Submitted:
30 August 2024
Posted:
02 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Area and Experiment Design

2.2. Estimation of Biological Traits
2.3. Sample Preparation and Metabolic Profiling
2.3.1. Sample Preparation and Extraction
2.3.2. UPLC-Conditions
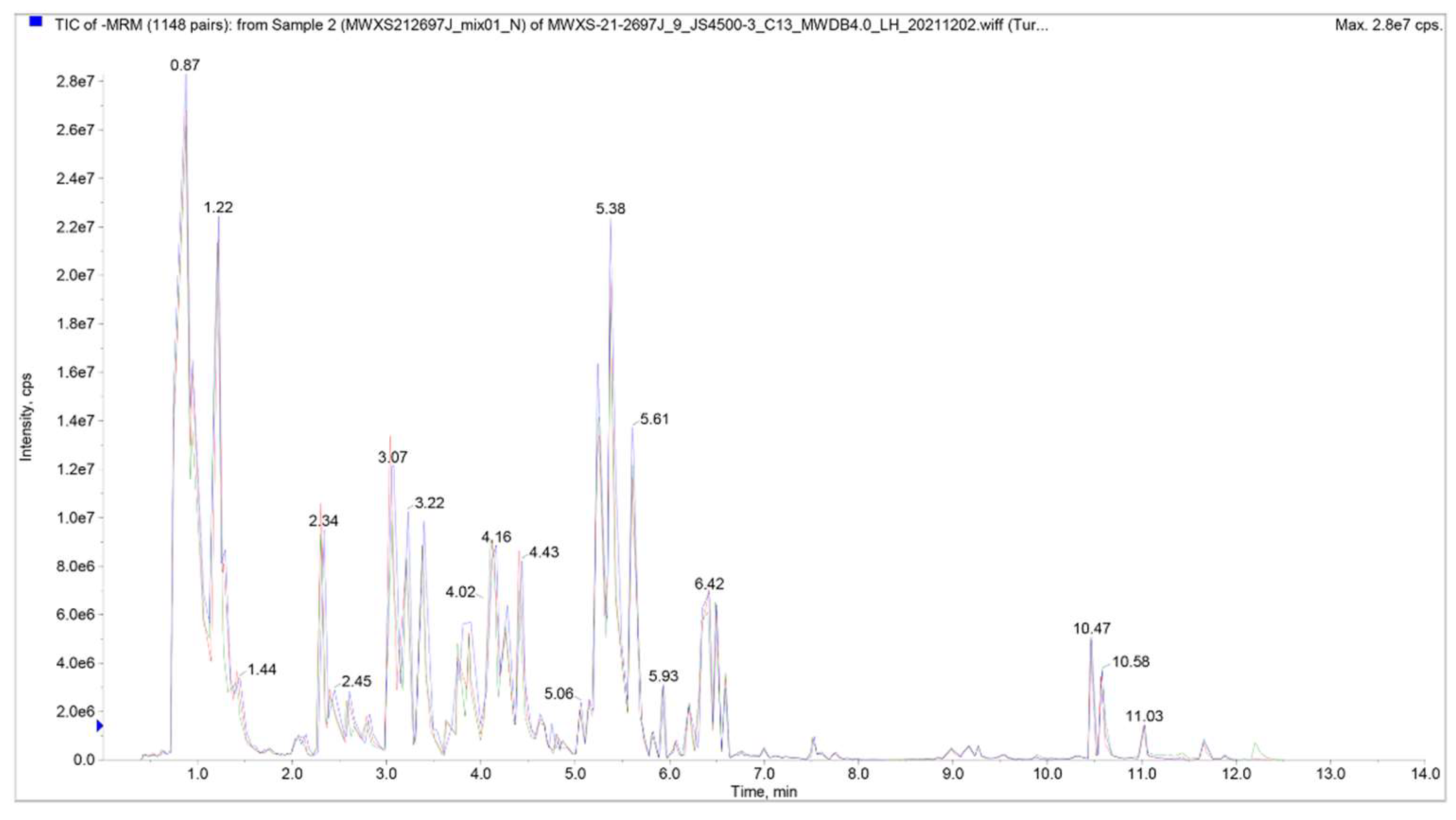
2.3.3. ESI-Q TRAP-MS/MS
