1. Introduction
Novel modalities to approach bone repair may address the burdens patients may face especially within aging populations and complications concerning bone density [
1]. One such avenue may be the usage of cold therapy, where intermittent short term cold exposure has been shown to lead to increased bone healing dynamics[
2,
3]. However, a major bottleneck in ideal application of cold exposure is lack of understanding the mechanistic action. Numerous in vivo studies and observations have shown that prolonged cold exposure impedes bone growth, repair, and morphology[
4,
5,
6], while short-term cold exposure exerts beneficial effects on bone healing and morphology[
3,
7,
8,
9]. In vitro investigations have demonstrated that long term cold exposure inhibits specific pathways associated with bone healing, such as osteoblastogenesis and hypoxia signaling[
4,
5,
10,
11,
12,
13]. Conversely, short term cold exposure induces an upregulation of these pathways but have yet to be prominently identified within an in-vivo bone injury model[
14,
15,
16].
Several investigations into short term cold application have elucidated its potential for augmenting bone development and repair, both in vitro and
in vivo. In vivo studies have consistently demonstrated enhanced bone formation post-injury- characterized by increased deposition of bone tissue at the injury site, concomitant with elevated levels of osteoblast activity biomarkers such as alkaline phosphatase (ALP)[
2,
3,
17]. Additionally, in vitro experiments have revealed that cold stimulation of mesenchymal stem cells (MSCs) and osteoblast progenitors promotes osteoblast differentiation[
14].
Collectively, cold exposure initiates a shift in cellular expression, especially within bone cells, stimulating anabolic bone formation pathways. Established literature has shown that cold exposure induces rapid cellular responses, largely mediated by the activation of cold shock proteins[
18,
19]. RNA binding motif protein 3 (RBM3) in particular has been established as the most representative protein expressed under cold shock[
20]. Similarly, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is a key regulator in bone homeostasis affecting primarily osteoblastogenesis as well as mitochondrial biogenesis[
21]. Both cold shock proteins hold a prominent role in cellular adaptation necessary for cellular survival [
22,
23] Recently, these proteins have garnered particular attention for potential impact on bone repair due to their prevalence in osteoblastic cells and pivotal roles in regulating osteoblastogenesis pathways[
19]. Additionally, it has been previously established that compromise of the vascular network in and around bone results in hypoxia, leading to the activation of a key mechanism in bone healing to restore the blood flow, i.e., angiogenesis[
24]. Angiogenesis is a critical part of the bone healing process in order to restore native blood flow following a bone injury[
25]. In-vivo bone injury studies have shown enhancement of vasculature following cold treatment- with angiogenic markers such as vascular endothelial growth factor (VEGF) being upregulated indicative of enhancement of angiogenic pathways. However, there is a lack of findings regarding the mechanism of action at the bone injury site[
3,
17]. Reports of short cold exposure have been shown to effect bone perfusion[
26]; for instance 10 min of immersion in 22°C water causes approximately 40% reduction of whole limb blood flow[
7].
The TRP (Transient Receptor Potential) family of receptors are expressed in sensory neurons and vascular endothelial cells. These play a crucial role in sensing various stimuli, including temperature changes[
27,
28]. Among the TRP family, TRP channels such as transient receptor potential cation channel subfamily M (TRPM8) and transient receptor potential ankyrin 1 (TRPA1) are particularly involved in the perception of cold temperatures and in the conduction of this vasoconstrictive pathway[
29]. TRPM8 channels are pivotal in coordinating responses to cold exposure within a temperature range typically from 8°C to 28°C[
30]. TRPA1 channels are activated by cold exposure within a range of approximately 8°C to 17°C causing vasoconstriction and changes in blood flow, essential for thermoregulation and tissue viability during cold exposure[
31]
.
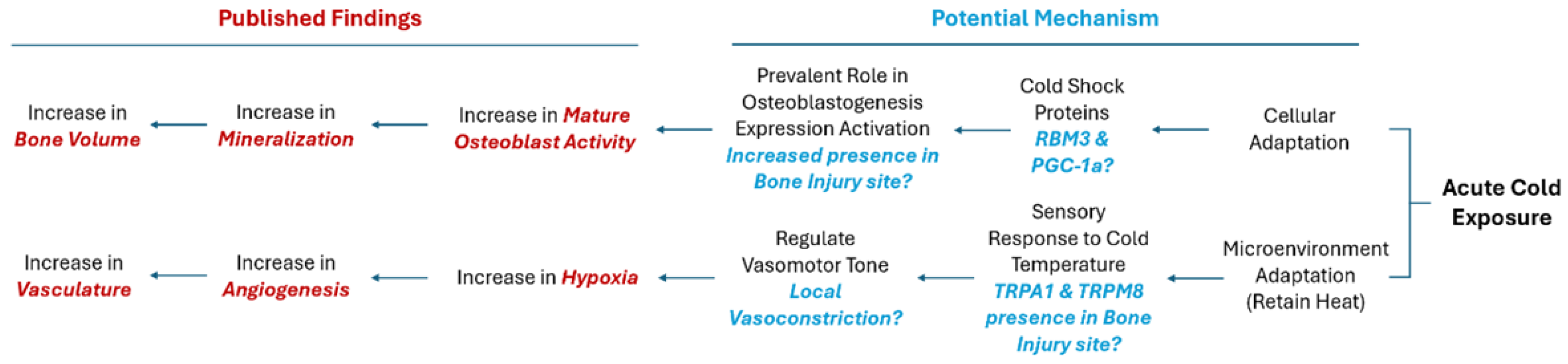
With this in mind, we hypothesized that with the addition of cold there may be an increase in the presence of RBM3 and PGC-1α within a bone injury site, which could facilitate the differentiation of osteoblasts and explain documented cases of increased mineralization of newly formed bone (
Figure 1). Additionally, exposure to cold requires a microenvironmental adaptation to maintain heat homeostasis that involves a sensory response to cold temperature leading to the activation of TRPA1 and TRPM8. This in turn may highlight localized vasoconstriction that explains the increase in hypoxia detected and subsequentially the enhanced vasculature network within bone injury sites (
Figure 1).
The objective of this study is to delineate the mechanism by which cold therapy affects bone formation in-vivo at the injury site. Building on the potential benefits of acute cold observed in our previous studies, we extended our investigation to the mechanisms through which cold exposure promotes osteogenesis and contributes to the formation of a bony callus. In this work, we implemented a simple and reproducible mouse defect model to demonstrate that localized application of cold temperature leads to elevated induction of hypoxia in the bone injury site indirectly by modifying the vasomotor tone through TRP related pathways in conjunction with increased detection of RBM3 and PGC-1a, responsible for upregulation of osteoblast differentiation (
Figure 1).
