1. Introduction
The Jurkat cell line has been used as “normal” T cell line and has generated extremely valuable knowledge, such as T cell signaling pathways. However, the use of Jurkat cells as a T cell model has decreased in the recent years [
1], because it is realized that Jurkat cells are quite abnormal compared to the normal T cells in many ways [
1]. Nevertheless, T cell lines are still playing key roles in research fields such as cell therapy and immune checkpoints to develop therapeutic products for variety of disease. Such applications include drug testing and disease modeling and so on.
Immortalized normal human T cell lines are critical for basic research and clinical product development. However, many immortalization methods, including SV40 large T antigen, oncogene transfection, and virus all generate abnormality of karyotype and phenotype. Telomerase reverse transcriptase (
TERT) gene overexpression is considered the immortalization method that changes the karyotype and phenotype the least. However, the uncontrolled insertion of the TERT transgene can cause chromosome instability of the immortalized cell lines. Several methods have been used to immortalize cell lines in general. One of the widely used methods is transfection of SV40 large T-antigen, which has immortalized many different types of cell lines, such as kidney cell line HEK293T, chondrocyte cell line[
2]. Due to the capability of binding to transcription co-factor p300 and CBP, and perturbation of retinoblastoma and p53 tumor suppressor proteins, SV40 large T-antigen immortalized cells often have cancer transformation property[
3,
4]. Oncogene transfection was also proved to be efficient for immortalizing human T cell lines from cancer patients or healthy donor[
5]. But whether the immortalized cell lines had any chromosome abnormality or mutations were not known. Epstein Bar Virus (EBV) can infect the T cells
in vivo and immortalize T cells
in vivo [
6]. Therefore, some immortalized T cells lines were isolated and established from the EBV infected patients [
7]. Human
TERT over expression became a widely used method currently. First, hTERT immortalized cell lines did not lead to tumor formation in immune deficient nude mice, in contrast, SV40 immortalized cell lines showed tumorigenicity[
8]. Second,
TERT immortalized cell lines showed relative chromosome stability and retained the expression of phenotypic markers[
8,
9,
10]. Human T cells were also reported to be immortalized by
TERT over expression [
11].
Despite the advantages of
TERT over expression immortalization method, most of the delivery method for the
TERT transgene utilize retrovirus [
11], lentivirus [
12] or plasmids [
8,
13]. These methods all have one major disadvantage resulting in uncontrolled insertion of the transgenes. Uncontrolled insertions may cause insertional mutation or chromosome instability. Recently, Zhao and colleagues used a new strategy of replacing CDKN2A (p16) exon 2 with
TERT transgene by CRISPR/Cas9 technology, which can over express human
TERT and inactivate the tumor suppressor gene CDKN2A (p16) at the same time [
12]. Zhao and colleagues successfully immortalized human prostate epithelial cell line. However, the immortalized cell lines had chromosome loss and gain. The authors used a lentivirus vector for CRISPR/Cas9 and guide RNA delivery, which may cause the consistent expression of CRISPR/Cas9 and guide RNA in the cell progenies and eventually lead to the chromosome abnormality.
In this study, we used a similar concept of over expressing TERT and CDKN2A inactivation to immortalize human primary T cells, however we utilized CRISPR/Cas9 with conditions to ideally insert inert single copies of the transgene at a specific gene locus minimizing the incidence of off target DNA changes. The immortalized human primary T cell line exhibited a phenotype consistent with of primary human T cells.
2. Results
2.1. Establishment of Immortalized Normal CD8+ T Cell Line
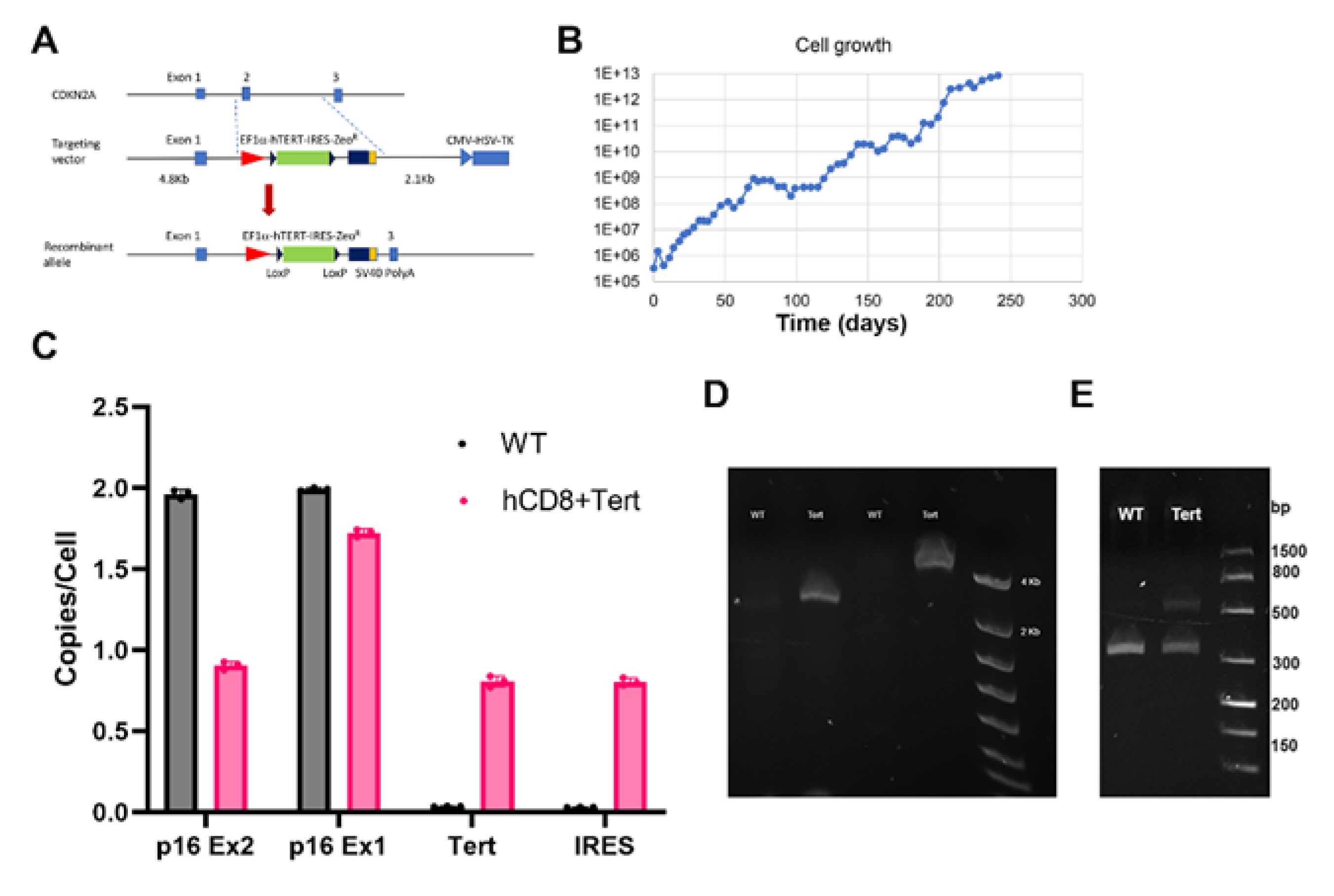
The electroporated CD8
+ T cells were selected with 5 μg/mL of Zeocin started from 2
nd passage and for over 10 passages. The live cell number was declined initially and recovered in the next passage. After 52 passages, the live cells expanded around 3 × 10
7 times (
Figure 1B). This data indicated that the CD8
+ T cells were immortalized. To confirm whether hTERT transgene was incorporated into CDKN2A (p16) gene exon 2, we first used ddPCR to detect the copy number of hTERT transgene per cell. We found that there were 1 copy of CDKN2A (p16) gene exon2 and 2 copies exon 1 (exon 1 was a part of 5’ recombinant arm of the donor DNA) in the immortalized CD8
+ T cells, and there were 2 copies of both p16 gene exon 1 and exon 2 in the WT CD8
+ T cells (Fig. 1C). One copy of hTERT transgene and IRES were also found in the immortalized CD8
+ T cells. This data indicated that the targeting donor DNA contained hTERT and Zeocin resistant genes was successfully replaced one copy of p16 gene exon 2. To further confirm the donor DNA was incorporated in the CDKN2A (p16) gene exon 2 as expected, we used 2 pairs of primers to detect the recombinant CDKN2A (p16 allele. Both pairs of primers had the forward primers located in the polyA signal of the transgene, and reverse primers located 3’ to the 3’ recombinant arm of donor DNA. Both pairs of primers were able to detect the recombinant allele (
Figure 1D). Since we only detected 1 copy of hTERT transgene and still had 1 copy of CDKN2A (p16) gene exon 2, we would expect that the CDKN2A (p16) exon 2 knockout was heterozygous. To test this hypothesis, we designed primers that can only detect WT CDKN2A (p16) allele and only detect p16 exon 2 knockout allele, namely forward primers only located in the replaced CDKN2A (p16) gene fragment or only located in the hTERT transgene cassette. The PCR data showed that both WT allele and knockout allele were detected (
Figure 1E), which confirmed that only 1 copy of hTERT transgene replaced the CDKN2A (p16) gene exon 2. We call this cell line hCD8
+ T-TERT hereafter.