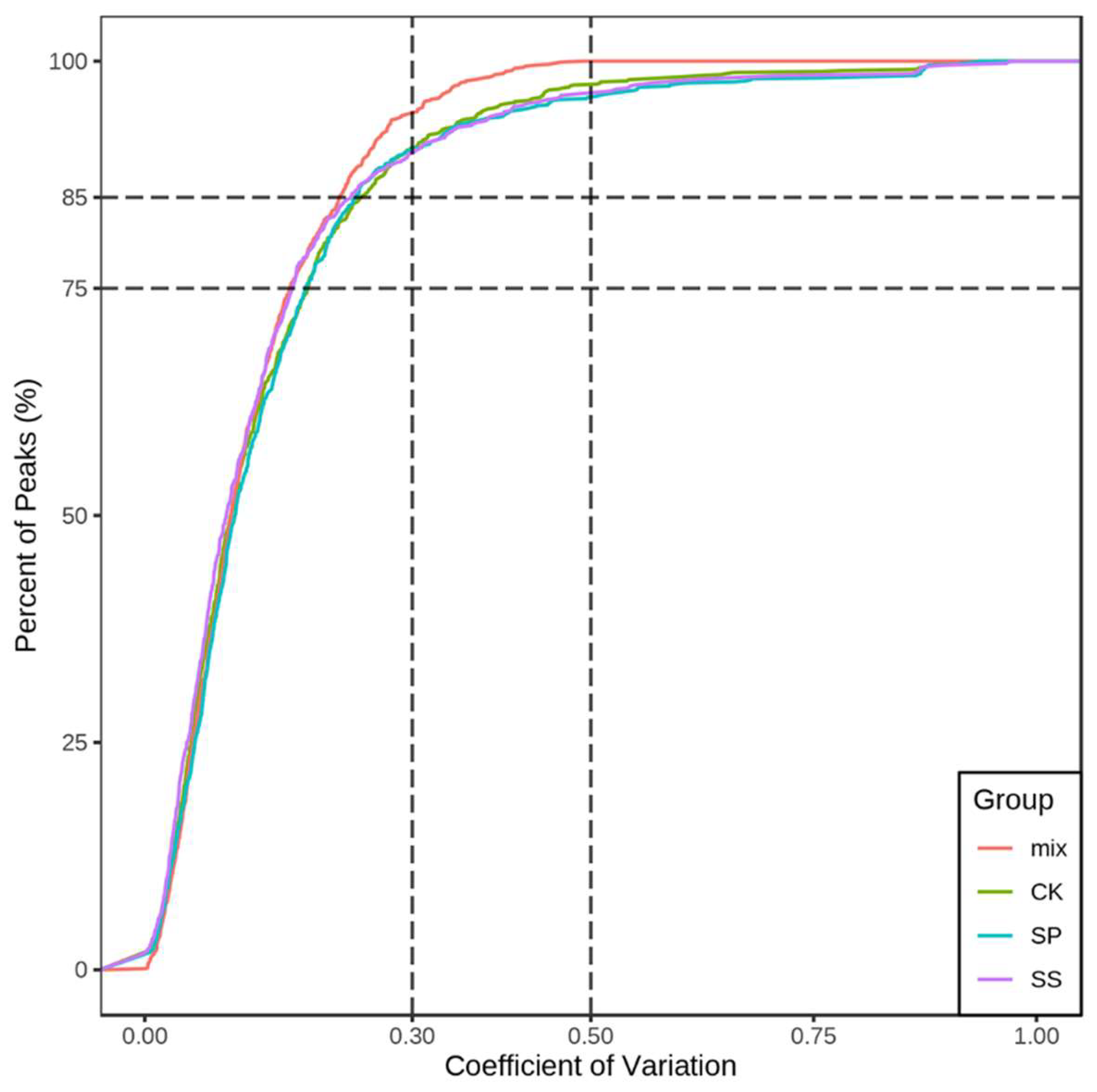
2.3.4. Quality Control Analysis (QC Analysis)
2.3.4. PCA Analysis
2.3.5. Differential