Submitted:
28 August 2024
Posted:
29 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Fungal Metabarcoding
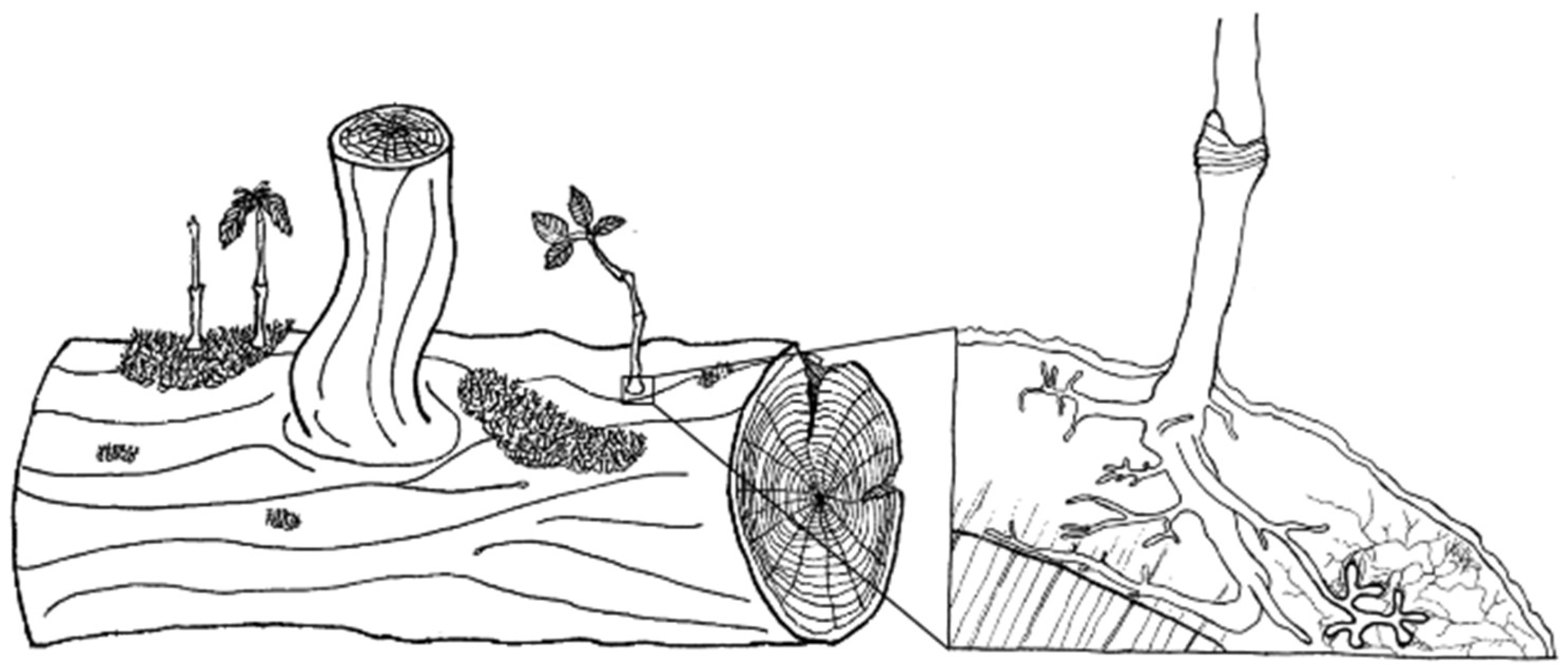
1.2. Case-Study: Deadwood Bark Ectomycorrhizae
2. Materials and Methods
2.1. Study Site and Log Selection
2.2. Root Tip Morphotypification and Sanger Barcoding
2.3. Nanopore Metabarcoding
2.3.1. Mock Community
2.3.2. Bark Sampling
2.3.3. Wet Lab
2.4. Bioinformatics
2.4.1. Reference Database
2.4.3. SH-Approach
2.4.4. OTU-Approach
2.4.5. Minimum Quality Evaluation
2.4.6. Bark Metabarcoding
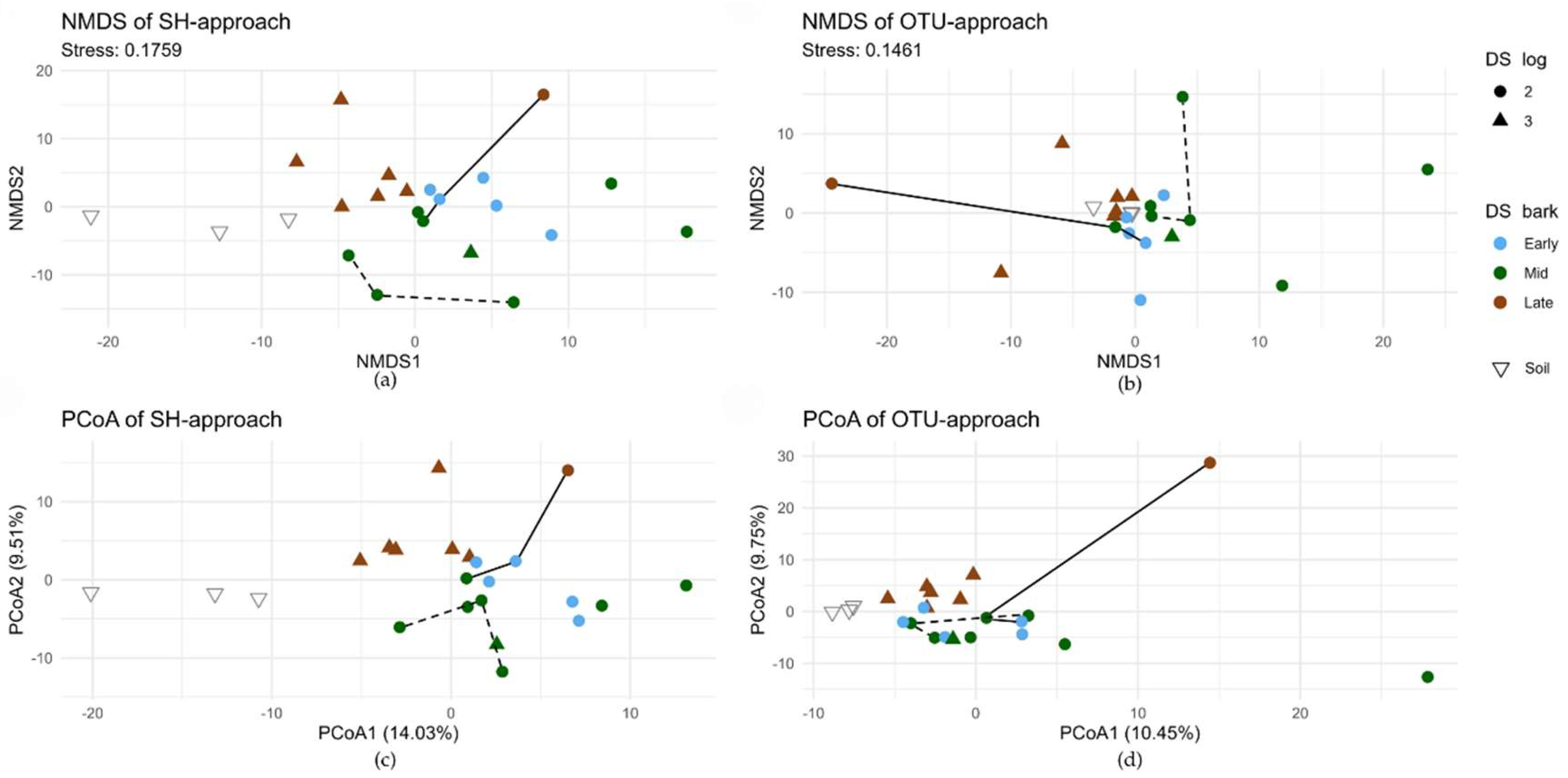
3. Results
3.1. Morphotyping
3.2. Metabarcoding
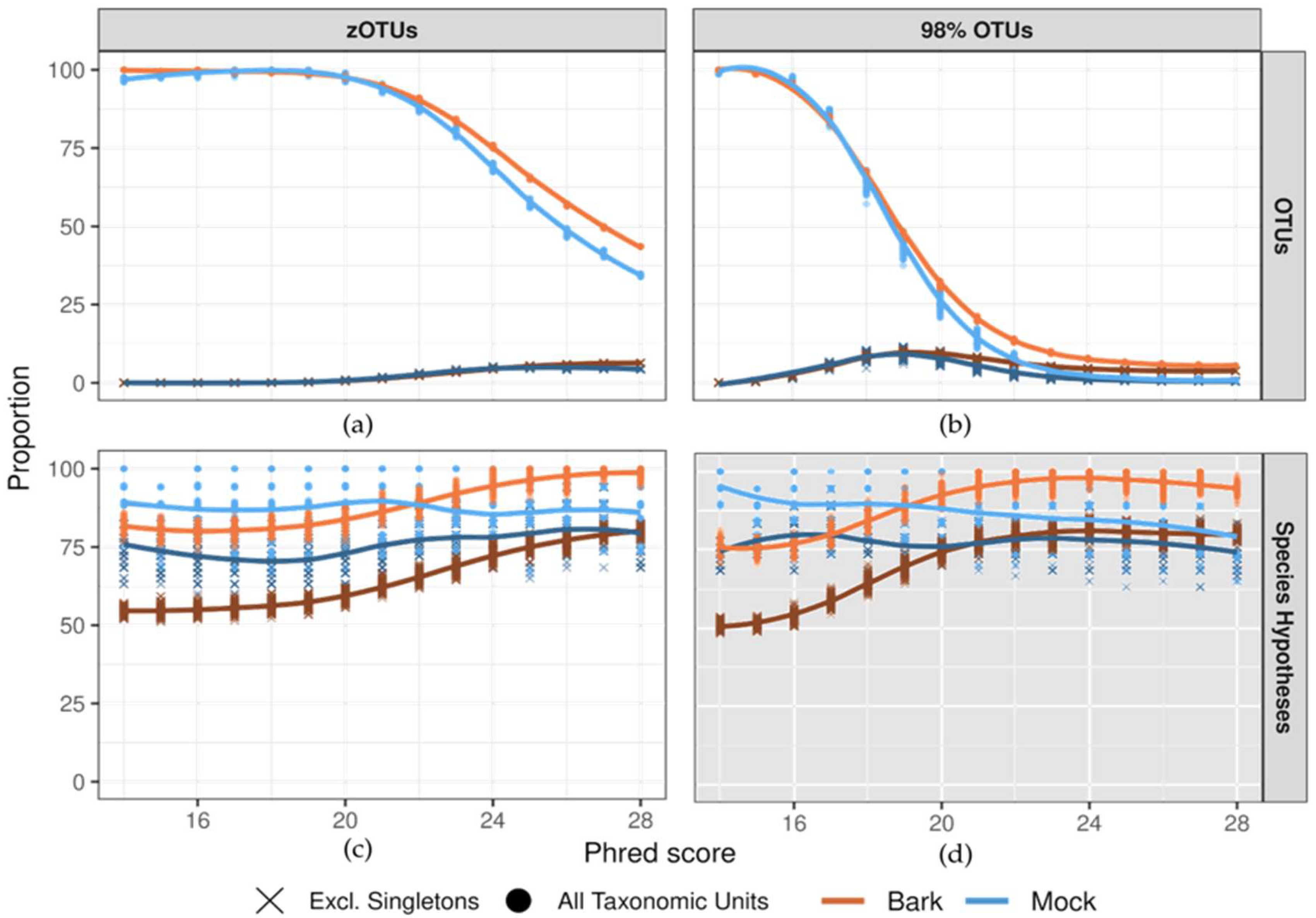
3.2.1. Minimum Quality Evaluation
