1. Introduction
The epidemic of obesity has reached elevated numbers worldwide and results in many comorbidities such as type 2 diabetes mellitus (T2D), cardiovascular disease, chronic kidney disease, and cancer, which impacts patient´s life quality [
1]. This condition is associated with a chronic state of inflammation including peripheral tissues and hypothalamus, a central area of nervous system that regulates food intake [
2,
3].
Leptin and insulin are the main anorexigenic hormones acting via leptin and insulin receptors expressed in the central nervous system (CNS), especially in hypothalamus [
4,
5,
6]. These two hormones suppress the activity of the orexigenic NPY/AgRP neurons, while stimulating the anorexigenic POMC/CART neurons [
7,
8], thus controlling food intake. Resistance to the central actions of leptin or insulin is linked to the increases of obesity and diabetes [
9].
In this context, bariatric surgery has emerged as a potent tool in the prevention and treatment of obesity and DM2[
10,
11]. Nowadays, the most common bariatric surgery procedures are Roux-en-Y gastric bypass (RYGB) and Sleeve Gastrectomy (SG) [
12,
13]. Both surgeries reduce body weight and improve insulin signaling, as well as glucose homeostasis. In male Sprague-Dawley rats, RYGB increases mRNA levels of orexigenic genes AgRP and NPY, with no alterations in anorexigenic genes CART and POMC. Moreover, RYGB down regulates dopaminergic transmission markers [
14]. However, the mechanism by which SG reduces body weight is not completely understood.
Once the mechanism by which SG regulates body weight in this model remains unknown, this study aimed to evaluate food intake, as well as the expression of orexigenic and anorexigenic hypothalamic genes in diet-induced obese mice submitted to SG.
2. Results
