Submitted:
29 August 2024
Posted:
30 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Isolate Sampling, DNA Preparation, Genome Sequencing and Published Genome Collection
2.2. Identification of Single-Nucleotide Polymorphism
2.3. Characterization of Genetic Diversity and Population Genetic Structures
2.4. Phylogeographic Analysis of M. oryzae
3. Results
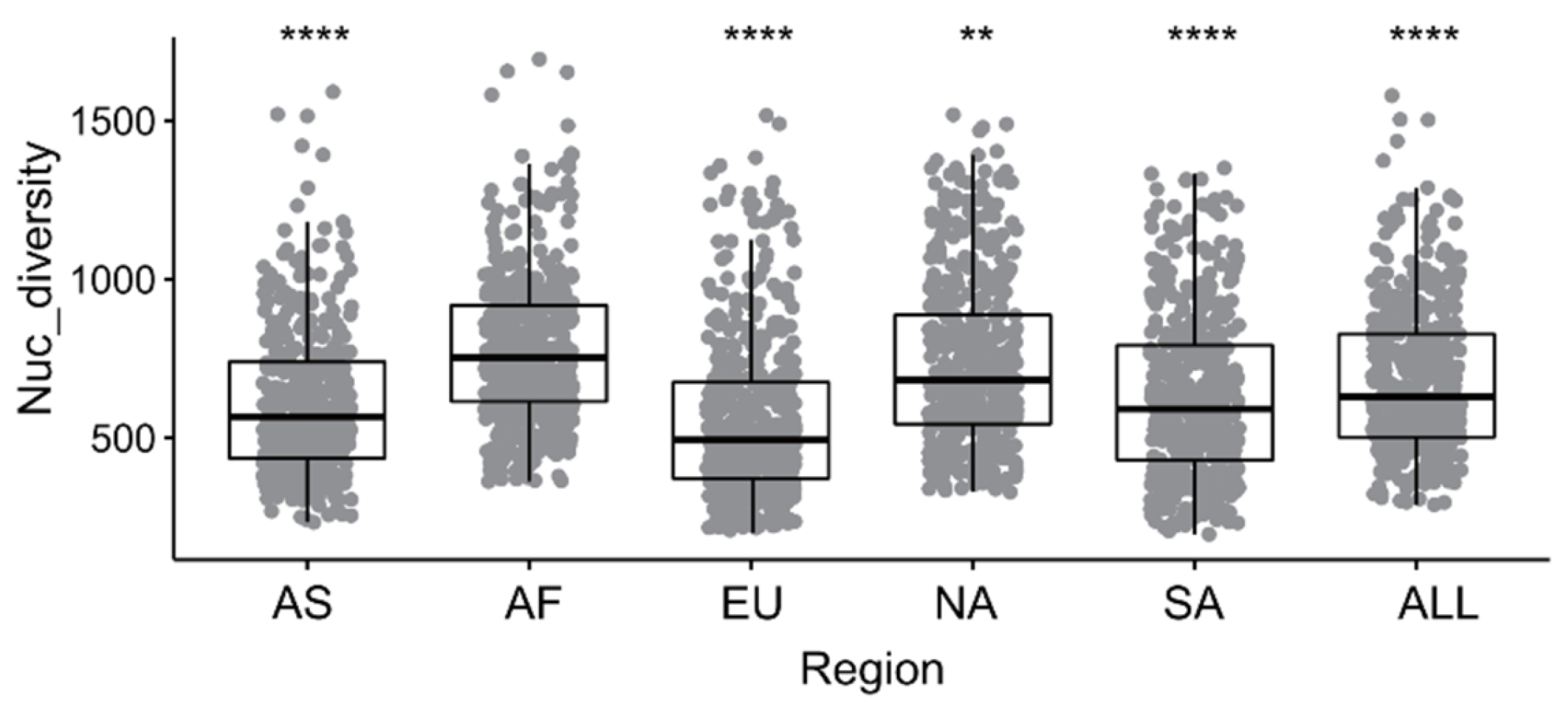
3.1. Spatiotemporal Dynamics of Genetic Diversity in M. oryzae Populations
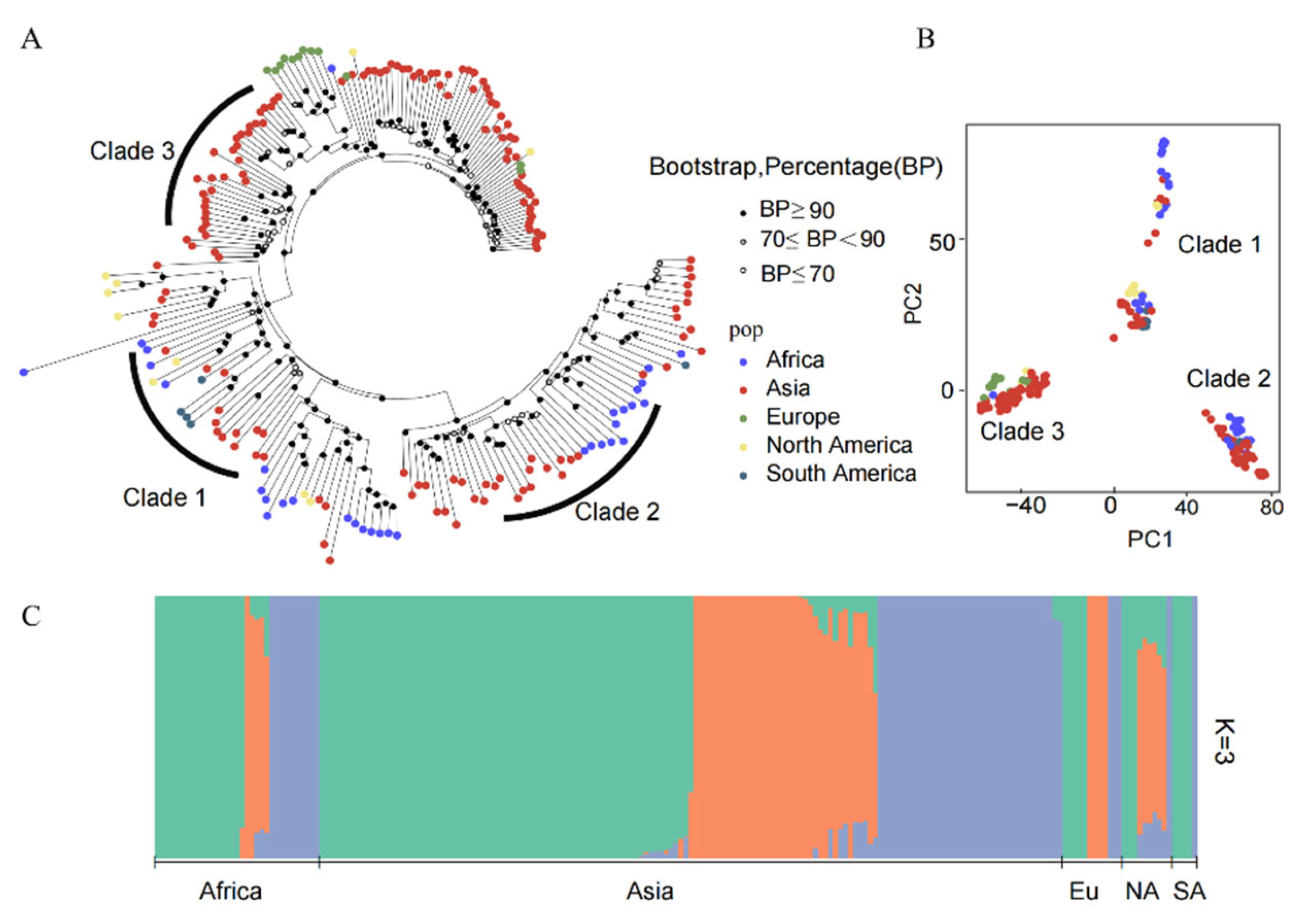
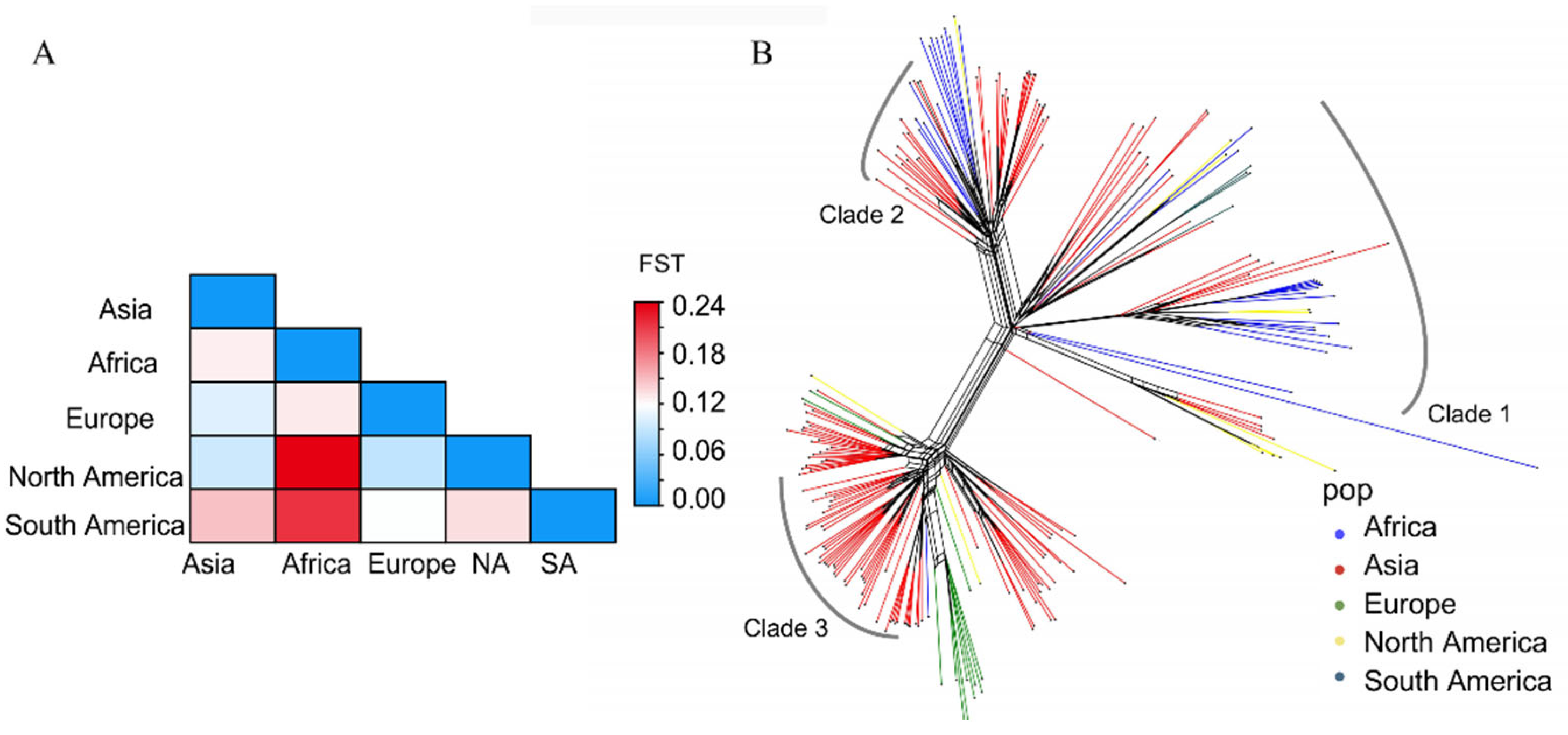
3.2. Genetic Structures of Worldwide Geographic Populations