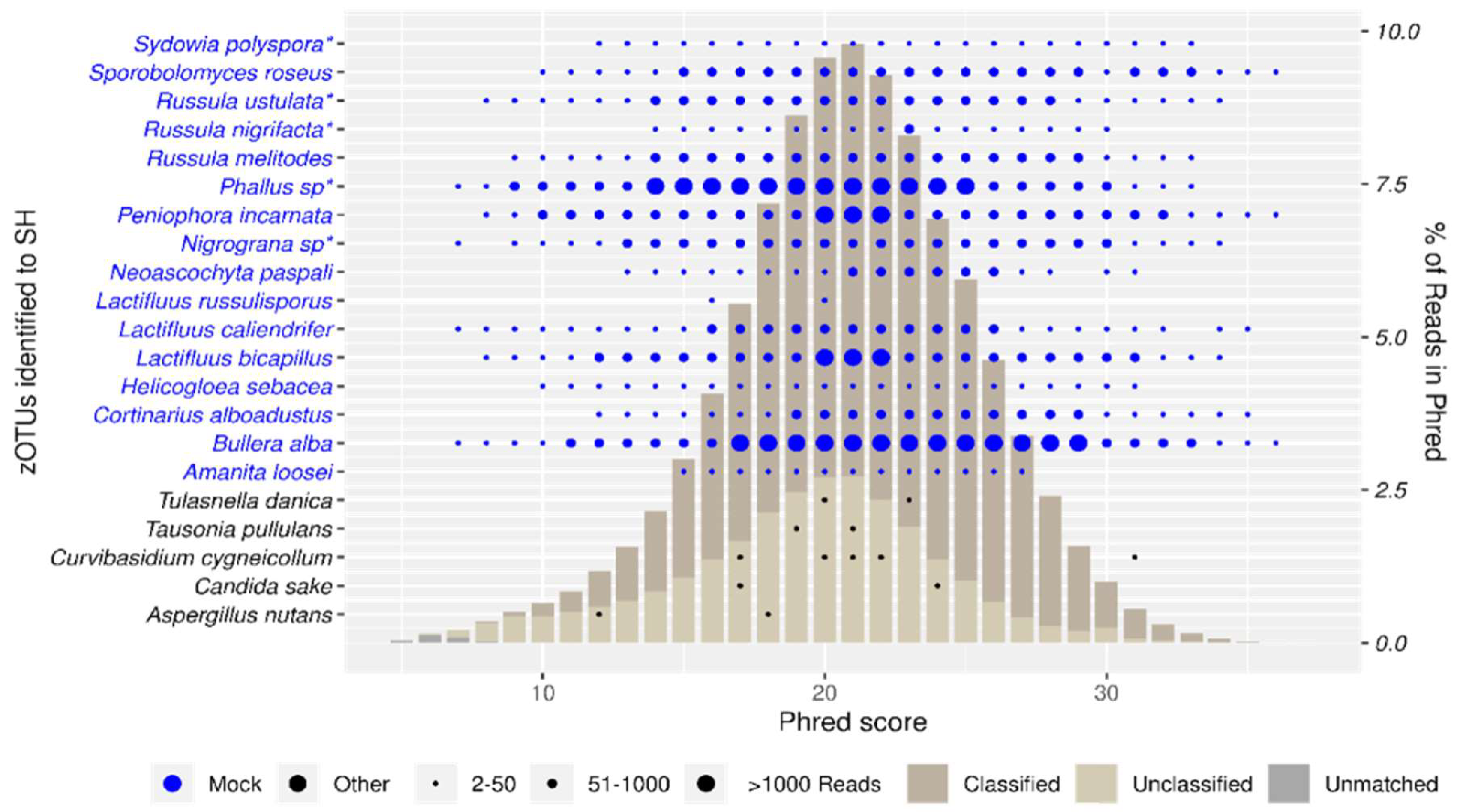
3.2.2. Mock Community
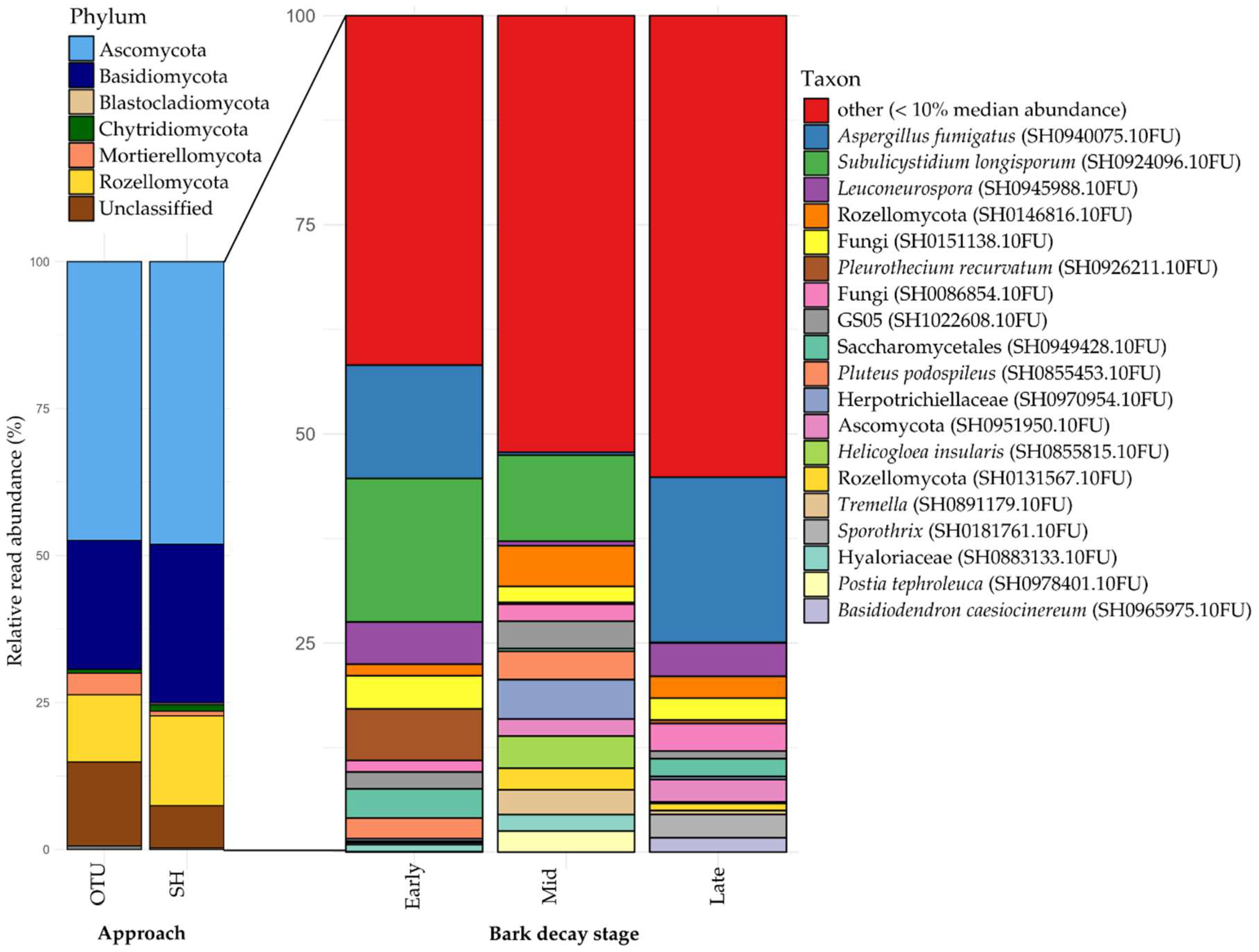
3.2.2. Bark Substrate Community
3.2.2. Mycorrhizae in Decaying Beech Inner Bark
4. Discussion
4.1. Minimum Quality Evaluation
4.2. Mock Community
4.3. Inner Bark Community
Ectomycorrhizal Colonization of Beech Deadwood
Database
Nanopore Metabarcoding
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tedersoo, L.; Bahram, M.; Zinger, L.; Nilsson, R.H.; Kennedy, P.G.; Yang, T.; Anslan, S.; Mikryukov, V. Best Practices in Metabarcoding of Fungi: From Experimental Design to Results. Molecular Ecology 2022, 31, 2769–2795. [CrossRef]
- Nilsson, R.H.; Tedersoo, L.; Ryberg, M.; Kristiansson, E.; Hartmann, M.; Unterseher, M.; Porter, T.M.; Bengtsson-Palme, J.; Walker, D.M.; De Sousa, F.; et al. A Comprehensive, Automatically Updated Fungal ITS Sequence Dataset for Reference-Based Chimera Control in Environmental Sequencing Efforts. Microbes and environments 2015, 30, 145–150. [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Bolchacova, E.; Voigt, K.; Crous, P.W.; et al. Nuclear Ribosomal Internal Transcribed Spacer (ITS) Region as a Universal DNA Barcode Marker for Fungi. Proceedings of the National Academy of Sciences 2012, 109, 6241–6246. [CrossRef]
- Oxford Nanopore Technologies Available online: https://nanoporetech.com/platform/accuracy/simplex (accessed on 4 January 2024).
