INTRODUCTION
Hyperlipidemia, marked by elevated plasma cholesterol and triglycerides, can be primary, due to inherited conditions, or secondary, with both types increasing the risk of cardiovascular disease. The mechanism underlying pregnancy-associated hyperlipidemia remains unclear. However, nutrition and hormones, particularly estrogens, are known to influence serum lipid levels [
1].
Lipids are essential components of body tissues, playing crucial roles in various biological functions. They serve as hormones or hormone precursors, aid in digestion, provide storage and metabolic fuels, function as structural components in biomembranes, and offer insulation for nerve conduction and heat retention. The causative relationship between plasma lipids, lipoproteins, and atherosclerosis has been conclusively established over the past decade. Lipoproteins transport dietary lipids to tissues for energy and fat storage, and significant changes in lipid and lipoprotein levels can alter their functions [
2].
Cholesterol is vital for normal fetal development, contributing to cell membrane formation, maintaining membrane integrity, and supporting cholesterol-rich domains essential for membrane-associated signaling cascades, including sonic hedgehog signaling [
3]. Excessive accumulation of major lipids in the plasma increases the risk of coronary heart disease (CHD), arterial and venous thrombosis, and other cardiovascular complications. Although changes in total cholesterol, triglycerides, LDL, and HDL levels have been documented during pregnancy, the extent of these changes varies across studies [
4,
5,
6].
Hyperlipidemia is one of the most significant biological changes during normal pregnancy, with hypertriglyceridemia being the most prominent feature, although the increase in total cholesterol levels is less dramatic. Some studies suggest an increased risk of coronary disease with multiple pregnancies. Pregnancy presents additional challenges in cases of preexisting hyperlipidemia, whether familial or secondary [
7].
Therefore, this study aimed to assess serum lipid profile levels in healthy Sudanese pregnant women, specifically during the second and third trimesters. The study also investigated the relationship between the number of pregnancies (parity) and serum lipid and lipoprotein levels.
MATERIALS AND METHODS
A cross-sectional study included 60 healthy Sudanese pregnant women, divided equally between the second and third trimesters (30 in each trimester), and 30 healthy non-pregnant women as a control group. A specifically designed questionnaire was used to select healthy Sudanese pregnant women and exclude those with diabetes mellitus, thyroid disorders, obstructive liver disease, kidney disease, hypertension, or those on medication for any condition.
The study received ethical clearance from the Research Ethical Committee of the Ministry of Health (Sudan). Data and samples were collected after obtaining informed consent from the participants, who were informed about the study's purpose and significance.
Clinical assessments of the test and control groups were performed by a consultant obstetrician at Umbada Hospital. Serum concentrations of total cholesterol, triglycerides, LDL, and HDL were measured using commercial kits from Biosystem Company (Spain) and a Biosystem BTS-310 spectrophotometer. The methods were validated for precision and accuracy by including commercially prepared control sera in each batch analysis.
Data were analyzed using SPSS software. Means and standard deviations of serum concentrations of total cholesterol, triglycerides, LDL, and HDL were determined for both the test and control groups. A t-test was used for comparison, with a p-value of < 0.05 considered significant. Linear regression analysis was conducted to assess the correlation between parity and serum lipid levels.
Results were presented in tables and figures, comparing the means of serum lipid profiles between the second and third trimesters and the control group, highlighting significant differences.
RESULTS
Serum concentrations of total cholesterol, triglycerides, LDL-cholesterol, and HDL-cholesterol were measured in 60 healthy Sudanese women during the second and third trimesters of pregnancy (30 in each trimester) and in a control group of 30 healthy non-pregnant Sudanese women matched for age. The collected data were analyzed using SPSS software. The mean and standard deviation of serum total cholesterol, triglycerides, LDL, and HDL levels were determined for the second and third trimesters of pregnancy. Comparisons between these groups were performed using a t-test with a p-value of < 0.05 considered statistically significant. Linear regression analysis was employed to assess the correlation between parity and serum concentrations of total cholesterol, triglycerides, LDL, and HDL.
