1. Introduction
Obesity is a global health crisis associated with numerous comorbidities, such as diabetes, cardiovascular diseases, and musculoskeletal disorders [
1,
2,
3]. Bariatric surgery is a widely recognized treatment for severe obesity, resulting in significant weight loss and a reduction in obesity-related health risks [
4,
5]. Despite these benefits, many patients continue to struggle with impaired quality of life due to residual or new health issues after surgery [
5].
Health-related quality of life (HRQoL) is a comprehensive measure of the overall well-being of individuals, encompassing physical, psychological, and social domains [
6,
7,
8]. HRQoL is particularly significant in clinical and public health settings, as it reflects the impact of health status, healthcare interventions and patient-reported outcomes [
9]. For bariatric patients, improving HRQoL is as important as achieving weight loss. Given the overlapping health challenges, the Sarcopenia Quality of Life (SarQoL®) questionnaire, which is specifically designed to assess quality of life in individuals with sarcopenia, needs to be adapted to evaluate HRQoL in individuals with obesity [
10].
Sarcopenia, a progressive and generalized loss of skeletal muscle mass and strength, is a significant public health concern, especially among the elderly population, and has significantly impacted physical performance in bariatric patients [
11]. It is associated with adverse outcomes such as physical disability, poor quality of life, and increased mortality. The condition not only impairs physical function but also affects psychological well-being, leading to a diminished HRQoL. Early detection is critical for proper management, making it essential to have criteria that can be routinely used in clinical practice. The European Working Group on Sarcopenia in Older People (EWGSOP) proposed an updated diagnostic pathway known as the EWGSOP2 criteria [
12]. Although initially developed for older adults, these criteria can also be relevant for bariatric patients, who often face similar muscle deterioration challenges [
13].
Recent data indicate that the prevalence of sarcopenia in the general population is approximately 11%, with a range from 3.2% to 26.3% [
14,
15]. This condition is particularly concerning because of its negative impact on quality of life, increased complication rates, and additional pressure on health systems [
16]. Concurrently, the prevalence of obesity remains high, and sarcopenia commonly coexists with obesity, creating a complex clinical entity known as sarcopenic obesity (SO) [
12,
17]. This condition triggers pathophysiological mechanisms, including insulin resistance, systemic inflammation, and oxidative stress. Sarcopenia and obesity mutually exacerbate each other, leading to a compounded negative effect on muscle mass and strength and increasing the risk of comorbidities such as type 2 diabetes, osteoporosis, cognitive impairment, and all-cause mortality [
18].
Despite the EWGSOP's concern over sarcopenic obesity, specific diagnostic pathways for SO were not immediately established, resulting in different diagnostic approaches in research [
19]. However, the European Society for Clinical Nutrition and Metabolism (ESPEN) and the European Association for the Study of Obesity (EASO) have recently published the first screening and diagnostic criteria with specific cutoff values for SO [
12]. This instrument aims to facilitate early diagnosis and establish the clinical importance of SO and its functional implications and impact on patients' quality of life.
Individuals with sarcopenic obesity generally have a poorer quality of life than those with obesity alone. Previous studies comparing the quality of life in individuals with SO versus those with sarcopenia alone have shown mixed results. Some studies indicate no significant differences or even better quality of life in people with SO [
20,
21]. These studies, however, used different diagnostic criteria than those recently established by ESPEN/EASO, highlighting the need for further research using new diagnostic standards [
12].
Evaluating the quality of life of bariatric patients with sarcopenic obesity should employ a specific tool, such as the SarQoL instrument. This tool has demonstrated good structural and psychometric properties across various cultural versions. The SarQoL questionnaire is a disease-specific instrument designed to evaluate HRQoL in individuals with sarcopenia. It covers various domains, including physical and mental health, daily activities, and social functioning, providing a comprehensive assessment of the impact of sarcopenia on quality of life [
22,
23].
The primary objective of this study was to assess the eventual impact of a structured exercise program on the HRQoL of individuals with sarcopenia after bariatric surgery, as evaluated by the SarQoL questionnaire. By determining the effectiveness of exercise interventions, this research aims to provide evidence-based recommendations for improving the quality of life in this population.
2. Materials and methods
Study Design
This randomized controlled trial (RCT) included patients with sarcopenia obesity who underwent gastric bypass (RYGB). The study was conducted over a period of six months at a Portuguese hospital.
The invitation to participate was made in the context of the preoperative evaluation, and participants who agreed to participate in the study were given the free and informed consent form previously approved by the University and Hospital Ethics Committee (HESE_CE_1917/21) (supplementary material).
The participants were randomly assigned to either the intervention group (IG), which received a structured exercise program, or the control group (CG), which received standard care without additional exercise intervention. Exercise training began one month after surgery and was conducted three times per week for 16 weeks, for a maximum of 55 minutes per session.
Eligibility Criteria
Patients were enrolled for bariatric surgery at the hospital; were diagnosed with sarcopenia on the basis of the European Association for the Study of Obesity/European Society for Clinical Nutrition and Metabolism (EASO/ESPEN) criteria, which include low muscle mass and low muscle strength without contraindications to exercise; and agreed to participate in the study. Patients who reported problems with locomotion, other previous bariatric surgery, or bariatric surgery complications were excluded.
