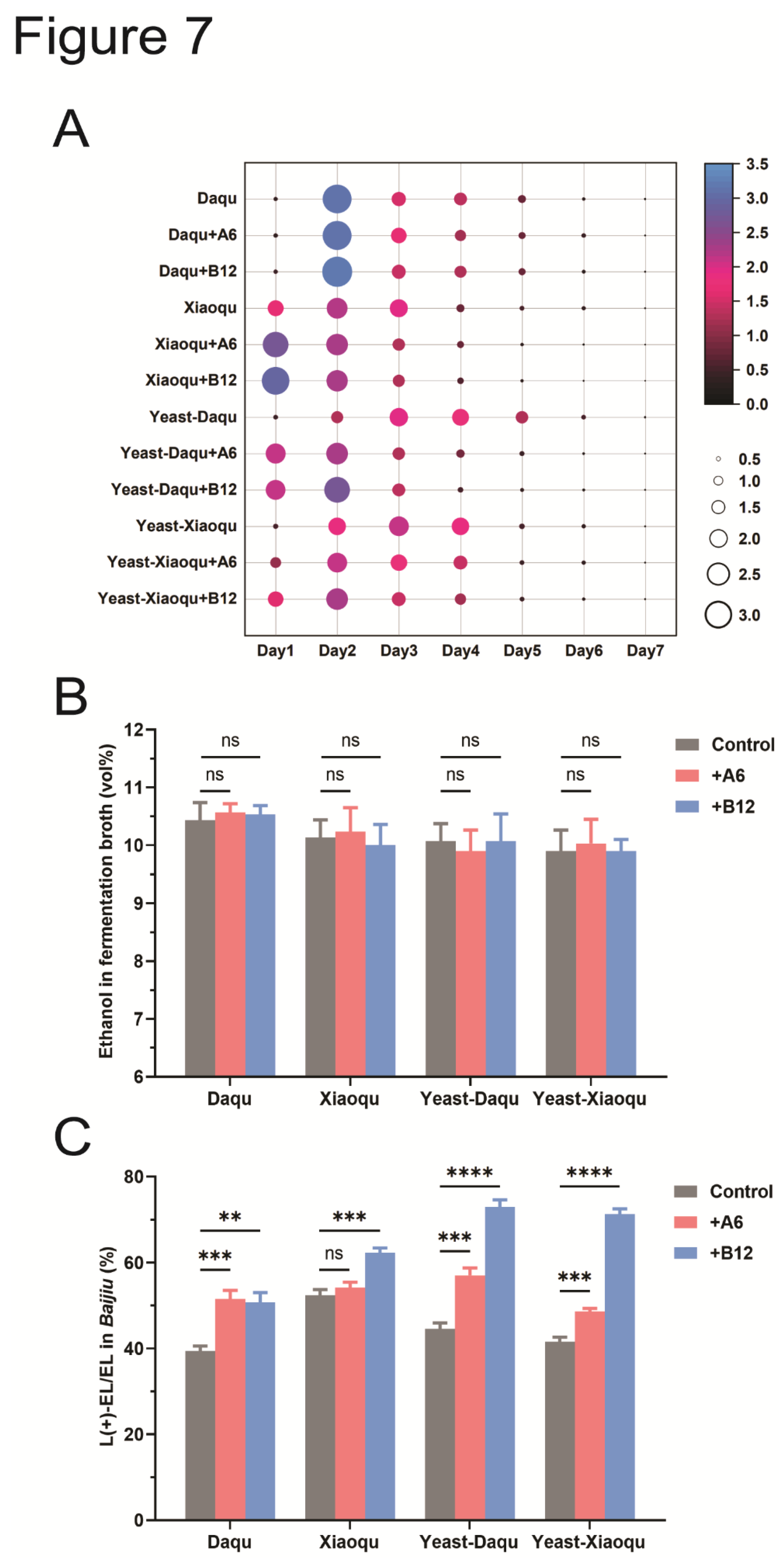
3.7. Application of A6 and B12 Strains in Liquid Fermentation Models
To assess whether the isolated A6 and B12 strains influence Baijiu fermentation, they were added to various liquid fermentation models. Four different models were used based on the source and abundance of the starting microorganisms: yeast from Xiaoqu (Yeast-Xiaoqu), yeast from Daqu (Yeast-Daqu), Xiaoqu, and Daqu. The fermentation process was monitored by measuring the weight loss of fermentation tanks.
