1. Introduction
The problem that reduced chili cultivation in Thailand is the severe outbreak of chili disease, both bacterial and fungal diseases. Farmers therefore use chemicals in the prevention and eradication of pepper disease to solve the problem. This causes higher production costs, lower yields, and pesticide residues in production and make farmers suffer from loss anthracnose disease (caused by infection Colletotrichum sp.) was an important disease and epidemic was found in all areas of chili production. Which may cause damage both in the planting field and after harvest (Boonyawadee et al., 2016). This fungus can penetrate the immature stage. But still do not show symptoms, will show symptoms when the peppers begin to ripen, it will be called this kind of latent infection. Most control methods are chemically controlled, such controls may cause side effects such as: the problem of chemical residues in production and the environment. Nowadays, consumers are more concerned about their health. Biological control of plant disease is another important alternative for anthracnose prevention in peppers (Wongcharee, 2015), for example, utilizing beneficial microorganisms in disease control anthracnose in peppers, such as Sariah (1994), was studied using Bacillus sp. to control anthracnose of chili caused by C. capsici and C. gloeosporioides. It was found that the antagonistic bacteria were able inhibited the growth of mycelium and spore germination as well as causing the hypha to have an abnormal shape, thickened walls, and voids are formed because of bacteria creating antibiotics.
Today, many countries around the world are increasingly interested in the use of microbes in the management of sustainable agriculture. This is an agricultural system that is in line with the development framework of the year 2016-2030 of the United Nations meeting with the goal of sustainable development (Arun, 2008; Jamsavang, 2011). As well as the trend of health-loving people who are interested in consuming more products that are safe from chemicals. Farmers therefore must find new methods to prevent disease and pests by avoiding the use of chemicals. And one of the most effective methods is to promote resistance or rapid growth, survive the infestation of pathogens. This was especially true for seedling diseases such as root and root rot of Cruciferae and Solanaceae, and low germination problems of Gramineae plants. It was found that several beneficial microorganisms were able to promote plant growth by stimulating plants to accumulate growth-related hormones. Some microorganisms can produce hormones necessary for the growth of plants such as bacteria Pseudomonas fluorescens SP007s that can produce the hormone auxin and gibberellin, etc. (Wilawan and Dusit, 2014) and reduced the complexity of the use of microorganisms. There is a research study reduced such limitations by developing a bio-product that is convenient for use, convenient transportation, light weight, and long shelf life (Jiradej, 2011).
As a result of this limitation, the objective of this research was to screen beneficial bacteria from chili do not shown symptoms of any disease. The efficacy was tested for the control of anthracnose in chili caused by C. capsici and developed a microbe-multiplication formula to develop a water-soluble powder bio-product with the properties of C. capsica and promoting growth in the seedling stage of plants. The development a formulation of the product to be suitable in terms of price, easy of transportation, storage during the sale, and maintaining the efficiency of beneficial bacteria as well as well as being able to expand the production scale at the industrial level.
2. Materials and Methods
2.1. Pathogen Isolation and Pathogenicity Test
Isolation of C. capsici from symptom-causing anthracnose of capsicum was isolated by Tissue transplanting technique (Agrios, 1978). The isolates were cultured on potato dextrose agar (PDA) for 5-7 days. Pathogenicity was tested by using Cork borers to penetrate the hypha tip of C. capsici, the agar chili juice was upside down on the chili fruit, incubated at room temperature inside a damp plastic box with ≥80%RH for 7 days. The wound size that occurred on the chili fruit was compared to the disease severity of the isolates.
2.2. Isolation of Beneficial Bacteria
The beneficial bacteria isolated from the Super-hot chili cultivar showed no symptoms of any disease with the Leaf wash technique modified from Wilawan (2008), by placing 10 grams of chili fruit in 90 ml distilled water. Drop 1-2 drops of surfactant, then shake it on the shaker at a speed of 120 rpm for 5 minutes to expose bacteria to the water and use it as a stock suspension to dilute the concentration. Ten-fold serial dilution method was used distributed over the surface of NGA (Riker and Riker, 1936), incubated at room temperature (28±3°C) for 24-48 h. The colonies of bacteria that grew on the NGA were selected and purified for further experiment.