- Wilson, A.W.; Eberhardt, U.; Nguyen, N.; Noffsinger, C.R.; Swenie, R.A.; Loucks, J.L.; Perry, B.A.; Herrera, M.; Osmundson, T.W.; DeLong-Duhon, S.; et al. Does One Size Fit All? Variations in the DNA Barcode Gaps of Macrofungal Genera. Journal of Fungi 2023, 9, 788. [CrossRef]
- Davidov, K.; Iankelevich-Kounio, E.; Yakovenko, I.; Koucherov, Y.; Rubin-Blum, M.; Oren, M. Identification of Plastic-Associated Species in the Mediterranean Sea Using DNA Metabarcoding with Nanopore MinION. Sci Rep 2020, 10, 17533. [CrossRef]
- Loit Kaire; Adamson Kalev; Bahram Mohammad; Puusepp Rasmus; Anslan Sten; Kiiker Riinu; Drenkhan Rein; Tedersoo Leho Relative Performance of MinION (Oxford Nanopore Technologies) versus Sequel (Pacific Biosciences) Third-Generation Sequencing Instruments in Identification of Agricultural and Forest Fungal Pathogens. Applied and Environmental Microbiology 2019, 85, e01368-19. [CrossRef]
- Theologidis, I.; Karamitros, T.; Vichou, A.-E.; Kizis, D. Nanopore-Sequencing Metabarcoding for Identification of Phytopathogenic and Endophytic Fungi in Olive (Olea Europaea) Twigs. Journal of Fungi 2023, 9, 1119. [CrossRef]
- Langsiri, N.; Worasilchai, N.; Irinyi, L.; Jenjaroenpun, P.; Wongsurawat, T.; Luangsa-ard, J.J.; Meyer, W.; Chindamporn, A. Targeted Sequencing Analysis Pipeline for Species Identification of Human Pathogenic Fungi Using Long-Read Nanopore Sequencing. IMA Fungus 2023, 14, 18. [CrossRef]
- Groben, G.; Clarke, B.B.; Kerkhof, L.J.; Bonos, S.A.; Meyer, W.A.; Qu, Y.; Luo, J.; Walsh, E.; Zhang, N. Mycobiome Analysis of Tall Fescue Grass Under Drought Stress Using the Illumina MiSeq and Oxford Nanopore Technology MinION. Phytobiomes Journal 2023, 7, 413–423. [CrossRef]
- Lysenko, L.; Griem, E.; Wagener, P.; Langer, E.J. Fungi Associated with Fine Roots of Fraxinus Excelsior Affected by Ash Dieback Detected by Next-Generation Sequencing. J Plant Dis Prot 2024. [CrossRef]
- Oosterbroek, S.; Doorenspleet, K.; Nijland, R.; Jansen, L. Decona: From Demultiplexing to Consensus for Nanopore Amplicon Data. ACA 2021, 4, e65029. [CrossRef]
- Maestri, S.; Cosentino, E.; Paterno, M.; Freitag, H.; Garces, J.M.; Marcolungo, L.; Alfano, M.; Njunjić, I.; Schilthuizen, M.; Slik, F.; et al. A Rapid and Accurate MinION-Based Workflow for Tracking Species Biodiversity in the Field. Genes 2019, 10, 468. [CrossRef]
- Stock, W.; Rousseau, C.; Dierickx, G.; D’hondt, S.; Martínez, L.A.; Dittami, S.M.; Loos, L. van der; Clerck, O.D. Breaking Free from References: A Consensus-Based Approach for Community Profiling with Long Amplicon Nanopore Data 2024, 2024.07.04.602031. [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010.