2.1. SG Reduces Body Weight, Fasting Glycemia, Insulinemia, and Leptinemia in Diet-Induced Obese Mice
Four weeks after SG, HFD-SG mice presented a reduction in body weight (38.22 ± 1.31) when compared to HFD and HFD-SHAM (44.19 ± 0.47 and 43.51 ± 0.71, respectively) (
Table 1). HFD-SG mice also presented reduced fasting glycemia (93.43 ± 4.67), insulinemia (0.93 ± 0.05), and leptinemia (1.43 ± 0.35) when compared to HFD (115.0 ± 4.60, 1.77 ± 0.15, 5.86 ± 1.38, respectively) and HFD-SHAM (122.4 ± 3.48, 1.92 ± 0.27, 6.44 ± 1.51, respectively) (
Table 1).
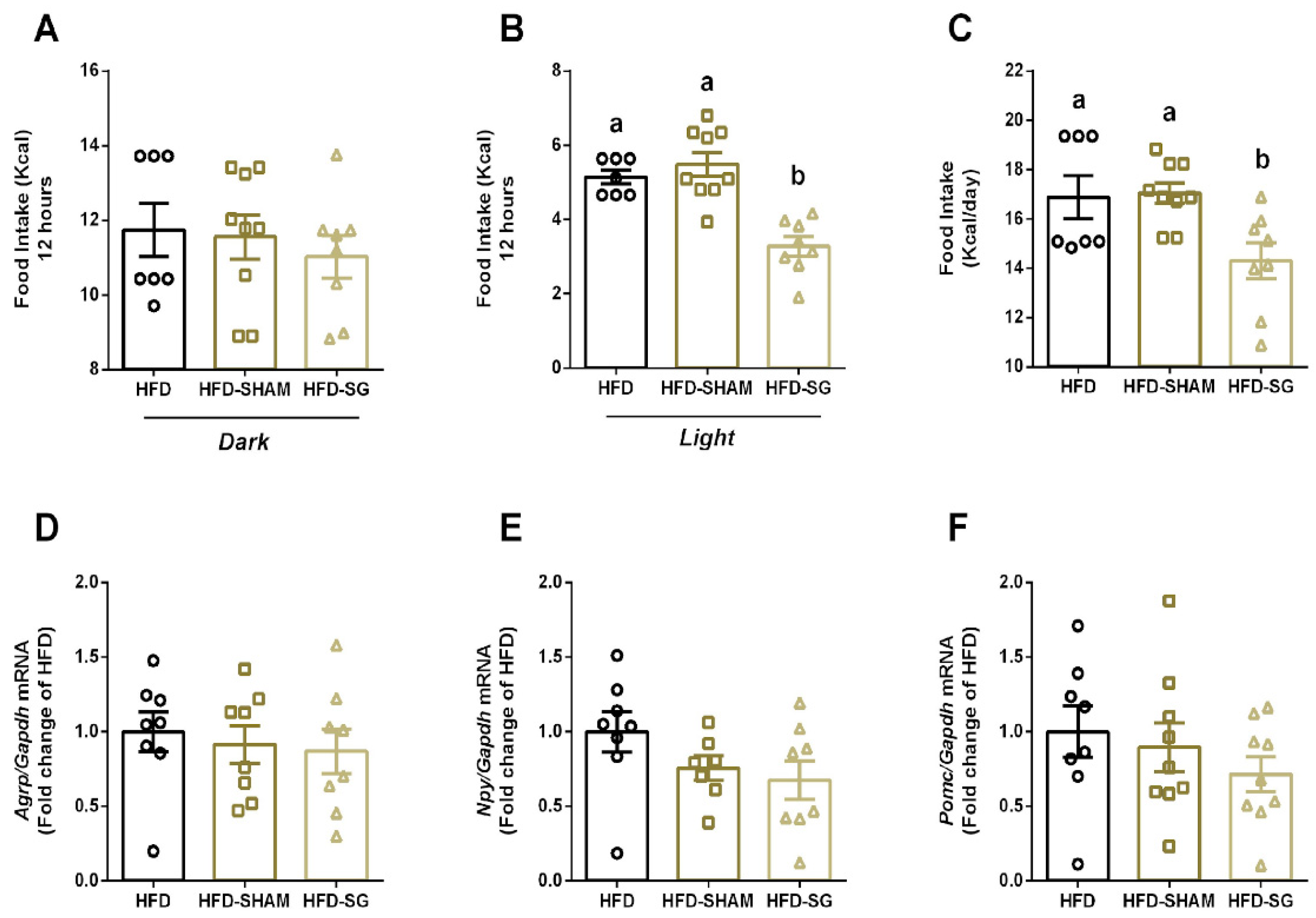
2.2. SG Reduces Food Intake with No Alterations on Hypothalamic Anorexigenic and Orexigenic Genes in diet-Induced Obese Mice
Once bariatric surgery reduces body weight, we aimed to evaluate food intake and regulation in mice. No differences were observed in food intake during the dark period between HFD, HFD-SHAM and HFD-SG (11.74 ± 0.71; 11.56 ± 0.59; 11.02 ± 0.57, respectively) (
Figure 1A). However, HFD-SG mice presented reduced food intake in the light period (3.28 ± 0.26) (
Figure 1B), as well as reduced total calorie intake (14.30 ± 0.73) (
Figure 1C) when compared to both HFD (5.15 ± 0.18; 16.88 ± 0.88) and HFD-SHAM mice (5.49 ± 0.32; 17.05 ± 0.42). Expression of orexigenic and anorexigenic genes in hypothalamus was similar in all groups. (
Figure 1D, E, F).
3. Discussion
In the present study, we verified that SG reduces insulin and leptin levels, as well as body weight. This effect is associated with reduced dark/light cycle-dependent food intake, with no alterations in hypothalamic genes that regulate food intake. Thus, understanding the molecular mechanisms by which SG reduces body weight and improves glucose homeostasis contributing to improve quality of life, is important in order to find new targets for obesity treatment.