2.3. Efficiency of Beneficial Bacteria Inhibit the Growth of C. capsici
2.3.1. Testing on Solid Culture Medium
To test the inhibitory activity of C. capsici P-TU008 by dual culture method on solid culture medium according to the modified method of Koomen and Jeffries (1993), the experiment was designed with CRD. The P-TU008 was cultured on PDA for 5-7 days, using Cork borers to penetrate the hypha tip of P-TU008 and the inoculum was placed upside-down distance 2 cm of the margins of the plate. The antagonistic bacteria cultured on the NGA for 24-48 hours were then crossed in the opposite of the fungus at 2 cm from the margins too, and incubation at room temperature for 24-48 hours. Efficiency was assessed by measuring the diameter of P-TU008 fungal colonies, comparing the efficiency with control with distilled water. Selected highly effective beneficial bacteria in the control to grow C. capsici P-TU008 for further experiments. The results were according to the research reported of Salah and Abbasi (2013) that reported isolation, characterization, and formulation of antagonistic bacteria for the management of seedlings damping-off and root rot disease of cucumber.
2.3.2. Testing in Liquid Culture Medium
Inhibition of P-TU008 by dual culture assay in nutrient yeast dextrose broth medium. The experiments were planned with CRD by culturing P-TU008 on PDA culture media, cutting off the end of hypha with Cork borer size 0.5 cm. Three agar chunks were placed in a 100 ml NYDB and filled with a bacterial cell suspension, 1 ml at a concentration of 1x108 cfu/ml of each strain was applied. Incubate a 120 rpm with shaker at room temperature for 72 hours. The results of the inhibition efficacy were recorded by weighing the hypha obtained from the strainer obtained in each process with the No. 1 filter paper compared with the control treatment using distilled water as negative control and the chemical carbendazim as positive control.
2.4. Develop a Media Formula to Increase the Population of Beneficial Bacteria
Study the type, composition, and ratio of nutrients by selecting the important nutrient sources of microorganisms including both carbon and nitrogen sources. By choosing a fish and soybean meal as nitrogen and molasses as carbon source and provide energy to beneficial bacteria. Development into 2 formulas: Formula 1 fish meal: molasses: CMC ratio 8: 5: 1 g/liter of water and Formula 2 soybean meal: molasses sugar: CMC ratio 8: 5: 1 g/liter of water. The formula was developed based on suitability and contains nutrients (C: N) suitable for the growth of beneficial bacteria. The culture’s medium was able to grow and multiply rapidly (Preecha and Prathuangwong, 2009) compared with nutrient glucose broth (NGB). Comparing the efficacy of formula mediums to increase the beneficial bacteria content by culturing the beneficial bacteria. There are 3 strains: SP-TU3, SP-TU6, and SP-TU37, incubated at room temperature for 48 hours, counting the number of beneficial bacteria populations in each recipe by dilution plate count method (Chuaboon and Prathuangwong, 2008).
2.5. Development of Water-Soluble Powder Bioproduct
2.5.1. Beneficial Bacteria Preparations
Each strain of beneficial bacteria (SP-TU3, SP-TU6, and SP-TU37) was cultured in NGB for 24 hours, then 5 ml/1 liters was added to the media formula as the most efficacy to the increasing the population of bacteria (from item 4). Incubated on a shaker at 150 rpm for 48 hours, then centrifuged at a speed of 8000 rpm for 5 minutes, adjust concentration at 0.7 O.D. with Spectrophotometer at 600 nm wavelength (approximately population 1x1015 cfu/ml). When examined by Dilution plate count to be mixed with carrier and nutrients in the production process of water-soluble powder bioproducts.
2.5.2. Selection of Substances and Nutrients
Selection of various nutrients and the optimal ratios for the viability and efficacy of beneficial bacteria. Various properties were assessed to select the type with water solubility and dispersed in water is talcum, calcium carbonate, CMC, and corn starch. The properties were assessed for both single and mixed formulas as follows: Formula 1 Talc: calcium carbonate: CMC ratio 9.75: 1.5: 1 Formula 2 corn starch: talc: calcium carbonate: CMC ratio 10: 87.5: 1.5: 1 Formula 3 corn starch: talc: calcium carbonate: CMC ratio 20: 87.5: 1.5: 1.