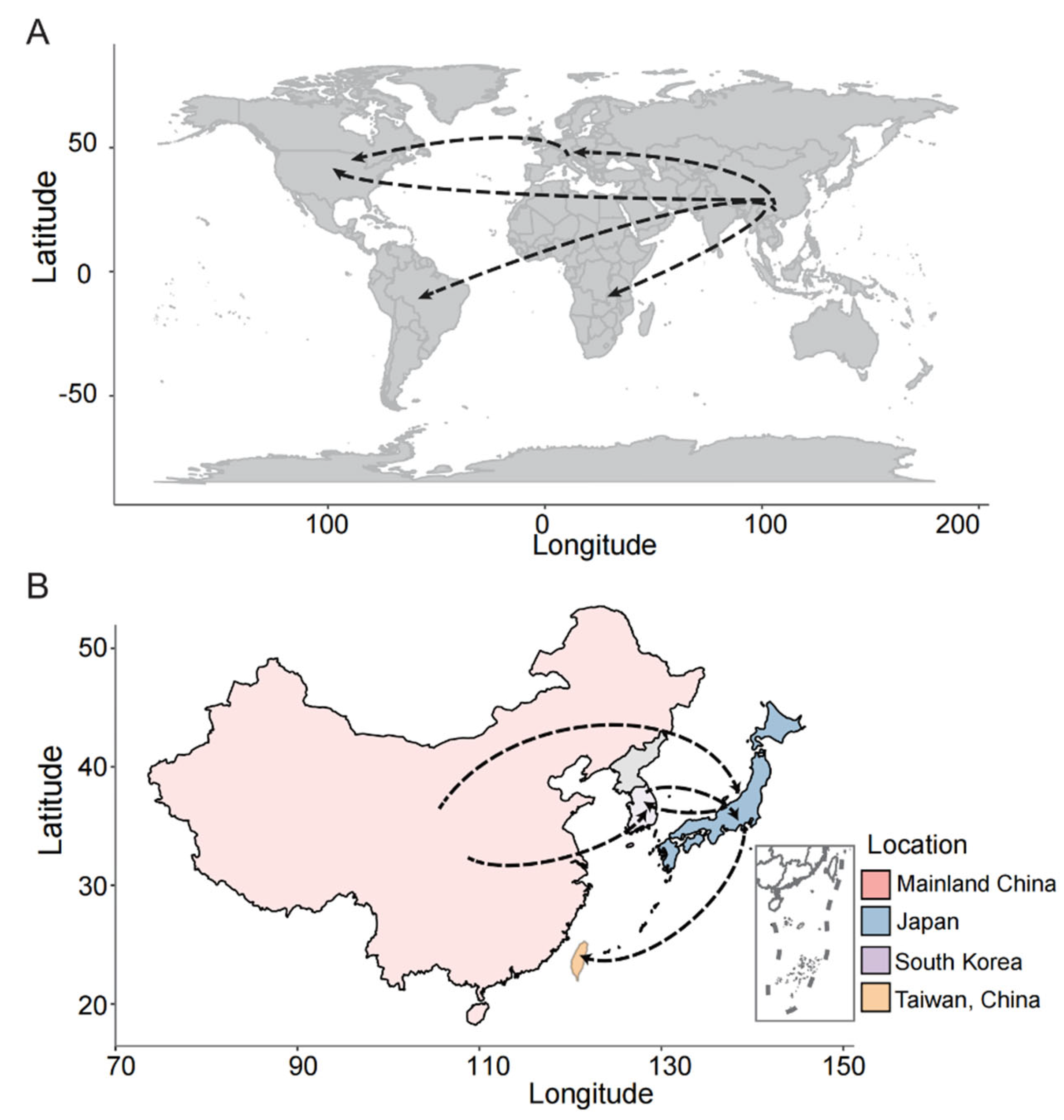
3.3. Migration Patterns of M. oryzae
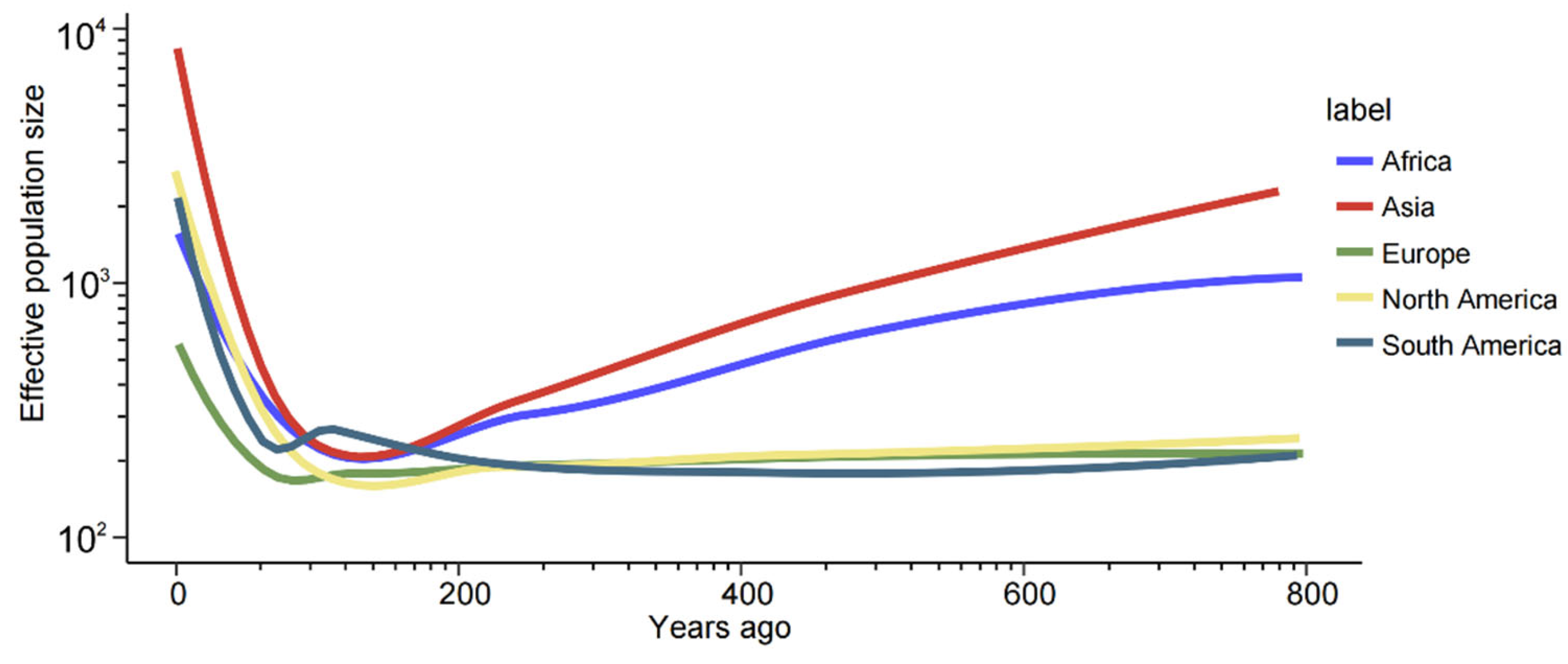
3.4. Demographic History of M. oryzae Populations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Talbot, N.J. On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea. Annu Rev Microbiol 2003, 57, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Khang, C.H.; Park, S.Y.; Lee, Y.H.; Valent, B.; Kang, S. Genome organization and evolution of the AVR-Pita avirulence gene family in the Magnaporthe grisea species complex. Mol Plant Microbe