- Christy, E.J.; Sollins, P.; Trappe, J.M. First-Year Survival of Tsuga Heterophylla without Mycorrhizae and Subsequent Ectomycorrhizal Development on Decaying Logs and Mineral Soil. Can. J. Bot. 1982, 60, 1601–1605. [CrossRef]
- Baldrian, P.; Zrůstová, P.; Tláskal, V.; Davidová, A.; Merhautová, V.; Vrška, T. Fungi Associated with Decomposing Deadwood in a Natural Beech-Dominated Forest. Fungal Ecology 2016, 23, 109–122. [CrossRef]
- Kubartová, A.; Ottosson, E.; Dahlberg, A.; Stenlid, J. Patterns of Fungal Communities among and within Decaying Logs, Revealed by 454 Sequencing. Molecular Ecology 2012, 21, 4514–4532. [CrossRef]
- Rajala, T.; Peltoniemi, M.; Hantula, J.; Mäkipää, R.; Pennanen, T. RNA Reveals a Succession of Active Fungi during the Decay of Norway Spruce Logs. Fungal Ecology 2011, 4, 437–448. [CrossRef]
- Mäkipää, R.; Rajala, T.; Schigel, D.; Rinne, K.T.; Pennanen, T.; Abrego, N.; Ovaskainen, O. Interactions between Soil- and Dead Wood-Inhabiting Fungal Communities during the Decay of Norway Spruce Logs. ISME J 2017, 11, 1964–1974. [CrossRef]
- Rajala, T.; Tuomivirta, T.; Pennanen, T.; Mäkipää, R. Habitat Models of Wood-Inhabiting Fungi along a Decay Gradient of Norway Spruce Logs. Fungal Ecology 2015, 18, 48–55. [CrossRef]
- Bödeker, I.T.M.; Nygren, C.M.R.; Taylor, A.F.S.; Olson, A.; Lindahl, B.D. ClassII Peroxidase-Encoding Genes Are Present in a Phylogenetically Wide Range of Ectomycorrhizal Fungi. ISME J 2009, 3, 1387–1395. [CrossRef]
- Lindahl, B.D.; Tunlid, A. Ectomycorrhizal Fungi–Potential Organic Matter Decomposers, yet Not Saprotrophs. New Phytologist 2015, 205, 1443–1447. [CrossRef]
- Lindahl, B.; Stenlid, J.; Olsson, S.; Finlay, R. Translocation of 32P between Interacting Mycelia of a Wood-Decomposing Fungus and Ectomycorrhizal Fungi in Microcosm Systems. The New Phytologist 1999, 144, 183–193. [CrossRef]
- Tedersoo, L.; Suvi, T.; Jairus, T.; Kõljalg, U. Forest Microsite Effects on Community Composition of Ectomycorrhizal Fungi on Seedlings of Picea Abies and Betula Pendula. Environmental Microbiology 2008. [CrossRef]
- Poznanovic, S.K.; Lilleskov, E.A.; Webster, C.R. Sharing Rotting Wood in the Shade: Ectomycorrhizal Communities of Co-Occurringbirch and Hemlock Seedlings. Mycorrhiza 2015, 25, 153–164. [CrossRef]
- Kropp, B.R. Fungi from Decayed Wood as Ectomycorrhizal Symbionts of Western Hemlock. Can. J. For. Res. 1982, 12, 36–39. [CrossRef]
- Orrego, G. Western Hemlock Regeneration on Coarse Woody Debris Is Facilitated by Linkage into a Mycorrhizal Network in an Old-Growth Forest. master thesis, THE UNIVERSITY OF BRITISH COLUMBIA: Vancouver, 2018.
- Tedersoo, L.; Kõljalg, U.; Hallenberg, N.; Larsson, K.-H. Fine Scale Distribution of Ectomycorrhizal Fungi and Roots across Substrate Layers Including Coarse Woody Debris in a Mixed Forest. New Phytologist 2003, 159, 153–165. [CrossRef]
- Kluting, K.; Strid, Y.; Six, D.; Rosling, A. Forest Fire Influence on Tomicus Piniperda-Associated Fungal Communities and Phloem Nutrient Availability of Colonized Pinus Sylvestris. Microb Ecol 2023, 86, 224–239. [CrossRef]
- Rumpf, S.; Schönfelder, E.; Ahrends, B. 3 Biometrische Schätzmodelle für Nährelementgehalte in Baumkompartimenten. 2018.
- Mussche, S.; Bussche, B.; De Schrijver, A.; Neirynck, J.; Nachtergale, L.; Lust, N. Nutrient Uptake of a Mixed Oak/Beech Forest in Flanders (Belgium). SG 1998, 63. [CrossRef]
- André, F.; Jonard, M.; Ponette, Q. Biomass and Nutrient Content of Sessile Oak (Quercus Petraea (Matt.) Liebl.) and Beech (Fagus Sylvatica L.) Stem and Branches in a Mixed Stand in Southern Belgium. Science of The Total Environment 2010, 408, 2285–2294. [CrossRef]
- Ahrends, B.; Von Wilpert, K.; Weis, W.; Vonderach, C.; Kändler, G.; Zirlewagen, D.; Sucker, C.; Puhlmann, H. Merits and Limitations of Element Balances as a Forest Planning Tool for Harvest Intensities and Sustainable Nutrient Management—A Case Study from Germany. Soil Systems 2022, 6, 41. [CrossRef]
- Vandekerkhove, K.; Deforce, K.; Bastiaens, J. Historic-ecological position of beech in the area of the Sonian Forest and an overview of beech-forest- related biodiversity present in the forest; Instituut voor Natuur- en Bosonderzoek, 2018; [CrossRef]
- De Keersmaeker, L.; Esprit, M.; Goessens, S.; Anja, L.; Thomaes, A.; Van de Kerckhove, P.; Vandekerkhove, K. Monitoring Programme on Strict Forest Reserves in Flanders (Belgium) - Site Level Stand Structure, Regeneration and Vegetation Data 2023. [CrossRef]
- Renvall, P. Community Structure and Dynamics of Wood-Rotting Basidiomycetes on Decomposing Conifer Trunks in Northern Finland. Karstenia 1995, 35, 1–51. [CrossRef]
- Agerer, R. Studies on Ectomycorrhizae II. Introducing Remarks on Characterisation and Identification. Mycotaxon 1986, 26, 473–492.
- Agerer, R. Studies on Ectomycorrhizae III. Mycorrhizae Formed by Four Fungi in the Genera Lactarius and Russula on Spruce. Mycotaxon 1986, 27, 1–59.