Sample Size and Randomization
A total of 35 participants were enrolled in the study, with 19 in the IG and 16 in the CG. Patients proposed for bariatric surgery (gastric bypass-RYGB) were randomly assigned at the time of proposal by a systematic random process to usual care (CG) or usual care with an exercise program (IG).
Intervention
The program lasted 16 weeks, three times a week, for up to 55 minutes per session, starting one month after surgery. Each session started with 5 minutes of warm-up and ended with 10 minutes of cool-down [
24]. The intervention was a progressive combined exercise program based on the FITT-VP (frequency, type, intensity, time, type, duration, volume, and progression) prescription [
24,
25] (
Table 1).
Intervention Group: The intervention group participated in a structured exercise program designed to improve muscle strength, endurance, and overall physical function. A certified exercise physiologist supervised each session to ensure proper technique and safety. The program included:
Resistance training: weeks 1--4
Hypertrophy training: weeks 5--10
Strength training: weeks 11--16
Control Group: Participants in the control group received standard care, including regular health check-ups and nutritional counseling, but did not participate in any additional structured exercise program.
Outcomes
Anthropometry and body composition
Weight was measured with a scale with the patients wearing no shoes or heavy clothing. Height was determined by a manual stadiometer. BMI was calculated (weight/height
2), and the abdominal circumference was determined with a measuring tape [
26,
27]. To evaluate body composition, we used dual-energy X-ray absorptiometry (DEXA) (DXA, Hologic QDR, Hologic, Inc., Bedford, MA, USA) [
28].
Muscle strength
To evaluate the muscle strength of the upper limbs, a handgrip strength test was conducted via manual pressure dynamometry (handgrip). The participants were instructed to stand with their elbows fully relaxed and straight. Each hand was tested twice, and the maximum grip strength value obtained was recorded as the muscle strength test value [
29,
30].
The muscle strength of the lower limbs was evaluated via the sit-to-stand test, in which participants were instructed to stand and sit for 30 s as many times as possible [
31]. The timed chair stand test is a variation that counts how many times a patient can rise and sit in the chair over a 30-second interval [
32,
33]. Because the chair stand test evaluates both strength and endurance, it offers a reliable yet practical measure of strength but may be confounded by changes in weight after surgery.
Muscle mass
Muscle quantity or mass is evaluated by dual-energy X-ray absorptiometry (DEXA) because it is a common method for measuring skeletal muscle mass [
13]. Skeletal muscle mass refers to the amount of muscle that is attached to the skeleton and helps in systemic movement and maintaining posture, which means that the sum of the muscle masses of the four limbs is defined as the appendicular skeletal muscle mass (ASMM) [
34].
To calculate appendicular skeletal muscle mass (ASMM), we used the sum of the muscle masses of the upper and lower limbs (muscle mass of the arms [kg] + muscle mass of the legs [kg]). ASMM was divided by weight (meters) to diagnose sarcopenia (ASMM/weight) [
12,
35]. The ASMM score has been used to assess sarcopenic obesity [
36].
Health-related quality of life - SarQoL
The primary outcome measure was the SarQoL questionnaire, a validated tool. The primary outcome measure was the SarQoL questionnaire, a validated tool specifically designed to assess the quality of life of individuals with sarcopenia [
10,
37]. The SarQoL questionnaire is a self-administered tool developed in 2013 that aims to assess quality of life specifically related to sarcopenia; it comprises 55 items condensed into 22 questions, which are rated on a 4-point Likert scale organized into seven domains of quality of life. Scores range from 0 to 100, with higher scores indicating better quality of life [
10].
These domains include the following: 1. Physical and Mental Health: This domain assesses the overall physical and mental well-being of individuals with sarcopenia. It includes questions related to physical symptoms, emotional well-being, and overall satisfaction with health. 2. Locomotion: This domain focuses on an individual's ability to move and perform daily activities. It includes questions about mobility, balance, and the ability to perform tasks such as walking, climbing stairs, and getting in and out of chairs. 3. Body composition: This domain examines an individual's body composition, including muscle mass and body fat percentage. 4. Functionality: This domain assesses an individual's ability to perform basic functional tasks, such as dressing, bathing, and toileting. 5. Activities of Daily Living: This domain evaluates an individual's ability to perform activities that are essential for daily living, such as eating, grooming, and managing medications. 6. Leisure Activities: This domain focuses on an individual's engagement in leisure activities and hobbies. It includes questions about participation in recreational activities, hobbies, social interactions, and overall satisfaction with leisure time. 7. Fears: This domain assesses the individual's fears and concerns related to sarcopenia, such as fear of falling or fear of losing independence [
32].
Physical Performance
The 400-m walk test was used to measure walking ability and endurance. The participants were asked to complete 20 laps of 20 meters each as fast as possible and were allowed up to two rest stops during the test [
38,
39]. Low physical performance was considered when the test was not completed or when it took more than 6 minutes to complete [
40].