2.2. TERT Gene Expression was Elevated in hCD8+ T-TERT Cells
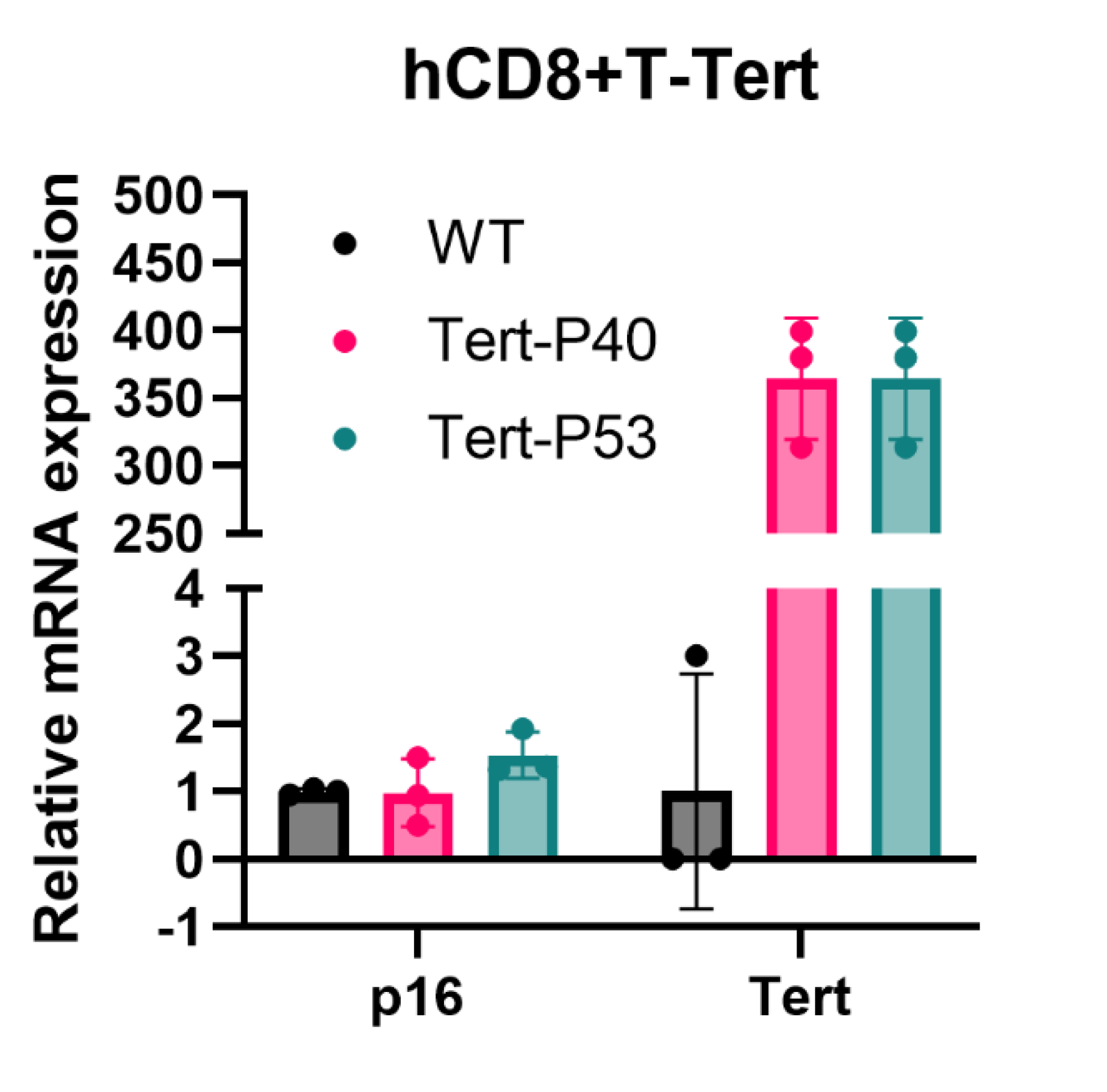
HCD8
+ T-TERT cells were cultured with Zeocin for all the passages and were resistant to Zeocin, which indicated that the Zeocin resistant gene was expressed in the cells. The hTERT gene was designed at the upstream of Zeocin resistant gene. To test whether hTERT transgene was expressed in hCD8
+ T-TERT cells, we isolated total RNA from 2 different passages of the cells (passage 40 and passage 53) and control WT CD8
+ T cells. Direct RT-ddPCR data revealed that hTERT gene expression was over 350 folds elevated in the immortalized cells, but it was minimally detected in WT CD8
+ T cells (
Figure 2). This data indicated that hCD8
+ T-TERT cells had the hTERT transgene replaced CDKN2A (p16) exon 2 locus, and the TERT transgene was highly expressed. Since there was one copy of CDKN2A (p16) gene exon 2 was replaced with hTERT transgene, we asked whether p16 mRNA expression in the hCD8
+ T-TERT cells was reduced compared to WT cells. DdPCR data revealed that the p16 mRNA expression remained almost same as compared with WT CD8
+ T cells (
Figure 2).