3. Results
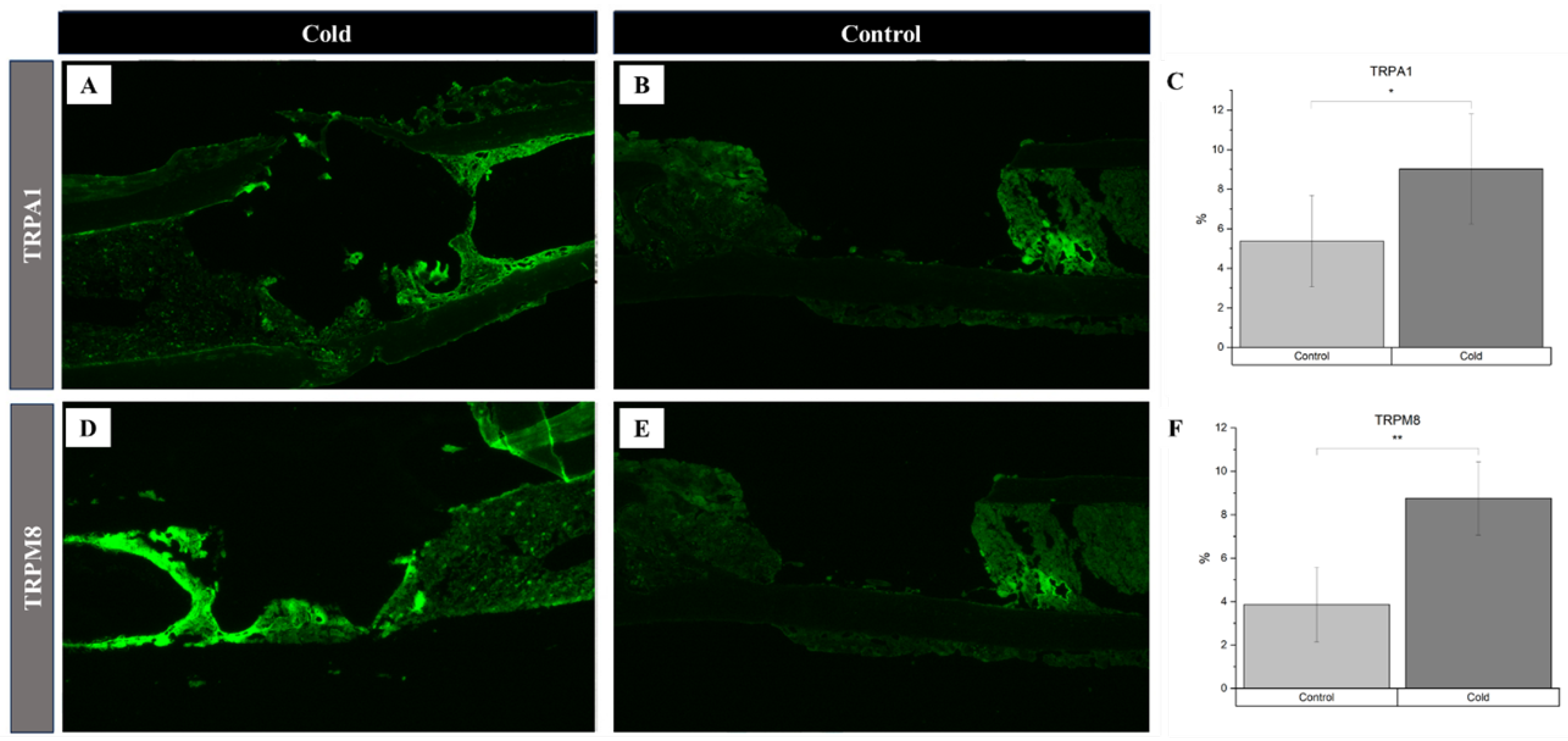
The assessment of vasoconstriction receptors revealed a significant increase (
Figure 2C) in TRPA1 presence following cold treatment (p = 0.012), with levels rising to 9.03% ± 2.78 in the cortical defect region, compared to 5.38 ± 2.32 in untreated controls (
Figure 2A vs.
Figure 2B). Similarly, TRPM8 levels were significantly elevated (p < 0.001) after cold exposure, showing an increase to 8.75 ± 1.68 in treated femurs versus 3.86 ± 1.72 in untreated ones (
Figure 2D vs.
Figure 2E). Elevation of receptors responsible for triggering a vasoconstrictive response confirms the detection of cold exposure within the microenvironment of the bone injury site.
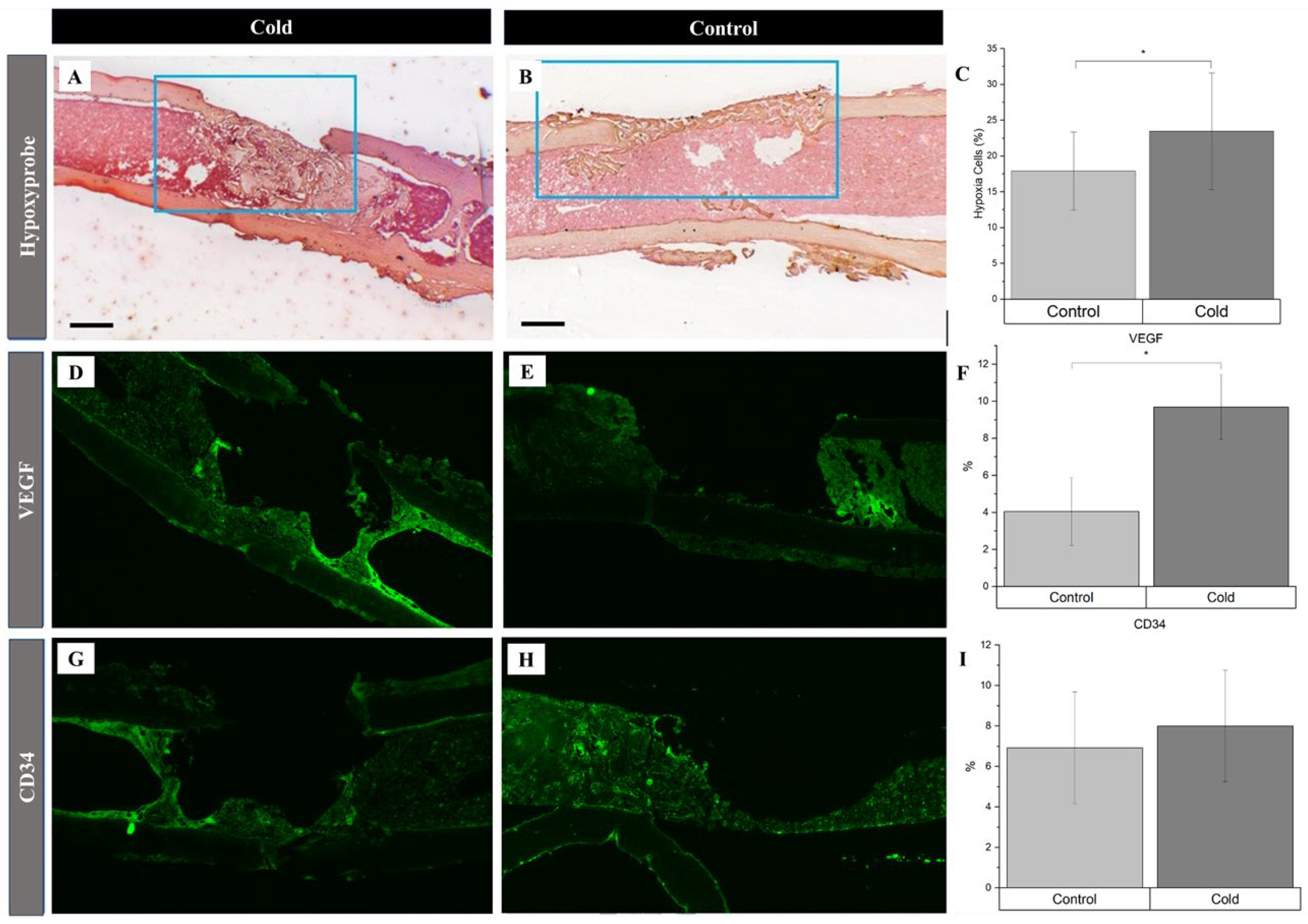
There was a 5.6% increase in the number of hypoxic cells within and around the cortical bone defect of experimental (
Figure 3A) hindlegs (23.5% ± 5.8) compared to untreated (
Figure 3B) controls (17.9% ± 3.8) following exposure to an acute cold stimulus (p-value<0.01) reflecting an increase in local hypoxia following the application of cold exposure (
Figure 3C). Investigating cold-induced cortical defect repair revealed significant angiogenic changes, particularly for Vascular Endothelial Growth Factor (VEGF) expression. VEGF expression in the cold-treated group (
Figure 3D) notably surpassed the control group (
Figure 3E), with levels at 9.69% ± 1.75 and 4.05% ± 1.83, respectively (
Figure 3F; p<0.001), suggesting accelerated angiogenesis. Endothelial cells within the cortical defect were identified using the endothelial-specific marker CD34. There was no statistically significant change (
Figure 3I) in the presence of these cells in the cold-treated group (8.00% ± 2.76;
Figure 3G) compared to the control group (6.92% ± 2.77;
Figure 3H).