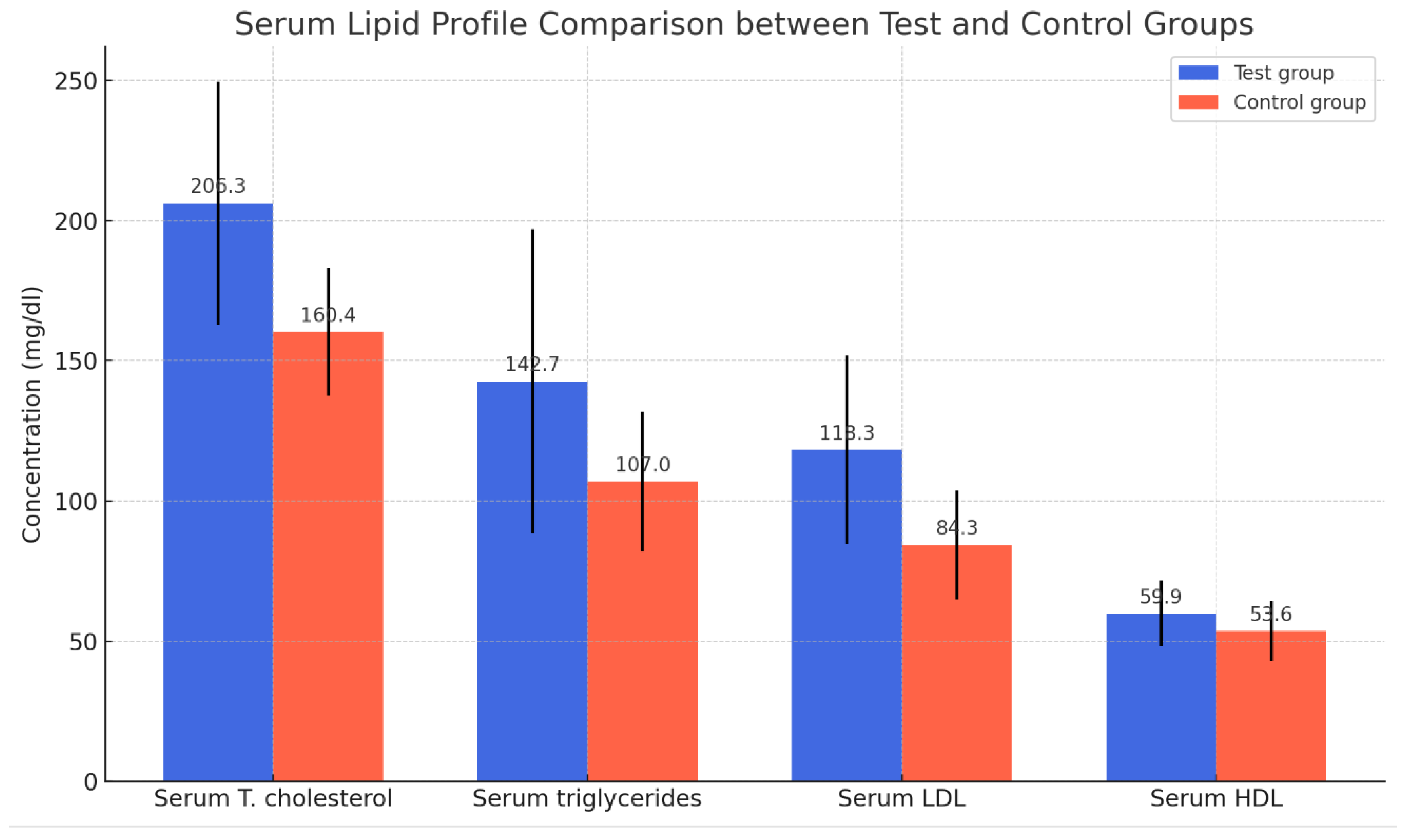
The results indicated a significant difference in the mean serum levels of total cholesterol, triglycerides, LDL, and HDL between the pregnant women (n = 60) and the control group (n = 30). As shown in
Table 1 and
Figure 1, all measured lipid concentrations (total cholesterol, triglycerides, LDL, and HDL) were significantly higher in the test group compared to the control group. The differences in means are statistically significant, suggesting that the condition of pregnancy has a notable impact on lipid levels, as shown in
Table 1 and
Figure 1.
The table displays the mean ± SD and probability (P). A t-test was used for comparison. P < 0.05 was considered significant.
Figure 1: Mean Lipid Concentrations in Test and Control Groups.