Here, we observed that SG reduces food and Kcal intake during the light cycle (
Figure 1B). It is known that rodents display different feeding behaviors than humans due to light/dark cycles, being more active in the night period [
15]. However, when mice are exposed to a high-fat diet, they consume more food in their less active period (light period). This effect is associated with alterations on the expression of circadian rhythm genes in the liver and hypothalamus [
16]. Furthermore, in middle-to-older aged adults’ rats, the timing of food intake is associated with obesity development [
17]. Also, in a rat model of night work, shifting food intake to a more natural period, which is the active phase (dark cycle) prevents obesity development [
18]. Interestingly, our results showed that SG reduces food intake during the light period in obese mice, thus, improving metabolism.
Hypothalamus plays a central role in food intake by the expression of orexigenic and anorexigenic genes [
3]. However, no differences were observed in the expression of these genes when mice were submitted to SG (
Figure 1D, E, F). In contrast, RYGB surgery alters orexigenic genes expression with no differences observed in anorexigenic genes [
14]. Thereby SG modulates body weight, at least in part, by an orexigenic/anorexigenic independent manner.
Recent studies have elucidated the effects of leptin and insulin interaction actions in the body. For instance, leptin resistance leads to the inhibition of insulin signaling, whereas insulin resistance alters leptin signaling in a hypothalamic cell line [
19]. Conversely, another study revealed that insulin potentiates leptin-induced STAT3 phosphorylation, a critical transcription factor involved in a major signaling pathway exerting leptin anti-obesity effects [
20]. Thus, it is possible that leptin and insulin may act synergistically to reduce body weight and food intake. Our data show that SG surgery reduces leptin and insulin levels (
Table 2) as well as improves insulin sensitivity [
21], which may be associated with reduced food intake.
Recently, it was demonstrated that SG reduces body weight and improves glucose homeostasis by FGF15/19 pathway [
21]. FGF15/19 is an intestinal hormone secreted from ileal enterocytes in response to a meal [
22]. FGF19 plays a well-established role in bile acid metabolism, however, it also regulates energy and glucose homeostasis in rodents [
23,
24,
25,
26]. In obese mice, SG improves insulin sensitivity along with reduced beta cell insulin secretion. Also, SG reduces gene expression of inflammatory and ER stress markers in pancreatic beta cells [
21]. This altered FGF15/19 pathway improves glucose homeostasis by reducing beta cell overload induced by high-fat diet (HFD), which modulates pancreatic alpha cells, reducing glucagon secretion. This mechanism can contribute to avoid type 2 diabetes development [
21]. Another study also demonstrated that FGF19 reduces food intake and body weight, whereas the administration of an FGF-receptor inhibitor leads to increased food intake and impaired glucose tolerance, suggesting a physiological central role for FGF19 signaling pathway [
27].
Foraging for food precedes food consumption and it is an important component of the overall metabolic program regulating feeding. Foraging is governed by the neuronal circuits from the central nervous system [
28,
29,
30]. Nevertheless, how this mechanism is influenced by diet and/or hormonal signals is still not well understood. Huang and colleagues have demonstrated that FGF19 suppresses foraging-like behaviors [
31], suggesting that the intestinal hormone FGF15/19 signals a satiating state to the brain, thereby suppressing foraging-like behaviors. However, further studies still have to be done to investigate the role of FGF15/19 in controlling food intake and foraging-like behaviors in our mice model.
Finally, SG surgery seems to be an important strategy in the treatment of obesity and its comorbidities. Even though this procedure does not alter anorexigenic and orexigenic hypothalamic genes, it was able to reduce food intake during the light period and body weight. Such effect may be due to reduced insulin and leptin levels as well as, increased FGF15/19 levels, which all together may contribute to reduced food intake and altered foraging-like behaviors, contributing to improve metabolism and reduce body weight.
4. Materials and Methods
4.1. Animals
4-week-old male C57BL/6 mice obtained from the animal facility of the University of Campinas were maintained on a 12h light-dark cycle, in a temperature-controlled facility with free access to food and water. The Ethics Committee at the University of Campinas (License Number: 5242-1/2019) approved all experimental procedures involving mice, which were conducted in accordance with the last revision of the National Institutes of Health (NIH) guide for the care and use of laboratory animals.