2.5.3. Water Solubility Property Test
The water solubility of the nutrient, carrier, and formula mixed was developed according to the ratio (in item 5.2). Ten grams of carrier and nutrients in 900 ml of water, timer for water solubility immediately after pouring the mixture in water. Test the water solubility of the carrier repeated 3 replications in CRD. The water solubility property test was divided into 5 levels of assessments based on the dissolution time of the carrier: Level 1, Immediate dissolution in 1-5 minutes (best level), Level 2, Dissolves water within 5-10 minutes (good level), level 3, water softener within 11-30 minutes (medium level), level 4, instant water solubility within 30-60 minutes (fair level), and level 5, Water-insoluble carriers cling to the top of the water surface (below the standard level) (Pupakdeepan, 2011).
2.5.4. Spension and Sedimentation Properties Test
The suspension and sedimentation properties of the nutrient, carrier, and formula mixed were developed according to the ratio (in item 5.2). Ten grams of carrier and nutrients in 900 ml of water and mix well with a shaker at 150 rpm for 5 min. The evaluation was conducted into 5 levels based on time of suspension and sedimentation of the carrier are Level 1 being the suspended carrier and fully precipitated within 12-24 hours (best level), Level 2 was the suspended carrier and the complete sedimentation within 30-60 minutes (good level), Level 3 is suspended carrier and complete sedimentation within 11-30 minutes (moderate level), Level 4 is suspended carrier and complete sedimentation within 5-10 minutes (fair level), and level 5 is the suspended carrier and is completely precipitated within 1-5 minutes (below the standard level) (Pupakdeepan, 2011).
2.5.5. The Proportion of the Nutrient and Carrier for Beneficial Bacteria
The appropriate ratio from the tests in 5.2 mixed according to the following ratios: Formula 1 talc: calcium carbonate: CMC (ratio 9.75: 1.5: 1), Formula 2 corn starch: talc: calcium carbonate: CMC (ratio 10: 87.5: 1.5:1), Formula 3 corn starch: talc: calcium carbonate: CMC (ratio 20: 87.5: 1.5: 1). Comparison of water solubility, suspension, and sedimentation properties test compared to single of talc calcium carbonate, CMC, and corn starch powder. Mixing ratio of 900 g of carrier substances (
Section 2.1) mixed with 100 ml of beneficial bacteria cells. Then, it was put to heat at 50°C for 3 hours. The bioproduct was divided into 5 g each foil bag, sealed tightly, and stored in room temperature conditions. The bioproduct of each formulation was assessed by examining the survival of beneficial bacterial populations every month using the Dilution plate count method on the NGA for 3 months.
2.5.6. Performance Testing of Bio-Products
The target economic crops consisted of Cruciferae (guangdong and kale), Gramineae (ruzi grass), and Solanaceae (chili) with blotter test for seed germination. The powdered product in the ratio of 1 kg of seed / 10 g of biochemical powder was compared with the sterilization control process. The seed germination was assessed after incubation at room temperature for 7 days and added water daily. Record the percentage of germination, plant length and root length, and growth parameter (Chuaboon et al., 2007). All data were analyzed for the variance and the difference of mean values by statistical DMRT by computer program.
- 2.
Disease inhibition
The efficacy of bioproduct to inhibit growth of C. capsici P-TU008 by dual culture method mention above. Using the bioproduct 1 g mixed with water 1 liter on solid culture medium, the experiment was designed with CRD. The P-TU008 was cultured on PDA for 5-7 days, using cork borers to penetrate the hypha tip of P-TU008 and the inoculum was placed upside-down distance 2 cm of the margins of the plate. The antagonistic bacteria cultured on the NGA for 24-48 hours were then crossed in the opposite of the fungus at 2 cm from the margins too, and incubation at room temperature for 24-48 hours. Efficiency was assessed by measuring the diameter of P-TU008 fungal colonies, comparing the efficiency with control with distilled water. Selected highly effective beneficial bacteria in the control to grow C. capsici P-TU008 for further experiments. The Materials and Methods should be described with sufficient details to allow others to replicate and build on the published results. Please note that the publication of your manuscript implicates that you must make all materials, data, computer code, and protocols associated with the publication available to readers. Please disclose at the submission stage any restrictions on the availability of materials or information. New methods and protocols should be described in detail while well-established methods can be briefly described and appropriately cited.