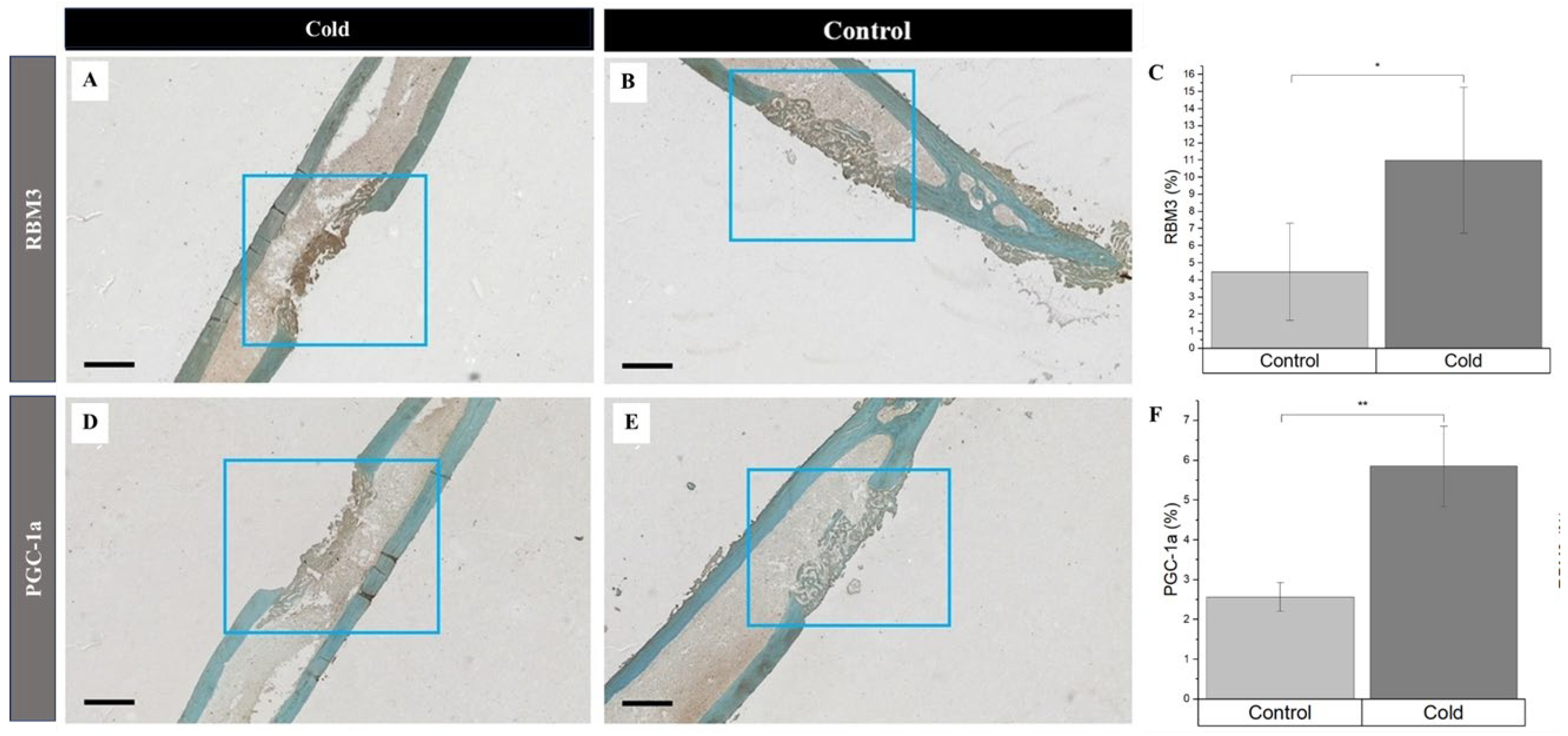
Simultaneously, increased detection of PGC-1a and RBM3 were prevalent in newly forming bone cells and the surrounding matrix within cortical defects following cold exposure. RBM3 significantly (p-value<0.008) increased
(Figure 4C) within cold
(Figure 4A) treated femora (11.0% ± 4.26%) compared to nontreated
(Figure 4B) femora (4.47% ± 2.84%)). Synonymously, PGC-1a was significantly (p-value = 0.039) elevated
(Figure 4F) in cold
(Figure 4D) treated cortical defects (5.85% ± 1.01%) in comparison to nontreated
(Figure 4E) defects (2.57% ± 0.36%). Increase in presence of RBM3 and PGC-1a indicates that cells within the bone injury site are affected by cold exposure resulting in the stimulation of proteins responsible for cellular adaptation required for cell survival [
19,
33,
34]. Furthermore, the percentage of PGC-1a and RBM3 positive cells marked by DAB staining (brown) within and around the cortical bone defect in the hindlimbs of mice exposed to a cold stimulus is higher in comparison to the control hindlimbs is of particular interest in part due to the prominent roles RBM3 and PGC-1a have in osteoblastogenesis pathways.
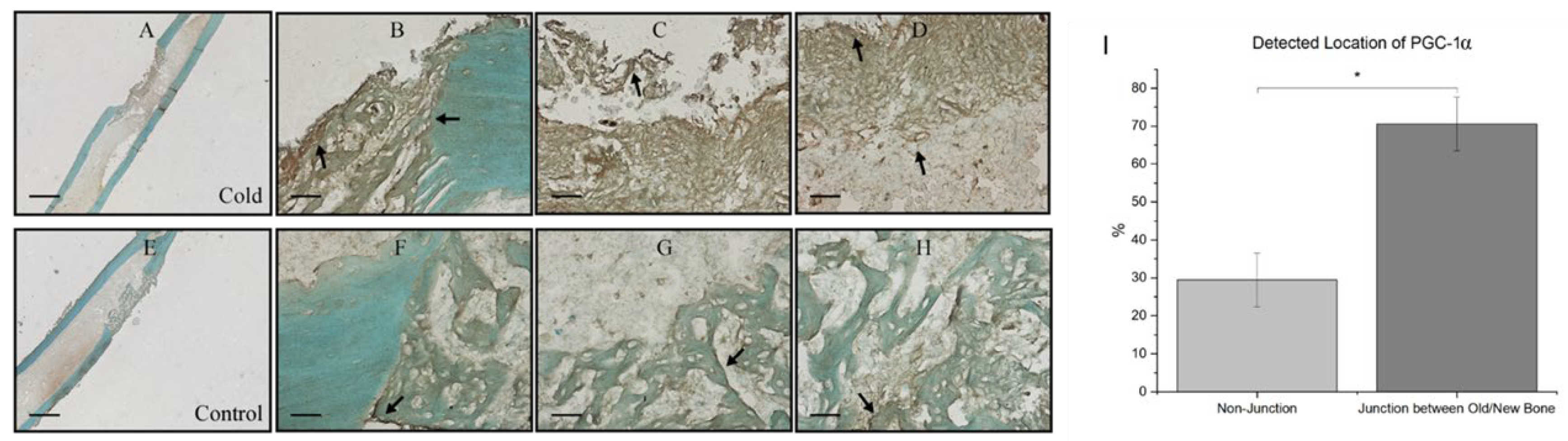
At the junction of old bone and new bone formation, there was a distinct observational contrast in the prevalence of PGC-1a within the new bone formation region within cold treated femora (
Figure 5A) and non-treated femora (
Figure 5E). Within both cold and control treated femora, 70.56% ± 7.08 of total PGC-1a was detected at the junction of new and old bone (
Figure 5B-D) corresponding with PGC-1a’s role as a cold shock protein and deterring necessary cellular changes as well as PGC-1a’s prominence in the bone repair process as this finding was consistently found within non-treated legs (
Figure 5F-H)
Assessment of the differentiation of osteoblasts following cold therapy shows that there was a statistically significant facilitative impact on the osteogenic pathway. ALP, an early marker of immature osteoblasts, was shown to be significantly elevated (p-value = 0.021) following cold treatment at day 5 (
Figure 6A) compared to non-treated femurs (2.72 U/L ± 0.85 vs. 0.74 U/L (µmol/min/L) ± 0.35). This similar trend was prevalent at day 10 (
Figure 6B) as well where ALP was significantly elevated (p-value < 0.001) following cold exposure (2.25 U/L ± 0.04 vs. 1.03 U/L ± 0.74). OCN, a marker for mature osteoblasts capable of mineralization, was not significant at day 5 (p-value = 0.29;
Figure 6C).