Metabolites Selected
2.3.6. KEGG Annotation and Enrichment Analysis
3. Results
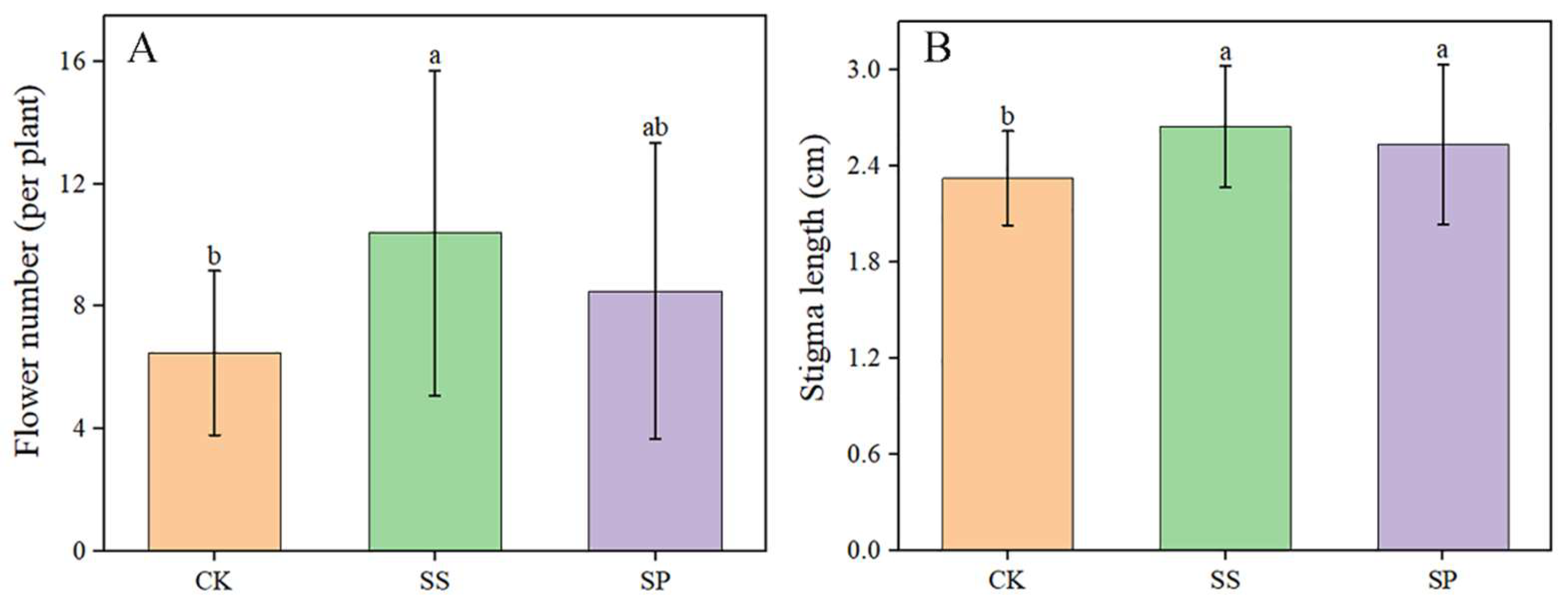