2.3. HCD8+ T-TERT Cells Exhibited Partial Normal Karyotype at High Passages
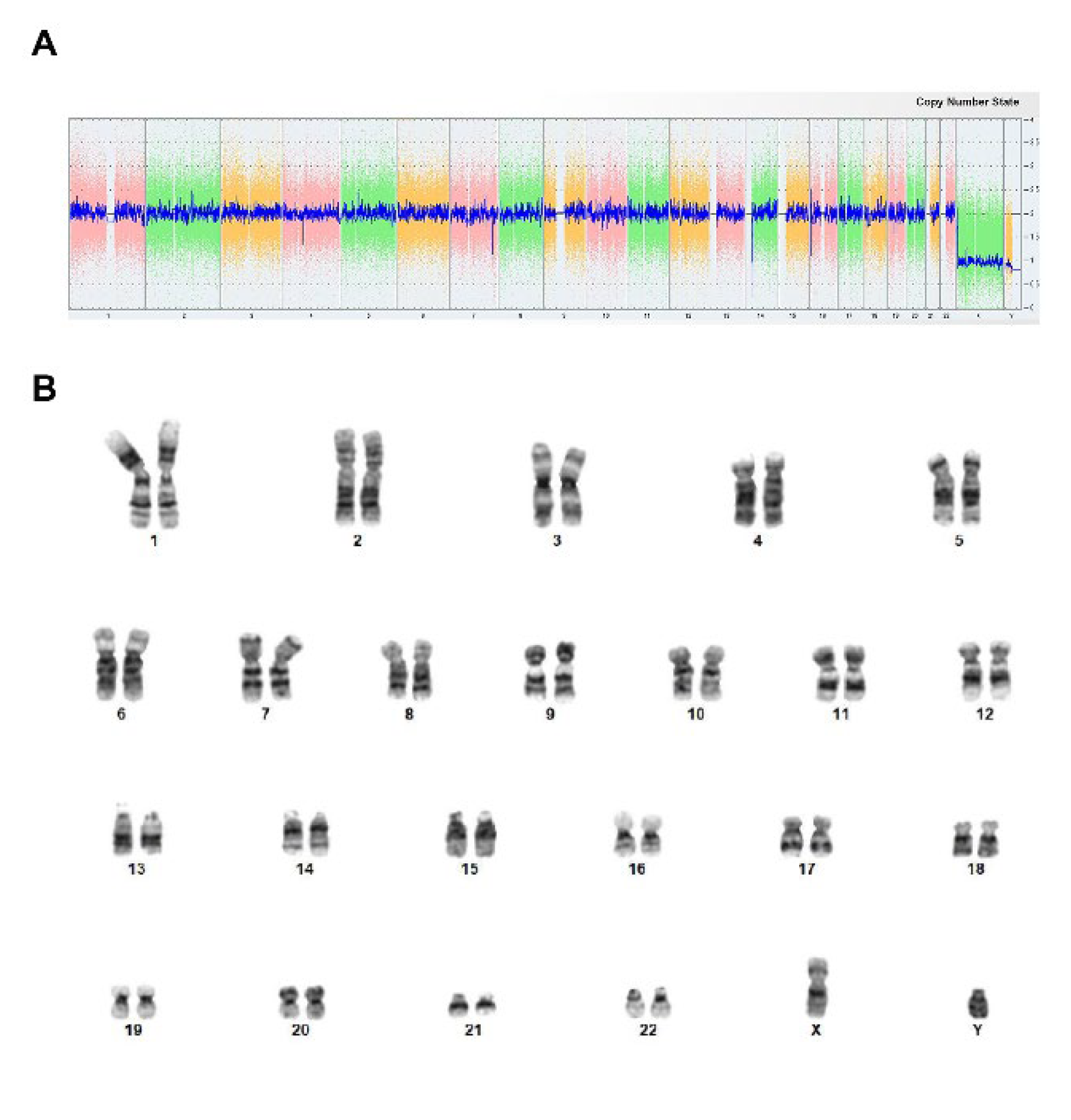
One of the goals of this immortalization method was to have very little alteration of the genome of T cells. With this minimal alteration, we expected that the hCD8
+ T-TERT cell line retained normal karyotype at high passages. The hCD8
+ T-TERT cells were analyzed by 2 different methods at passage 47. First, Karyostat
+ Karyotyping (microarray analysis, ThermoFisher Scientific) data showed that there were 23 pairs of chromosomes, and no obvious chromosome loss and gain was observed (
Figure 3A). Cytogenic karyotyping results also showed that at least 45% (9 out of 20) of the cells had normal 23 pairs of chromosomes and no obvious chromosome loss and gain (
Figure 3B) (Karyologic, NC). There were some small alterations observed such as deletion of chromosome q11.1 (5 out of 20), translocation of small part of chromosome 10 and 13 (2 out of 20) (data not shown). But chromosome alterations were not observed in Karyostat
+ analysis. This data indicated that our immortalization method largely retained normal karyotype as WT CD8
+ T cells.
2.4. HCD8+ T-TERT Cells Retained the Dependence of IL-2 and CD3/CD28 Activator for Survival and Expansion