Interact 2008, 21, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Zeigler, R.S. Recombination in Magnaporthe grisea. Annu Rev Phytopathol 1998, 36, 249. [Google Scholar] [CrossRef]
- Valent, B. The Impact of Blast Disease: Past, Present, and Future. Methods Mol Biol 2021, 2356, 1–18. [Google Scholar]
- Notteghem, J.L.; Silué, D. Distribution of the mating type alleles in Magnaporthe grisea populations pathogenic on rice. Phytopathology 1992, 82, 421–424. [Google Scholar] [CrossRef]
- Dayakar, B.V.; Narayanan, N.N.; Gnanamanickam, S.S. Cross-Compatibility and Distribution of Mating Type Alleles of the Rice Blast Fungus Magnaporthe grisea in India. Plant Dis 2000, 84, 700–704. [Google Scholar] [CrossRef]
- Maciel, J.L.; Ceresini, P.C.; Castroagudin, V.L.; Zala, M.; Kema, G.H.; McDonald, B.A. Population structure and pathotype diversity of the wheat blast pathogen Magnaporthe oryzae 25 years after its emergence in Brazil. Phytopathology 2014, 104, 95–107. [Google Scholar] [CrossRef]
- Tredway, L.P.; Stevenson, K.L.; Burpee, L.L. Mating type distribution and fertility status in Magnaporthe grisea populations from turfgrasses in georgia. Plant Dis 2003, 87, 435–441. [Google Scholar] [CrossRef]
- Consolo, V.F.; Cordo, C.A.; Salerno, G.L. Mating-type distribution and fertility status in Magnaporthe grisea populations from Argentina. Mycopathologia 2005, 160, 285–290. [Google Scholar] [CrossRef]
- Zhan, J.; Thrall, P.H.; Burdon, J.J. Achieving sustainable plant disease management through evolutionary principles. Trends Plant Sci 2014, 19, 570–575. [Google Scholar] [CrossRef]
- Markert JA, Champlin DM, Gutjahr-Gobell R, Grear JS, Kuhn A, McGreevy TJ Jr; et al. Population genetic diversity and fitness in multiple environments. BMC Evol Biol 2010, 10, 205.