- Agerer, R. (ed. ) Colour Atlas of Ectomycorrhizae.; 1st–11th ed.; Einhorn-Verlag: Schwäbisch Gmünd, 1987;
- Agerer, R. Characterisation of Ectomycorrhiza. In Methods in Microbiology; Norris, J.R., D.J., R., Varma, A.K., Eds.; Academic Press Limited, 1991; Vol. 23, pp. 25–73. [CrossRef]
- Agerer, R. Anatomical Characteristics of Identified Ectomycorrhizas: An Attempt towards a Natural Classification.; Mycorrhiza: structure, function, molecular biology and biotechnology.; Springer-Verlag: Berlin Heidelberg, 1995; [CrossRef]
- Agerer, R.; R.M., D.; S., E.; Ingleby K., L. Descriptions of Ectomycorrhizae.; Einhorn-Verlag: Schwäbisch Gmünd, 1996;
- Nuytinck, J.; Verbeken, A. Lactarius Sanguifluus versus Lactarius Vinosus — Molecular and Morphological Analyses. Mycol Progress 2003, 2, 227–234. [CrossRef]
- Tedersoo, L.; Bahram, M.; Ryberg, M.; Otsing, E.; Koljalg, U.; Abarenkov, K. Global Biogeography of the Ectomycorrhizal /Sebacina Lineage (Fungi, Sebacinales) as Revealed from Comparative Phylogenetic Analyses. Molecular ecology 2014, 23, 4168–4183. [CrossRef]
- Edgar, R.C. SINTAX: A Simple Non-Bayesian Taxonomy Classifier for 16S and ITS Sequences 2016, 074161. [CrossRef]
- Frøslev, T.G.; Kjøller, R.; Bruun, H.H.; Ejrnæs, R.; Brunbjerg, A.K.; Pietroni, C.; Hansen, A.J. Algorithm for Post-Clustering Curation of DNA Amplicon Data Yields Reliable Biodiversity Estimates. Nat Commun 2017, 8, 1188. [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PloS one 2013, 8, e61217. [CrossRef]
- Mikryukov, V. metagMisc: Miscellaneous Functions for Metagenomic Analysis. View Article 2019.
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘Vegan.’ Community ecology package, version 2013, 2, 1–295.
- Corcoran, D.; Corcoran, M.D. Package ‘AICcPermanova.’ 2023.
- De Caceres, M.; Jansen, F.; De Caceres, M.M. Package ‘Indicspecies.’ indicators 2016, 8.
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An Open Annotation Tool for Parsing Fungal Community Datasets by Ecological Guild. Fungal ecology 2016, 20, 241–248. [CrossRef]
- Karst, S.M.; Ziels, R.M.; Kirkegaard, R.H.; Sørensen, E.A.; McDonald, D.; Zhu, Q.; Knight, R.; Albertsen, M. Enabling High-Accuracy Long-Read Amplicon Sequences Using Unique Molecular Identifiers with Nanopore or PacBio Sequencing 2019. [CrossRef]
- Lin, X.; Waring, K.; Tyson, J.; Ziels, R.M. High-Accuracy Meets High-Throughput for Microbiome Profiling with near Full-Length 16S rRNA Amplicon Sequencing on the Nanopore Platform 2023, 2023.06.19.544637. [CrossRef]
- Baloğlu, B.; Chen, Z.; Elbrecht, V.; Braukmann, T.; MacDonald, S.; Steinke, D. A Workflow for Accurate Metabarcoding Using Nanopore MinION Sequencing. Methods in Ecology and Evolution 2021, 12, 794–804. [CrossRef]
- Oxford Nanopore Announces Breakthrough Technology Performance to Deliver Complete Human Genome Assemblies and Richer Multiomic Data in London Calling Available online: https://nanoporetech.com/news/oxford-nanopore-announces-breakthrough-technology-performance-to-deliver-complete-human-genomes-and-richer-multiomic-data-in-london-calling-tech-update (accessed on 26 August 2024).
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat Methods 2016, 13, 581–583. [CrossRef]
- Dierickx, G.; Froyen, M.; Halling, R.; Wisitrassameewong, K.; Delgat, L.; De Crop, E.; Verbeken, M. Updated Taxonomy of Lactifluus Section Luteoli: L. Russulisporus from Australia and L. Caliendrifer from Thailand. Mycokeys 2019, 56, 13–32. [CrossRef]
- Palmer, J.M.; Jusino, M.A.; Banik, M.T.; Lindner, D.L. Non-Biological Synthetic Spike-in Controls and the AMPtk Software Pipeline Improve Mycobiome Data. PeerJ 2018, 6, e4925. [CrossRef]
- Schoutteten, N.; Yurkov, A.; Leroux, O.; Haelewaters, D.; Van Der Straeten, D.; Miettinen, O.; Boekhout, T.; Begerow, D.; Verbeken, A. Diversity of Colacosome-Interacting Mycoparasites Expands the Understanding of the Evolution and Ecology of Microbotryomycetes Available online: https://www.ingentaconnect.com/content/wfbi/sim/pre-prints/content-a2_sim_vol106_art2;jsessionid=1tl834f1c8ks.x-ic-live-03# (accessed on 3 August 2023).
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; May, T.W.; Frøslev, T.G.; Pawlowska, J.; Lindahl, B.; Põldmaa, K.; Truong, C.; et al. The UNITE Database for Molecular Identification and Taxonomic Communication of Fungi and Other Eukaryotes: Sequences, Taxa and Classifications Reconsidered. Nucleic Acids Research 2024, 52, D791–D797. [CrossRef]
- Malysheva, V.; Spirin, V.; Schoutteten, N.; De Lange, R.; Pennanen, J.; Larsson, K.-H. New and Noteworthy Species of Helicogloea (Atractiellomycetes, Basidiomycota) from Europe. Annales Botanici Fennici 2020, 57, 1. [CrossRef]
- Walleyn, R.; Vandekerkhove, K. Diversiteit, ecologie en indicatorwaarde van paddestoelen op groot dood beukenhout in het bosreservaat kersselaerspleyn (zoniënwoud). 2002.