However, OCN was significantly (p-value = 0.019) more prominent within cold-treated femurs (0.84 mg/mL ± 0.17 vs. 0.35mg/mL ± 0.10) at day 10 (
Figure 6D) indicating the presence of mature osteoblasts capable of mineralization. ARS further confirmed the detection on calcium deposits with a statistically significant increase (p-value = 0.030) in the amount of mineralization from isolated osteoblasts at day 14 (
Figure 6G) following cold exposure (
Figure 6E) in comparison to non-treated (
Figure 6F) samples (1.04% ± 0.40 vs. 0.27% ± 0.025).
4. Discussion
The biological mechanism that promotes reparative processes and osteogenic differentiation upon cold exposure is multifaceted as different pathways related to bone repair are upregulated. Short-term cold exposure elicits a complex physiological response involving vasoconstriction, impacting bone healing through modulation of angiogenesis and vascular responses[
7,
27]. The hypoxic microenvironment ensuing from diminished blood flow elicits the production of angiogenic factors such as vascular endothelial growth factor (VEGF)[
35]. Adaptations to cold exposure lead to activation of cell hemostasis regulators within bone cells that corroborate the stimulation of osteogenic pathways and bone formation.
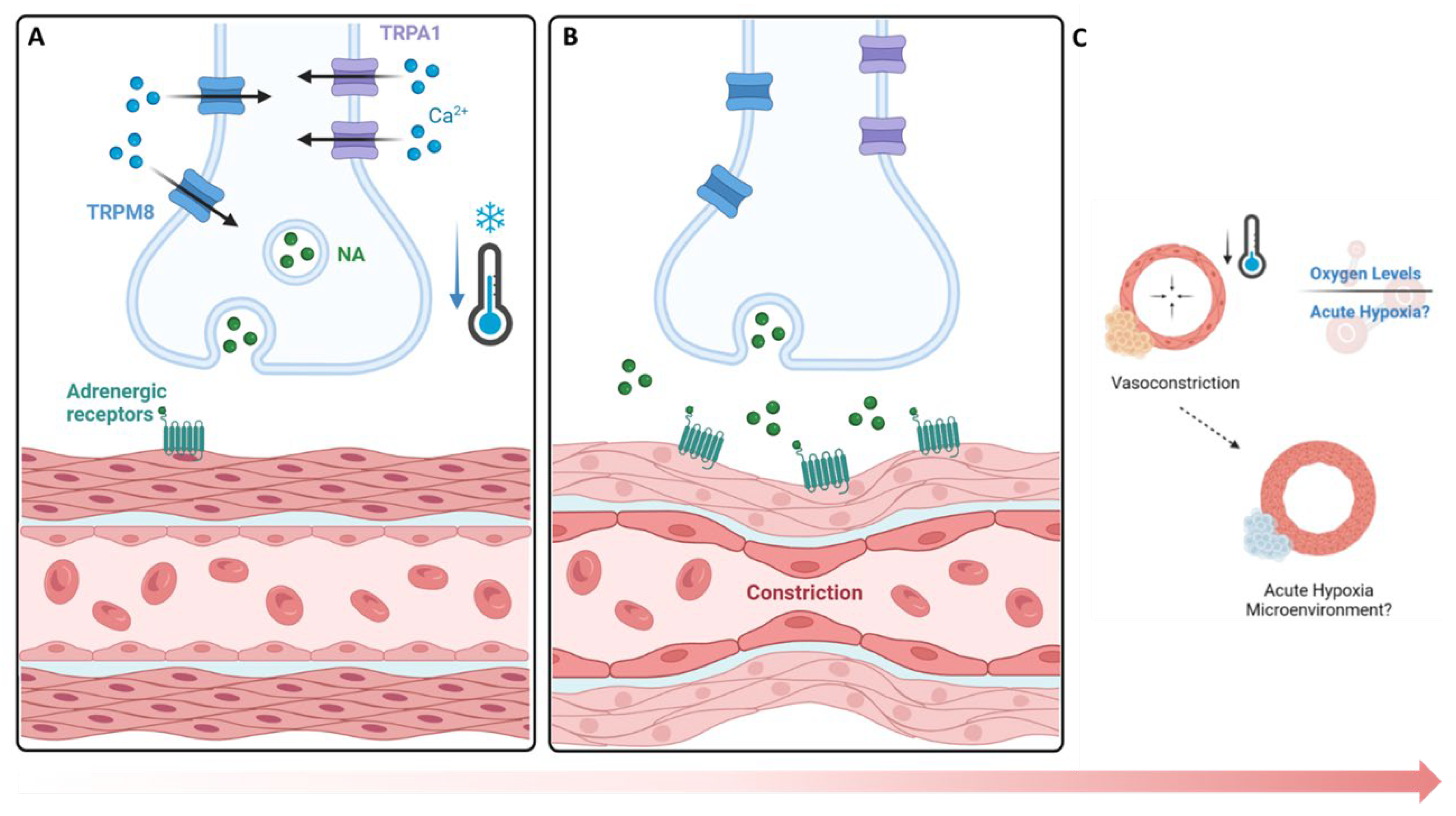
The findings of this study show that an upregulation of TRPA1 (3.65% increase,
Figure 2) and TRPM8 (4.89% increase,
Figure 2) followed by a hypoxic environment (5.0% increase,
Figure 3) upon cold application. Given their role in sensory perception, TRPA1 and TRPM8 cold stimulated upregulation within cortical defects signifies an amplified sensory response to lower temperature[
27]. The vasoconstriction, a pivotal process for blood flow regulation, is induced by the sympathetic nervous system through activation of TRPA1 and TRPM8 that release norepinephrine, activating α-adrenergic receptors on vascular smooth muscle cells (
Figure 7A)[
31,
36]. Then, an intracellular signaling cascade occurs with the release of intracellular calcium ions causes the contraction of vascular smooth muscle cells (
Figure 7B), ultimately resulting in vasoconstriction and reduced blood flow to peripheral tissue leading to hypoxia (
Figure 7C)[
27]. To measure the acute hypoxic microenvironment, we developed a novel approach based on pimonidazole based staining [
37] to measure hypoxic cells within a bone injury site
(Supplementary Figure 1A; Supplementary Figure 4) as the short half-life of the Hypoxia Inducible Factor 1-α protein is impractical to directly analyze the level of hypoxia[
10].