4.2. Obesity Induction
Mice received a 45 kcal% saturated high-fat diet (HFD) (Prag Soluções; SP, Brazil) for 10 weeks. Then, mice were randomly divided into three body weight-matched groups prior to surgery: 1) high-fat diet group (HFD), 2) HFD submitted to sham operation (HFD-SHAM), and 3) HFD submitted to Sleeve Gastrectomy (HFD-SG).
4.3. Sleeve Gastrectomy and Sham Operations
Sleeve Gastrectomy (SG) and sham operations were performed under anesthesia as previously described [
21]. For SG, the incision was performed from His angle, and 80% of the stomach was removed, including complete resection of the gastric fundus. Sham surgery consisted of laparotomy only. Mice were kept on a liquid diet for 5 days after surgery. On day 6, mice were given doughy HFD, and on day 12, they returned to solid HFD.
4.4. Glycemia, Insulin, and Leptin Evaluation
Four weeks after the postoperative recovery period, mice were weighed and glycemia was verified by a glucometer (Accu-chek®, Roche, Basileia, Switzerland) in fed and 12h fasted states. Mice were euthanized by decapitation after isoflurane inhalation, the hypothalamus was removed for gene expression analysis and plasm was collected for insulin and leptin measurement by ELISA kits (Mouse insulin, Catalog #10-1247-1, Mercodia, Sylveniusgatan, Sweden).
4.5. Food Intake
At the 3rd week after the postoperative recovery period, mice were maintained individually, in home cages for 24h of adaptation. After that, food consumption was measured during 3 consecutive days and was calculated by the difference between the food weight at 7 p.m. vs. 7 a.m. Food intake was then determined as the mean food consumption of this period [
32].
4.6. mRNA Extraction and Real Time Quantitative PCR (qRT-PCR
The total RNA content of hypothalamus was extracted using TRIzol reagent (Life Technologies, Gaithersburg, MD, USA), following phenol-chloroform RNA extraction, according to the manufacturer’s recommendations. RNA concentration was measured by Nanodrop (Nanodrop Thermo Scientific, Wilmington, DE, USA). cDNA was prepared using 2 µg of total RNA and the High Capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). The LuminoCt qPCR read mix (Sigma-Aldrich, Burlington, MA, USA) was used in the PCR reactions. Quantification was performed using the 7500 Fast Real-time PCR System (Applied Biosystems, Foster City, CA, USA). The relative expression of mRNAs was determined after normalization with the housekeeping gene Gapdh (Applied Biosystems, Foster City, CA, USA), using the 2
-ꕔꕔCt method. The qRT-PCR target assays (IDT DNA Technologies, Ann Arbor, MI, USA) are shown in
Table 2.
4.7. Statical Analysis
The data are presented as the mean ± standard error of the mean (SEM). To evaluate data normality, we applied the Shapiro–Wilk test. When normal, we used One-Way ANOVA with an unpaired Tukey’s post-hoc test; otherwise, Kruskal-Wallis with an unpaired Dunn’s post-hoc test was adopted. The difference between groups was considered statistically significant if p ≤ 0.05.
Author Contributions
Conceptualization, S.L.B, L.A.V and G.B.S. ; methodology, S.L.B, G.M.S, G.A.B and J.M.; software, G.M.S and J.M.; validation, G.M.S and J.M..; formal analysis, G.M.S.; investigation, G.M.S, S.L.B., E.M.C, A.C.B; resources, S.L.B, L.A.V and G.B.S.; data curation, S.L.B and G.B.S.; writing—original draft preparation, J.F.V, A.M.F.J., G.M.S and S.L.B.; writing—review and editing, J.F.V, A.M.F.J., G.M.S, G.A.B and S.L.B.; visualization, J.F.V, A.M.F.J., G.M.S and S.L.B.; supervision E.M.C., A.C.B and L.A.V.; project administration, S.L.B; funding acquisition, E.M.C., A.C.B and L.A.V. All authors have read and agreed to the published version of the manuscript.”
Funding
This research was funded by the São Paulo Research Foundation (FAPESP), grant numbers #2013/07607-8, #2015/12611-0, #2019/00728-0, and #2021/04664-7.