The introduction of A6 or B12 strains had varying effects on the fermentation process. In the Daqu fermentation model, the addition of A6 or B12 did not have a significant impact. This may be due to the high abundance of yeast and bacteria in Daqu, which limited the effect of the added strains. Conversely, the addition of A6 or B12 notably affected the Xiaoqu fermentation model. Compared to the control, the presence of A6 or B12 increased weight loss during the early stages of fermentation and shortened the overall fermentation time (
Figure 7A). This effect may be attributed to the interaction between the added bacteria (A6 or B12) and the yeast in the system, which likely stimulated yeast metabolic activity and accelerated the fermentation process. Similar effects were observed in the Yeast-Xiaoqu and Yeast-Daqu fermentation models (
Figure 7A).
Despite these variations in fermentation process effects across the models, the final ethanol content in the fermentation broth did not significantly differ between the test groups and the controls, indicating that A6 and B12 strains primarily influence the fermentation rate rather than the final ethanol production (
Figure 7B).
The fermentation broth was distilled to obtain
Baijiu samples, and the proportion of L(+)-EL to total ethyl lactate was analyzed. In all four fermentation models, the addition of strain B12 increased the proportion of L(+)-EL significantly compared to the control (
Figure 7C). Furthermore, the addition of strain A6 notably increased the proportion of L(+)-EL in the Yeast-Xiaoqu, Yeast-Daqu, and Xiaoqu fermentation models. Notably, the addition of A6 or B12 did not affect the overall production of ethyl lactate (
Figure S2A). Overall, incorporating high L(+)-LA-producing strains A6 or B12 into liquid fermentation models effectively enhances the proportion of L(+)-EL in
Baijiu, with B12 showing a superior effect compared to A6.
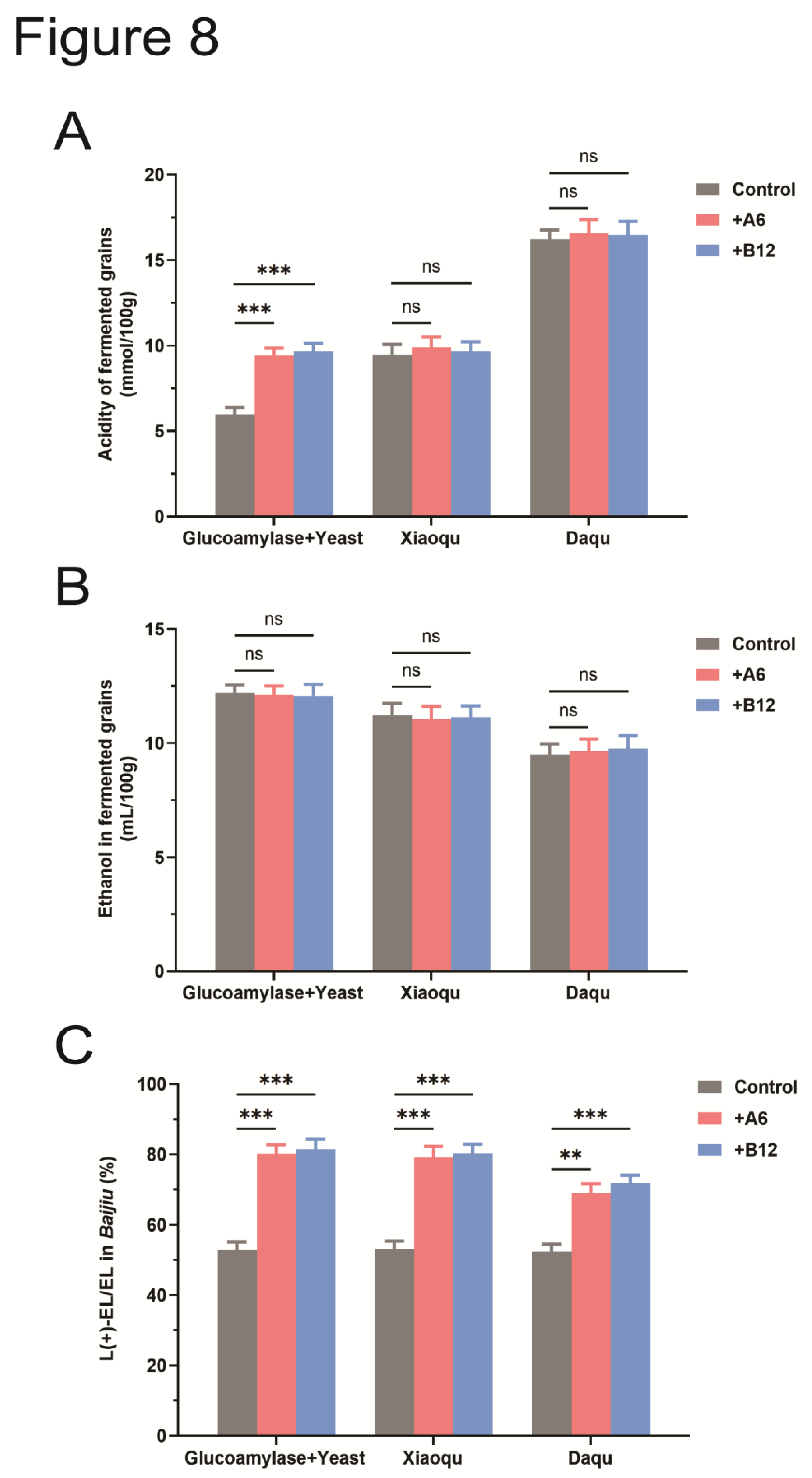
3.8. Application of A6 and B12 Strains in Solid-State Fermentation