- Ódor, P.; Heilmann-Clausen, J.; Christensen, M.; Aude, E.; van Dort, K.W.; Piltaver, A.; Siller, I.; Veerkamp, M.T.; Walleyn, R.; Standovár, T.; et al. Diversity of Dead Wood Inhabiting Fungi and Bryophytes in Semi-Natural Beech Forests in Europe. Biological Conservation 2006, 131, 58–71. [CrossRef]
- Heilmann-Clausen, J.; Christensen, M. Fungal Diversity on Decaying Beech Logs - Implications for Sustainable Forestry. Biodiversity and Conservation - BIODIVERS CONSERV 2003, 12, 953–973. [CrossRef]
- Abrego, N. Wood-Inhabiting Fungal Communities: Opportunities for Integration of Empirical and Theoretical Community Ecology. Fungal Ecology 2022, 59, 101112. [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella Species as the Plant Growth-Promoting Fungi Present in the Agricultural Soils. Agriculture 2021, 11, 7. [CrossRef]
- Beatrice Schol-Schwarz, M. Rhinocladiella, Its Synonym Fonsecaea and Its Relation to Phialophora. Antonie van Leeuwenhoek 1968, 34, 119–152. [CrossRef]
- Vicente, V.A.; Attili-Angelis, D.; Pie, M.R.; Queiroz-Telles, F.; Cruz, L.M.; Najafzadeh, M.J.; de Hoog, G.S.; Zhao, J.; Pizzirani-Kleiner, A. Environmental Isolation of Black Yeast-like Fungi Involved in Human Infection. Studies in Mycology 2008, 61, 137–144. [CrossRef]
- Marčiulynas, A.; Marčiulynienė, D.; Mishcherikova, V.; Franić, I.; Lynikienė, J.; Gedminas, A.; Menkis, A. High Variability of Fungal Communities Associated with the Functional Tissues and Rhizosphere Soil of Picea Abies in the Southern Baltics. Forests 2022, 13, 1103. [CrossRef]
- Roberts, P. Exidiopsis Species from Devon, Including the New Segregate Genera Ceratosebacina, Endoperplexa, Microsebacina, and Serendipita. Mycological Research 1993, 97, 467–478. [CrossRef]
- Réblová, M.; Nekvindová, J. New Genera and Species with Chloridium-like Morphotype in the Chaetosphaeriales and Vermiculariopsiellales. Studies in Mycology 2023, 106, 199–258. [CrossRef]
- Cai, F.; Druzhinina, I.S. In Honor of John Bissett: Authoritative Guidelines on Molecular Identification of Trichoderma. Fungal Diversity 2021, 107, 1–69. [CrossRef]
- Burke, D.J.; López-Gutiérrez, J.C.; Smemo, K.A.; Chan, C.R. Vegetation and Soil Environment Influence the Spatial Distribution of Root-Associated Fungi in a Mature Beech-Maple Forest. Applied and Environmental Microbiology 2009, 75, 7639–7648. [CrossRef]
- Tedersoo, L.; Gates, G.; Dunk, C.W.; Lebel, T.; May, T.W.; Kõljalg, U.; Jairus, T. Establishment of Ectomycorrhizal Fungal Community on Isolated Nothofagus Cunninghamii Seedlings Regenerating on Dead Wood in Australian Wet Temperate Forests: Does Fruit-Body Type Matter? Mycorrhiza 2009, 19, 403–416. [CrossRef]
- Vincenot, L.; Nara, K.; Sthultz, C.; Labbé, J.; Dubois, M.; Tedersoo, L.; Martin, F.; Selosse, M. Extensive Gene Flow over Europe and Possible Speciation over Eurasia in the Ectomycorrhizal Basidiomycete Laccaria Amethystina Complex. Molecular Ecology 2012, 21, 281–299. [CrossRef]
- Holec, J.; Kučera, T.; Běťák, J.; Hort, L. Macrofungi on Large Decaying Spruce Trunks in a Central European Old-Growth Forest: What Factors Affect Their Species Richness and Composition? Mycol Progress 2020, 19, 53–66. [CrossRef]
- Holec, J.; Kučera, T. Richness and Composition of Macrofungi on Large Decaying Trees in a Central European Old-Growth Forest: A Case Study on Silver Fir (Abies Alba). Mycol Progress 2020, 19, 1429–1443. [CrossRef]
- Fiore-Donno, A.-M.; Martin, F. Populations of Ectomycorrhizal Laccaria Amethystina and Xerocomus Spp. Show Contrasting Colonization Patterns in a Mixed Forest. New Phytologist 2001, 152, 533–542. [CrossRef]
- Boeraeve, M.; Everts, T.; Vandekerkhove, K.; De Keersmaeker, L.; Van de Kerckhove, P.; Jacquemyn, H. Partner Turnover and Changes in Ectomycorrhizal Fungal Communities during the Early Life Stages of European Beech (Fagus Sylvatica L.). Mycorrhiza 2021, 31, 43–53. [CrossRef]
- Grebenc, T.; Christensen, M.; Vilhar, U.; Cater, M.; Martin, M.P.; Simoncic, P.; Kraigher, H. Response of Ectomycorrhizal Community Structure to Gap Opening in Natural and Managed Temperate Beech-Dominated Forests. Can. J. For. Res. 2009, 39, 1375–1386. [CrossRef]
- Hortal, S.; Trocha, L.K.; Murat, C.; Chybicki, I.J.; Buée, M.; Trojankiewicz, M.; Burczyk, J.; Martin, F. Beech Roots Are Simultaneously Colonized by Multiple Genets of the Ectomycorrhizal Fungus Laccaria Amethystina Clustered in Two Genetic Groups. Molecular Ecology 2012, 21, 2116–2129. [CrossRef]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piche, Y. Ink and Vinegar, a Simple Staining Technique for Arbuscular-Mycorrhizal Fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [CrossRef]
- Lilleskov, E.A.; Bruns, T.D. Spore Dispersal of a Resupinate Ectomycorrhizal Fungus, Tomentella Sublilacina, via Soil Food Webs. Mycologia 2005, 97, 762–769. [CrossRef]
- Persson, Y.; Ihrmark, K.; Stenlid, J. Do Bark Beetles Facilitate the Establishment of Rot Fungi in Norway Spruce? Fungal Ecology 2011, 4, 262–269. [CrossRef]
- Seibold, S.; Müller, J.; Baldrian, P.; Cadotte, M.W.; Štursová, M.; Biedermann, P.H.W.; Krah, F.-S.; Bässler, C. Fungi Associated with Beetles Dispersing from Dead Wood – Let’s Take the Beetle Bus! Fungal Ecology 2019, 39, 100–108. [CrossRef]
- Frank, A. Die Bedeutung Der Mykorrhiza-Pilze Für Die Gemeine Kiefer. Forstwissenschaftliches Centralblatt 1894, 185–190. [CrossRef]
- Kuyper, T.W. De rol van ectomycorrhiza-schimmels in de nutriëntenkringloop. Coolia 1990, 33, 32–37.