Diagnosis of Sarcopenia Obesity
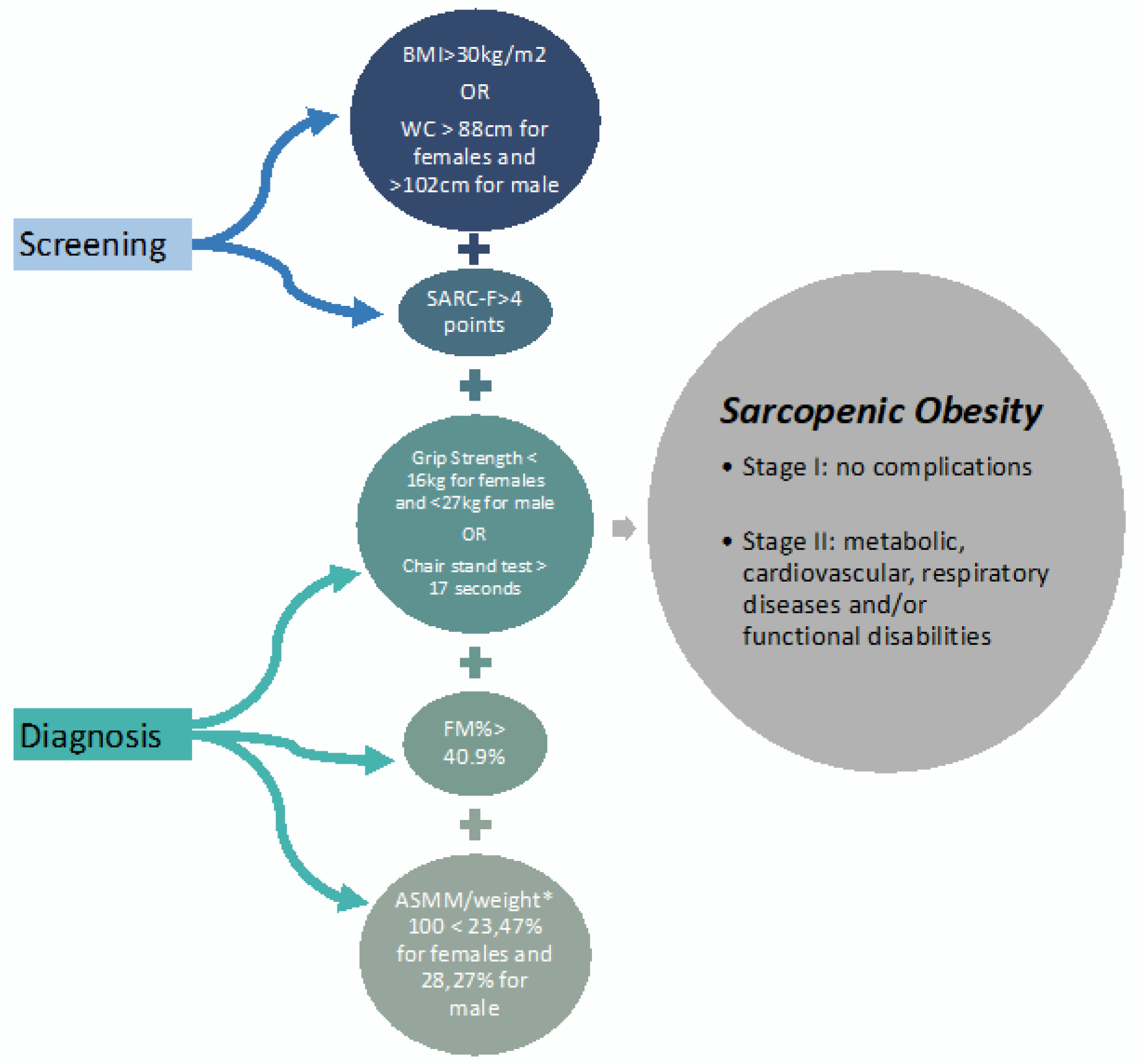
Sarcopenia is diagnosed and considered severe when a high BMI or waist circumference combined with low muscle mass, low muscle strength and low physical performance are identified (
Figure 1).
The first diagnostic criterion for sarcopenia is low muscle strength. Low muscle strength was defined as a handgrip strength of <27 kg for males and <16 kg for females [
41] and >17 s for the chair stand test [
12,
17,
42].
Data collection
Data were collected at baseline and post-intervention by the same team, consisting of one specialist nurse, a surgeon, and an exercise physiologist. The participants completed the SarQoL questionnaire and underwent physical performance and muscle strength assessments at each time point.
Statistical methods
Data analysis was performed via Jamovi (version 1.6). Descriptive statistics were used to summarize the baseline characteristics. Categorical variables are expressed as frequencies and percentages, and continuous variables are expressed as the means and standard deviations. Data normality was assessed with the Shapiro‒Wilk test and an independent t test or Mann‒Whitney test to examine group differences. Differences between the intervention and control groups were assessed via independent t tests for continuous variables and chi-square tests for categorical variables. Reliability was analyzed according to internal consistency and considered acceptable when Cronbach’s alpha was ≥ 0.7. Changes in SarQoL scores and secondary outcomes were analyzed via repeated-measures ANOVA. Correlation analyses explored the relationships between changes in SarQoL scores and physical performance measures. Statistical significance was set at p < 0.05.
3. Results
The baseline characteristics of the participants are presented in
Table 1. The mean age of the participants was 46.9 years, with no significant difference between the intervention and control groups (p = 0.071). The sex distribution was 77.1% female and 22.9% male. Baseline SarQoL scores, physical function, muscle mass, and muscle strength were comparable between the two groups, indicating successful randomization.