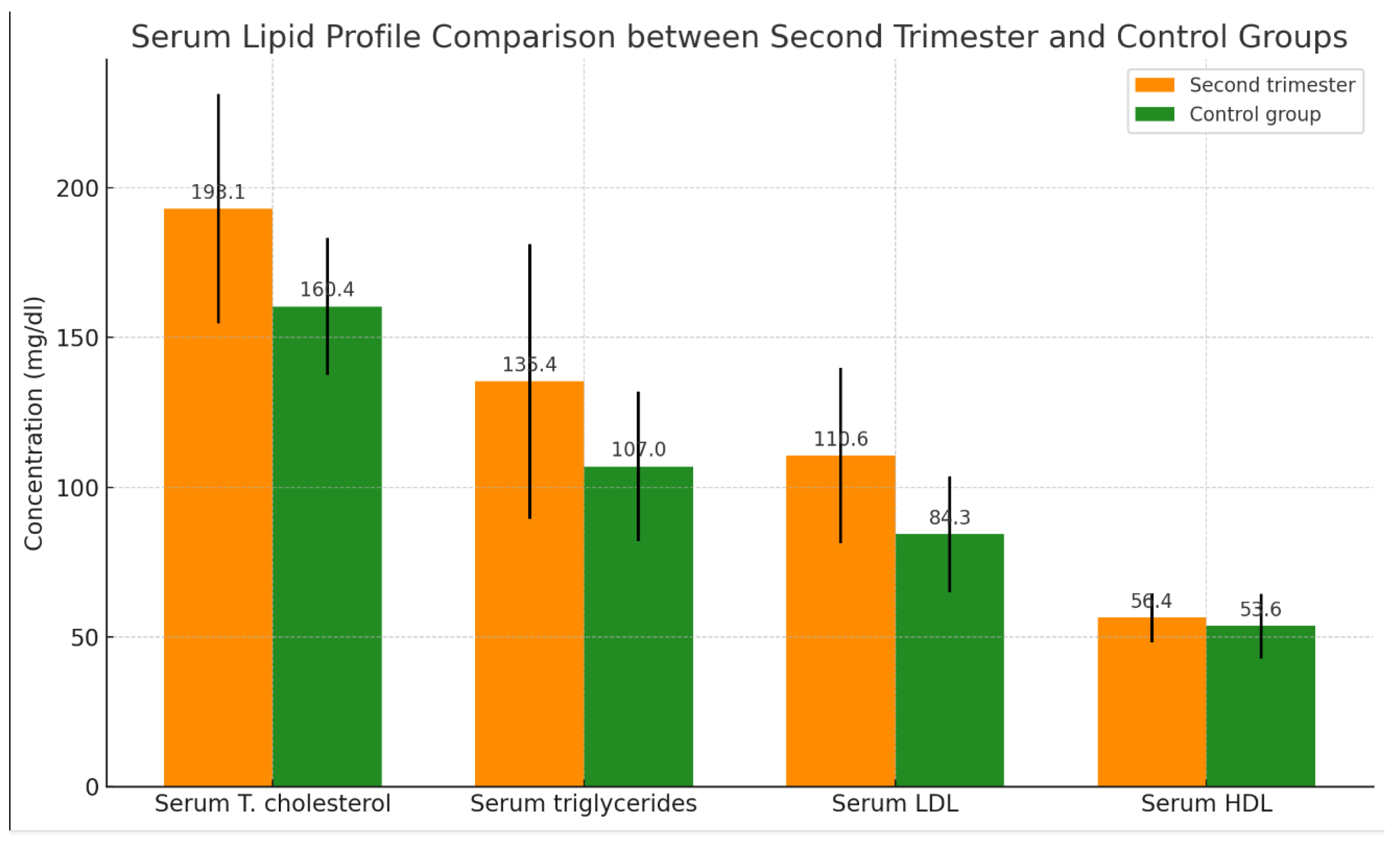
Table 2 presents a significant difference in the mean serum levels of total cholesterol, triglycerides, LDL, and HDL between women in their second trimester of pregnancy (n = 30) and the control group (n = 30), as shown in
Table 2 and
Figure 2.
The table presents the means ± SD with ranges in brackets and probability (P). A t-test was employed for the comparisons. A P-value of < 0.05 is considered significant.
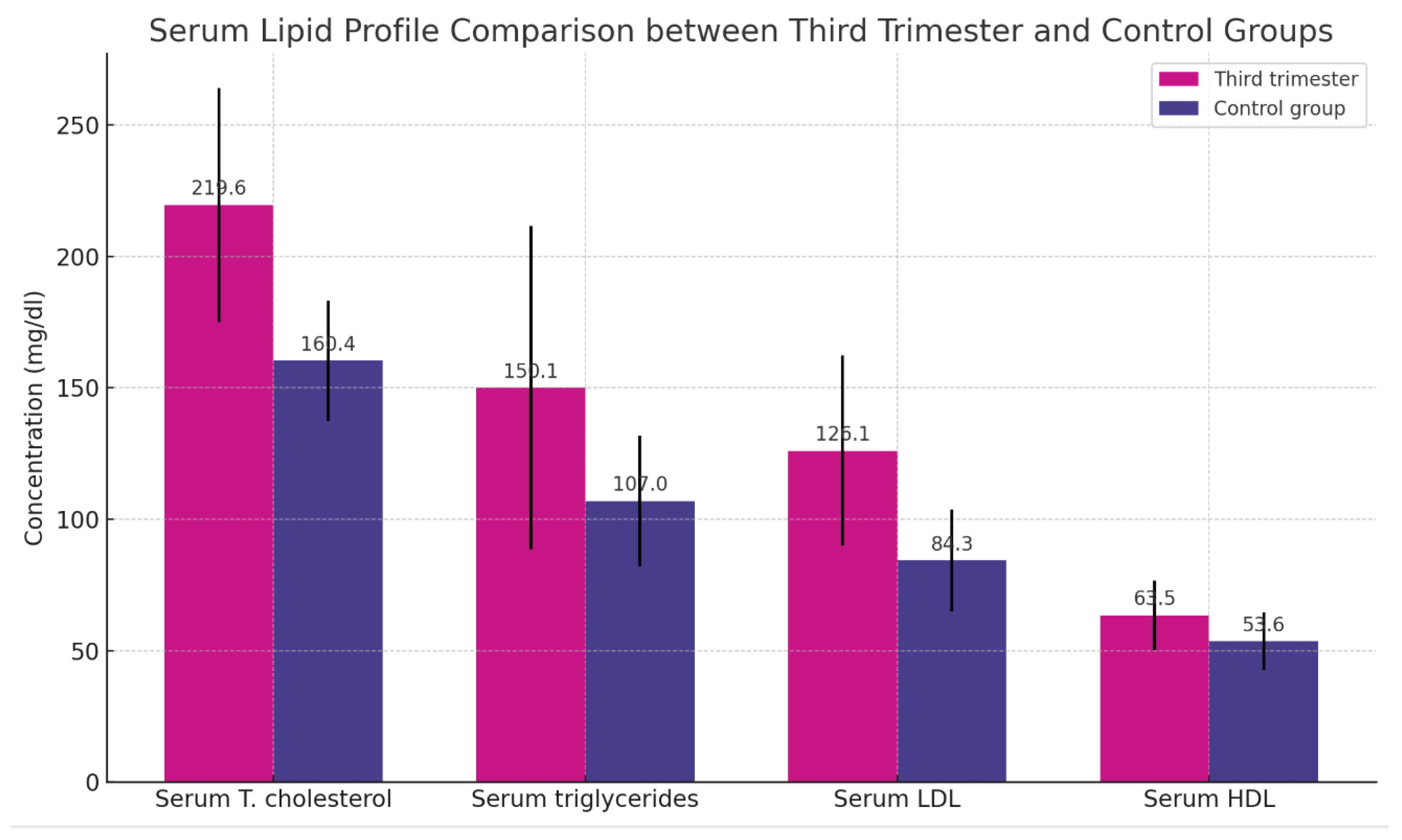
Table 3 illustrates a significant difference in the mean serum levels of total cholesterol, triglycerides, LDL, and HDL between women in their third trimester of pregnancy (n = 30) and the control group (n = 30), as shown in
Table 3 and
Figure 3.