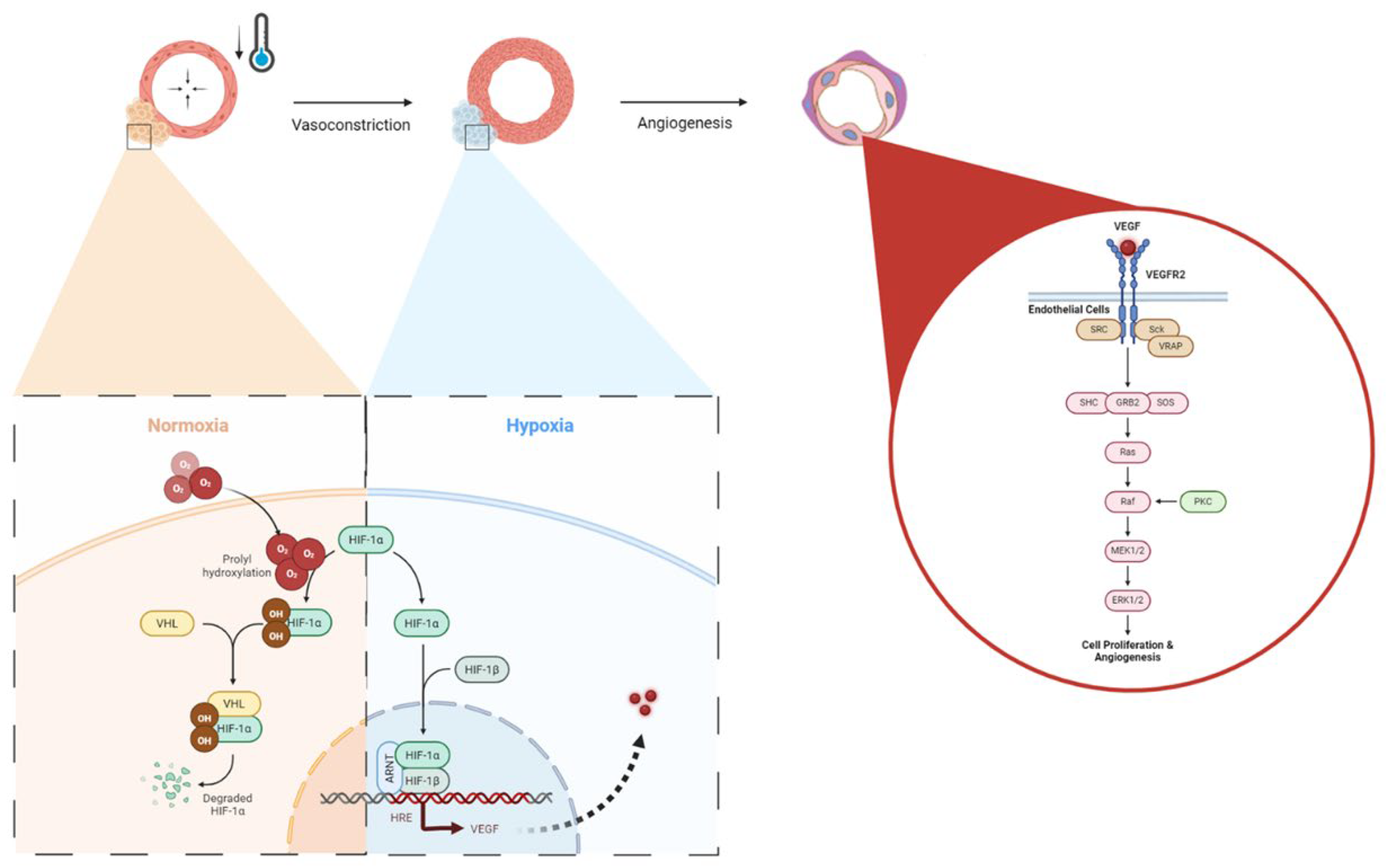
In the context of bone healing, angiogenesis is essential for supplying oxygen, nutrients, and growth factors to the healing site, facilitating the formation of new bone tissue[
11]. Here, we have shown that the application of localized non-invasive cold therapy for a short duration has demonstrated an elevation of baseline hypoxia within a bone injury site
(23.5%,
Figure 3) and upregulation of VEGF (9.69%,
Figure 3) in cortical defect following cold therapy. Under normal conditions, Hypoxia Inducible Factor 1-alpha (HIF-1α) undergoes degradation because oxygen presence induces its prolyl hydroxylation, marking it for targeted destruction but, in a localized acute hypoxic microenvironment, HIF-1α is stabilized and forms a dimer with HIF-1β to activate the expression of VEGF through the Hypoxia-Response Element (HRE) pathway (
Figure 8). The resulting VEGF is released to trigger 1) endothelial cell activation through its receptor vascular endothelial growth factor receptor 2 (VEGFR2), initiating signaling pathways such as PI3K-Akt and MAPK, which are crucial for endothelial cell proliferation and migration (
Figure 8)[
38,
39] and 2) TRPA1 activation which directs the development of the direction of vessel elongation[
40]. Furthermore, elevated VEGF following short term cold exposure has been shown to be consistent with enhanced maturation of vasculature within bone injury models [
2,
3,
17] especially within calluses where heightened presence of VEGF overlaps in the same regions of elevated presence of CD34, a biomarker of mature endothelial cells essential for vasculature [
2]. Within cortical defects specifically, previous results have shown that bone vessel volume over tissue volume (BVV/TV) was found to be higher in the cold group compared with control (7.8% vs. 6.7% p<0.001) [
3].
Nonetheless, TRPM8’s role in angiogenesis is less defined but appears to be implicated in influencing endothelial function, suggesting its potential contribution to the angiogenic process[
40,
41]. Consequently, observed upregulation of VEGF suggests an augmented angiogenic response, essential for fostering bone regeneration within cortical defects[
3,
17]. Moreover, the collaborative action of VEGF, TRPA1, and TRPM8 indirectly supports osteogenic processes by securing an adequate blood supply to regenerating tissue, facilitating osteoblast proliferation, and thereby promoting bone formation and maturation within cortical defects.
While vasomotor tone and hypoxia plays an important role in regulating angiogenesis in bone repair following cold exposure, the expression of essential cell hemostasis regulators such as RBM3 and PGC-1α are elevated[
42,
43]. Initially, cold denaturation of proteins results in proliferation of heat shock proteins (HSPs) for stabilization and proper folding. Dissociation of the HSP/heat shock factor-1 (HSF1) dimer releases free HSF1, which becomes phosphorylated and nuclear localized, leading to HSP expression
(Figure 9A). Heat shock protein 70 (HSP70) activates Toll-like receptor 4 (TLR4), which triggers the MyD88/NF-kB signaling pathway. The phosphorylated and nuclear-localized NF-kB dimer is crucial for RBM3 expression
(Figure 9B) [
44,
45], RBM3 activates the MAPK/ERK pathway, leading to the expression of runx2 and osteocalcin (OCN), essential for osteoblast differentiation and consistent with preceding findings showing that RBM3 has previously shown to stimulate the osteoblast differentiation via the ERK signalling pathway and activates two osteogenic genes, Runx2 and OCN
(Figure 9C)[
14,
42]. Runx2 has been shown to be a critical gene in inducing osteoblast differentiation[
46], whereas OCN has an important role in calcifying new bone tissue to reform the mechanical integrity of native bone at an injury site[
47]. Several in-vitro studies have shown elevated expression of Runx2 following cold exposure stimulation indicating Runx2 as a crucial gene important in the osteogenic pathway impacted by cold exposure [
14,
48]. Within fractures, this is important in transitioning from a fibrogenesis callus to a bony callus[
49]. The increased expression of RBM3 confirms the activation of osteoblast differentiation associated with elevation of ALP levels at days 5 and 10 (2.72U/L, 2.25U/L,
Figure S4) and calcium-binding pathways, which are aligned with enhanced bone formation following acute cold therapy. This is consistent with in-vitro studies that have shown elevated levels of ALP and OCN following short term cold exposure indicating activation of osteoblastogenesis pathways essential for osteoblast maturation [
14]. ‘Under the same cold treatment conditions, ALP levels have been shown to increase in the callus of femoral fractures, correlating with enhanced mineralized bone formation. These results are consistent with similar observations in cortical defects, further supporting the role of cold exposure in stimulating bone repair pathways [
2,
3,
17].