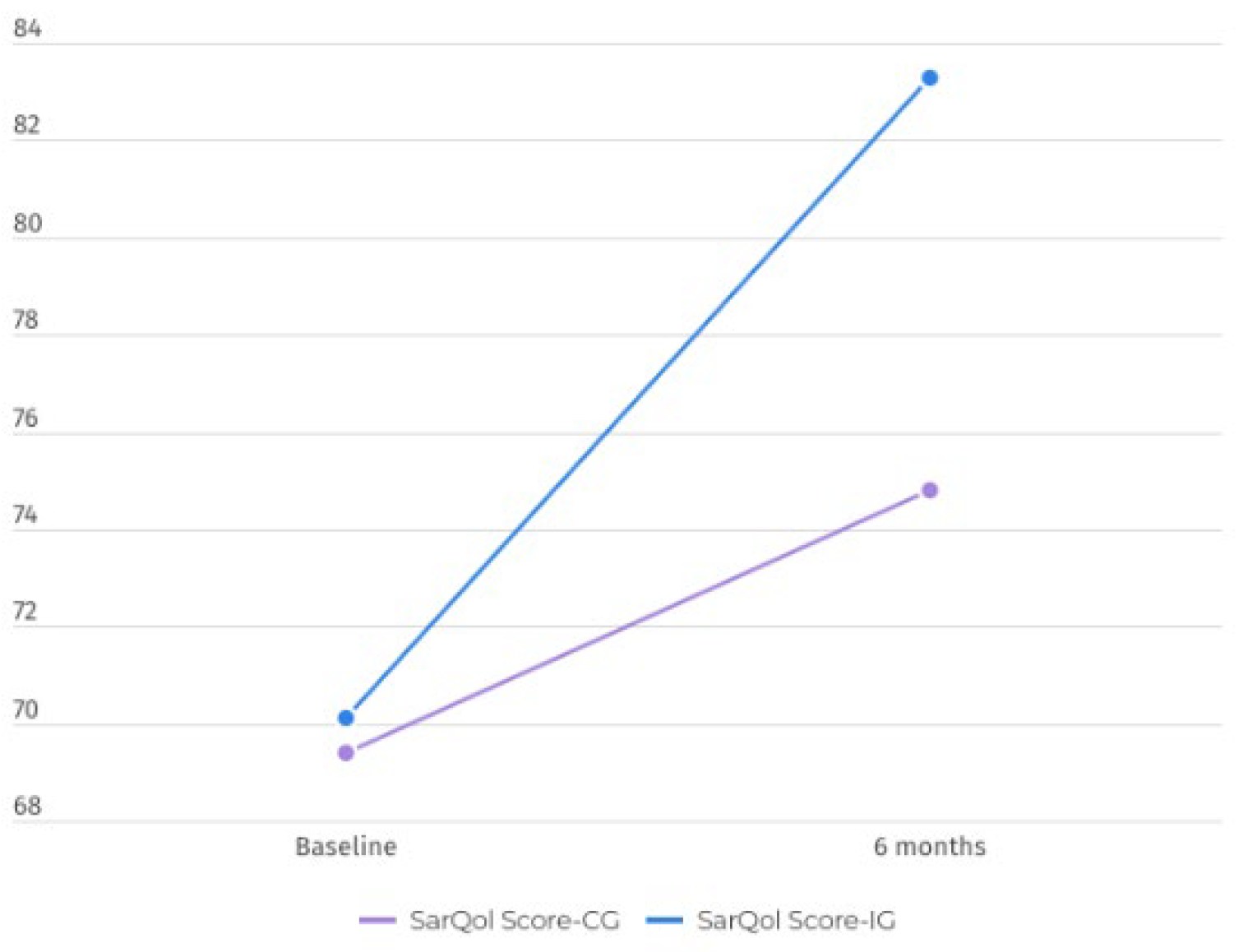
The primary outcome measure was the change in the SarQoL score from baseline to post-intervention. Reliability was analyzed according to internal consistency, and Cronbach’s alpha was considered excellent (0.946). Compared with the control group, the intervention group presented significant improvements in SarQoL scores (p < 0.001) (p=0.103). The mean increase in the SarQoL score for the intervention group was 13.2 points, whereas the control group had a mean increase of 5.4 points. The group differences were significant, with a large effect size (p=0.038; η2=0.125) (
Table 2).
The comparative analysis of the changes after the exercise program (
Table 3) revealed that participants in the intervention group demonstrated significant improvements in physical performance, as measured by the sit-to-stand and 400-m walk tests. The mean sit-to-stand score increased by 1.68 points in the IG compared with 0.41 points in the CG (p=0.014), and the 400-m walk test score increased by -1.18 points in the IG versus -0.04 points in the CG (p=0.014).
Handgrip strength improved significantly in the intervention group, with a mean increase of 2.39 kg, whereas the control group showed a mean increase of 1.29 kg (p=0.012).
The SarQoL questionnaire has different metric properties for all the items assessed. The overall SarQoL score significantly differed after the exercise program (p=0.038) (
Figure 2) but also differed in two domains: domain 2—locomotion (p=0.094, η2=0.189) and domain 5—activities of daily living (p=0.005, η2=0.125), with a large effect size in both.
4. Discussion
This study aimed to explore the impact of exercise on HRQoL in bariatric patients via the SarQoL questionnaire.