The table displays the means ± SD ranges in brackets and probability (P). A t-test was utilized for the comparisons. A P-value of < 0.05 is deemed significant.
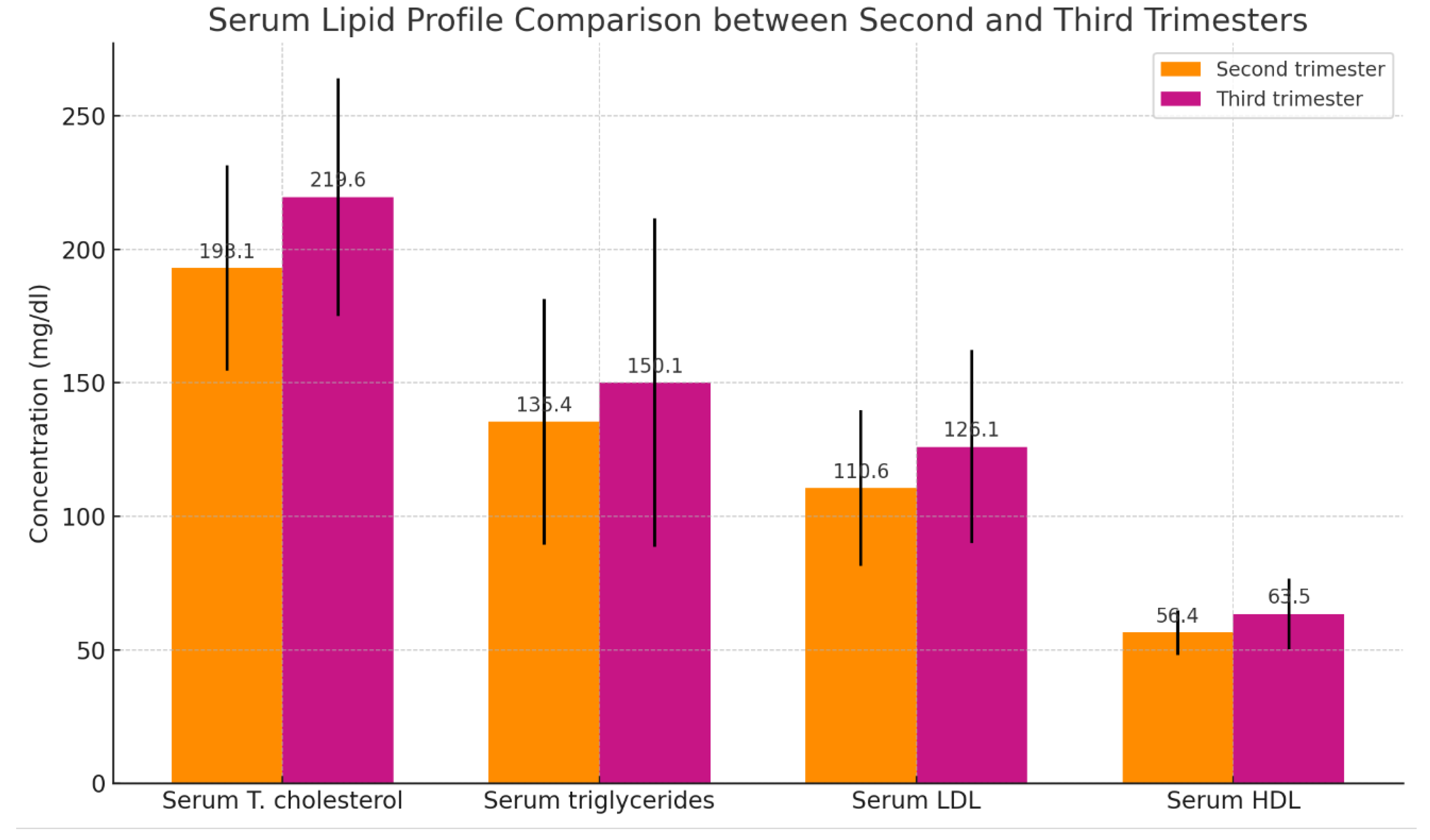
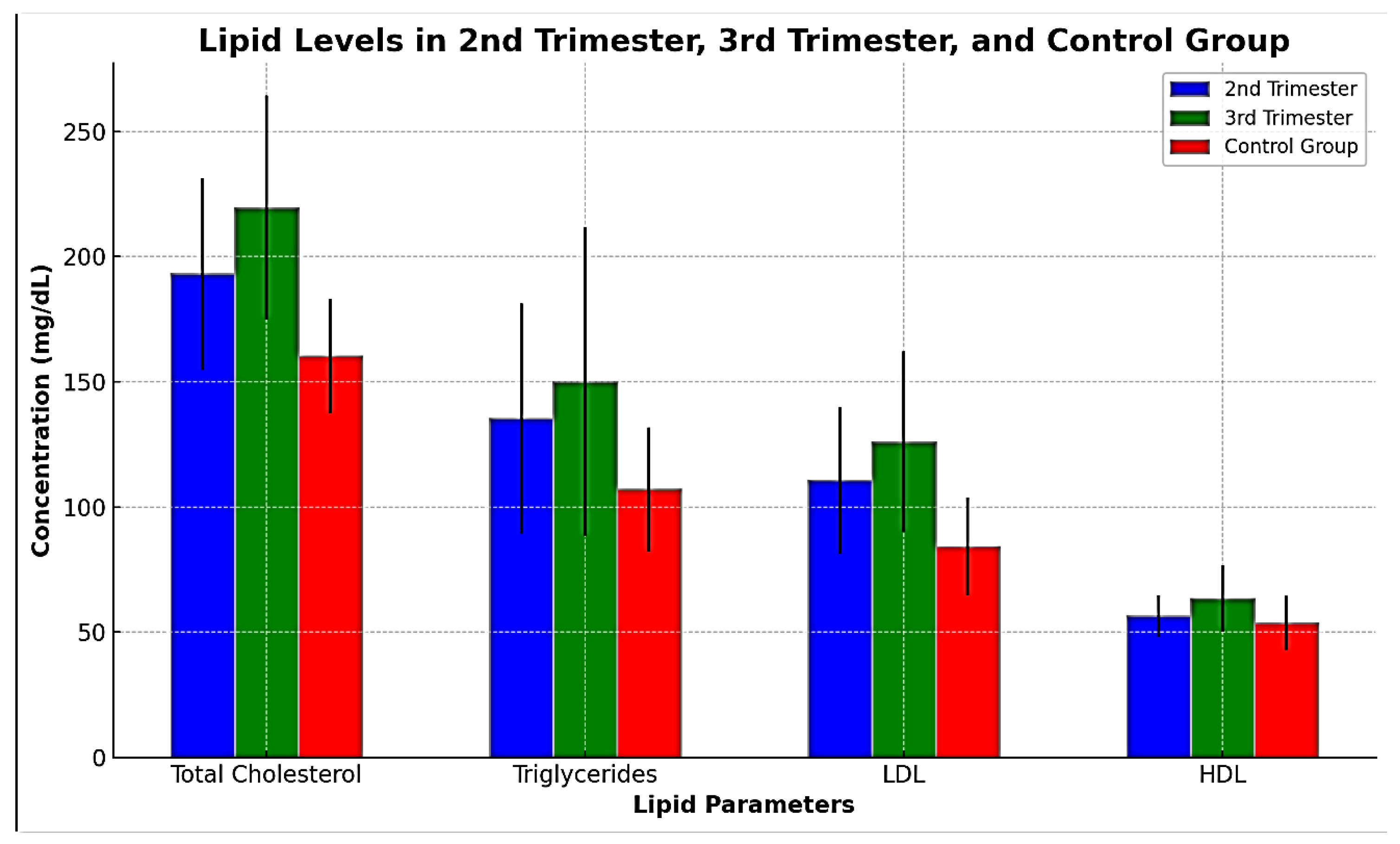
Table 4 demonstrates a significant difference in the mean serum levels of total cholesterol, triglycerides, LDL, and HDL between the third trimester (n = 30) and the second trimester (n = 30) of pregnancy, as shown in
Table 4 and
Figure 4.
The table presents the means ± SD with ranges in brackets and probability (P). A t-test was conducted for the comparison. A P-value of < 0.05 is regarded as significant.