3. Results and Discussion
3.1. Isolate and Pathogenic Test
Twelve strains of C. capsici were isolated and when tested for pathogenicity on chili peppers, it was found that strain P-TU008 had the highest ability to cause anemia. The anthracnose in 3 types of test peppers, consisting of super-hot peppers, white peppers, and red peppers, were symptomatic within 3 days of inoculation. characterized by a rapid spread of invasion and found that green peppers exhibited slower symptoms than red peppers. This is consistent with Adikaram et al. (1982) who found that green peppers produced more capsicannol than red peppers. It was found that ripe red chilies had a lower concentration of the substance until it was insufficient to inhibit the growth of fungi, causing ripe chilies to show symptoms of disease.
3.2. Isolate Beneficial Bacteria
By isolating beneficial bacteria from 20 samples of fertile peppers showing no signs of any disease, 182 strains of beneficial bacteria were isolated and could be grouped into 8 groups by colony: 1) Glossy orange-yellow colonies, convex, smooth edge 2) White glossy, flat-rounded colonies, smooth edge 3) Light yellow, glossy, convex, smooth-edged colonies 4) Glossy dark yellow colonies Glossy, round, flat, edge smooth; 5) matt white colony, round flat, smooth edge; 6) glossy opaque colony, jagged edge; 7) Glossy red colony, convex, smooth edge, and 8) Yellow colony. Glossy, opaque, convex, smooth edges as reported by Hebbar et al. (1991), antagonistic microorganisms were isolated from leaves, roots, and parts of sunflower plants. The isolated pathogens belong