Submitted:
29 August 2024
Posted:
30 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. The Importance of the Disease
1.2. Fungicides Used in the Control of CLS until the Presents
1.2.1. Resistance
1.3. The Picolinamides (Qil – FRAC Group 21)
2. Objectives
3. Materials and Methods
3.1. Trial Sites and Used Cultivars
3.2. Trial Design
3.3. Test Items
3.4. Field Trials
3.5. Trial Assessment
3.6. Statistics and Data Analysis
4. Results
4.1. Infection Level (AUDPC)
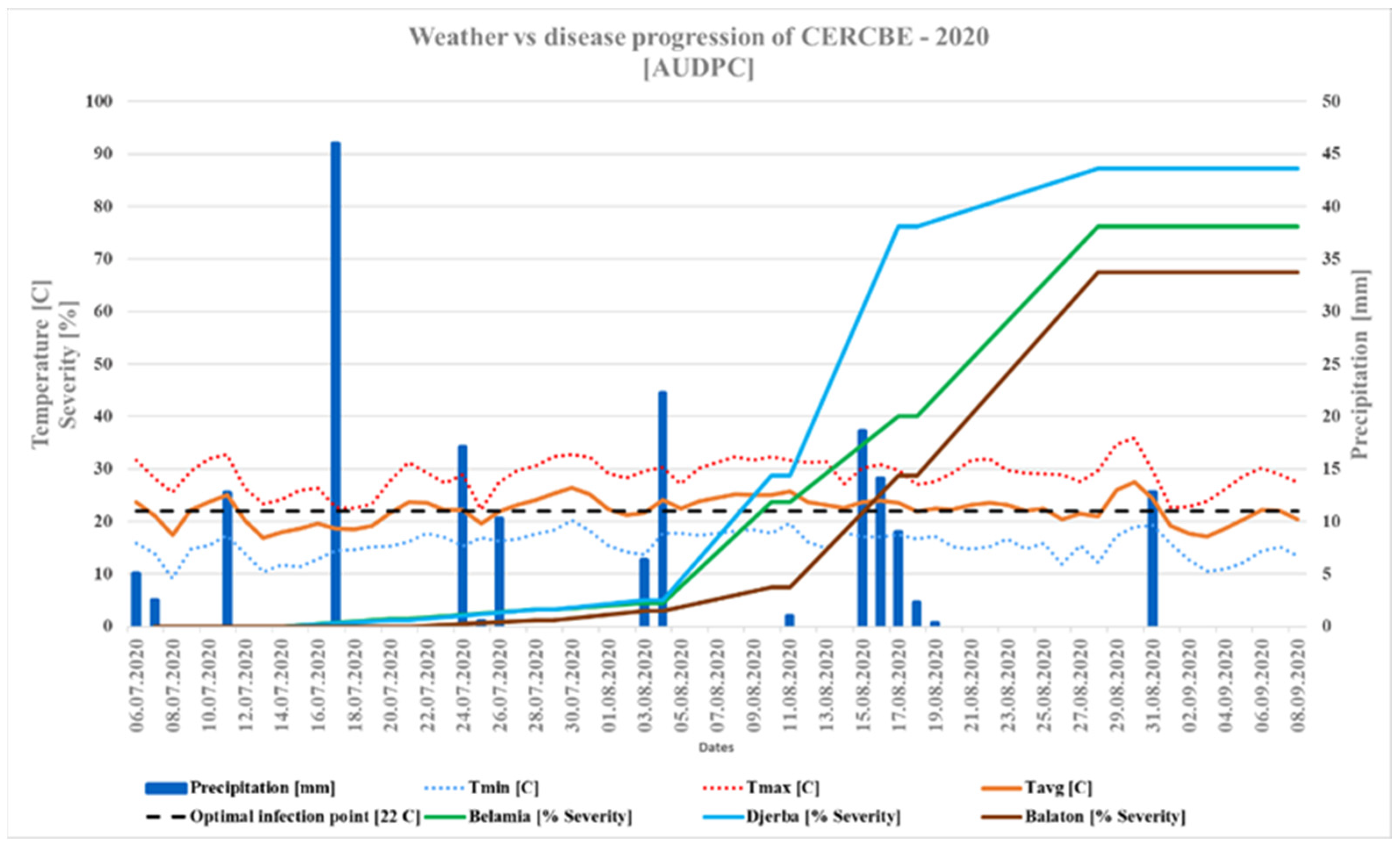
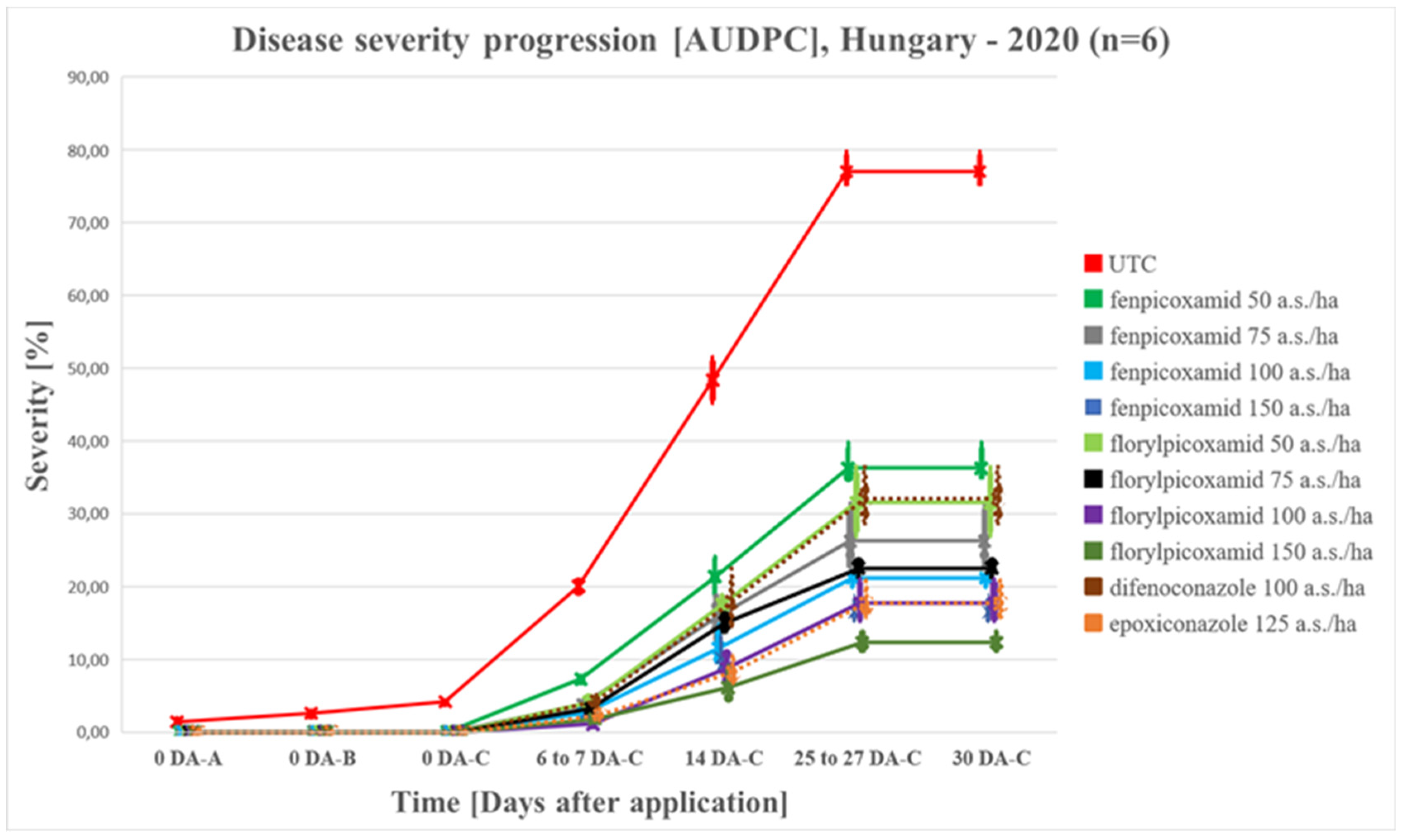
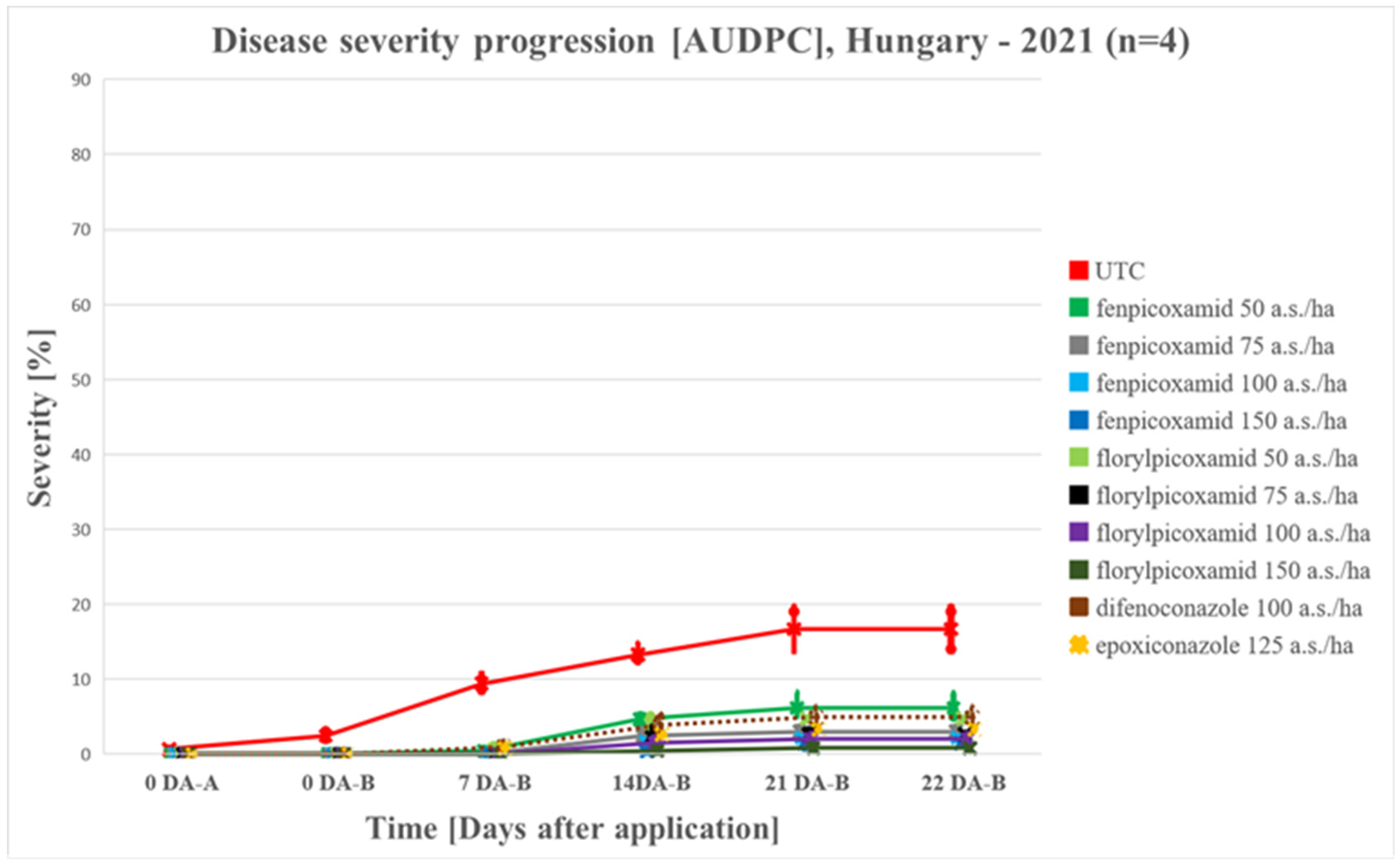
4.1.1. Disease Progress in 2020 vs 2021
4.2. Efficacy of Fungicides
4.2.1. Efficacy for Fenpicoxamid (Inatreq™)
4.2.2. Efficacy for Florylpicoxamid (Adavelt™)
5. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Saccardo, P.A. Fungi Veneti novi vel critici. Series V. Nuovo Giornale Botanico Italiano. 1876, 8(2):161-211.
- Ruppel, E.G. Foliar diseases caused by fungi. Whitney & JE Duffus (ed.). APS Press, St Paul, Minnesota. 1986, 8-9.
- Lartey, R. T., Weiland, L. Panella, P., Crous, C. Windels, Cercospora Leaf Spot of Sugar Beet and Related Species. St. Paul, MN, USA: American Phytopathological Society. 2010, 296.