Institutional Review Board Statement
All experiments were approved by the Committee for Ethics in Animal Experimentation of the University of Campinas under the protocol CEUA/IB/UNICAMP #5242-1/2019.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Thanks to all the technical support provided by employees of the institutions involved.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Frontiers in Endocrinology 2021, 12. [CrossRef]
- Sonnefeld, L.; Rohmann, N.; Geisler, C.; Laudes, M. Is human obesity an inflammatory disease of the hypothalamus? European Journal of Endocrinology 2023, 188 (3), R37-R45. [CrossRef]
- Timper, K.; Brüning, J. C. Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Disease Models & Mechanisms 2017, 10 (6), 679-689. [CrossRef]
- Baskin, D. G.; Breininger, J. F.; Schwartz, M. W. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes 1999, 48 (4), 828-833. [CrossRef]
- Cheung, C. C.; Clifton, D. K.; Steiner, R. A. Proopiomelanocortin Neurons Are Direct Targets for Leptin in the Hypothalamus. Endocrinology 1997, 138 (10), 4489-4492. [CrossRef]
- Baskin DG, Wilcox BJ, Figlewicz DP, Dorsa DM. Insulin and insulin-like growth factors in the CNS. Trends Neurosci. 1988;11(3):107-11. [CrossRef]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289-95. [CrossRef]
- Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature (2000) 404(6778):661–71. [CrossRef]
- Thon, M.; Hosoi, T.; Ozawa, K. Possible Integrative Actions of Leptin and Insulin Signaling in the Hypothalamus Targeting Energy Homeostasis. Frontiers in Endocrinology 2016, 7. [CrossRef]
- Roth AE, Thornley CJ, Blackstone RP. Outcomes in Bariatric and Metabolic Surgery: an Updated 5-Year Review. Curr Obes Rep. 2020;9(3):380-389. [CrossRef]
- Ji, Y.; Lee, H.; Kaura, S.; Yip, J.; Sun, H.; Guan, L.; Han, W.; Ding, Y. Effect of Bariatric Surgery on Metabolic Diseases and Underlying Mechanisms. Biomolecules 2021, 11 (11), 1582. [CrossRef]
- Buchwald H. The evolution of metabolic/bariatric surgery. Obes Surg. 2014;24(8):1126-1135. [CrossRef]
- Eisenberg, D.; Shikora, S. A.; Aarts, E.; Aminian, A.; Angrisani, L.; Cohen, R. V.; De Luca, M.; Faria, S. L.; Goodpaster, K. P. S.; Haddad, A.; et al. 2022 American Society for Metabolic and Bariatric Surgery (ASMBS) and International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): Indications for Metabolic and Bariatric Surgery. Surgery for Obesity and Related Diseases 2022, 18 (12), 1345-1356. [CrossRef]
- Barkholt P, Pedersen PJ, Hay-Schmidt A, Jelsing J, Hansen HH, Vrang N. Alterations in hypothalamic gene expression following Roux-en-Y gastric bypass. Mol Metab. 2016;5(4):296-304. [CrossRef]
- Desmet, L.; Thijs, T.; Mas, R.; Verbeke, K.; Depoortere, I. Time-Restricted Feeding in Mice Prevents the Disruption of the Peripheral Circadian Clocks and Its Metabolic Impact during Chronic Jetlag. Nutrients 2021, 13 (11), 3846. [CrossRef]
- Kohsaka, A.; Laposky, A. D.; Ramsey, K. M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F. W.; Bass, J. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metabolism 2007, 6 (5), 414-421. [CrossRef]
- Xiao, Q.; Garaulet, M.; Scheer, F. A. J. L. Meal timing and obesity: interactions with macronutrient intake and chronotype. International Journal of Obesity 2019, 43 (9), 1701-1711. [CrossRef]