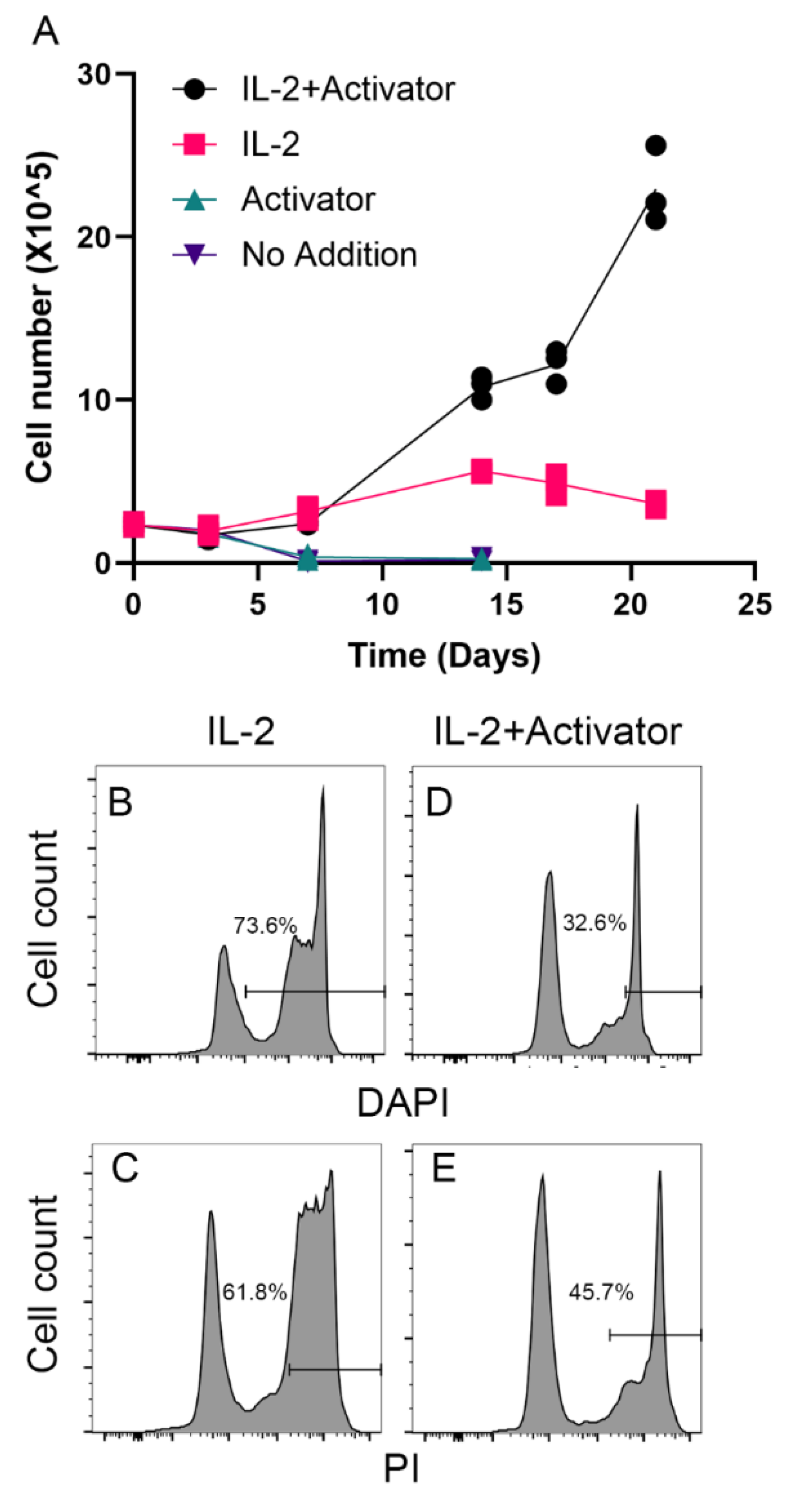
To determine whether the immortalized hCD8
+ T-TERT cell line retained the dependence of IL-2 and CD3/CD28 activator for survival and expansion as the parental cells, we cultured the immortalized T cells in medium with or without IL-2 and/or CD3/CD28 activator. As shown in
Figure 4A, the cells started to grow after thawed out from the cryopreserved vial in a week in the medium contained both IL-2 and CD3/CD28 activator. However, the cells only survived and expanded in the first few passages in expansion medium contains IL-2 but no CD3/CD28 activator (
Figure 4A). But in the medium without IL-2 (activator alone or no addition), the cells died in a week (
Figure 4A). We further confirmed that large proportion of the cells were dead detected as DAPI positive (73.6%,
Figure 4B) and PI positive (61.8%,
Figure 4C). The cells cultured with IL-2 and CD3/CD28 activator had much fewer dead cells (32.6% DAPI positive and 45.7% PI positive), which indicated that the immortalized cells retained the dependence of IL-2 and CD3/CD28 activator for
in vitro survival and expansion.
2.5. HCD8+ T-TERT Cells Retained Normal CD8+ T Cell Phenotypic Surface Markers