- Xu X, Yang W, Tian K, Zheng J, Liu X, Li K; et al. Genetic diversity and pathogenicity dynamics of Magnaporthe oryzae in the wuling mountain area of china. Eur J Plant Pathol 2019, 153, 731–742. [CrossRef]
- Xu X, Tang X, Han H, Yang W, Liu X, Li K; et al. Pathogenicity, mating type distribution and avirulence gene mutation of Magnaporthe oryzae populations in the wuling mountain region of china. Physiol Mol Plant Pathol 2021, 116, 101716. [CrossRef]
- Li, Y.; Liu, E.M.; Dai, L.Y.; Li, C.Y.; Liu, L. Genetic diversity among populations as related to pathotypes for Magnaporthe grisea in hunan province. Chin J Rice Sci 2007, 21, 304. [Google Scholar]
- Yadav MK, Aravindan S, Raghu S, Prabhukarthikeyan SR, Keerthana U, Ngangkham U; et al. Assessment of genetic diversity and population structure of Magnaporthe oryzae causing rice blast disease using ssr markers. Physiol Mol Plant P 2019, 106, 157–165. [CrossRef]
- Jagadeesh, D.; Kumar, M.K.P.; Amruthavalli, C.; Devaki, N.S. Genetic diversity of Magnaporthe oryzae, the blast pathogen of rice in different districts of karnataka, india determined by simple sequence repeat (ssr) markers. Indian Phytopathology 2020, 73, 713–723. [Google Scholar] [CrossRef]
- Longya, A.; Talumphai, S.; Jantasuriyarat, C. Morphological characterization and genetic diversity of rice blast fungus, Pyricularia oryzae, from thailand using issr and srap markers. J Fungi (Basel) 2020, 6, 38. [Google Scholar] [CrossRef]
- Lopez, A.L.C.; Cumagun, C.J.R. Genetic structure of Magnaporthe oryzae populations in three island groups in the philippines. Eur J Plant Pathol 2019, 153, 101–118. [Google Scholar] [CrossRef]
- Odjo T, Diagne D, Adreit H, Milazzo J, Raveloson H, Andriantsimialona D; et al. Structure of african populations of Pyricularia oryzae from rice. Phytopathology 2021, 111, 1428–1437. [CrossRef]
- Roumen, E.; Levy, M.; Notteghem, J.L. Characterisation of the european pathogen population of Magnaporthe grisea by DNA fingerprinting and pathotype analysis. Eur J Plant Pathol 1997, 103, 363–371. [Google Scholar] [CrossRef]
- Pagliaccia D, Urak RZ, Wong F, Douhan LI, Greer CA, Vidalakis G; et al. Genetic structure of the rice blast pathogen (Magnaporthe oryzae) over a decade in North Central California rice fields. Microb. Ecol 2018, 75, 310–317. [CrossRef] [PubMed]
- Onaga G, Suktrakul W, Wanjiku M, Quibod IL, Entfellner JBD, Bigirimana J; et al. Magnaporthe oryzae populations in sub-saharan africa are diverse and show signs of local adaptation. BioRxiv, 2020. [CrossRef]
- Zhong Z, Chen M, Lin L, Han Y, Bao J, Tang W; et al. Population genomic analysis of the rice blast fungus reveals specific events associated with expansion of three main clades. ISME J 2018, 12, 1867–1878. [CrossRef]
- Saleh, D.; Milazzo, J.; Adreit, H.; Fournier, E.; Tharreau, D. South-East Asia is the center of origin, diversity and dispersion of the rice blast fungus, Magnaporthe oryzae. New Phytol 2014, 201, 1440–1456. [Google Scholar] [CrossRef] [PubMed]
- Gladieux P, Ravel S, Rieux A, Cros-Arteil S, Adreit H, Milazzo J; et al. Coexistence of multiple endemic and pandemic lineages of the rice blast pathogen. mBio 2018, 9, e01806–17.
- Taheri, P.; Irannejad, A. Genetic structure of various Magnaporthe oryzae populations in iran and uruguay. Aus Plant Pathol 2014, 43, 287–297. [Google Scholar] [CrossRef]
- Duan G, Bao J, Chen X, Xie J, Liu Y, Chen H; et al. Large-scale genome scanning within exonic regions revealed the contributions of selective sweep prone genes to host divergence and adaptation in Magnaporthe oryzae species complex. Microorganisms 2021, 9, 562. [CrossRef]
- Gladieux P, Condon B, Ravel S, Soanes D, Maciel JLN, Nhani A Jr; et al. Gene Flow between Divergent Cereal- and Grass-Specific Lineages of the Rice Blast Fungus Magnaporthe oryzae. mBio 2018, 9, e01219–17.
- Lande, R. Neutral theory of quantitative genetic variance in an island model with local extinction and colonization. Evolution 1992, 46, 381–389. [Google Scholar] [CrossRef]
- Kimura, M.; Weiss, G.H. The stepping stone model of population structure and the decrease of genetic correlation with distance. Genetics 1964, 49, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Meng JW, He DC, Zhu W, Yang LN, Wu EJ, Xie JH; et al. Human-mediated gene flow contributes to metapopulation genetic structure of the pathogenic fungus Alternaria alternata from potato. Front Plant Sci 2018, 9, 00198. [CrossRef]
- Gao, F.; Chen, C.; Li, B.; Weng, Q.; Chen, Q. The gene flow direction of geographically distinct Phytophthora infestans populations in China corresponds with the route of seed potato exchange. Front Microbiol 2020, 11, 1077. [Google Scholar] [CrossRef]
- Tharreau D, Fudal I, Andriantsimialona D, Utami D, Fournier E, Lebrun MH; et al. World population structure and migration of the rice blast fungus, Magnaporthe oryzae. Advances in genetics, genomics and control of rice blast disease, Springer 2009; pp:209-15.