to the group Actinomycetes, Pseudomonad and Bacillus, etc. In addition, Sutthisa and Namsang (2017) reported that antagonistic bacteria were isolated from tomato leaf surfaces for 46 isolates, 40 of which were obtained by washing the leaves and 6 isolates from leaf printing methods.
3.3. The Efficacy of Beneficial Bacteria in Inhibiting the Growth of C. capsici
3.3.1. Test on Solid Medium
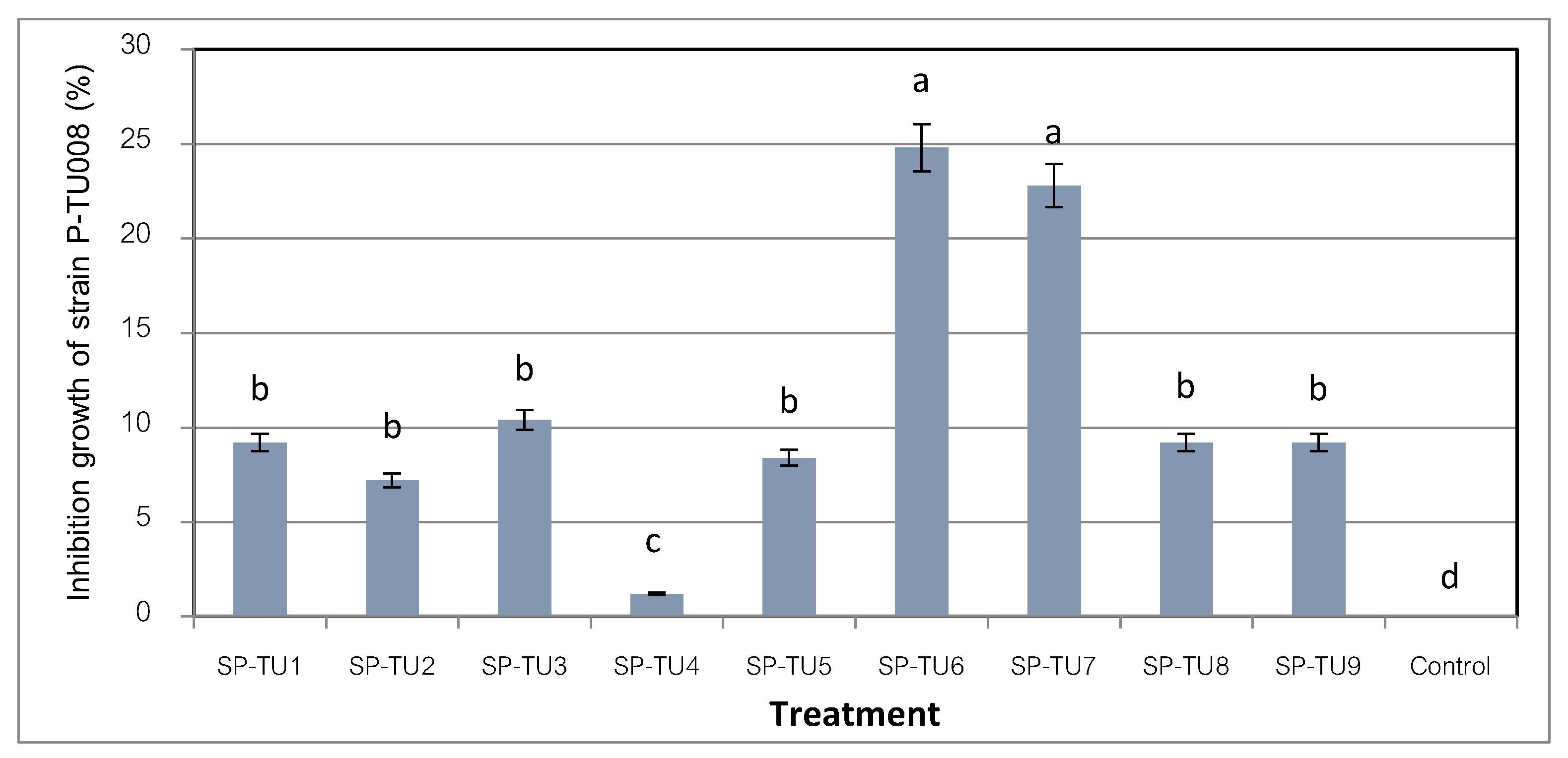
The efficacy test of the beneficial bacteria in inhibiting fungal growth of strains P-TU008 showed that the beneficial bacteria strains SP-TU7 and SP-TU6 had the highest efficiency. The inhibition percentages were 24.8 and 22.8 percent, respectively, followed by the SP-TU3 strain with 10.4 percent inhibitory efficiency (P=0.05) compared to the distilled water control treatment (
Figure 1). This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.3.2. Test in Liquid Medium
The inhibition efficacy of mycotoxins P-TU008 in NYDB medium was tested by selecting the most effective bacteria in inhibiting the growth of P-TU008 in 3 strains of solid media, strain SP-TU3, SP-TU6, and SP-TU7 found that these bacteria were still as effective in inhibiting mycelial growth as carbendazim chemical control. The inhibition percentages were 98.93, 95.69 and 94.62%, respectively (P=0.05) compared to the autoclave control. Consistent with research by Montesinos and Bonatera (1996), the bacteria Erwinia herbicola and Pseudomonas fluorescens were isolated from the roots of the plant. Spore germination and filamentous growth of Stemphylium vesicarium were inhibited with antibiotics.