With normal karyotype revealed by 2 different karyotyping methods, we further investigated whether the hCD8
+ T-TERT cells had retained phenotypic surface markers expression as WT CD8
+ T cells. We first analyzed the T cell lineage surface markers, CD3, CD4, and CD8.
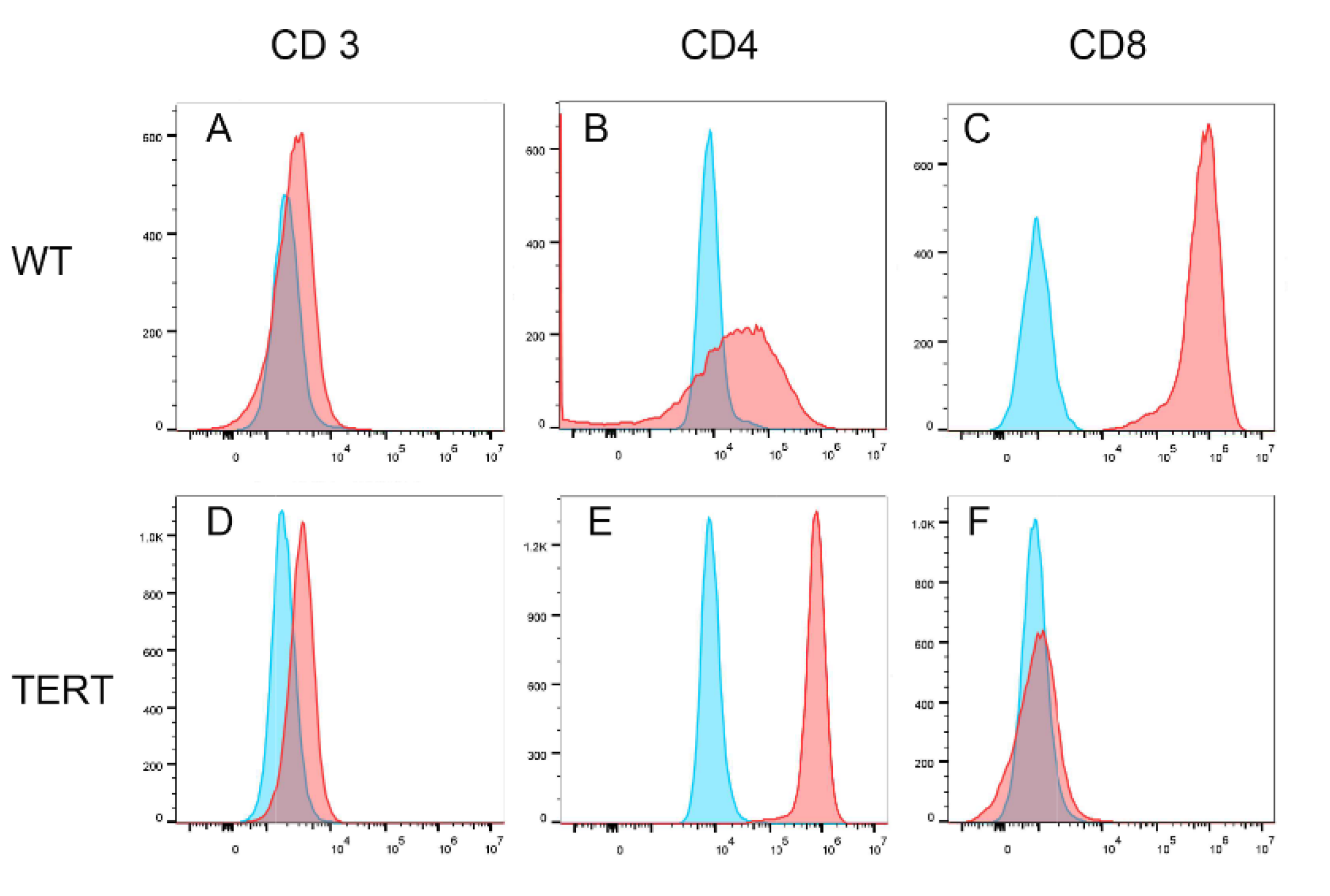
Figure 5 showed that the hCD8
+ T-TERT cells retained CD3 and CD4 surface marker expression at P50 as WT CD8
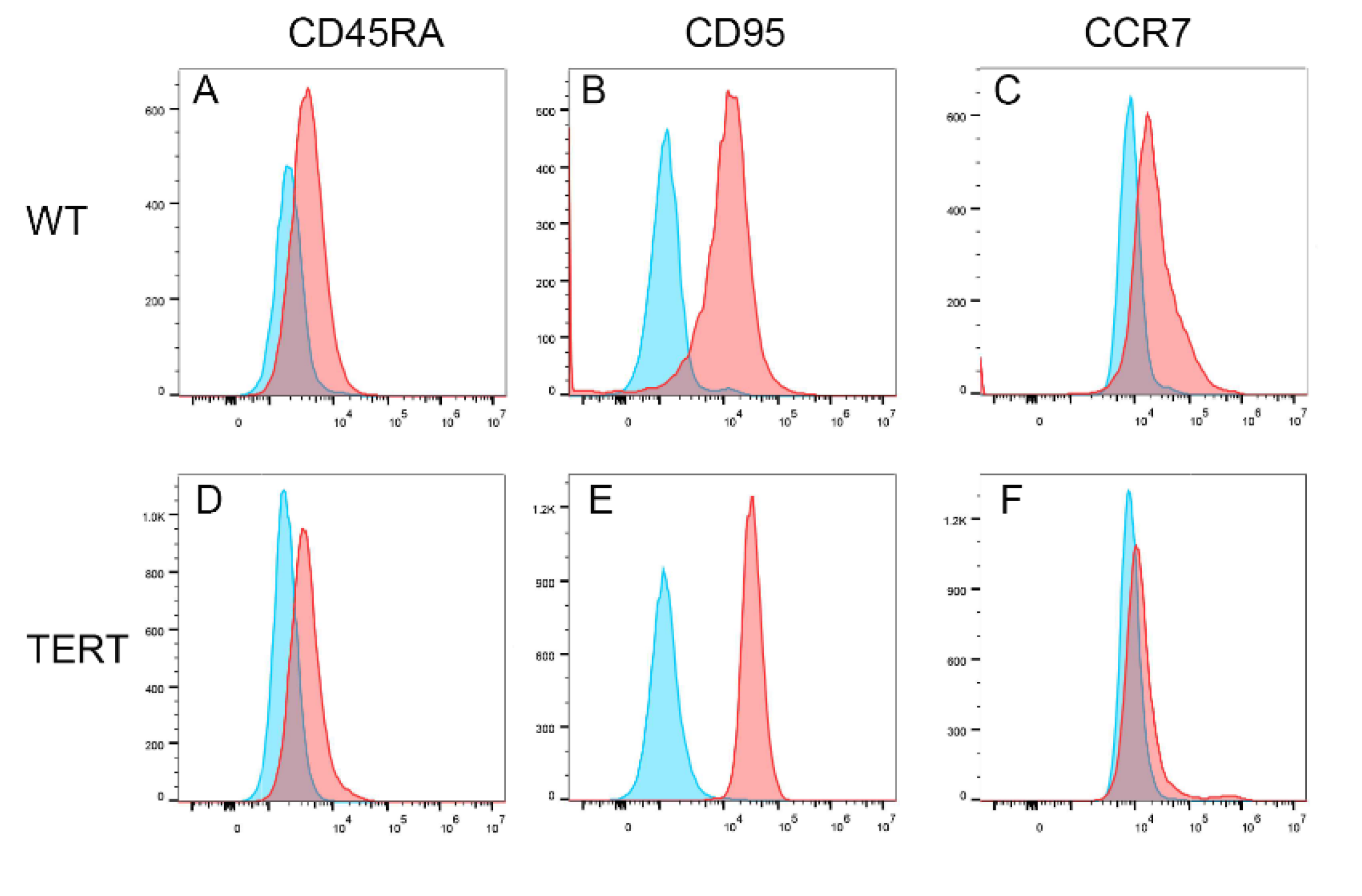
+ T cells, but lost CD8 surface marker expression. Differentiation markers such as CD45RA and CD95 were also expressed on the hCD8
+ T-TERT cell surface as WT T cells (
Figure 6A,B,D,E), but CCR7 expression level was lower on the hCD8
+ T-TERT cells than that of WT T cells (
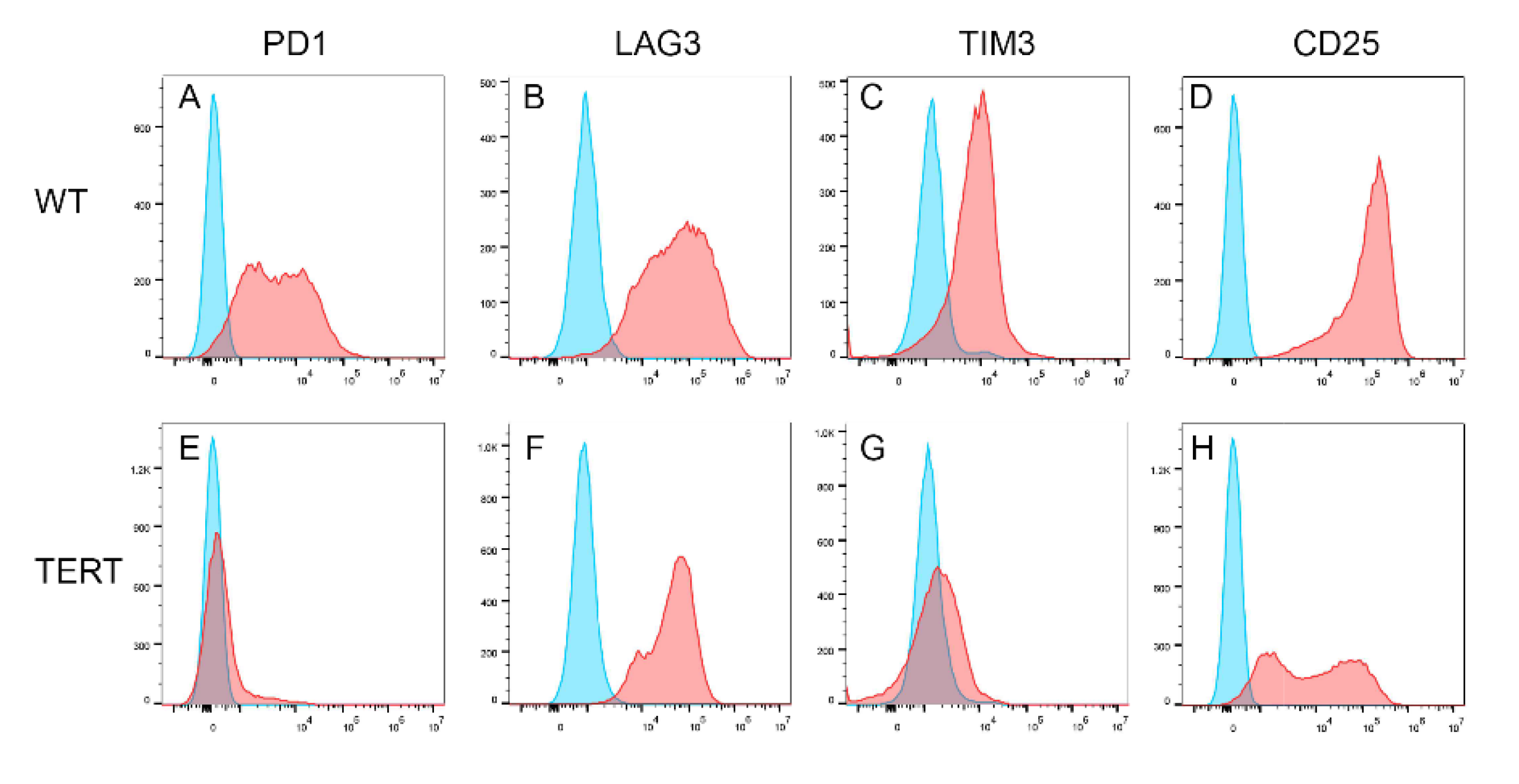
Figure 6C,F). Equivalent expression of T cell activation surface marker CD25 was observed on hCD8
+ T-TERT and WT CD8
+ T cell surface (
Figure 7D,H). Both hCD8
+ T-TERT and WT T cells had positive expression of exhaustion marker TIM3, LAG3 on the surface (
Figure 7B,C,F,G), but hCD8
+ T-TERT cells exhibited less PD1 expression than WT T cells (
Figure 7A,E). The presence of the cell surface markers detected by FACS was summarized in
Table 1.