Bariatric surgery, a significant intervention for treating severe obesity, aims not only to reduce weight but also to enhance overall quality of life [
43]. The effectiveness of bariatric surgery extends beyond physical health improvements to encompass psychological and social dimensions [
44]. Patient-reported outcome measures (PROMs), particularly HRQoL metrics, are essential tools for assessing these dimensions [
5]. PROMs are instruments used to capture patients' perspectives on their health status, treatment efficacy, and overall well-being. These self-reported measures provide invaluable insights that complement clinical evaluations. HRQoL specifically focuses on aspects of quality of life directly related to health conditions and treatments, including physical functioning, mental health, and social interactions [
7].
HRQoL measures capture improvements in physical health, which include increased mobility, reduced pain, and increased energy levels. Patients often report significant gains in their ability to perform daily activities and exercise, which are critical positive indicators of surgery [
45].
HRQoL is a comprehensive measure of the overall well-being of individuals, encompassing physical, psychological, and social domains. For bariatric patients, improving HRQoL is as important as achieving weight loss [
46].
The results of this study indicate that a structured exercise program significantly improves HRQoL in bariatric patients diagnosed with sarcopenia, as measured by the SarQoL questionnaire [
10]. Compared with the control group, the intervention group experienced a substantial improvement in SarQoL scores, highlighting the positive impact of regular physical activity on various dimensions of quality of life, including physical and mental health, functionality, and social engagement.
These findings are consistent with previous research demonstrating the benefits of exercise in older adults and those with chronic health conditions. Exercise has been shown to enhance muscle strength, physical performance, and overall well-being, likely contributing to the observed HRQoL improvements [
47].
Several mechanisms may explain the beneficial effects of exercise on HRQoL in sarcopenic bariatric patients. First, resistance training increases muscle mass and strength, which are critical for maintaining physical function and reducing the risk of disability. Improved muscle function enables individuals to perform daily activities more efficiently and with less fatigue, leading to increased independence and quality of life [
48].
Second, aerobic exercise improves cardiovascular fitness and endurance, which can reduce the sensation of fatigue and improve overall energy levels [
49]. This increase in physical capacity may also enhance participation in social and recreational activities, contributing to better mental health and social well-being [
50].
Third, flexibility and balance exercises help prevent falls and related injuries, which are common concerns in older adults with sarcopenia. By reducing the risk of falls, these exercises contribute to a greater sense of security and confidence in daily activities [
51].
Finally, regular exercise is associated with various psychological benefits, including reduced symptoms of depression and anxiety, improved mood, and better stress management. These mental health improvements likely play a significant role in the overall increase in HRQoL observed in the intervention group [
52,
53].
Obesity is frequently associated with psychological issues, including depression, anxiety, and low self-esteem [
8]. After surgery, many patients experience improvements in these areas, which are effectively captured through HRQL metrics. By evaluating changes in mental health status, PROMs help in understanding the psychological benefits of bariatric surgery, such as increased self-confidence, better body image, and reduced depression symptoms [
54,
55].
The social implications of obesity, such as social stigma and isolation, can be profound. HRQL assessments after bariatric surgery often reveal improvements in social interactions and relationships [
56]. Patients may experience increased social participation, better interpersonal relationships, and improved overall life satisfaction. These improvements are crucial for obtaining a holistic understanding of the impact of surgery on patients' lives [
57]. PROMs provide an evaluation of bariatric surgery outcomes. By capturing the subjective experiences of patients, healthcare providers can tailor follow-up care and interventions to address specific needs and concerns. This personalized approach ensures that the treatment is not only clinically effective but also aligns with the patient's quality of life goals [
58].
The use of PROMs in post-surgery evaluations allows for long-term monitoring of patients' well-being. Regular HRQL assessments can help identify emerging issues or declining trends in health-related quality of life, prompting timely interventions [
59,
60]. Continuous monitoring supports sustained improvements and helps in managing any complications or psychosocial challenges that may arise. PROMs, particularly those measuring HRQL, are indispensable in evaluating the comprehensive outcomes of bariatric surgery. They provide critical insights into the physical, psychological, and social improvements experienced by patients, facilitating a holistic understanding of the impact of surgery [
55]. By integrating PROMs into post-surgical care, healthcare providers can enhance personalized care, ensure long-term support, and ultimately improve the overall success of bariatric interventions.
5. Conclusions
In conclusion, this study demonstrated that a structured exercise program significantly improved health-related quality of life in bariatric patients diagnosed with sarcopenia, as evaluated by the SarQoL questionnaire. These findings underscore the importance of incorporating regular physical activity into the management of sarcopenia to increase overall well-being and quality of life. Healthcare providers should prioritize the promotion and integration of exercise programs for sarcopenic populations to address this growing public health concern effectively.
The findings of this study have significant implications for clinical practice and public health policy. Given the substantial improvements in HRQoL observed with exercise interventions, healthcare providers should consider incorporating structured exercise programs into standard care for bariatric patients with sarcopenia. Exercise regimens should be tailored to individual capabilities and preferences, ensuring safety and adherence.
The healthcare system should support the development and implementation of community-based exercise programs for older adults and individuals with sarcopenia. Providing accessible and affordable exercise options can help improve the overall quality of life in this population and reduce healthcare costs associated with sarcopenia-related complications. Additionally, research should examine the cost-effectiveness of exercise interventions in improving HRQoL and reducing healthcare utilization in sarcopenic populations, providing further evidence to support the widespread implementation of exercise programs.