- Shane, W., Teng, P., Impact of Cercospora leaf spot on root weight, sugar yield, and purity of Beta vulgaris. Plant Disease, 1992, 76. 812–820.
- Holtschulte, B., Cercospora beticola. In: Asher M., Holtschulte, B., 2000, 5-16.
- Smith, G., Ruppel, E., Association of Cercospora leaf spot, gross sucrose, percentage sucrose, and root weight in sugar beet. Canadian Journal of Plant Science, 1973, 53, 695–696.
- Wolf, P. F., Verreet, J. A., Factors affecting the onset of Cercospora leaf spot epidemics in sugar beet and establishment of disease-monitoring thresholds. Phytopathology. 2005, 95. 269–274.
- Kimmel, J. A cukorrépa lombvédelme az elmúl évtizedben. Cukoripar. 1999, 52. 2. 71-74.
- Fischl, G. A cukorrépa betegségei. PATE, Keszthely. 1992, 154.
- Pool, V. W., McKay, M., Climatic conditions as related to Cercospora beticola. Journal of Agricultural Research, 1916, 6. 21–60.
- Pundhir, V. S., Mukhopahyay, A. N., Recurrence of Cercospora leafspot of sugarbeet. Indian Journal of Agricultural Sciences. 1987, 57. 186-189.
- Canova, A., Richerce su la biologia e l’epidemiologia della Cercospora beticola Sacc., Parte IV. Annali Della Sperimentazione Agraria, N. S. 1959, 13. 685-776.
- Rossi, V., Battilani, P., CERCOPRI: a forecasting model for primary infections of Cercospora leaf spot of sugarbeet 1. EPPO Bulletin, 1991, 21, 527–531.
- Windels, C. E., Lamey, H. A., Hilde, D., Widner, J., Knudsen, T., A Cerospora leaf spot model for sugar beet: In practice by an industry. Plant Disease. 1998, 82. 716–726.
- Pitblado, R., Nichols, I., The implementation of BEETCAST-a weather-timed fungicide spray program for the control of Cercospora leaf spot, in Ontario and Michigan. Journal of Sugarbeet Research. 2005, 42. 53–54.
- Racca, P., Jörg, E., CERCBET 3 – a forecaster for epidemic development of Cercospora beticola. EPPO Bulletin, 2007, 37, 344–349.
- Shane, W., Teng, P., Cercospora beticola infection prediction model presented. Sugar Producer. 1984, 10(3). 14-19.
- Collins, D. P., Jacobsen, B. J., Optimizing a Bacillus subtilis isolate for biological control of sugar beet Cercospora leaf spot. Biological Control, 2003, 26, 153–161.
- Galletti, S., Burzi, P. L., Cerato, C., Marinello, S., Sala, E., Trichoderma as a potential biocontrol agent for Cercospora leaf spot of sugarbeet. Bio Control. 2008, 53. 917-930.
- Kusstatscher, P., Cernava, T., Harms, K., Maier, J., Eigner, H., Berg, G. et al., Disease incidence in sugar beet fields is correlated with microbial diversity and distinct biological markers. Phytobiomes Journal. 2019, 3. 22–30.
- FRAC, www.FRAC.info, 2024.
- Giannopolitis, C., Occurrence of strains of Cercospora beticola resistant to triphenyltin fungicides in Greece. Plant Disease Report, 1978, 62. 205–208.
- Secor, G. A., Rivera, V.V., Khan, M. F. R., Gudmestad, N. C., Monitoring fungicide sensitivity of Cercospora beticola of sugarbeet for disease management decisions. PlantDisease. 2010, 94.1272–1282.
- Rosenzweig, N., Hanson, L. E., Mambetova, S., Jiang, Q., Guza, C., Stewart, J. et al., Temporal population monitoring of fungicide sensitivity in Cercospora beticola from sugarbeet (Beta vulgaris) in the Upper Great Lakes. Canadian Journal of Plant Pathology. 2020, 10.
- Dekker, J., Preventing and Managing Fungicide Resistance. US National Research Council Committee on Strategies for the Management of Pesticide-Resistant Pest Populations, Pesticide resistance: Strategies and Tactics for Management. Washington, DC: National Academy Press. 1986, 347–354.
- Ioannidis, P., Fungicides chemicals and techniques for controlling Cercospora beticola Sacc. in Greece. Presented at the Proceedings of Mediterranean Commitee Meeting of IIRB, Thessaloniki, Greece, 1994, 139–151.