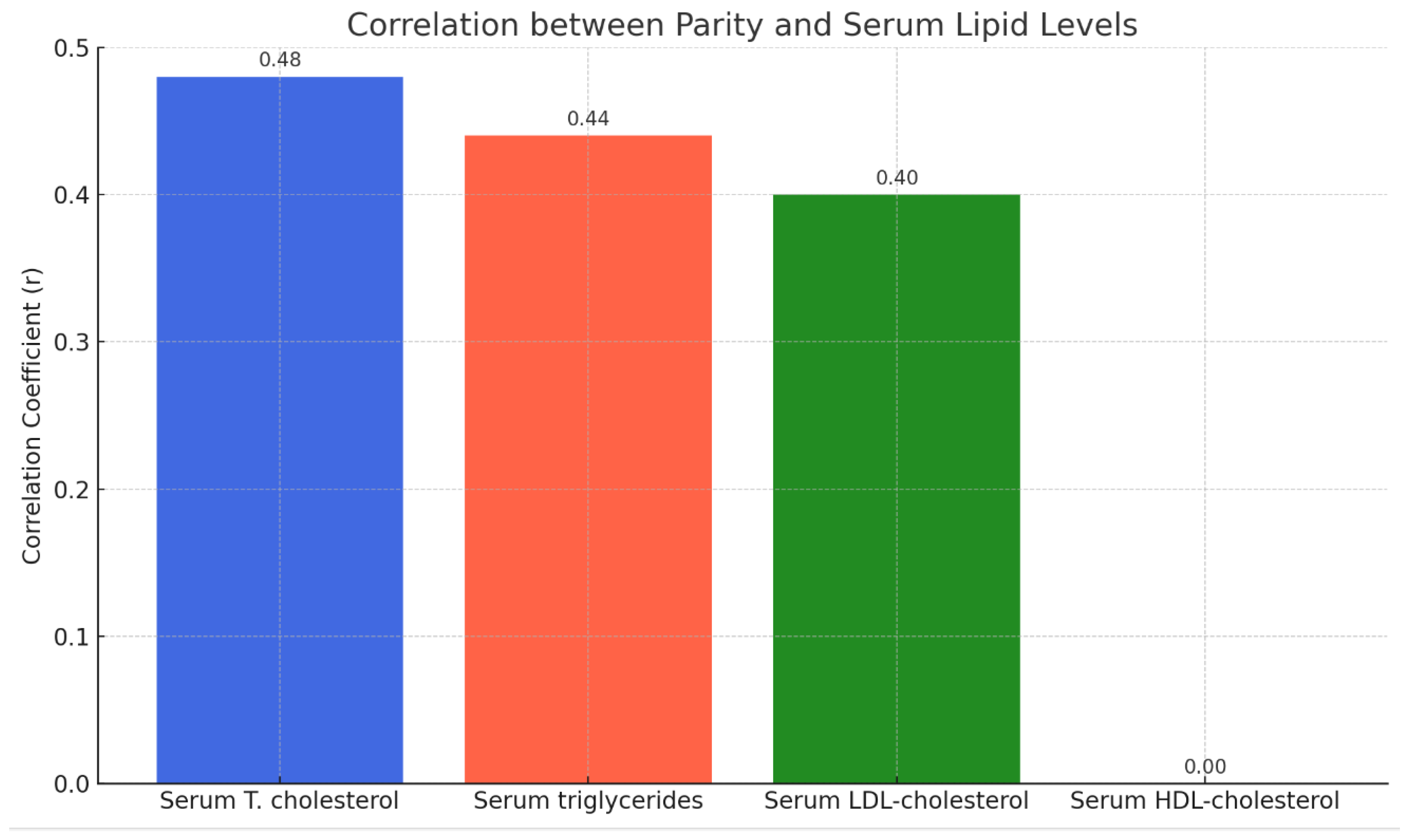
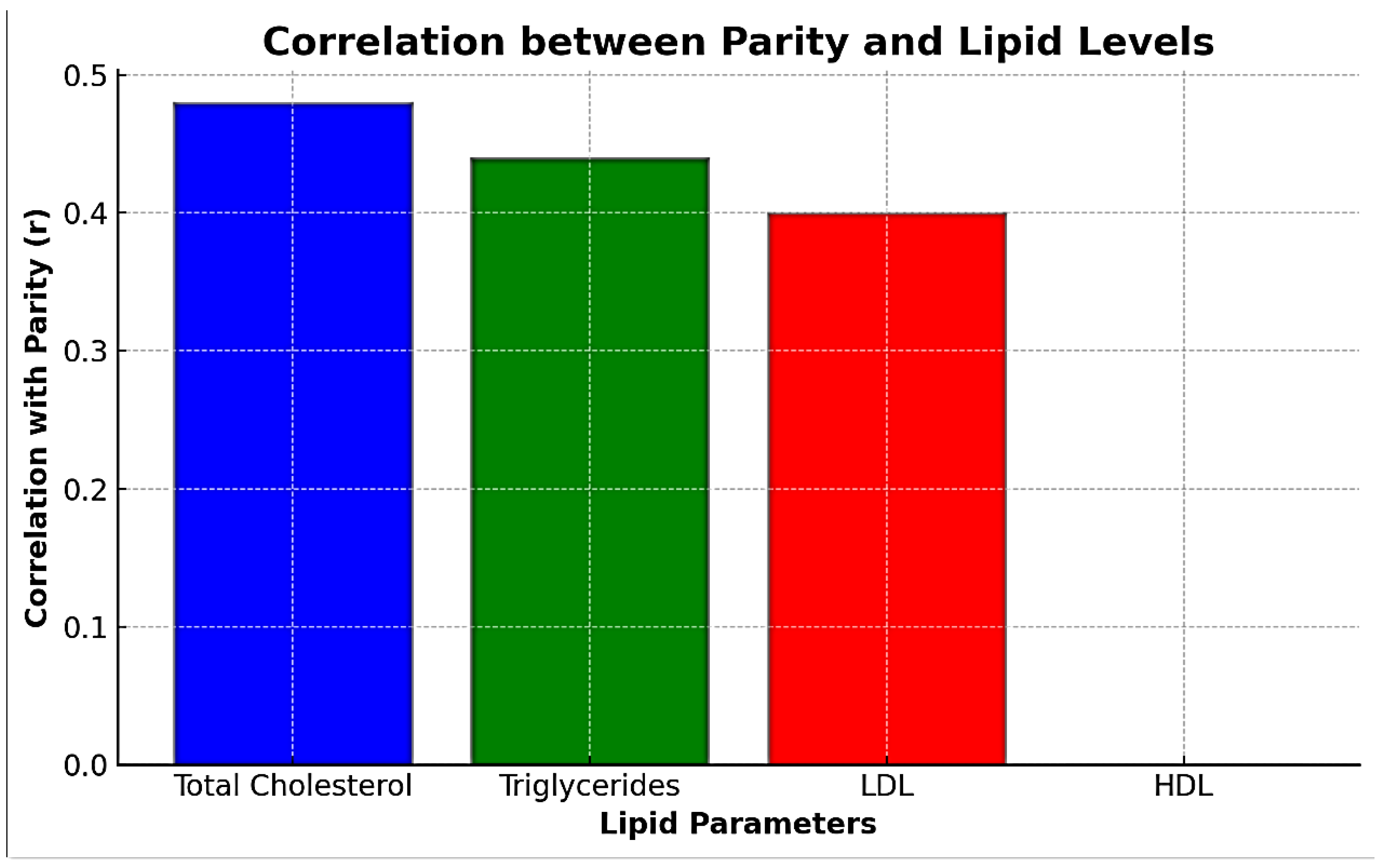
Table 5 indicates a weak positive correlation between pregnancy parity and serum levels of total cholesterol, triglycerides, and LDL but no correlation with serum HDL levels, as shown in
Table 5 and
Figure 5.