Studies have already demonstrated that cold exposure leads to activation of PGC-1α via the PKA/CaMKII pathway where cold activation of adrenergic receptors and transient receptor proteins (TRPs) stimulates cAMP and calcium influx, leading to cAMP-response element binding protein (CREB) phosphorylation via the PKA/Ca2+/CaMK pathway
(Figure 9D)[
50,
51]. PGC-1α has synergistic properties with ERRα which is highly expressed in osteoblasts and osteoclasts, and interacts cooperatively with PGC-1α to amplify the levels of the key proteins in bone formation, including OCN, Runx2, and osteopontin (OPN) necessary for mineralization
(Figure 9E)[
52]. For example, OPN facilitates blood vessel maturation following angiogenesis and is essential for early callus formation.[
11] Furthermore, in-vitro studies have demonstrated upregulation of OPN essential for osteoblast differentiation following cold exposure resulting from Runx2 stimulation [
48]. Distinctive contrast of PGC-1α detected at the junction between old bone and new bone formed further reinforces its importance in being responsible for establishing the baseline for new bone formed by osteoblasts to interconnect with old bone [
53]
(Figure 5). These osteoblasts then secrete further OPN and create a positive feedback cycle for bone regeneration [
53]. Additionally, PGC-1alpha activation stimulates the transcription of mitochondrial genes by forming a complex with nuclear respiratory factor 1 & 2 (NRF-1 and NRF-2), activating Mitochondrial transcription factor A (TFAM) and NCMP expression [
50], that are crucial for mitochondrial biogenesis and intracellular calcium storage, enhancing oxidative phosphorylation, and energy production (
Figure 9F)[
19]. This also induces genes involved in calcium homeostasis, leading to increased calcium storage in mitochondrial granules, which is crucial for osteoblasts and bone formation[
54]. Adequate calcium levels are essential for the mineralization process in osteoblasts. This is aligned with elevated alizarin red staining in osteoblasts from cold-treated bone injury samples at day 14 (1.04%,
Figure S5) indicating an enhanced extracellular matrix mineralization, reflecting improved osteoblast functionality and bone formation. This may be due to cold exposure influencing cellular metabolism and energy homeostasis via RBM3 and PGC-1α, supporting osteoblast differentiation and matrix mineralization (
Figure S4). By boosting mitochondrial function and calcium storage, PGC-1alpha activation via cold therapy supports osteoblastic activity and bone formation. This dual action improves mitochondrial efficiency and ensures sufficient calcium availability, facilitating proper osteoblast function and differentiation [
54]. Future studies can further explore the influence of mitochondrial biogenesis on bone healing and matrix mineralization.
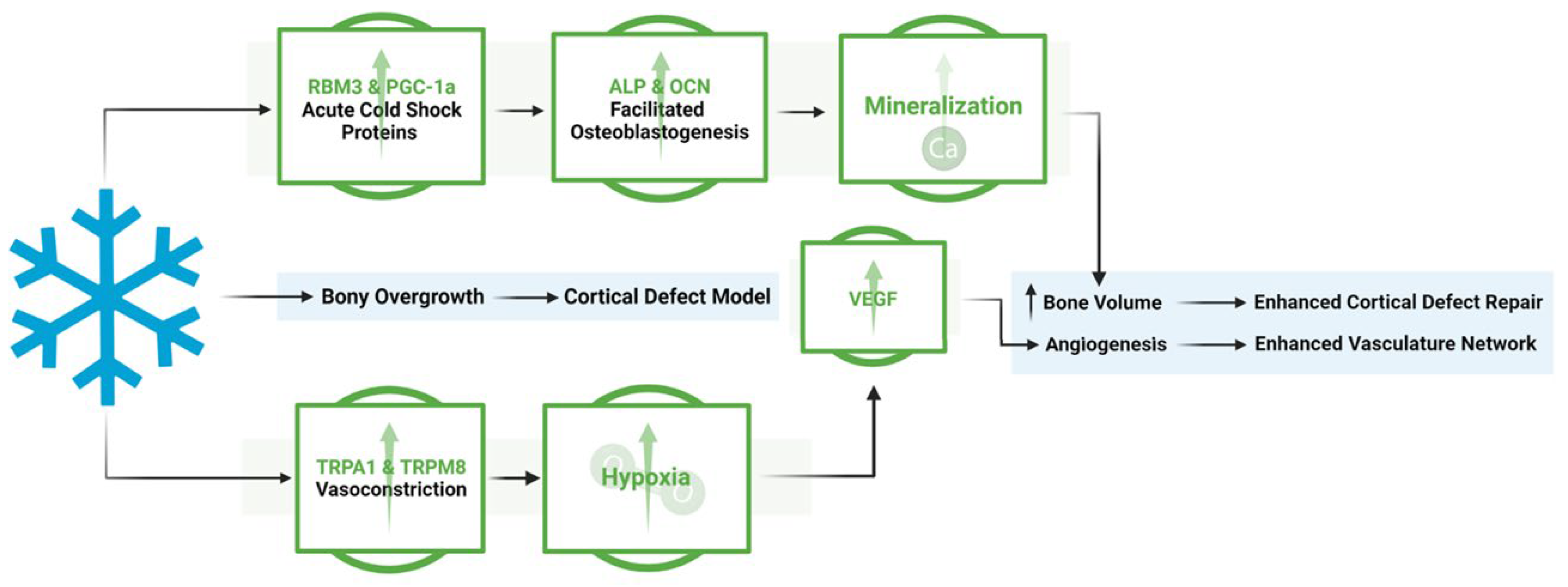
This work shows the dual effect of cold exposure on 1) bone and vasculature morphology and 2) on pathways involved in bone repair (
Figure 10). Upregulation of TRPA1 and TRPM8 in the injury region induces vasoconstriction resulting in a heightened acute hypoxic microenvironment, leading to elevated VEGF levels and activation of angiogenic pathways, promoting a robust vasculature network in cold-treated bone injuries. Elevated RBM3 and PGC-1α levels at the injury site support osteoblast differentiation necessary for new bone mineralization, thereby enhancing bone production following cold exposure.
Figure 1.
Potential mechanisms activated in response to cold exposure.
Figure 1.
Potential mechanisms activated in response to cold exposure.
Figure 2.
Identification of receptor proteins involved in vasoconstriction within a cortical defect following cold exposure. TRPM8 and TRPA1 detection (bright green) within the cortical defect region (4x magnification). Scale bar represents 500 µm. A, D) Non-treated femurs serving as baseline. B, E) Cold treated femurs. C) TRPA1 Staining Analysis Expression (n=8) of positive staining for TRPA1 in the control group was 5.38 ± 2.32 while in the experimental group it was 9.03% ± 2.78 (*p-value = 0.012). F) TRPM8 Staining Analysis Expression (n=8) of positive staining for TRPM8 in the control group was 3.86% ± 1.72 while in the experimental group it was 8.75% ± 1.68 (**p-value < 0.001).
Figure 2.