- Onaga, G.; Wydra, K.; Koopmann, B.; Séré, Y.; von Tiedemann, A. Population structure, pathogenicity, and mating type distribution of Magnaporthe oryzae isolates from east Africa. Phytopathology 2015, 105, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Milgroom, M.; Han, S.; Kang, S.; Lee, Y.H. Genetic differentiation of Magnaporthe oryzae populations from scouting plots and commercial rice fields in Korea. Phytopathology 2008, 98, 436–442. [Google Scholar] [CrossRef]
- Kawakubo S, Gao F, Li S, Tan Z, Huang YK, Adkar-Purushothama CR; et al. Genomic analysis of the brassica pathogen turnip mosaic potyvirus reveals its spread along the former trade routes of the Silk Road. Proc Natl Acad Sci U S A 2021, 118, e2021221118. [CrossRef] [PubMed]
- Zhong Z, Norvienyeku J, Chen M, Bao J, Lin L, Chen L; et al. Directional selection from host plants is a major force driving host specificity in Magnaporthe species. Sci Rep 2016, 6, 25591. [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ; et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 2005, 434, 980–986. [CrossRef]
- Li, F.; Fast, R. Accurate short read alignment with burrows-wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A; et al. The genome analysis toolkit: A mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res 2010, 20, 1297–1303. [CrossRef]
- Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158.
- Delcher, A.L.; Phillippy, A.; Carlton, J.; Salzberg, S.L. Fast algorithms for large-scale genome alignment and comparison. Nucleic Acids Res 2002, 30, 2478–2483. [Google Scholar] [CrossRef]
- Pfeifer, B.; Wittelsbürger, U.; Ramos-Onsins, S.E.; Lercher, M.J. PopGenome: An efficient Swiss army knife for population genomic analyses in R. Mol Biol Evol 2014, 31, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal or partially clonal reproduction. PeerJ 2013, 2, e281. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res 2009, 19, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Francis, RM. pophelper: An R package and web app to analyse and visualize population structure. Mol Ecol Resour 2017, 17, 27–32. [Google Scholar] [CrossRef]
- Huson, D.H.; Bryant, D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 2006, 23, 254–267. [Google Scholar] [CrossRef]
- Lemey, P.; Rambaut, A.; Drummond, A.J.; Suchard, M.A. Bayesian phylogeography finds its roots. PLoS Comput Biol 2009, 5, e1000520. [Google Scholar] [CrossRef]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol 2018, 4, vey016. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. Modelfinder: Fast model selection for accurate phylogenetic estimates. Nat Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Ortiz EJUhdoz. Vcf2phylip v2. 0: Convert a vcf matrix into several matrix formats for phylogenetic analysis. URL https://doi org/105281/zenodo 2019;2540861.
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in bayesian phylogenetics using tracer 1.7. Syst Biol 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Bielejec, F.; Baele, G.; Vrancken, B.; Suchard, M.A.; Rambaut, A.; Lemey, P. SpreaD3: Interactive visualization of spatiotemporal history and trait evolutionary processes. Mol Biol Evol 2016, 33, 2167–2169. [Google Scholar] [CrossRef]
- Li, Q.; Xu, K.; Yu, R.J. Genetic variation in chinese hatchery populations of the japanese scallop (patinopecten yessoensis) inferred from microsatellite data. Aquaculture 2007, 269, 211–219. [Google Scholar] [CrossRef]
- Edwards, A.W. The genetical theory of natural selection. Genetics 2000, 154, 1419–1426. [Google Scholar] [CrossRef]
- Zhan, J. Population genetics of plant pathogens. In B. McDonald (eds) eLS. John Wiley & Sons, Ltd 2016. [CrossRef]
- McDonald, B.A.; McDermott, J.M. Population genetics of plant pathogenic fungi. Bioscience 1993, 43, 311–319. [Google Scholar] [CrossRef]
- Gutaker RM, Groen SC, Bellis ES, Choi JY, Pires IS, Bocinsky RK; et al. Genomic history and ecology of the geographic spread of rice. Nat Plants 2020, 6, 492–502. [CrossRef]
- Gross, B.L.; Zhao, Z. Archaeological and genetic insights into the origins of domesticated rice. Proc Natl Acad Sci U S A 2014, 111, 6190–6197. [Google Scholar] [CrossRef]
- Thon MR, Pan H, Diener S, Papalas J, Taro A, Mitchell TK; et al. The role of transposable element clusters in genome evolution and loss of synteny in the rice blast fungus Magnaporthe oryzae. Genome Biol 2006, 7, R16.