LIPID LEVELS IN PREGNANT WOMEN BY TRIMESTER AND CONTROL GROUP: as shown in
Table 6 and
Figure 6.
CORRELATION BETWEEN PARITY AND LIPID LEVELS
The document provides the following correlation coefficients (r) between parity and serum lipid levels; as shown in
Table 7 and
Figure 7.
Total Cholesterol: A moderate positive correlation (r = 0.48) suggests that as the number of pregnancies increases, the total cholesterol levels tend to rise.
Triglycerides: A moderate positive correlation (r = 0.44) indicates a similar trend, with triglyceride levels increasing with higher parity.
LDL: A moderate positive correlation (r = 0.40) shows that LDL levels also tend to increase as the number of pregnancies increases.
HDL: No correlation (r = 0.00) means that the number of pregnancies does not significantly affect HDL levels.
DISCUSSION
The present study aimed to investigate the serum lipid profile changes during the second and third trimesters of pregnancy in healthy Sudanese women. The findings reveal significant alterations in lipid metabolism, characterized by elevated levels of total cholesterol, triglycerides, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) as pregnancy progresses. These results align with previous research indicating pregnancy-induced hyperlipidemia, emphasizing the impact of physiological and hormonal changes on lipid metabolism.
Pregnancy is accompanied by substantial hormonal shifts, including increased levels of estrogen, progesterone, and placental lactogen. These hormones are crucial for maintaining pregnancy but also significantly influence lipid metabolism. Estrogen, in particular, is known to induce hepatic biosynthesis of endogenous triglycerides, leading to hypertriglyceridemia, a hallmark of pregnancy. The observed rise in serum lipid levels, particularly in the third trimester, supports the notion that hormonal modulation plays a pivotal role in lipid alterations during pregnancy.
The study demonstrates a progressive increase in lipid levels from the second to the third trimester. This trend is consistent with previous studies indicating that lipid concentrations peak in the third trimester. The marked increase in triglycerides, as observed in our study, is particularly noteworthy. Triglyceride levels can be two to three times higher in the third trimester compared to non-pregnant women, driven by estrogen-mediated hepatic biosynthesis. This hypertriglyceridemia serves as a physiological adaptation to ensure an adequate energy supply to the growing fetus.
Elevated lipid levels during pregnancy are physiologically necessary for fetal development and energy provision. Cholesterol, for instance, is vital for cell membrane formation, membrane integrity maintenance, and involvement in membrane-associated signaling pathways. However, excessive lipid accumulation poses potential risks. Hyperlipidemia, particularly elevated LDL levels, is associated with an increased risk of cardiovascular disease. Pregnant women with pre-existing hyperlipidemia or those experiencing pronounced lipid increases may be at higher risk for complications such as gestational diabetes, pre-eclampsia, and future cardiovascular issues.
The study also explored the relationship between parity and lipid profile changes. A weak positive correlation was observed between the number of pregnancies (parity) and levels of total cholesterol, triglycerides, and LDL, while no significant correlation was found with HDL levels. This finding suggests that repeated pregnancies may have a cumulative effect on lipid metabolism, potentially increasing the risk of cardiovascular disease in multiparous women. Previous research has indicated similar trends, with multiparous women exhibiting slightly altered lipid profiles compared to primiparous women.
The clinical implications of these findings are multifaceted. Monitoring lipid levels in pregnant women, especially those with multiple pregnancies or pre-existing lipid abnormalities, is crucial for early identification and management of potential complications. Dietary and lifestyle interventions may help mitigate the risks associated with hyperlipidemia during pregnancy. Further research is warranted to elucidate the underlying mechanisms of pregnancy-induced lipid changes and to develop targeted strategies for managing hyperlipidemia in pregnant women.