Author Contributions
Conceptualization, C.M. and M.C.; methodology, C.M.; software, C.M., A.R.; validation, A.R., S.M. and M.C.; formal analysis, C.M.; investigation, C.M. and M.C.; resources, A.R.; data curation, M.C.; writing—original draft preparation, C.M. and M.C.; writing—review and editing, M.C., A.R., J.B.; visualization, J.B.; supervision, S.M.; project administration, C.M.; funding acquisition, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethics Committee (HESE_CE_1917/21).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- H. Yuan et al., “A comparison of interferential current efficacy in elderly intervertebral disc degeneration patients with or without sarcopenia: a retrospective study,” BMC Musculoskelet Disord, vol. 25, no. 1, Dec. 2024. [CrossRef]
- L. B. Sardinha et al., “Prevalence of overweight, obesity, and abdominal obesity in a representative sample of Portuguese adults,” PLoS One, vol. 7, no. 10, p. e47883, Oct. 2012. [CrossRef]
- A. Morabia and T. Abel, “The WHO report ‘Preventing chronic diseases: a vital investment’ and us,” Soz Praventivmed, vol. 51, no. 2, p. 74, Apr. 2006. [CrossRef]
- P. R. Schauer and F. Rubino, “International Diabetes Federation position statement on bariatric surgery for type 2 diabetes: Implications for patients, physicians, and surgeons,” Surgery for Obesity and Related Diseases, vol. 7, no. 4, pp. 448–451, Jul. 2011. [CrossRef]
- M. Raoof et al., “Health-Related Quality-of-Life (HRQoL) on an Average of 12 Years After Gastric Bypass Surgery,” Obes Surg, vol. 25, no. 7, pp. 1119–1127, Jul. 2015. [CrossRef]
- D. H. Griauzde, A. M. Ibrahim, N. Fisher, A. Stricklen, R. Ross, and A. A. Ghaferi, “Understanding the psychosocial impact of weight loss following bariatric surgery: a qualitative study,” BMC Obes, vol. 5, no. 1, Dec. 2018. [CrossRef]
- K. D. Coulman, F. MacKichan, J. M. Blazeby, and A. Owen-Smith, “Patient experiences of outcomes of bariatric surgery: a systematic review and qualitative synthesis,” Obes Rev, vol. 18, no. 5, pp. 547–559, May 2017. [CrossRef]
- J. F. Brazil et al., “Improved Quality of Life, Fitness, Mental Health and Cardiovascular Risk Factors with a Publicly Funded Bariatric Lifestyle Intervention for Adults with Severe Obesity: A Prospective Cohort Study,” Nutrients 2021, Vol. 13, Page 4172, vol. 13, no. 11, p. 4172, Nov. 2021. [CrossRef]
- C. Santos, M. Carvalho, L. Oliveira, A. Palmeira, L. M. Rodrigues, and J. Gregório, “The Long-Term Association between Physical Activity and Weight Regain, Metabolic Risk Factors, Quality of Life and Sleep after Bariatric Surgery,” Int J Environ Res Public Health, vol. 19, no. 14, p. 8328, Jul. 2022. [CrossRef]
- C. Beaudart et al., “Validation of the SarQoL®, a specific health-related quality of life questionnaire for Sarcopenia,” J Cachexia Sarcopenia Muscle, vol. 8, no. 2, pp. 238–244, Apr. 2017. [CrossRef]
- A. J. Cruz-Jentoft et al., “Sarcopenia: European consensus on definition and diagnosis,” Age Aging, vol. 39, no. 4, pp. 412–423, Apr. 2010. [CrossRef]
- L. M. Donini et al., “Consensus Statement Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement,” 2022. [CrossRef]
- E. Ramirez, R. Salas, C. Bouzas, R. Pastor, and J. A. Tur, “Comparison between Original and Reviewed Consensus of European Working Group on Sarcopenia in Older People: A Probabilistic Cross-Sectional Survey among Community-Dwelling Older People,” Gerontology, vol. 68, no. 8, pp. 869–876, Aug. 2022. [CrossRef]
- M. L. Endalifer and G. Diress, “Epidemiology, Predisposing Factors, Biomarkers, and Prevention Mechanism of Obesity: A Systematic Review,” J Obes, vol. 2020, 2020. [CrossRef]
- S. Wei, T. T. Nguyen, Y. Zhang, D. Ryu, and K. Gariani, “Sarcopenic obesity: epidemiology, pathophysiology, cardiovascular disease, mortality, and management,” Front Endocrinol (Lausanne), vol. 14, 2023. [CrossRef]
- O. Ethgen, C. Beaudart, F. Buckinx, O. Bruyère, and J. Y. Reginster, “The Future Prevalence of Sarcopenia in Europe: A Claim for Public Health Action,” Calcif Tissue Int, vol. 100, no. 3, pp. 229–234, Mar. 2017. [CrossRef]
- C. Tsigos et al., “Criteria for EASO-Collaborating Centers for Obesity Management,” Obes Facts, vol. 4, no. 4, p. 329, Aug. 2011. [CrossRef]
- N. N. Crispim Carvalho, V. J. B. Martins, J. M. Filho, A. da C. P. de Arruda Neta, F. C. F. Pimenta, and J. L. de Brito Alves, “Effects of preoperative sarcopenia-related parameters on the musculoskeletal and metabolic outcomes after bariatric surgery: a one-year longitudinal study in females,” Sci Rep, vol. 13, no. 1, Dec. 2023. [CrossRef]