- Georgopoulos, S., Dovas, C., A serious outbreak of strains of Cercospora beticola resistant to benzimidazole fungicides in Northern Greece. Plant Disease Report, 1973, 5. 321–324.
- Ruppel, E., Scott, P., Strains of Cercospora beticola resistant to benomyl in the USA. Plant Disease Report, 1974, 58, 434–436.
- Dafang, H., Shuzhi, W. X. Z., Studies on resistance of Cercospora beticola to benzimidazole fungicides. Journal of Plant Protection, 1982, 2. 11.
- Pal, V., Mukhopadhyay, A., Occurrence of strains of Cercospora beticola resistance to carbendazim (MBC) in India. Indian Journal of Mycology and Plant Pathology, 1983, 13. 333–334.
- Kimmel, J., A Cercospora beticola fungicid rezisztenciája. Cukoripar. 2003, 56. 2. 67-70.
- Davidson, R., Hanson, L., Franc, G., Panella, L., Analysis of β-tubulin gene fragments from benzimidazole-sensitive and -tolerant Cercospora beticola. Journal of Phytopathology. 2006, 154, 321–328.
- Trkulja, N., Ivanović, Ž., Pfaf-Dolovac, E., Dolovac, N., Mitrović, M., Toševski, I. et al., Characterisation of benzimidazole resistance of Cercospora beticola in Serbia using PCR-based detection of resistance-associated mutations of the β-tubulin gene. European Journal of Plant Pathology. 2013, 135. 889–902.
- Brown, M., Waller, C., Charlet, C., Palmieri, R., The use of flutriafol based fungicides for the control of sugar beet diseases in Europe. Presented at the 1986 British Crop Protection Conference Pests and Diseases, 1986, 3. 1055–1061.
- Karaoglanidis, G., Ioannidis, P., Thanassoulopoulos, C., Influence of fungicide spray schedules on the sensitivity of Cercospora beticola to the sterol demethylation-inhibiting fungicide flutriafol. Crop Protection, 2001, 20. 941–947.
- El Housni, Z. S., Tahiri Ezrari, A., Ouijja, A., Lahlali, R., First report of benzimidazole, DMI and QoI-insensitive Cercospora beticola in sugar beet in Morocco. New Disease Reports. 2018, 38. 17.
- Trueman, C., Hanson, L., Somohano, P., Rosenzweig, N., First report of DMI-insensitive Cercospora beticola on sugar beet in Ontario, Canada. New Disease Reports, 2017, 36. 20.
- Bolton, M. D., Birla, K., Rivera, V. V., Rudolph, K. D., Secor, G.A., Characterization of CbCyp51 from field isolates of Cercospora beticola. Phytopathology, 2012a, 102. 298–305.
- Karaoglanidis, G., Ioannidis, P., Fungicide resistance of Cercospora beticola in Europe. In: Lartey R., Weiland J., Panella L., Crous P., Windels C. (Eds.) (2010): Cercospora Leaf Spot of Sugar Beet and Related Species. St. Paul, MN, USA: American Phytopathological Society. 2010, 189–211.
- Bolton, M.D., Rivera, V. V., del Río Mendoza, L. E., Khan, M. F., Secor, G. A., Efficacy of variable tetraconazole rates against Cercospora beticola isolates with differing in vitro sensitivities to DMI fungicides. Plant Disease, 2012b, 96. 1749–1756.
- Bolton, M. D., Ebert, M. K., Faino, L., Rivera-Varas, V., de Jonge, R., Van de Peer, Y., RNA-sequencing of Cercospora beticola DMI-sensitive and -resistant isolates after treatment with tetraconazole identifies common and contrasting pathway induction. Fungal Genetics and Biology. 2016, 92. 1–13.
- Secor, G., Rivera, V., Bolton, M., Sensitivity of Cercospora beticola to Foliar Fungicides in 2017. The Sugarbeet Research and Education Board of Minnesota and North Dakota. 2017, 161–168.
- Karaoglanidis, G., Thanassoulopoulos, C., Cross-resistance patterns among sterol biosynthesis inhibiting fungicides (SBIs) in Cercospora beticola. European Journal of Plant Pathology, 2003, 109. 929–934.