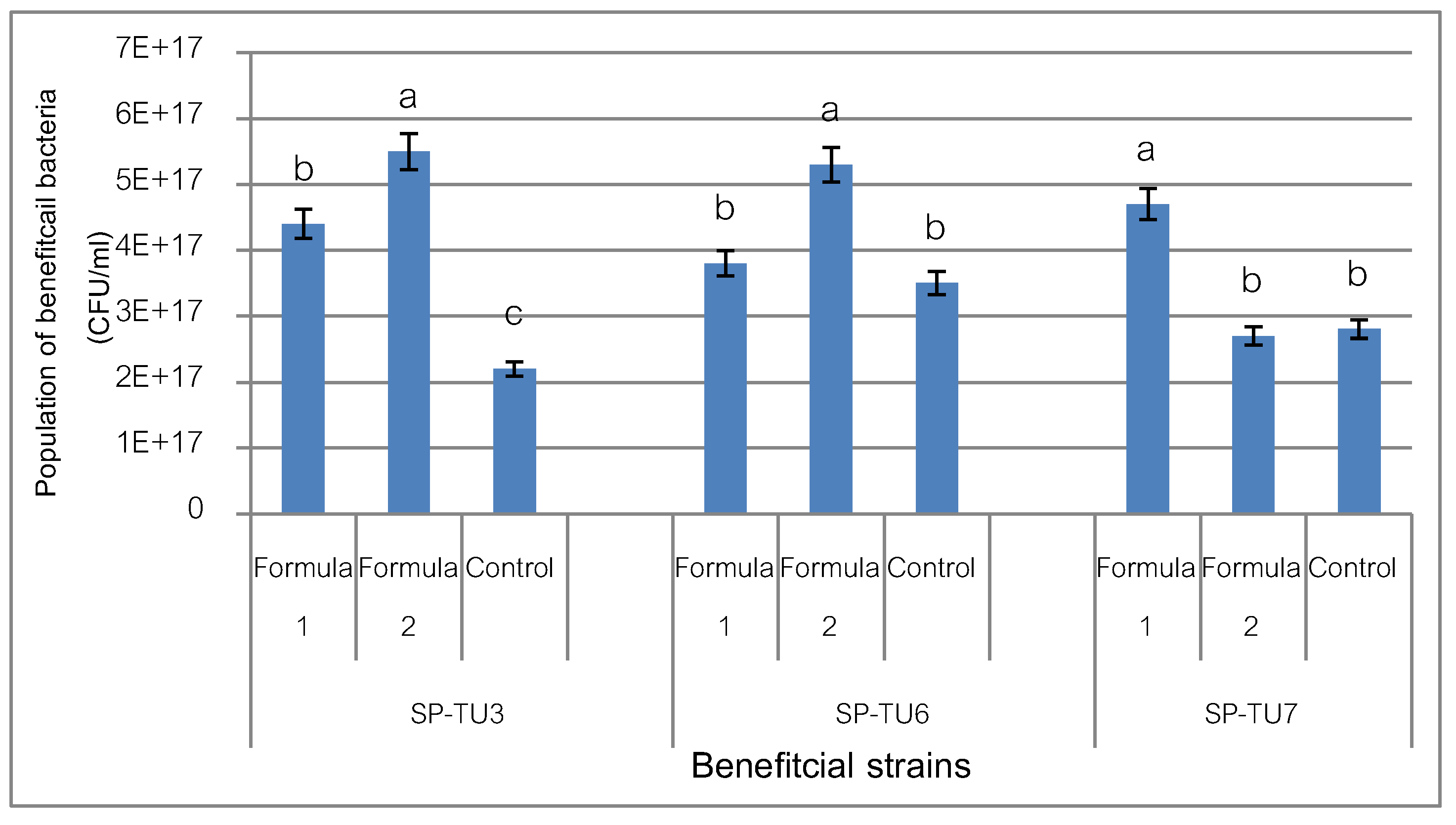
3.4. Develop a Formula to Increase the Number of Beneficial Bacteria Cells
From the experiment, it was found that Beneficial bacteria strains SP-TU3 and SP-TU6 thrived in the second formula (Formula 2) containing soy meal: molasses: CMC (8:5:1 g in water 1. l) with a population of 5.5x10
17cfu/ml, and strain SP-TU7 had the highest growth in the formula 1 (Formula 1) containing fishmeal: molasses: CMC (8:5: 1 g in 1 liter of water) with a population of 5.3x10
17 cfu/ml. Compared with the growth in NGB medium (
Figure 2), the experiment showed that different nutrient sources had different effect on the growth of beneficial bacteria. By developing a formula for increasing the amount using soybean meal. Or fish meal instead of beef extract and peptone, which is extracted from fat-free meat, contains carbohydrates nitrogen organic compounds water-soluble vitamins (Ralph, 2007) and use molasses as an alternative energy source for glucose. The enhanced formula developed was a formula with a nutrient composition that was readily available, cheap, and as effective as the standard NGA formula.