3. Discussion
Immortalized T cells have broad applications in both basic research and industry. Jurkat cells has been working as T cell model for decades and generating extensive amount of useful knowledge. However, the limitation of Jurkat cells is now realized simply because they are cancer cells not normal T cells [
1]. There had been several T cell lines immortalized by using virus, such as leukemia virus or Herpesvirus [
14,
15,
16,
17]. But the virus immortalized, or SV40 large T antigen immortalized cells usually have oncogenic phenotype. Normal T cell lines can fill in the gap that Jurkat cell line was not able to, such as gene and cell therapies. To keep immortalized cell as normal as possible is very challenging, gain and/or deletion of chromosomes were often observed even in the TERT immortalized cell lines [
12,
18,
19,
20,
21]. Recently, Zhao and colleagues introduced a method of immortalization human primary epithelial cells with controlled TERT transgene copy number [
12]. The authors used CRISPR/Cas9 to introduce TERT transgene in the CDKN2A (p16) exon2 locus, which resulted in over expression of TERT and knockout CDKN2A (p16) at the same time. However, they used Lentivirus vector to deliver the Cas9 and sgRNA, which will integrate into the genome of the immortalized cells. To avoid CRISPR/Cas9 off target, and continuously making double stranded DNA breaks on and off targets, we used CRISPR/Cas9 protein and synthetic sgRNA in this study. To make more precise donor DNA recombination, we used traditional gene knockout strategy of have long 5’ and 3’ recombinant arms. We were able to immortalize human primary CD8
+ T cells with 500 copies of donor DNA/cell. The single copy of TERT transgene and knockout an allele of CDKN2A (p16) was sufficient to immortalize human CD8
+ T cells. Moreover, the immortalized T cells largely kept phenotypic surface markers. Although we did see some chromosome small alterations in cytogenic karyotyping, but the Karyostat
+ analysis did not detect these changes. In addition, the finding of the immortalized hCD8
+ T-TERT cells are still dependent on cytokines and antigen stimulation for proliferation, suggesting that these T cells were not transformed. We plan to obtain clonal cell line from this immortalized human CD8
+ T cell line for the further modified cell lines development, such as CAR-T mode cell lines. To our knowledge, this is the first that established a normal human T cell line with single TERT transgene insertion that will be highly useful to for T cell research studies.
4. Materials and Methods
4.1. CD8+ T Cells Isolation and Culture
Human primary peripheral blood mononuclear cells (PBMC) were purchased from ATCC (Cat# PCS-800-011). The cryopreserved PBMC were thawed in 37°C water bath. CD8+ T cells were isolated immediately from the PBMC by using EasySep Human CD8+ T Cell Isolation kit (StemCell Technologies, Cat# 17953) and following manufacture’s protocol. The purified CD8+ cells were then cultured in ImmunoCult-XF T cell Expansion Medium (StemCell Technologies, Cat# 10981) supplied with ImmunoCult Human CD3/CD28 T Cell Activator (StemCell Technologies, Cat# 10991) and 100 ng/mL of human recombinant IL-2 (R&D Systems, Cat# BT-002-100) at 37°C, 5% CO2.
4.2. Immortalization Donor DNA Design and Single Guide RNA
Our initial experiments utilized the strategy of immortalizing human primary T cells, as previously reported by replace CDKN2 gene with human TERT transgene. To ensure the precise recombination occur at the designed locus, we also adapted the traditional knockout strategy of using 5’ and 3’ recombinant arms. As shown in Fig.1A, the donor DNA contained 4.8 Kb 5’ recombinant arm from upstream of CDKN2A gene exon 2 (ends at Chr9:21974421). The donor DNA also contained EF1α promoter driven TERT gene followed by internal ribosomal entry site (IRES) and Zeocin resistant gene. We designed the 2.1 Kb 3’ recombinant arm starts from Chr9:21969794 (intron 2) to make the replaced sequence (4.5Kb) similar size to the transgene (4.6Kb). At the end of the 3’ recombinant arm, we added a CMV promoter driven Herpes simplex virus thymidine kinase (HSV-Tk) gene for negative selection of random inserted cells. The tradition knockout homologous recombination efficiency was very low for the initial experiments. The second set of experiments used CRISPR/Cas9 to make double strand DNA break to increase the recombinant efficiency. We also want to avoid the uncontrolled insertion of Cas9 expressing sequence, we used Cas9 protein (Integrated DNA Technologies, Cat# 1081058) instead of Cas9 expressing vectors (to reduce off target cleavage effects). Single guide RNA (sgRNA) sequence was designed on Integrated DNA Technologies website and ordered from Integrated DNA Technologies. The sequence of sgRNA was mC*mA*mG*rArUrGrArUrGrCrCrArCrGrCrArCrArArUrGrUrUrUrUrArGrArGrCrUrArGrArArArUrArGrCrArArGrUrUrArArArArUrArArGrGrCrUrArGrUrCrCrGrUrUrArUrCrArArCrUrUrGrArArArArArGrUrGrGrCrArCrCrGrArGrUrCrGrGrUrGrCmU*mU*mU*rU (Phosphorothioated 2’-O-methyl RNA bases are entered as ‘m_*’).
4.3. CD8+ T Cell Immortalization
The CD8+ T cells were electroporated with Neon Transfection System (Life Technologies) by following manufacture’s protocol. Briefly, after the purified CD8+ T cells were cultured in expansion medium for 2-3 days, the cells were spin down at 250 × g for 5 min at room temperature and washed once with PBS without Ca2+ and Mg2+. The cells were then resuspended in Buffer R at the concentration of 3 × 106/mL. In a microcentrifuge tube, 100 μL of cells were mixed with 0.7 μL of prepared RNP (mixture of Cas9 protein and guide RNA) and 500 copies/cell donor DNA. The mixture of cells and RNP was loaded into the electroporation tip and electroporated at 1600v 10ms for 3 pulses. The cells were then cultured in 0.5 mL T cell expansion medium in 24-well plate at 37 °C, 5% CO2 for 2 days. The cells were passaged and further cultured in T cell expansion medium supplied with 5 μg/mL Zeocin for selection of resistant cells. Genomic DNA was extracted from the cells at the indicated passage for charactering the copy number of transgene and CDKN2A (p16) gene.
4.4. Genomic DNA Extraction and PCR
Genomic DNA was extracted from the immortalized cells by using Zymo Quick-DNA kit (Zymo Research, Cat# D3025) and following manufacture’s protocol. Around 1 ng of genomic DNA was used as template for amplifying recombinant CDKN2A (p16) allele in a polymerase chain reaction (PCR) by using PCR kit (Takara Bio, Cat# R050A). The sequence of the primers was listed in
Table 1. The amplification of PCR products was visualized in a FlashGel (Lonza, Cat# 57023).