- Leroux, P., Albertini, C., Gautier, A., Gredt, M., Walker, A. S., Mutations in the CYP51 gene correlated with changes in sensitivity to sterol 14α-demethylation inhibitors in field isolates of Mycosphaerella graminicola. Pest Management Science. 2007, 63, 688–698.
- Ziogas, B. N., Malandrakis, A. A., Sterol biosynthesis inhibitors: C14 demethylation (DMIs). In: Ishii, H. and Hollomon, D. (Eds.) (2015): Fungicide Resistance in Plant Pathogens. Tokyo, Japan: Springer. 2015, 199–216.
- Trkulja, N. R., Milosavljević, A. G., Mitrović, M. S., Jović, J. B., Toševski, I. T., Khan, M. F. et al., Molecular and experimental evidence of multi-resistance of Cercospora beticola field populations to MBC, DMI and QoI fungicides. European Journal of PlantPathology, 2017, 149. 895-910.
- Shrestha, S., Neubauer, J., Spanner, R., Natwick, M., Rios, J., Metz, N., Rapid detection of Cercospora beticola in sugar beet and mutations associated with fungicide resistance using LAMP or probe-based qPCR. Plant Disease. 2020, 104. 1654–1661.
- Fernández-Ortuño, D., Torés, J. A., De Vicente, A., Pérez-García, A., Mechanisms of resistance to QoI fungicides in phytopathogenic fungi. International Microbiology, 2008, 11. 1-9.
- Birla, K., Rivera, V. V., Secor, G. A., Khan, M. F., Bolton, M. D., Characterization of cytochrome b from European field isolates of Cercospora beticola with quinone outside inhibitor resistance. European Journal of Plant Pathology. 2012, 34. 475–488.
- Bolton, M. D., Rivera, V. V., Secor, G., Identification of the G143A mutation associated with QoI resistance in Cercospora beticola field isolates from Michigan, United States. Pest Management Science, 2013, 69. 35–39.
- Piszczek, J., Pieczul, K., Kiniec, A., First report of G143A strobilurin resistance in Cercospora beticola in sugar beet (Beta vulgaris) in Poland. Journal of Plant Diseases and Protection, 2018, 125. 99–101.
- Kayamori, M., Shimizu, M., Yamana, T., Komatsu, T., Minako, S., Shinmura, A. et al., First report of QoI resistance in Cercospora beticola in sugar beet in Japan. Journal of General Plant Pathology. 2020, 86. 149–153.
- Trueman, C., Hanson, L., Rosenzweig, N., Jiang, Q., Kirk, W. First report of QoI insensitive Cercospora beticola on sugarbeet in Ontario, Canada. PlantDisease. 2013, 97. 1255.
- Kirk, W., Hanson, L., Franc, G., Stump, W., Gachango, E., Clark, G. et al., First report of strobilurin resistance in Cercospora beticola in sugar beet (Beta vulgaris) in Michigan and Nebraska, USA. New Disease Reports, 2012, 26. 3.
- Owen, W. J., Yao, Ch., Myung, K., Kemmit, G., Leader, A., Meyer, K. G., Bowling, A. J., Slanec, T., Kramer, V., Biological characterization of fenpicoxamid, a new fungicide with utility in cereals and other crops. Pest Management Science, 2017, 73(10). [CrossRef]
- Meyer, K. G., Yao, Ch., Lu, Y., Bravo-Altamirano, K., Buchan, Z., Daeuble, J. F., DeKorver, K., DeLorbe, J., Heemstra, R., Herrick, J., Jones, D., Loy, B. A., Rigoli, J., Wang, N. X., Wilmot, J., Young, D., Chapter 28 – The discovery of florylpicoxamid, a new picolinamide for disease control. Recent Highlights in the Discovery and Optimization of Crop Protection Products. 2021, 433-442.
- Yao, Ch., Meyer, K. G., Gallup, C., Bowling, A. J., Hufnagl, A., Myung, K., Lutz, J., Slanec, T., Pence, H. E., Delgado, J., Wang, N. X., Florylpicoxamid, a new picolinamide fungicide with broad spectrum activity. Pest management science. 2021. [CrossRef]
- PPDB: Pesticide Properties DataBase, 2024. University of Hertfordshire. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/3073.htm; https://sitem.herts.ac.uk/aeru/ppdb/en/Reports/3191.htm.
- EPPO Global Database, Cercospora beticola (CERCBE), 2024. Available online: https://gd.eppo.int/taxon/CERCBE.