- A. J. Cruz-Jentoft et al., “Erratum: Sarcopenia: Revised European consensus on definition and diagnosis (Age and Aging DOI: 10.1093/aging/afy169),” Age Aging, vol. 48, no. 4, p. 601, Jul. 2019. [CrossRef]
- R. H. Liu and J. D. Irwin, “Understanding the postsurgical bariatric experiences of patients two or more years after surgery,” Quality of Life Research, vol. 26, no. 11, pp. 3157–3168, Nov. 2017. [CrossRef]
- J. A. Batsis, T. A. Mackenzie, L. K. Barre, F. Lopez-Jimenez, and S. J. Bartels, “Sarcopenia, sarcopenic obesity and mortality in older adults: Results from the National Health and Nutrition Examination Survey III,” Eur J Clin Nutr, vol. 68, no. 9, pp. 1001–1007, Sep. 2014. [CrossRef]
- C. Beaudart et al., “Validation of the SarQoL®, a specific health-related quality of life questionnaire for Sarcopenia,” J Cachexia Sarcopenia Muscle, vol. 8, no. 2, pp. 238–244, Apr. 2017. [CrossRef]
- C. Beaudart et al., “Sarcopenia in daily practice: assessment and management,” BMC Geriatr, vol. 16, no. 1, pp. 1–10, Oct. 2016. [CrossRef]
- B. A. Bushman, “Determining the i (Intensity) for a FITT-VP aerobic exercise prescription,” ACSMs Health Fit J, vol. 18, no. 3, pp. 4–7, 2014. [CrossRef]
- L. M. Burke, G. J. Slater, J. J. Matthews, C. Langan-Evans, and C. A. Horswill, “ACSM Expert Consensus Statement on Weight Loss in Weight-Category Sports,” Curr Sports Med Rep, vol. 20, no. 4, pp. 199–217, Apr. 2021. [CrossRef]
- K. I. Norton, “Standards for Anthropometry Assessment,” Kinanthropometry and Exercise Physiology, pp. 68–137, Sep. 2018. [CrossRef]
- K. Devonshire-Gill, “The Exercise-Health Paradigm: A historical perspective. In Kinanthropometry and Exercise Physiology.,” Kinanthropometry and Exercise Physiology, p. 28, 2018.
- M. Pekař, A. Pekařová, M. Bužga, P. Holéczy, and M. Soltes, “The risk of sarcopenia 24 months after bariatric surgery – assessment by dual energy X-ray absorptiometry (DEXA): a prospective study,” Videosurgery and Other Miniinvasive Techniques, vol. 15, no. 4, pp. 583–587, Mar. 2020. [CrossRef]
- H. C. Roberts et al., “A review of the measurement of grip strength in clinical and epidemiological studies: Toward a standardized approach,” Age Aging, vol. 40, no. 4, pp. 423–429, Jul. 2011. [CrossRef]
- R. Cooper, D. Tomlinson, M. Hamer, and S. M. Pinto Pereira, “Lifetime body mass index and grip strength at age 46 years: the 1970 British Cohort Study,” J Cachexia Sarcopenia Muscle, vol. 13, no. 4, pp. 1995–2004, Aug. 2022. [CrossRef]
- A. Soriano-Maldonado et al., “Physical Exercise following bariatric surgery in women with Morbid obesity: Study protocol clinical trial (SPIRIT compliant),” Medicine (United States), vol. 99, no. 12, 2020. [CrossRef]
- C. Beaudart et al., “Sarcopenia in daily practice: assessment and management,” BMC Geriatr, vol. 16, no. 1, pp. 1–10, 2016. [CrossRef]
- M. Cesari et al., “Added Value of Physical Performance Measures in Predicting Adverse Health-Related Events: Results from the Health, Aging and Body Composition Study,” J Am Geriatr Soc, vol. 57, no. 2, pp. 251–259, Feb. 2009. [CrossRef]
- S. A. Studenski et al., “The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates,” The Journals of Gerontology: Series A, vol. 69, no. 5, pp. 547–558, May 2014. [CrossRef]
- H. Gould, S. L. Brennan, M. A. Kotowicz, G. C. Nicholson, and J. A. Pasco, “Total and appendicular lean mass reference ranges for Australian men and women: the Geelong osteoporosis study,” Calcif Tissue Int, vol. 94, no. 4, pp. 363–372, 2014. [CrossRef]
- C. Liu et al., “The role of obesity in sarcopenia and the optimal body composition to prevent against sarcopenia and obesity,” Front Endocrinol (Lausanne), vol. 14, p. 1077255, 2023. [CrossRef]
- A. Geerinck, M. Locquet, O. Bruyère, J. Y. Reginster, and C. Beaudart, “Evaluating quality of life in frailty: applicability and clinimetric properties of the SarQoL® questionnaire,” J Cachexia Sarcopenia Muscle, vol. 12, no. 2, pp. 319–330, Apr. 2021. [CrossRef]
- L. Baroudi, M. W. Newman, E. A. Jackson, K. Barton, K. A. Shorter, and S. M. Cain, “Estimating Walking Speed in the Wild,” Front Sports Act Living, vol. 0, p. 166, Nov. 2020. [CrossRef]