To evaluate the impact of A6 and B12 strains on solid-state fermentation, they were tested in three different fermentation models: a simplified model with glucoamylase and Saccharomyces cerevisiae (Glucoamylase+Yeast), Xiaoqu, and Daqu. The acidity of the fermented grains was measured at the end of fermentation for each model.
In the simplified fermentation model, the acidity of the fermented grains in the control group was 5.9 mmol/100g. The addition of A6 or B12 strains increased the acidity to 9.4 and 9.6 mmol/100g, respectively, which was significantly higher than that of the control (P<0.001) (
Figure 8A). This increase in acidity is attributed to the bacterial acid production by A6 and B12, whereas the control group, lacking these bacteria, produced less acid during fermentation. However, the addition of A6 or B12 did not significantly alter the acidity in the Xiaoqu and Daqu solid-state fermentation models (P>0.05). Since Daqu contains a greater number and variety of bacteria, the acidity of fermented grains in the Daqu solid-state fermentation model is significantly higher than that of the Glucoamylase+Yeast and Xiaoqu solid-state fermentation models, underscoring the role of bacteria as the primary contributors to acid production.
The ethanol content in fermented grains was measured at the end of fermentation. Consistent with the results from liquid fermentation, the addition of A6 and B12 strains did not affect ethanol production. No significant differences in ethanol content were observed between the test groups and the control in all three solid-state fermentation models (P>0.05) (
Figure 8B).
Additionally, the proportion of L(+)-EL to total ethyl lactate in the
Baijiu was analyzed. Adding A6 or B12 to the solid-state fermentation models greatly improves the proportion of L(+)-EL to total ethyl lactate in
Baijiu. In the Glucoamylase+Yeast and Xiaoqu models, the proportion of L(+)-EL significantly increased from 52% to 81% (P<0.01) (
Figure 8C). Similar to their effects in liquid fermentation, the addition of A6 or B12 did not affect the total ethyl lactate production (
Figure S2B). In summary, the addition of A6 or B12 strains effectively enhances the proportion of L(+)-EL in
Baijiu across both liquid and solid-state fermentation models.