CONCLUSION
In conclusion, this study highlights significant changes in serum lipid profiles during the second and third trimesters of pregnancy in healthy Sudanese women. The findings underscore the impact of hormonal and physiological changes on lipid metabolism, with notable increases in total cholesterol, triglycerides, LDL, and HDL levels as pregnancy progresses. Understanding these alterations is essential for managing the potential risks associated with hyperlipidemia during pregnancy and ensuring maternal and fetal health. Future studies should focus on the long-term cardiovascular implications of pregnancy-induced hyperlipidemia and explore effective interventions for lipid management in pregnant women.
FUTURE DIRECTIONS
Future research should focus on a more comprehensive analysis of the underlying mechanisms that contribute to the changes in lipid profiles during pregnancy. Longitudinal studies could provide deeper insights into how these lipid alterations impact both maternal and fetal outcomes in the long term. Additionally, exploring the role of different dietary and lifestyle interventions in managing hyperlipidemia during pregnancy could offer valuable guidelines for clinical practice. Further research could also investigate the genetic factors that may predispose certain women to more significant lipid changes during pregnancy and assess the potential long-term cardiovascular risks associated with these alterations.
Acknowledgments
The authors would like to express their gratitude to the participants who volunteered for this study, as well as the staff at Umbada Hospital for their assistance in data collection and clinical assessments. Special thanks to the Research Ethical Committee of the Ministry of Health, Sudan, for their guidance and approval. The authors also appreciate the technical support provided by the Basic Science Department, Prince Sattam Bin Abdulaziz University.
Conflict of Interest
The authors declare no conflict of interest in the conduct and publication of this study. All findings and conclusions are based solely on the data collected and analyzed during the research.
References
- Punnon, R. The relationship between serum oestradiol levels and serum triglycerides, cholesterol, and phospholipids levels in normal human pregnancy. British Journal of Obstetrics and Gynaecology 1977, 84, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Winkler, K.; Wetzka, B. Low-density lipoprotein (LDL) subfraction during pregnancy. Journal of Clinical Endocrinology & Metabolism 2000, 85, 4543–4550. [Google Scholar] [CrossRef]
- Woollett, L.A. Where does fetal and embryonic cholesterol originate and what does it do? Annual Review of Nutrition 2008, 28, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Ness, R.B.; Harris, T. Number of pregnancies and the subsequent risk of cardiovascular disease. The New England Journal of Medicine 1993, 328, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, J.A.; Xi, A.R. Hyperlipidemia of normal pregnancy in Karachi-Bakistan. Kaohsiung Journal of Medical Sciences 1999, 15, 529–535. [Google Scholar] [PubMed]
- Bizzi, P.; Tonolo, G.; Esposito, F. Lipoprotein metabolism during normal pregnancy. American Journal of Obstetrics and Gynecology 1999, 181, 430–434. [Google Scholar] [CrossRef] [PubMed]
- DeJager, S.; Turpin, G. Hyperlipidemia in pregnancy. Lancet 1996, 25, 1839–1845. [Google Scholar] [CrossRef]
- Lockitch, G. (1993). Handbook of diagnostic biochemistry and hematology in normal pregnancy. CRC Press.
- Desoye, G.; Schmeditsch, M.; Pfeifer, K.P.; Zechner, R.; Kostner, G.M. Correlation of hormones with lipid and lipoprotein levels during normal pregnancy and postpartum. Journal of Clinical Endocrinology & Metabolism 1987, 64, 704–712. [Google Scholar] [CrossRef]
- Alvarez, J.; Montelongo, A.; Iglesias, A.; Lasunción, M.A. Longitudinal study on lipoprotein profile, high-density lipoprotein subclass, and postheparin lipases during gestation in women. Journal of Lipid Research 1996, 37, 299–308. [Google Scholar] [CrossRef]
- Knopp, R.H.; Bergelin, R.O.; Wahl, P.W. Population-based lipoprotein lipid reference values for pregnant women compared to nonpregnant women classified by sex hormone usage. American Journal of Obstetrics and Gynecology 1982, 15, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Halstead, A. C., Lockitch, G., Vallance, H., Wadsworth, L., & Wittmann, B. K. (1993). Handbook of diagnostic biochemistry and hematology in normal pregnancy. CRC Press.
- Husain, F.; Latif, S.; Uddin, M.; Nessa, A. Lipid profile changes in the second trimester of pregnancy. Mymensingh Medical Journal 2008, 17, 17–21. [Google Scholar] [PubMed]
- Chiang, A.N.; Yang, M.L.; Hung, J.H.; Shyn, S.K. Alterations of serum lipid levels and their biological relevance during and after pregnancy. Life Sciences 1995, 57, 2367–2375. [Google Scholar] [CrossRef]
- Glueck, C.J.; Fallet, R.W.; Scheel, D. Effects of oestrogenic compounds on triglyceride kinetics. Metabolism 1975, 24, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Rebuffé-Scrive, M.; Enk, L.; Crona, N.; Lönnroth, P. Fat cell metabolism in different regions in women: Effect of menstrual cycle, pregnancy, and lactation. The Journal of Clinical Investigation 1985, 75, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Ashwood ER (2001) Clinical chemistry of pregnancy In, C.A. Burtis & E. R. Ashwood (Eds.), Tietz fundamentals of clinical chemistry (5th ed., pp. 900–921). W.B. Saunders Company.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).