Identification of receptor proteins involved in vasoconstriction within a cortical defect following cold exposure. TRPM8 and TRPA1 detection (bright green) within the cortical defect region (4x magnification). Scale bar represents 500 µm. A, D) Non-treated femurs serving as baseline. B, E) Cold treated femurs. C) TRPA1 Staining Analysis Expression (n=8) of positive staining for TRPA1 in the control group was 5.38 ± 2.32 while in the experimental group it was 9.03% ± 2.78 (*p-value = 0.012). F) TRPM8 Staining Analysis Expression (n=8) of positive staining for TRPM8 in the control group was 3.86% ± 1.72 while in the experimental group it was 8.75% ± 1.68 (**p-value < 0.001).
Figure 3.
Identification of angiogenic related factors and endothelial cells within a cortical defect following cold exposure. VEGF and CD34 (bright green) detection within the cortical defect region (4x magnification). Scale bar represents 500 µm. A,D,G) Cold treated femurs. B,E,H) Non-treated femurs serving as baseline C) Hypoxia Staining Analysis Expression (n=10) of positive staining for hypoxia in the control group was 17.9% ± 3.8 while in the experimental group it was 23.5% ± 05.8 (*p-value <0.01). F) VEGF Staining Analysis Expression (n=8) of positive staining for VEGF in the control group was 4.05% ± 1.83 while in the experimental group it was 9.69% ± 1.75 (*p-value<0.001). I) CD34 Staining Analysis Expression (n=8) of positive staining for CD34 in the control group was 6.92% ± 2.77 while in the experimental group it was 8.00% ± 2.76 (p-value = 0.42).
Figure 3.
Identification of angiogenic related factors and endothelial cells within a cortical defect following cold exposure. VEGF and CD34 (bright green) detection within the cortical defect region (4x magnification). Scale bar represents 500 µm. A,D,G) Cold treated femurs. B,E,H) Non-treated femurs serving as baseline C) Hypoxia Staining Analysis Expression (n=10) of positive staining for hypoxia in the control group was 17.9% ± 3.8 while in the experimental group it was 23.5% ± 05.8 (*p-value <0.01). F) VEGF Staining Analysis Expression (n=8) of positive staining for VEGF in the control group was 4.05% ± 1.83 while in the experimental group it was 9.69% ± 1.75 (*p-value<0.001). I) CD34 Staining Analysis Expression (n=8) of positive staining for CD34 in the control group was 6.92% ± 2.77 while in the experimental group it was 8.00% ± 2.76 (p-value = 0.42).
Figure 4.
Identification of cold shock proteins and hypoxia in regenerating bone following cold exposure. PGC-1a (brown), RBM3 (brown), and hypoxia (brown) detection within the cortical defect region (4x magnification). Scale bar represents 500 µm. (A, D) Cold treated femurs. B, E) Non-treated femurs serving as baseline C) RBM3 Staining Analysis Expression (n=8) of positive staining for RBM3 in the control group was 4.47% ± 2.84 while in the experimental group it was 11.0% ± 4.26 (**p-value<0.008). F) PGC-1a Staining Analysis Expression (n=8) of positive staining for PGC-1a in the control group was 2.57% ± 0.36 while in the experimental group it was 5.85% ± 1.01 (*p-value = 0.039).
Figure 4.
Identification of cold shock proteins and hypoxia in regenerating bone following cold exposure. PGC-1a (brown), RBM3 (brown), and hypoxia (brown) detection within the cortical defect region (4x magnification). Scale bar represents 500 µm. (A, D) Cold treated femurs. B, E) Non-treated femurs serving as baseline C) RBM3 Staining Analysis Expression (n=8) of positive staining for RBM3 in the control group was 4.47% ± 2.84 while in the experimental group it was 11.0% ± 4.26 (**p-value<0.008). F) PGC-1a Staining Analysis Expression (n=8) of positive staining for PGC-1a in the control group was 2.57% ± 0.36 while in the experimental group it was 5.85% ± 1.01 (*p-value = 0.039).
Figure 5.
Prevalence of PGC-1a at the junction of old and new bone formation following cold therapy treatment. A, E) 2.5x Magnification. Scale bar represents 500 µm. B-D, F-H) 20x Magnification. Scale Bar represents 50 µm. PGC-1a has been shown to be upregulated (I) at the junction of new and old bone (F-H) Furthermore, PGC-1a expression within these junctions appears to be elevated following cold exposure (B-D) indicating PGC-1a upregulation. I) Detected location of PGC-1α Staining Analysis Expression (n=8) of PGC-1α within cells at the junction of newly formed bone and old bone was 70.56% ± 7.08 while in non-junctional regions it was 29.44% ± 7.08 (*p-value <0.00001).
Figure 5.
Prevalence of PGC-1a at the junction of old and new bone formation following cold therapy treatment. A, E) 2.5x Magnification. Scale bar represents 500 µm. B-D, F-H) 20x Magnification. Scale Bar represents 50 µm. PGC-1a has been shown to be upregulated (I) at the junction of new and old bone (F-H) Furthermore, PGC-1a expression within these junctions appears to be elevated following cold exposure (B-D) indicating PGC-1a upregulation. I) Detected location of PGC-1α Staining Analysis Expression (n=8) of PGC-1α within cells at the junction of newly formed bone and old bone was 70.56% ± 7.08 while in non-junctional regions it was 29.44% ± 7.08 (*p-value <0.00001).
Figure 6.
Detection of ALP and OCN at Day 5 and Day 10 within isolate osteoblasts following cold exposure and mineralization within isolate osteoblasts following cold exposure for 14 days. A) ALP Activity after 5 days of cold exposure was 2.72 U/L (µmol/min/L) ± 0.85 while in the non-treated group it was 0.74 U/L ± 0.35 (*p-value = 0.021). B) ALP Activity after 10 days of cold exposure was 2.25 U/L ± 0.04 while in the non-treated group it was 1.0301 U/L ± 0.24 (**p-value < 0.001) C) OCN levels after 5 days of cold exposure was 0.34 mg/mL ± 0.10 while in the non-treated group it was 0.43 mg/mL ± 0.10 (p-value = 0.29) D) OCN levels after 10 days of cold exposure was 0.84 mg/mL ± 0.17 while in the non-treated group it was 0.35 mg/mL ± 0.10 (***p-value = 0.019) ARS (red) detection within isolate osteoblasts (10x magnification). Scale bar represents 250 µm. E) Osteoblasts isolated from non-treated fractured femurs. F) Osteoblasts isolated from daily cold-treated fractured femurs. G) ARS Staining Analysis: Detection of ARS after 14 days of cold exposure was 1.04% ± 0.40 while in the non-treated group it was 0.27% ± 0.025 (***p-value = 0.030).
Figure 6.
Detection of ALP and OCN at Day 5 and Day 10 within isolate osteoblasts following cold exposure and mineralization within isolate osteoblasts following cold exposure for 14 days. A) ALP Activity after 5 days of cold exposure was 2.72 U/L (µmol/min/L) ± 0.85 while in the non-treated group it was 0.74 U/L ± 0.35 (*p-value = 0.021). B) ALP Activity after 10 days of cold exposure was 2.25 U/L ± 0.04 while in the non-treated group it was 1.0301 U/L ± 0.24 (**p-value < 0.001) C) OCN levels after 5 days of cold exposure was 0.34 mg/mL ± 0.10 while in the non-treated group it was 0.43 mg/mL ± 0.10 (p-value = 0.29) D) OCN levels after 10 days of cold exposure was 0.84 mg/mL ± 0.17 while in the non-treated group it was 0.35 mg/mL ± 0.10 (***p-value = 0.019) ARS (red) detection within isolate osteoblasts (10x magnification). Scale bar represents 250 µm. E) Osteoblasts isolated from non-treated fractured femurs. F) Osteoblasts isolated from daily cold-treated fractured femurs. G) ARS Staining Analysis: Detection of ARS after 14 days of cold exposure was 1.04% ± 0.40 while in the non-treated group it was 0.27% ± 0.025 (***p-value = 0.030).

Figure 7.