3.5. Development of Bio-Products in Water-Soluble Formulations to Promote the Growth of Economic Crops
Water solubility test suspension and precipitation of carrier substances. The properties of the 3 formulas of bio-products developed, it was found that all 3 formulas developed had the best water solubility at level 1, which was the best level by instant water-soluble carrier within 1- time. 5 min. As for the suspension and dispersion properties of the product, it was found that all 3 bioproduct formulations had the best suspension and sedimentation time of carriers. Level 1 was the suspension and precipitate within 12 -24 hours, as good as the solubility test and the single carrier sedimentation, which is also at its best.
3.6. Proper Ratio of Carriers and Nutrients that are Suitable for Maintaining the Viability of Beneficial Bacteria
From the development of 3 bio-formula formulas, namely Bioproduct Formula 1 (Bioproduct-1) contains talc: calcium carbonate: CMC ratio 9.75:1.5:1, Bioproduct Formula 2 (Bioproduct-2) cornstarch: talc: calcium carbonate: CMC Ratio 10:87.5:1.5:1 Formula 3 (Bioproduct-3) cornstarch: talc: calcium carbonate: CMC ratio 20:87.5:1.5:1 found that formula 1 bioproduct was suitable for maintaining the viability of bacteria with beneficial strains SP-TU3 and SP-TU6 with inoculum populations of 4.5x10
12 and 3.2x10
13 CFU/gram of bioproduct, respectively, and beneficial bacteria SP-TU7 strain were able to maintain viability in formulation 2 bioproduct. the highest with a population of 2.4x10
12 CFU/g bioproduct, when counting the cultured population after 3 months of storage (
Table 1).
3.7. Seed Germination Stimulation Test
The results showed that seed dressing with beneficial bacteria strain SP-TU6 in formula 3 (cornstarch: talc: calcium carbonate: CMC ratio 20:87.5:1.5:1) had the highest efficiency in seed treatment. Promote germination and growth indicators of Guangdong and kale were 98, 88% and 3.62, 88, respectively. The SP-TU7 strain in the second formula (cornstarch: talc: calcium carbonate: CMC ratio 10:87.5:1.5:1) had the highest germination-promoting and ruzi growth indicators at 62% and 6.51, respectively, while the SP-TU3 in Formula 1 (talc: calcium carbonate: CMC ratio 9.75:1.5:1) had the highest efficiency in promoting germination and growth indicators of chili peppers, 95 and 1.86, respectively (
Table 2). It helps bacteria to flourish around the roots of plants to promote plant growth and increase the efficiency of plant disease control. This is because it keeps the antagonist near the seed and can multiply along with the seedling to protect the plant as soon as it germinates (El-Hassan and Gowen, 2006).
4. Conclusions
From the experiment, all 182 strains of beneficial bacteria were isolated, divided into 8 groups according to colony characteristics. In anthracnose, the three beneficial bacterial strains with the best percentage of inhibition were identified as strains SP-TU3, SP-TU6, and SP-TU7, both tested on solid and liquid food. It is as effective as the chemical control process with carbendazim. From the experimental results, it can be concluded that strains SP-TU3 and SP-TU6 had the highest growth in the second formula and SP-TU7 strain had the highest growth in the second formula. 1 Bio-product formulations in water-soluble powder form. All 3 formulas developed have the best water solubility within 1-5 minutes. The suspension and dispersion in the water of the products found that all 3 formulations of bio-products have the level of the best carrier suspension and sedimentation time was within 12-24 hours. Formula 1 bio-product had an optimal ratio of carrier and nutrients suitable for maintaining the viability of the beneficial bacteria of strain SP-TU3 and SP-TU6 and formulation 2 bioproducts contained the optimal ratio of carriers and nutrients suitable for maintaining the viability of strain SP-TU7 and SP-TU6 beneficial bacteria in formulation 3. It is most effective in promoting germination and growth indicators of Guangdong and kale. The strains of bacteria SP-TU7 in Formula 2 and bacteria SP-TU3 in Formula 1 had the highest efficiency in promoting germination and growth indicators of ruzi grass and chili, respectively.
Acknowledgments
We thank Thammasat University Center of Excellence in Agriculture Innovation Centre through Supply Chain and Value Chain and Major of Agricultural Technology, Faculty of Science and Technology, Thammasat University for providing experimental and laboratory facility. This work was supported by the Thailand Science Research and Innovation Fundamental Fund.