3.1. Biological Changes in Saffron under Three Cropping Patterns
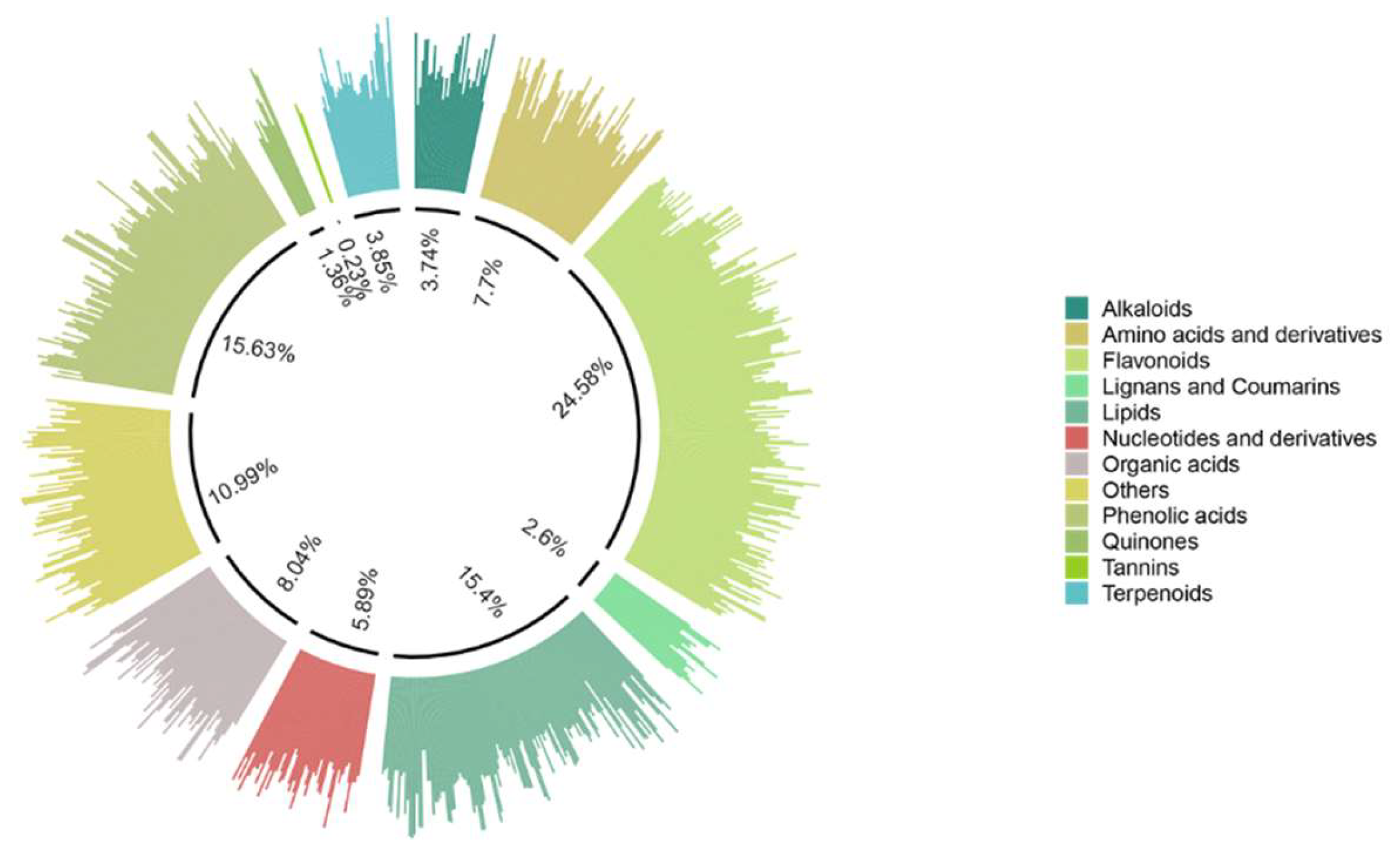