- EPPO Database on PP1 Standards. 2024. Available online: https://pp1.eppo.int/standards.
- Wolf, P. F., Verreet, J. A., The IPM sugar beet model—an integrated pest management system in Germany for the control of fungal leaf diseases in sugar beet. Plant Dis. 2002, 86. 336-344.
- Abbott, W. S., A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18: 265-267.
- Brillaud Cailliau, M., Gate, S., Guepet, G., Leader, A., Kemmit, G., InatreqTM active (fenpicoxamid), a new natural fungicide for the control of Zymoseporia tritici and the other diseases in cereals. 12e Conference Internationale sur les maladies des plantes tours – 10, 11 et 12 Décembre, 2018. Available online: https://www.cabidigitallibrary.org/doi/pdf/10.5555/20193089498.
- Gustafson, G. J., Delgado, J., Biró, A., Gallup, C., Use of a difluro-(2-Hydroxyprpyl) pyridine compound as a fungicide for control of leaf spot of sugar beets. Patent Publication number: US20210274787 (A1), 2021. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=6&ND=3&adjacent=true&locale=en_EP&FT=D&date=20210909&CC=US&NR=2021274787A1&KC=A1.
- Gallup, C. Gallup, C., Huang, Y-H., Biró, A., Yao, Ch., Meyer, K. G., Da Cunha, L. C. V., Fairfax, M., Husband, B., Richburg, J., Martin, M., Use of acyclic picolinamide compound as a fungicide for control of phytopathogenic fungi in row crops. Patent publication number: 20200077656; UA127713 (C2), 2020. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?FT=D&date=20231213&DB=EPODOC&locale=en_EP&CC=UA&NR=127713C2&KC=C2&ND=4.
- Cioni, F., Collina, M., Maines, G., Khan, M. F. R., Secor, G. A., Rivera, V. V., A New Integrated Pest Management (IPM) Model for Cercospora Leaf Spot of Sugar Beets in the Po Valley, Italy. Sugar Tech. Jan-Mar 2014. 2014, 16(1): 92-99. Available online: https://www.researchgate.net/publication/260159032. [CrossRef]
- Karadimos, D. A., Karaoglanidis, G.S., Comparative Efficacy, Selection of Effective Partners, and Application Time. Plant Disease. 2006, 90(6), 820-825. [CrossRef]
- Kempl, F., Tomasetig, C., Gotsmi, S., Effects of triazols and strobilurins on the spreading of cercospora. 73rd IIRB Congress. Brussels, 2012. Available online: https://www.researchgate.net/publication/284155978.

| Trial number | Country code | Trial location | Variety | Appl. date | Appl. Crop BBCH |
Initial CERCBE % inf. sev. |
|---|---|---|---|---|---|---|
| EA20F9B001F-AB01 | HUN | Jászberény | Smart Belamia | 20June 20 28June 20 03 Aug 20 |
38 38-39 39 |
1.5 |
| EA20F9B001F-AB02 | HUN | Jászberény | Smart Djerba | 21June 20 28June 20 03 Aug 20 |
38 38-39 39 |
2.5 |
| EA20F9B001F-AB03 | HUN | Jászberény | Balaton | 22June 20 08 Aug 20 |
38 39 |
3 |
| EA20F9B002F-AB01 | HUN | Jászberény | Smart Belamia | 20June 20 28June 20 03 Aug 20 |
38 38-39 39 |
1.5 |
| EA20F9B002F-AB02 | HUN | Jászberény | Smart Djerba | 21June 20 28June 20 03 Aug 20 |
38 38-39 39 |
2.5 |
| EA20F9B002F-AB03 | HUN | Jászberény | Balaton | 22June 20 08 Aug 20 |
38 39 |
3 |
| EA21F9B001F-AB01 | HUN | Jászberény | Smart Belamia | 24 Aug 21 07 Sep 21 |
39 39 |
0 |
| EA21F9B001F-AB02 | HUN | Jászberény | Smart Djerba | 24 Aug 21 07 Sep 21 |
39 39 |
1.25 |
| EA21G1C001F-AB01 | HUN | Jászberény | Smart Belamia | 24 Aug 21 07 Sep 21 |
39 39 |
0 |
| EA21G1C001F-AB02 | HUN | Jászberény | Smart Djerba | 24 Aug 21 07 Sep 21 |
39 39 |
1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