References
- Adikaram, N.K.B.; Brown, A.E.; Swinburne, T.R. Phytoalexin involvement in the latent infection of Capsicum annuum L. fruit caused by Glomerella cingulata (Stonem). Physiol. Plant Pathol 1982, 21, 161–170. [Google Scholar] [CrossRef]
- Agrios, G.N. 1978. Plant Pathology. Academic Press., New York. 703 p.
- Arun, A. 2016. Local Institutions and Climate Change Adaptation. In The Social Dimensions of Climate Change. Social Development Department, The World Bank, Washington DC.
- Chirawut, B., R. Suttayakom and W. Dejnumbunchacha. 2016. Control of sweet pepper anthracnose by generally recognized as safe (GRAS) substances in combination with heat treatment. Thai Agricultural Research Journal. 34 (2): 134-149.
- Chuaboon, W and S. Prathuangwong. 2008. Appropriate management practices, frequency and concentration of beneficial bacteria co-operation onto increasable control efficiency of economic diseases on cauliflower plant. In Proc. of 46th Kasetsart University Annual Conference: Plants. 29 January –1 February 2008.
- Chuaboon, W., Kasem, S., and S. Prathuangwong. 2007. Co-efficacy of beneficial bacteria combined with natural products for growth promoting and induced resistance against diseases of cauliflower farm production.
- EI-Hassan, S.A.; Gowen, S.R. Formulation and delivery of the bacterial antagonist Bacillus subtilis for management of lentil vascular wilt caused by Fusarium oxysporum f.sp. lentis. J. Phytopathol 2006, 154, 148–155. [Google Scholar] [CrossRef]
- Hebbar, P.B.; Heulin, O.; Singh, S.P. Bacterial antagonists of sunflower (Helianthus annuus L.) fungal pathogen. Plant Soil 1991, 133, 40–131. [Google Scholar] [CrossRef]
- Jamsavang, J. 2011. Bioproducts: The Concept of Producing Bioproducts in Business. Department of Plant Pathology, Faculty of Agriculture, Kasetsart University.
- Koomen, I.; Jeffries, P. Effects of antagonistic microorganisms on the post-harvest development of Colletrotrichum gloeosporioides on mango. Plant Pathol 1993, 42, 230–237. [Google Scholar] [CrossRef]
- Montesinos, E.; Bonatera, A. Dose-response models in biological control of plant pathogens: an empirical verification. Phytopathology 1996, 5(86), 464–472. [Google Scholar] [CrossRef]
- Preecha, C. and S. Prathuangwong. 2009. Development of Bacillus amyloliquefaciens KPS46 formulation for control of soybean disease. P 222. Abstracts: In Proc. of ISSAAS congress 2008 "agriculture for the 3 Es: economy, environment, and energy. Thailand.
- Pupakdeepan, W. 2011. Diversity of Xanthomonas oryzae pv. oryzae Causes of Dry Leaf Edge Disease of Rice and Biological Control of Disease. Master’s Thesis. Kasetsart University.
- Ralph, S.T. 2007. Cultivation of Bacteria and Fungi. In Manual of Environmental Microbiology, 3rd Eds. Book Editor(s): Christon, J.H., Ronald L.C., Jay L.G., David A. L., Aaron L. M. and D.S. Linda. [CrossRef]
- Salah, E. K. and P. A. Abbasi. 2013. Isolation, characterization, and formulation of antagonistic bacteria for the management of seedlings damping-off and root rot disease of cucumber. Southern Crop Protection and Food Research Center. Agriculture and Agri-Food Canada, Canada.
- Sariah, M. 1994. Potential of Bacillus spp. as biocontrol agent for anthracnose fruit rot of chilli. Malayian Applied Biol. 23 (1-2): 53-60.
- Sutthisa, W. and P. Namsang. 2017. Screening of phylloplane bacteria from tomato leaves to control Stemphylium sp., a causal agent of gray leaf spot disease. Burapha Science Journal. 22 (Special Volume 2017): 73-83.
- Wongcharee, D. 2015. Efficacy of an Encapsulated Antagonistic Bacillus in Controlling Chili Anthracnose. Master’s Thesis. Suranaree University of Technology.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).