- Salgado-Delgado, R.; Angeles-Castellanos, M.; Saderi, N.; Buijs, R. M.; Escobar, C. Food Intake during the Normal Activity Phase Prevents Obesity and Circadian Desynchrony in a Rat Model of Night Work. Endocrinology 2010, 151 (3), 1019-1029. [CrossRef]
- Nazarians-Armavil, A.; Menchella, J. A.; Belsham, D. D. Cellular Insulin Resistance Disrupts Leptin-Mediated Control of Neuronal Signaling and Transcription. Molecular Endocrinology 2013, 27 (6), 990-1003. [CrossRef]
- Thon, M.; Hosoi, T.; Ozawa, K. Insulin enhanced leptin-induced STAT3 signaling by inducing GRP78. Scientific Reports 2016, 6 (1), 34312. [CrossRef]
- Soares GM, Balbo SL, Bronczek GA, et al. Vertical sleeve gastrectomy improves glucose-insulin homeostasis by enhancing β-cell function and survival via FGF15/19. Am J Physiol Endocrinol Metab. 2024;326(2):E134-E147. [CrossRef]
- Guthrie G, Vonderohe C, Burrin D. Fibroblast growth factor 15/19 expression, regulation, and function: An overview. Mol Cell Endocrinol. 2022;548:111617. [CrossRef]
- Inagaki, T.; Choi, M.; Moschetta, A.; Peng, L.; Cummins, C. L.; Mcdonald, J. G.; Luo, G.; Jones, S. A.; Goodwin, B.; Richardson, J. A.; et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metabolism 2005, 2 (4), 217-225. [CrossRef]
- Zhou, M.; Luo, J.; Chen, M.; Yang, H.; Learned, R. M.; Depaoli, A. M.; Tian, H.; Ling, L. Mouse species-specific control of hepatocarcinogenesis and metabolism by FGF19/FGF15. Journal of Hepatology 2017, 66 (6), 1182-1192. [CrossRef]
- Potthoff, J., Matthew; Boney-Montoya, J.; Choi, M.; He, T.; Sunny, E., Nishanth; Satapati, S.; Suino-Powell, K.; Xu, E., H.; Gerard, D., Robert; Finck, N., Brian; et al. FGF15/19 Regulates Hepatic Glucose Metabolism by Inhibiting the CREB-PGC-1α Pathway. Cell Metabolism 2011, 13 (6), 729-738. [CrossRef]
- Kir, S.; Beddow, S. A.; Samuel, V. T.; Miller, P.; Previs, S. F.; Suino-Powell, K.; Xu, H. E.; Shulman, G. I.; Kliewer, S. A.; Mangelsdorf, D. J. FGF19 as a Postprandial, Insulin-Independent Activator of Hepatic Protein and Glycogen Synthesis. Science 2011, 331 (6024), 1621-1624. [CrossRef]
- Ryan, K. K.; Kohli, R.; Gutierrez-Aguilar, R.; Gaitonde, S. G.; Woods, S. C.; Seeley, R. J. Fibroblast Growth Factor-19 Action in the Brain Reduces Food Intake and Body Weight and Improves Glucose Tolerance in Male Rats. Endocrinology 2013, 154 (1), 9-15. [CrossRef]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14(3):351-355. [CrossRef]
- Krashes, M. J.; Koda, S.; Ye, C.; Rogan, S. C.; Adams, A. C.; Cusher, D. S.; Maratos-Flier, E.; Roth, B. L.; Lowell, B. B. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. Journal of Clinical Investigation 2011, 121 (4), 1424-1428. [CrossRef]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683-685. [CrossRef]
- Huang, A.; Maier, M. T.; Vagena, E.; Xu, A. W. Modulation of foraging-like behaviors by cholesterol-FGF19 axis. Cell & Bioscience 2023, 13 (1). [CrossRef]
- Soares, G. M.; Zangerolamo, L.; Costa-Júnior, J. M.; Vettorazzi, J. F.; Carneiro, E. M.; Saad, S. T.; Boschero, A. C.; Barbosa-Sampaio, H. C. Whole-Body ARHGAP21-Deficiency Improves Energetic Homeostasis in Lean and Obese Mice. Frontiers in Endocrinology 2019, 10. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).