- S. Vestergaard, K. V. Patel, S. Bandinelli, L. Ferrucci, and J. M. Guralnik, “Characteristics of 400-Meter Walk Test Performance and Subsequent Mortality in Older Adults,” Rejuvenation Res, vol. 12, no. 3, p. 177, Jun. 2009. [CrossRef]
- A. J. Cruz-Jentoft et al., “Sarcopenia: revised European consensus on definition and diagnosis,” Age Aging, vol. 48, no. 1, pp. 16–31, Jan. 2019. [CrossRef]
- R. M. Dodds et al., “Grip strength across the life course: normative data from twelve British studies,” PLoS One, vol. 9, no. 12, Dec. 2014. [CrossRef]
- M. Cesari et al., “Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study,” J Am Geriatr Soc, vol. 57, no. 2, pp. 251–259, Feb. 2009. [CrossRef]
- L. Sjöström, “Review of the key results from the Swedish Obese Subjects (SOS) trial – a prospective controlled intervention study of bariatric surgery,” J Intern Med, vol. 273, no. 3, pp. 219–234, Mar. 2013. [CrossRef]
- R. L. Kolotkin and J. R. Andersen, “A systematic review of reviews: exploring the relationship between obesity, weight loss and health-related quality of life,” Clin Obes, vol. 7, no. 5, pp. 273–289, Oct. 2017. [CrossRef]
- A. J. Budin, P. Sumithran, A. D. MacCormick, I. D. Caterson, and W. A. Brown, “Patient and healthcare practitioner evaluation of patient-reported outcomes in bariatric surgery – a modified Delphi study,” International Journal of Obesity 2024, pp. 1–9, Jul. 2024. [CrossRef]
- S. G. Engel et al., “Psychometric and cross-national evaluation of a Portuguese version of the Impact of Weight on Quality of Life-Lite (IWQOL-Lite) questionnaire,” European Eating Disorders Review, vol. 13, no. 2, pp. 133–143, Mar. 2005. [CrossRef]
- M. Steffl, R. W. Bohannon, L. Sontakova, J. J. Tufano, K. Shiells, and I. Holmerova, “Relationship between sarcopenia and physical activity in older people: A systematic review and meta-analysis,” Clin Interv Aging, vol. 12, pp. 835–845, May 2017. [CrossRef]
- M. E. Nelson et al., “Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association,” Med Sci Sports Exerc, vol. 39, no. 8, pp. 1435–1445, Aug. 2007. [CrossRef]
- A. B. Newman et al., “Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability,” JAMA, vol. 295, no. 17, pp. 2018–2026, 2006. [CrossRef]
- L. Sjöström et al., “Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery,” N Engl J Med, vol. 351, no. 26, pp. 2683–2693, Dec. 2004. [CrossRef]
- A. Shumway-Cook, W. Gruber, M. Baldwin, and S. Liao, “The effect of multidimensional exercises on balance, mobility, and fall risk in community-dwelling older adults,” Phys Ther, vol. 77, no. 1, pp. 46–57, 1997. [CrossRef]
- J. F. Kubik, R. S. Gill, M. Laffin, and S. Karmali, “The impact of bariatric surgery on psychological health,” J Obes, vol. 2013, 2013. [CrossRef]
- A. Harper et al., “Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group,” Psychol Med, vol. 28, no. 3, pp. 551–558, May 1998. [CrossRef]
- K. D. Coulman, T. Abdelrahman, A. Owen-Smith, R. C. Andrews, R. Welbourn, and J. M. Blazeby, “Patient-reported outcomes in bariatric surgery: a systematic review of standards of reporting,” Obes Rev, vol. 14, no. 9, pp. 707–720, Sep. 2013. [CrossRef]
- C. Mendes, M. Carvalho, C. Martins, L. M. Rodrigues, and J. Gregório, “Design and Development of a Nurse-Led Program for the Management of Bariatric Surgery Patients-The NURLIFE Program,” Prof Case Manag, vol. 29, no. 5, pp. 229–234, Sep. 2024. [CrossRef]
- L. Tolvanen, Å. Svensson, E. Hemmingsson, A. Christenson, and Y. T. Lagerros, “Perceived and Preferred Social Support in Patients Experiencing Weight Regain After Bariatric Surgery-a Qualitative Study,” Obes Surg, vol. 31, no. 3, pp. 1256–1264, Mar. 2021. [CrossRef]
- D. Wolf et al., “Can nurses impact patient outcomes using a patient-centered care model?,” J Nurs Adm, vol. 38, no. 12, pp. 532–540, Dec. 2008. [CrossRef]
- J. Camolas, O. Santos, P. Moreira, and I. do Carmo, “INDIVIDUO: Results from a patient-centered lifestyle intervention for obesity surgery candidates,” Obes Res Clin Pract, vol. 11, no. 4, pp. 475–488, Jul. 2017. [CrossRef]
- K. D. Coulman, F. MacKichan, J. M. Blazeby, J. L. Donovan, and A. Owen-Smith, “Patients’ experiences of life after bariatric surgery and follow-up care: a qualitative study,” BMJ Open, vol. 10, no. 2, Feb. 2020. [CrossRef]
- K. D. Coulman et al., “A Comparison of Health Professionals’ and Patients’ Views of the Importance of Outcomes of Bariatric Surgery,” Obes Surg, vol. 26, no. 11, pp. 2738–2746, Nov. 2016. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).