- Couch, B.C.; Kohn, L.M. A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia 2002, 94, 683–693. [Google Scholar] [CrossRef]
- Yoshida K, Saunders DG, Mitsuoka C, Natsume S, Kosugi S, Saitoh H; et al. Host specialization of the blast fungus Magnaporthe oryzae is associated with dynamic gain and loss of genes linked to transposable elements. BMC Genomics 2016, 17, 370.
- Chung H, Goh J, Han SS, Roh JH, Kim Y, Heu S; et al. Comparative pathogenicity and host ranges of Magnaporthe oryzae and related species. Plant Pathol J 2020, 36, 305–313. [CrossRef]
- Woolhouse, M.E.; Haydon, D.T.; Antia, R. Emerging pathogens: The epidemiology and evolution of species jumps. Trends Ecol Evol 2005, 20, 238–244. [Google Scholar] [CrossRef]
- Parada-Rojas, C.H.; Quesada-Ocampo, L.M. Phytophthora capsici populations are structured by host, geography, and fluopicolide sensitivity. Phytopathology 2022, 112, 1559–1567. [Google Scholar] [CrossRef]
- Diao Y, Zhang C, Xu J, Lin D, Liu L, Mtung'e OG; et al. Genetic differentiation and recombination among geographic populations of the fungal pathogen Colletotrichum truncatum from chili peppers in China. Evol Appl 2015, 8, 108–118. [CrossRef] [PubMed]
- Li, J.; Lu, L.; Jia, Y.; Wang, Q.; Fukuta, Y.; Li, C. Characterization of field isolates of Magnaporthe oryzae with mating type, DNA fingerprinting, and pathogenicity assays. Plant Dis 2016, 100, 298–303. [Google Scholar] [CrossRef]
- Onaga, G.; Wydra, K.; Koopmann, B.; Séré, Y.; von Tiedemann, A. Population structure, pathogenicity, and mating type distribution of Magnaporthe oryzae isolates from east Africa. Phytopathology 2015, 105, 1137–1145. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Barker, B.M. Use of population genetics to assess the ecology, evolution, and population structure of coccidioides. Emerg Infect Dis 2016, 22, 1022–1030. [Google Scholar] [CrossRef]
- Saleh, D.; Milazzo, J.; Adreit, H.; Fournier, E.; Tharreau, D. South-East Asia is the center of origin, diversity and dispersion of the rice blast fungus, Magnaporthe oryzae. New Phytol 2014, 201, 1440–1456. [Google Scholar] [CrossRef]
- Huang X, Wei X, Wang ZX, Wang A, Zhao Q; et al. A map of rice genome variation reveals the origin of cultivated rice. Nature 2012, 490, 497–501. [CrossRef] [PubMed]
- Londo, J.P.; Chiang, Y.C.; Hung, K.H.; Chiang, T.Y.; Schaal, B.A. Phylogeography of asian wild rice, Oryza rufipogon, reveals multiple independent domestications of cultivated rice, Oryza sativa. Proc Natl Acad Sci U S A 2006, 103, 9578–9583. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Han, B. Rice domestication occurred through single origin and multiple introgressions. Nat Plants 2015, 2, 15207. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Platts, A.E.; Fuller, D.Q.; Hsing, Y.I.; Wing, R.A.; Purugganan, M.D. The rice paradox: Multiple origins but single domestication in asian rice. Mol Biol Evol 2017, 34, 969–979. [Google Scholar] [CrossRef]
- Yang M, Smit S, de Ridder D, Feng J, Liu T, Xu J, et al. Adaptation of Fusarium Head Blight Pathogens to Changes in Agricultural Practices and Human Migration. Adv Sci (Weinh) 2024; 5:e2401899.
- Thrall PH, Oakeshott JG, Fitt G, Southerton S, Burdon JJ, Sheppard A; et al. Evolution in agriculture: The application of evolutionary approaches to the management of biotic interactions in agro-ecosystems. Evol Appl 2011, 4, 200–215. [CrossRef]
- Williams, P.D. Darwinian interventions: Taming pathogens through evolutionary ecology. Trends Parasitol 2010, 26, 83–92. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