- Fukasawa, Y.; Kitabatake, H. Which Is the Best Substrate to Regenerate? A Comparative Pot Experiment for Tree Seedling Growth on Decayed Wood and in Soil. Forests 2022, 13, 1036. [CrossRef]
- Abarenkov, K.; Kõljalg, U.; Nilsson, R.H. UNITE Species Hypotheses Matching Analysis. Biodiversity Information Science and Standards 2022, 6, e93856. [CrossRef]
- Haelewaters, D.; Quandt, C.A.; Bartrop, L.; Cazabonne, J.; Crockatt, M.E.; Cunha, S.P.; De Lange, R.; Dominici, L.; Douglas, B.; Drechsler-Santos, E.R.; et al. The Power of Citizen Science to Advance Fungal Conservation. Conservation Letters 2024, 17, e13013. [CrossRef]
- Heilmann-Clausen, J.; Bruun, H.H.; Ejrnæs, R.; Frøslev, T.G.; Læssøe, T.; Petersen, J.H. How Citizen Science Boosted Primary Knowledge on Fungal Biodiversity in Denmark. Biological Conservation 2019, 237, 366–372. [CrossRef]
- Curry, K.D.; Wang, Q.; Nute, M.G.; Tyshaieva, A.; Reeves, E.; Soriano, S.; Wu, Q.; Graeber, E.; Finzer, P.; Mendling, W.; et al. Emu: Species-Level Microbial Community Profiling of Full-Length 16S rRNA Oxford Nanopore Sequencing Data. Nat Methods 2022, 19, 845–853. [CrossRef]
- Vorst, V. van der; Thijssen, M.; Fronen, B.J.; Groot, A. de; Maathuis, M.A.M.; Nijhuis, E.; Polling, M.; Stassen, J.; Voorhuijzen-Harink, M.M.; Jak, R. PIMENTA: PIpeline for MEtabarcoding through Nanopore Technology Used for Authentication 2024, 2024.02.14.580249. [CrossRef]
- Doorenspleet, K.; Jansen, L.; Oosterbroek, S.; Kamermans, P.; Bos, O.; Wurz, E.; Murk, A.; Nijland, R. The Long and the Short of It: Nanopore Based eDNA Metabarcoding of Marine Vertebrates Works; Sensitivity and Specificity Depend on Amplicon Lengths 2023, 2021.11.26.470087.
- Tedersoo, L.; Tooming-Klunderud, A.; Anslan, S. PacBio Metabarcoding of Fungi and Other Eukaryotes: Errors, Biases and Perspectives. New Phytologist 2018, 217, 1370–1385. [CrossRef]
- Wurzbacher, C.; Larsson, E.; Bengtsson-Palme, J.; Van den Wyngaert, S.; Svantesson, S.; Kristiansson, E.; Kagami, M.; Nilsson, R.H. Introducing Ribosomal Tandem Repeat Barcoding for Fungi. Molecular ecology resources 2019, 19, 118–127. [CrossRef]
- Laver, T.W.; Caswell, R.C.; Moore, K.A.; Poschmann, J.; Johnson, M.B.; Owens, M.M.; Ellard, S.; Paszkiewicz, K.H.; Weedon, M.N. Pitfalls of Haplotype Phasing from Amplicon-Based Long-Read Sequencing. Sci Rep 2016, 6, 21746. [CrossRef]
- White, R.; Pellefigues, C.; Ronchese, F.; Lamiable, O.; Eccles, D. Investigation of Chimeric Reads Using the MinION. F1000Research 2017, 6. [CrossRef]
- Xu, Y.; Lewandowski, K.; Lumley, S.; Pullan, S.; Vipond, R.; Carroll, M.; Foster, D.; Matthews, P.C.; Peto, T.; Crook, D. Detection of Viral Pathogens With Multiplex Nanopore MinION Sequencing: Be Careful With Cross-Talk. Front. Microbiol. 2018, 9. [CrossRef]
- Makhoul, M.; Chawla, H.S.; Wittkop, B.; Stahl, A.; Voss-Fels, K.P.; Zetzsche, H.; Snowdon, R.J.; Obermeier, C. Long-Amplicon Single-Molecule Sequencing Reveals Novel, Trait-Associated Variants of VERNALIZATION1 Homoeologs in Hexaploid Wheat. Front. Plant Sci. 2022, 13. [CrossRef]

| Bark decay stage | Description |
|---|---|
| Early | Layer (incl. inner bark) difficult to penetrate with a metal spoon (force needed), almost all fibers intact, dark yellow in color. Corresponds to log decay stage (DS) 1 or early 2. |
| Intermediate | Layer crumbles and can be scooped up with a metal spoon. Crumbles with minimal force between fingers, some fibers intact, light brown in color. Corresponds to a late log DS 2 or early 3. |
| Late | Layer is almost fully decayed, penetrable without force, it easily disintegrates between fingertips – resembles soil, no fibers intact, (dark) brown in color. Corresponds to a late log DS 3 or 4. |
| DS Bark | lowest taxon name | fidelity | p | SH | OTU | |
|---|---|---|---|---|---|
| Early | Chaetosphaeriaceae (SH0980871.10FU) | 0.798 | 0,001 | ● | ● |
| Tulasnella (OTU 1097) | 0.728 | 0,003 | ● | ||
| Sordariales (SH0840221.10FU) | 0.779 | 0,008 | ● | ● | |
| Pleurothecium recurvatum (SH0926211.10FU)* | 0.950 | 0.007 | ● | ● | |
| Mid | Ganoderma adspersum (SH0762773.10FU) | 0.839 | 0,001 | ● | |
| Hydnodontaceae (OTU 448) | 0.729 | 0,004 | ● | ||
| Herpotrichiellaceae (SH0970954.10FU) | 0.751 | 0,005 | ● | ● | |
| Rhinocladiella (OTU 75 | SH0970950.10FU) | 0.697 | 0,005 | ● | ||
| Helotiales (OTU 474) | 0.729 | 0,006 | ● | ||
| Mortierella (SH0960682.10FU) | 0.745 | 0,008 | ● | ● | |
| Serendipita (SH0743656.10FU) | 0.730 | 0,008 | ● | ● | |
| Chaetosphaeria decastyla (SH0980872.10FU) | 0.655 | 0,010 | ● | ||
| Late | Hyaloscyphaceae (SH0973189.10FU) | 0.707 | 0,006 | ● | ● |
| Rozellomycota (SH0910702.10FU) | 0.707 | 0,006 | ● | ||
| Ilyonectria mors-panacis (SH1450888.10FU) | 0.707 | 0,007 | ● | ||
| Rozellomycota (OTU 1883) | 0.711 | 0,007 | ● | ||
| Pezizomycotina (SH0755218.10FU) | 0.808 | 0,008 | ● | ● | |
| Trichoderma (OTU 4045) | 0.707 | 0,008 | ● | ||
| Hypocreales (OTU 2917) | 0.707 | 0,009 | ● | ||
| Leotiomycetes (SH0948543.10FU) | 0.707 | 0,010 | ● | ● | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