4.5. Total RNA Isolation
One million of log growth phase immortalized CD8+ T cells and WT CD8+ T cells were aliquoted into a microcentrifuge tube and spun down at 300 × g for 5 min at room temperature. The supernatant was removed from the cells. RNA samples were isolated from the cells by using Qiagen RNeasy kit (Qiagen, Cat# 74104) and following the kit protocol.
4.6. Droplet Digital PCR
Copy numbers of the CDKN2A (p16) gene and h
TERT transgene were analyzed by droplet digital PCR. Ten to forty nanograms of genomic DNA was used in each reaction. Bio-Rad ddPCR Supermix for Probes (no dUTP) was purchased from Bio-Rad (Cat# 186-3024). Primers and probes were designed at NIST and purchased from ThermoFisher Scientific, and the sequences were listed in
Table 2. Droplets were generated by using Bio-Rad droplet generator (Bio-Rad). After PCR amplification, droplets were analyzed on Bio-Rad QX200 droplet reader. For relative mRNA expression, ddPCR was performed using 1-Step RT-ddPCR Advanced Kit for Probes (Bio-Rad, Cat# 1864022) and total RNA as template. GAPDH (ThermoFisher, Cat# 402869) was used as internal control.
4.7. Flow Cytometry
T cell surface markers were analyzed by fluorescent activated cell sorting (FACS) analysis. Immortalized CD8+ T cells after 45 passages and WT CD8+ T cells within 6 passages were spined down at 300 × g for 5 min at room temperature and washed once with FACS buffer (PBS supplied with 10% FBS). The cells were resuspended in antibodies cocktails mixed in FACS buffer. The conjugated antibodies were all purchased from BioLegend, CD3-pacific blue (cat# 300329), CD4-AlexaFluo-488 (Cat# 300519), CD8-PE (Cat# 344705), CD25-AlexaFluo-647 (Cat# 302617), CD45RA-Pacificblue (Cat# 304117), CCR7-AlexaFluo-488 (Cat#353205), CD95-PerCP-Cy5.5 (Cat# 305629), PD-1-AlexaFluo-647 (Cat# 143721), TIM-3-PerCP-Cy5.5 (Cat# 345015), and LAG-3-PE (Cat# 369305). The cells were incubated with antibodies cocktail on ice for 1 hr, and then washed with 1 mL FACS buffer. The cells were subjected to Cytoflex flow cytometer after resuspending the cells in 300 μL FACS buffer. For dead cell analysis, the cells were spun down and washed with FACS buffer and resuspended in 300 μL FACS buffer contained 1 μg/mL of 4’,6’-diamidino-2-phenylindole (DAPI) and propidine iodide (PI). The staining of DAPI and PI was analyzed on Cytoflex. The FACS data was analyzed on FlowJo software (FlowJo, LLC). Each cell sample cultured in different medium condition without DAPI or PI staining was used for DAPI and PI negative gating.
Abbreviations: WT wild type; hTERT human telomerase reverse transcriptase; IRES internal ribosomal entry site; ddPCR droplet digital polymerase chain reaction.
Consent for publication
The National Institute of Standards and Technology Research Protections Office reviewed the protocol for this project and determined it is “not human subjects research” as defined in 15CFR27, the Common Rule for the Protection of Human Subjects.
Disclaimers
Certain commercial equipment, instruments, and materials are identified to specify the experimental procedure. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment are necessarily the best available for the purpose.
Funding
This work was supported by NIST intramural fund.
Author contributions
Conceptualization, Z.H., K.C. and H-J. H.; methodology, Z.H. and H-J. H.; data collection and analysis, Z.H. and H-J.H.; writing and editing, Z.H., K.C. and H-J. H.; All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We thank Jerilyn Izac and Megan Cleaveland for the critical reading and suggestions for the manuscript.
Conflicts of Interest
The authors have no conflicts of interest.
References
- Gioia, L.; et al. , A genome-wide survey of mutations in the Jurkat cell line. BMC Genomics 2018, 19, 334. [Google Scholar] [CrossRef] [PubMed]
- Robbins, J.R.; et al. , Immortalized human adult articular chondrocytes maintain cartilage-specific phenotype and responses to interleukin-1beta. Arthritis Rheum 2000, 43, 2189–2201. [Google Scholar] [CrossRef] [PubMed]
- DeCaprio, J.A.; et al. , SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell 1988, 54, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, D., M. T. Saenz-Robles, and J.M. Pipas, SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene 2005, 24, 7729–7745. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; et al. , Improvement of a method to reproducibly immortalize human T cells by oncogene transfection. Cytotechnology, 2000. 33, P. 71-81.
- Fahad, A.S.; et al. , Immortalization and functional screening of natively paired human T cell receptor repertoires. Protein Eng Des Sel, 2022; 35. [Google Scholar]
- Groux, H.; et al. , Isolation and characterization of transformed human T-cell lines infected by Epstein-Barr virus. Blood 1997, 89, 4521–4530. [Google Scholar] [CrossRef] [PubMed]
- Toouli, C.D.; et al. , Comparison of human mammary epithelial cells immortalized by simian virus 40 T-Antigen or by the telomerase catalytic subunit. Oncogene 2002, 21, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; et al. , Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol Cancer Res 2003, 1, 729–738. [Google Scholar] [PubMed]
- Thi, M.M.; et al. , Characterization of hTERT-immortalized osteoblast cell lines generated from wild-type and connexin43-null mouse calvaria. Am J Physiol Cell Physiol 2010, 299, C994–C1006. [Google Scholar] [CrossRef] [PubMed]
- Hooijberg, E.; et al. , Immortalization of human CD8+ T cell clones by ectopic expression of telomerase reverse transcriptase. J Immunol 2000, 165, 4239–4245. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; et al. , Immortalization of human primary prostate epithelial cells via CRISPR inactivation of the CDKN2A locus and expression of telomerase. Prostate Cancer Prostatic Dis 2021, 24, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.W.; et al. , Comparison of early passage, senescent and hTERT immortalized endothelial cells. Exp Cell Res 2005, 309, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, R.; et al. , Role of human T-cell leukemia virus type 1 X region proteins in immortalization of primary human lymphocytes in culture. J Virol 1992, 66, 4570–4575. [Google Scholar] [CrossRef] [PubMed]
- Meinl, E.; et al. , Immortalization of human T cells by Herpesvirus saimiri. Immunol Today 1995, 16, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Troidl, B.; et al. , Karyotypic characterization of human T-cell lines immortalized by Herpesvirus saimiri. Int J Cancer 1994, 56, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Robek, M.D. and L. Ratner, Immortalization of CD4(+) and CD8(+) T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J Virol 1999, 73, 4856–4865. [Google Scholar] [CrossRef] [PubMed]
- Farwell, D.G.; et al. , Genetic and epigenetic changes in human epithelial cells immortalized by telomerase. Am J Pathol 2000, 156, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Varella-Garcia, M.; et al. , Karyotypic characteristics of human uterine leiomyoma and myometrial cell lines following telomerase induction. Cancer Genet Cytogenet 2006, 170, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; et al. , Molecular and cytogenetic changes involved in the immortalization of nasopharyngeal epithelial cells by telomerase. Int J Cancer 2006, 119, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, M.M.; et al. , The establishment of telomerase-immortalized cell lines representing human chromosome instability syndromes. Hum Mol Genet 2000, 9, 403–411. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Immortalization of human primary CD8+ T cells. A, schematic view of donor DNA design and knockout p16 exon 2 after recombination. B, growth of immortalized CD8+T-TERT cells. C, copy numbers of p16 exon2, exon 1, TERT transgene and IRES in the immortalized CD8+T-TERT cells. D, recombinant p16 allele was detected in CD8+T-TERT cells by 2 sets of PCR primers, but not in the WT CD8+ cells. E, PCR detected p16 WT and KO alleles in CD8+T-TERT cells but only detected p16 WT allele in WT CD8+ cells.