Cold based activation of vasoconstrictive pathways and hypoxic impact. A & B) TRPA1 and TRPM8 Mechanism Cold exposure leads to activation of TRPM8 (8 - 28°C) and TRPA1 (8 - 17°C) initiating a contractive response leading to vasoconstriction. C) Hypoxic Impacts. A reduced influx of blood supply coincides with a decrease in oxygen availability leading to the formation of an acute hypoxic microenvironment with respect to nearby cells. NA: Norepinephrine.
Figure 7.
Cold based activation of vasoconstrictive pathways and hypoxic impact. A & B) TRPA1 and TRPM8 Mechanism Cold exposure leads to activation of TRPM8 (8 - 28°C) and TRPA1 (8 - 17°C) initiating a contractive response leading to vasoconstriction. C) Hypoxic Impacts. A reduced influx of blood supply coincides with a decrease in oxygen availability leading to the formation of an acute hypoxic microenvironment with respect to nearby cells. NA: Norepinephrine.
Figure 8.
Hypoxic upregulation following vasoconstriction via local cold exposure and impact on angiogenic pathways. Under normal oxygen conditions, HIF-1a is degraded via von Hippel–Lindau (VHL) after prolyl hydroxylation, but in hypoxia, HIF-1a forms a dimer with HIF-1B, nucleolocalizes with aryl hydrocarbon receptor nuclear translocator (ARNT), leading to VEGF expression through the HRE pathway, which then activates VEGFR2 on endothelial cells, upregulating cellular proliferation and angiogenesis via the Ras/Raf signaling pathway.
Figure 8.
Hypoxic upregulation following vasoconstriction via local cold exposure and impact on angiogenic pathways. Under normal oxygen conditions, HIF-1a is degraded via von Hippel–Lindau (VHL) after prolyl hydroxylation, but in hypoxia, HIF-1a forms a dimer with HIF-1B, nucleolocalizes with aryl hydrocarbon receptor nuclear translocator (ARNT), leading to VEGF expression through the HRE pathway, which then activates VEGFR2 on endothelial cells, upregulating cellular proliferation and angiogenesis via the Ras/Raf signaling pathway.
Figure 9.
Cellular Adaptive Signaling Pathways activated by Cold Exposure.RBM3 activation and downstream osteogenic potential following short term cold exposure. A) Cold exposure triggers a cellular cold shock response, activating RBM3 via HSPs released by phosphorylated and nuclear-localized HSF1. B) HSP70 activates TLR4, initiating the MyD88/NF-kB pathway, with phosphorylated NF-kB essential for RBM3 expression. C) RBM3 activates the MAPK/ERK pathway, leading to runx2 and osteocalcin (OCN) expression, crucial for osteoblast differentiation.PGC-1a activation and downstream osteogenic potential following short term cold exposure. PGC-1α Activation and Osteogenic Potential Following Short Term Cold Exposure D) Cold activation of adrenergic receptors and TRPs stimulates cAMP and calcium influx, leading to CREB phosphorylation via the PKA/Ca2+/CaMK pathway. E) PGC-1α forms a complex with ERRα in osteoblastic cells, promoting differentiation via the RUNX2/OCN pathway for mineralization. F) PGC-1α and NRF-1/NRF-2 activate TFAM and NCMP, crucial for mitochondrial biogenesis and intracellular calcium storage.
Figure 9.
Cellular Adaptive Signaling Pathways activated by Cold Exposure.RBM3 activation and downstream osteogenic potential following short term cold exposure. A) Cold exposure triggers a cellular cold shock response, activating RBM3 via HSPs released by phosphorylated and nuclear-localized HSF1. B) HSP70 activates TLR4, initiating the MyD88/NF-kB pathway, with phosphorylated NF-kB essential for RBM3 expression. C) RBM3 activates the MAPK/ERK pathway, leading to runx2 and osteocalcin (OCN) expression, crucial for osteoblast differentiation.PGC-1a activation and downstream osteogenic potential following short term cold exposure. PGC-1α Activation and Osteogenic Potential Following Short Term Cold Exposure D) Cold activation of adrenergic receptors and TRPs stimulates cAMP and calcium influx, leading to CREB phosphorylation via the PKA/Ca2+/CaMK pathway. E) PGC-1α forms a complex with ERRα in osteoblastic cells, promoting differentiation via the RUNX2/OCN pathway for mineralization. F) PGC-1α and NRF-1/NRF-2 activate TFAM and NCMP, crucial for mitochondrial biogenesis and intracellular calcium storage.
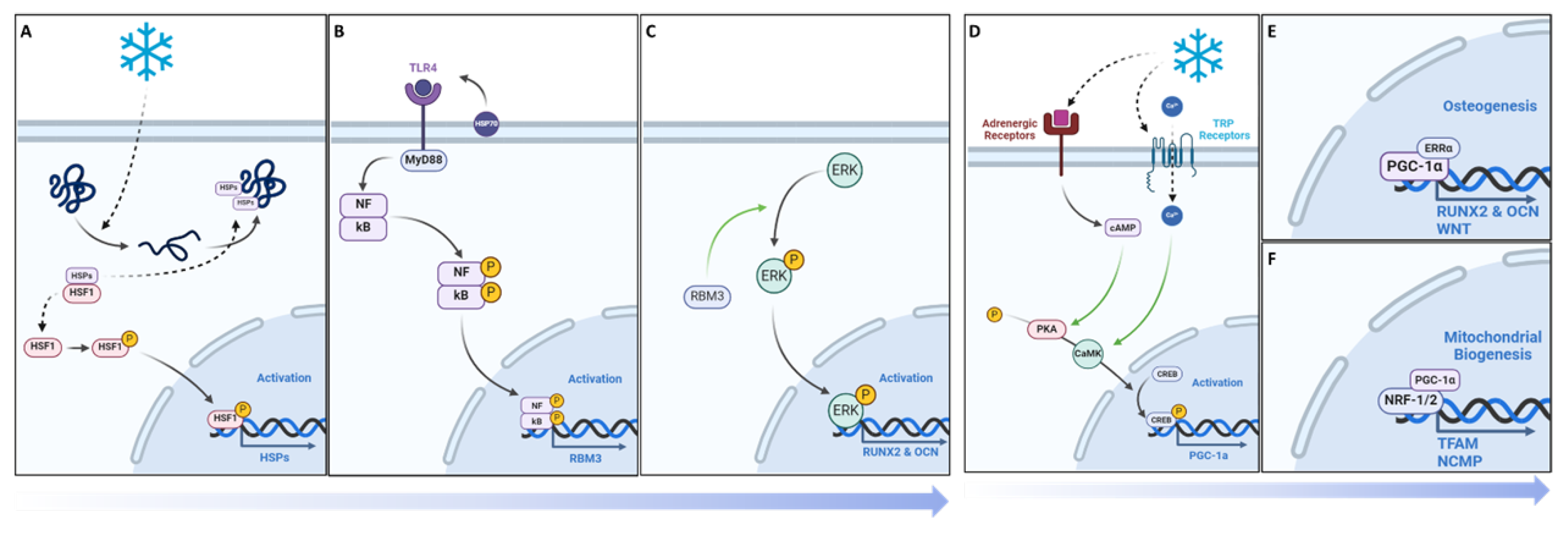
Figure 10.
Overview of the mechanistic propensities of short duration cold exposure and potential impacts in a bone injury site. Blue: Observed impacts of cold exposure on bone and vasculature morphology Green: Observed impacts of cold exposure on pathways involved in bone repair.
Figure 10.
Overview of the mechanistic propensities of short duration cold exposure and potential impacts in a bone injury site. Blue: Observed impacts of cold exposure on bone and vasculature morphology Green: Observed impacts of cold exposure on pathways involved in bone repair.