Figure 1.
Immortalization of human primary CD8+ T cells. A, schematic view of donor DNA design and knockout p16 exon 2 after recombination. B, growth of immortalized CD8+T-TERT cells. C, copy numbers of p16 exon2, exon 1, TERT transgene and IRES in the immortalized CD8+T-TERT cells. D, recombinant p16 allele was detected in CD8+T-TERT cells by 2 sets of PCR primers, but not in the WT CD8+ cells. E, PCR detected p16 WT and KO alleles in CD8+T-TERT cells but only detected p16 WT allele in WT CD8+ cells.
Figure 2.
Relative p16 and TERT mRNA expression in hCD8+T-TERT cells. HCD8+T-TERT cells express higher level of TERT and equivalent level of p16 mRNA compared to WT T cells.
Figure 2.
Relative p16 and TERT mRNA expression in hCD8+T-TERT cells. HCD8+T-TERT cells express higher level of TERT and equivalent level of p16 mRNA compared to WT T cells.
Figure 3.
Karyotype of hCD8+T-TERT cells. A, Karyostat+ showed normal Karyotype of hCD8+T-TERT cells. B, Cytogenic karyotype showed 45% of hCD8+T-TERT cells had normal karyotype.
Figure 3.
Karyotype of hCD8+T-TERT cells. A, Karyostat+ showed normal Karyotype of hCD8+T-TERT cells. B, Cytogenic karyotype showed 45% of hCD8+T-TERT cells had normal karyotype.
Figure 4.
Immortalized T cells retained dependence of IL-2 and CD3/CD28 activator. A, hCD8+ T-TERT cells growth in the media contained IL-2 + CD3/CD28 activator, IL-2, activator, or no addition. B, FACS plot showed the percentage of DAPI positive cells cultured in medium contained IL-2. C, FACS plot showed the percentage of PI positive cells cultured in medium contained IL-2. D, FACS plot showed the percentage of DAPI positive cells cultured in medium contained IL-2 and CD3/CD28 activator. E, FACS plot showed the percentage of PI positive cells cultured in medium contained IL-2 and CD3/CD28 activator.
Figure 4.
Immortalized T cells retained dependence of IL-2 and CD3/CD28 activator. A, hCD8+ T-TERT cells growth in the media contained IL-2 + CD3/CD28 activator, IL-2, activator, or no addition. B, FACS plot showed the percentage of DAPI positive cells cultured in medium contained IL-2. C, FACS plot showed the percentage of PI positive cells cultured in medium contained IL-2. D, FACS plot showed the percentage of DAPI positive cells cultured in medium contained IL-2 and CD3/CD28 activator. E, FACS plot showed the percentage of PI positive cells cultured in medium contained IL-2 and CD3/CD28 activator.
Figure 5.
T cell surface markers expression. A-B, CD3 and CD4 were expressed on portion of WT CD8+ T cells. C, CD8 was highly expressed on WT CD8+ T cells. D, CD3 was expressed on portion of hCD8+T-TERT cells. E, CD4 was highly expressed on all hCD8+T-TERT cells. F. CD8 was down regulated on hCD8+T-TERT cells surface.
Figure 5.
T cell surface markers expression. A-B, CD3 and CD4 were expressed on portion of WT CD8+ T cells. C, CD8 was highly expressed on WT CD8+ T cells. D, CD3 was expressed on portion of hCD8+T-TERT cells. E, CD4 was highly expressed on all hCD8+T-TERT cells. F. CD8 was down regulated on hCD8+T-TERT cells surface.
Figure 6.
T cell differentiation markers expression. A-F, CD45RA, CD95, and CCR7 all were expressed on both WT CD8+ T cells and hCD8+T-TERT cells.
Figure 6.
T cell differentiation markers expression. A-F, CD45RA, CD95, and CCR7 all were expressed on both WT CD8+ T cells and hCD8+T-TERT cells.
Figure 7.
T cell exhaustion and activation surface markers expression. A-C, most of the WT CD8+ T cells express PD1, LAG3, and TIM3 at P4. D, T cell activation marker was expressed on all WT CD8+ T cells. E, PD1 surface expression was down regulated on hCD8+T-TERT cells. F, LAG3 expression on hCD8+T-TERT cells was equivalent to WT CD8+ T cells. G, TIM3 expression was down regulated on hCD8+T-TERT cells. H, T cell activation marker CD25 was expressed on all the hCD8+T-TERT cells.
Figure 7.
T cell exhaustion and activation surface markers expression. A-C, most of the WT CD8+ T cells express PD1, LAG3, and TIM3 at P4. D, T cell activation marker was expressed on all WT CD8+ T cells. E, PD1 surface expression was down regulated on hCD8+T-TERT cells. F, LAG3 expression on hCD8+T-TERT cells was equivalent to WT CD8+ T cells. G, TIM3 expression was down regulated on hCD8+T-TERT cells. H, T cell activation marker CD25 was expressed on all the hCD8+T-TERT cells.
Table 1.
Summary of cell surface markers by FACS.
Table 1.
Summary of cell surface markers by FACS.
| Antigen |
WT |
T-TERT |
| CD3 |
+ |
+ |
| CD4 |
+ |
++ |
| CD8 |
+++ |
- |
| CD45RA |
+ |
+ |
| CD95 |
++ |
++ |
| CCR7 |
+/- |
+/- |
| PD1 |
+ |
+/- |
| LAG3 |
++ |
++ |
| TIM3 |
+ |
+/- |
| CD25 |
+++ |
+++/+ |
Table 2.
Primer and probe sequence.
Table 2.
Primer and probe sequence.
| Gene |
Forward primer |
Reverse primer |
Probe |
| Recombinant p16 |
CCATGACCGAGATCGGCGAG
|
GTGTCAGAAACGATGCTGTCTTC
|
N/A |
| Recombinant p16-2 |
CCGAGGAGCAGGACTGAATCGAA |
ACAACAGTGTCAGAAACGATGC |
N/A |
| WT p16 |
GTCTGCTGAAACTGCCAACA |
GAGGGGCCGAGTAAAGAAGA |
N/A |
| KO p16 |
GACTTCGTGGAGGACGACTT
|
|
N/A |
| TERT |
AGCCACGTCTCTACCTTGAC |
CTCATTCAGGGAGGAGCTCT |
GCCGTACATGCGACAGTTC |
| P16 exon2 |
CTTCCTGGACACGCTGGT |
CAGGTACCGTGCGACATC |
CTGAGGAGCTGGGCCATC |
| P16 exon1 |
GGGGAGTTTTCAGAAGGGGT |
TGGCTCCTCATTCCTCTTCC |
AATCACAGACCTCCTCCTGG |
| IRES |
CTTGGAATAAGGCCGGTGTG |
AAGAAGACAGGGCCAGGTTT |
TCTTTTGGCAATGTGAGGGC |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).