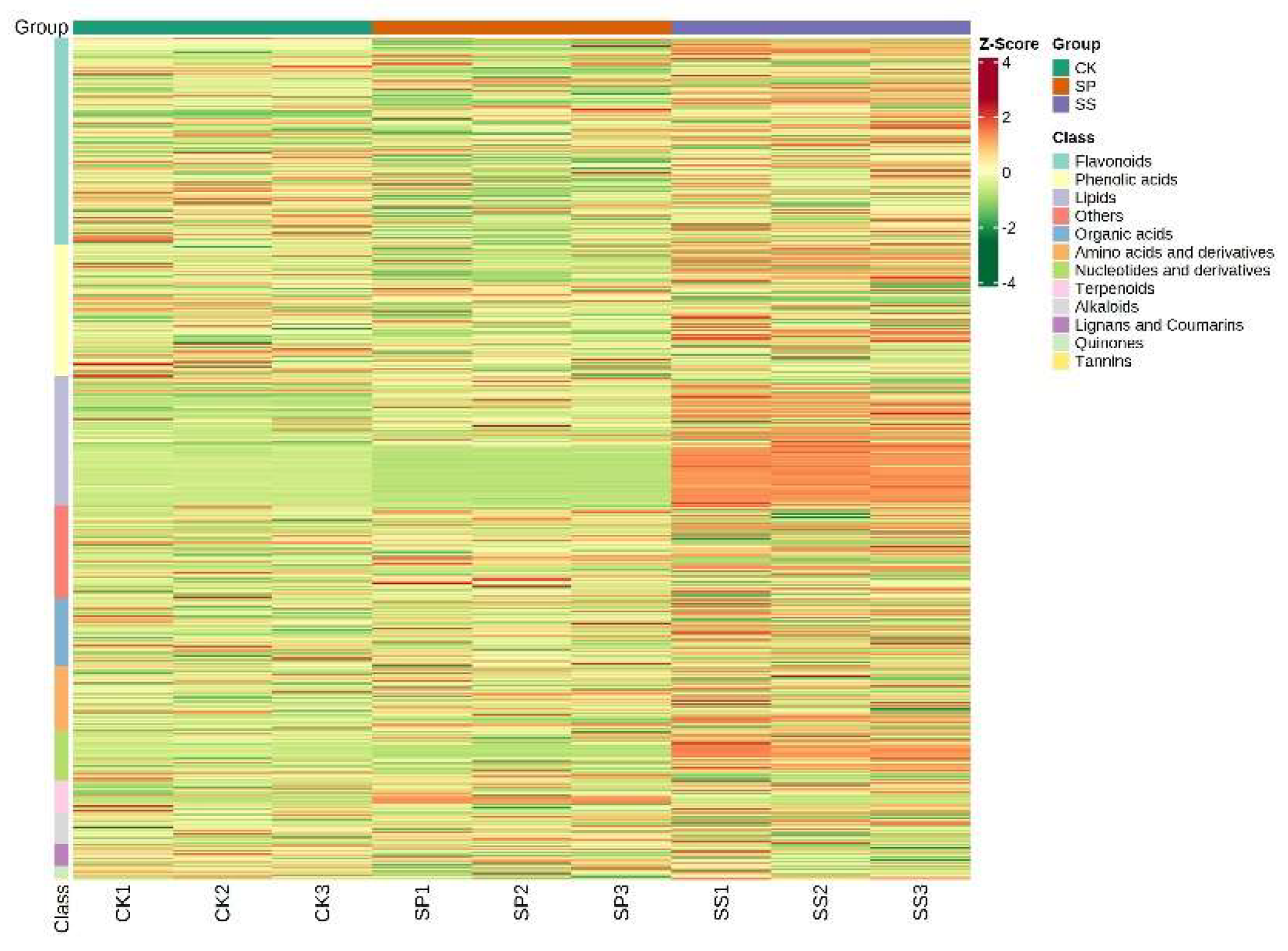
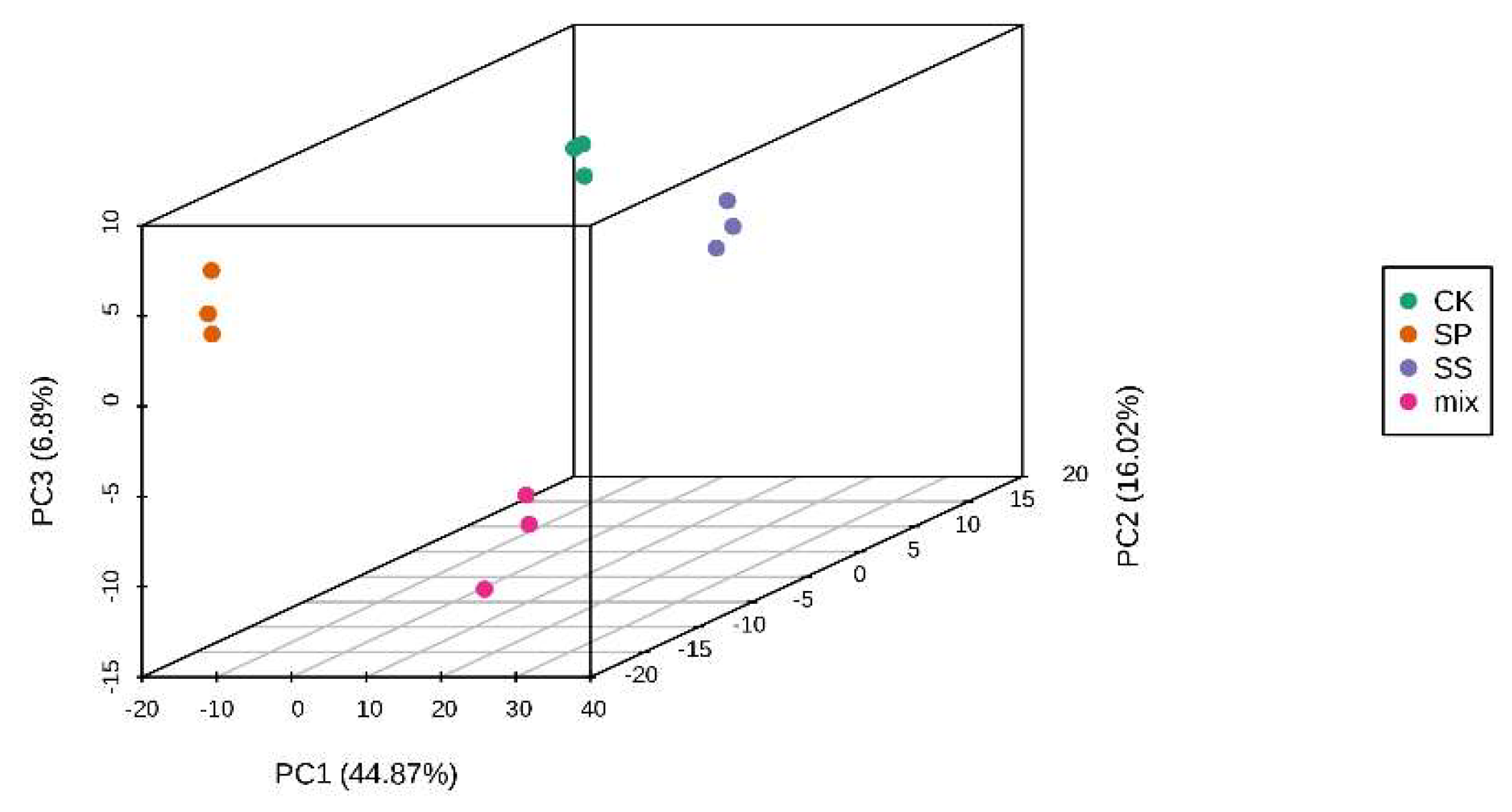
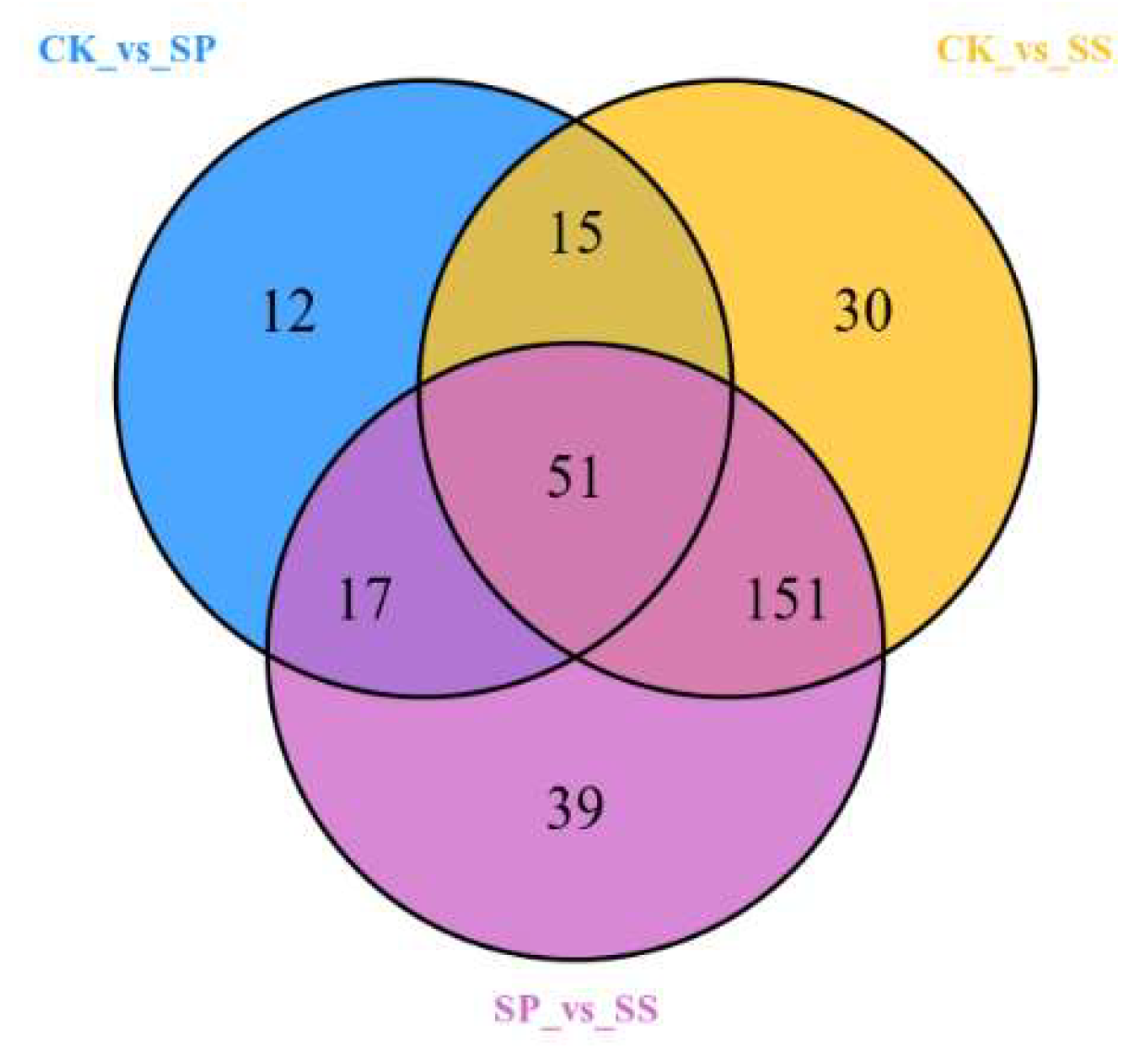
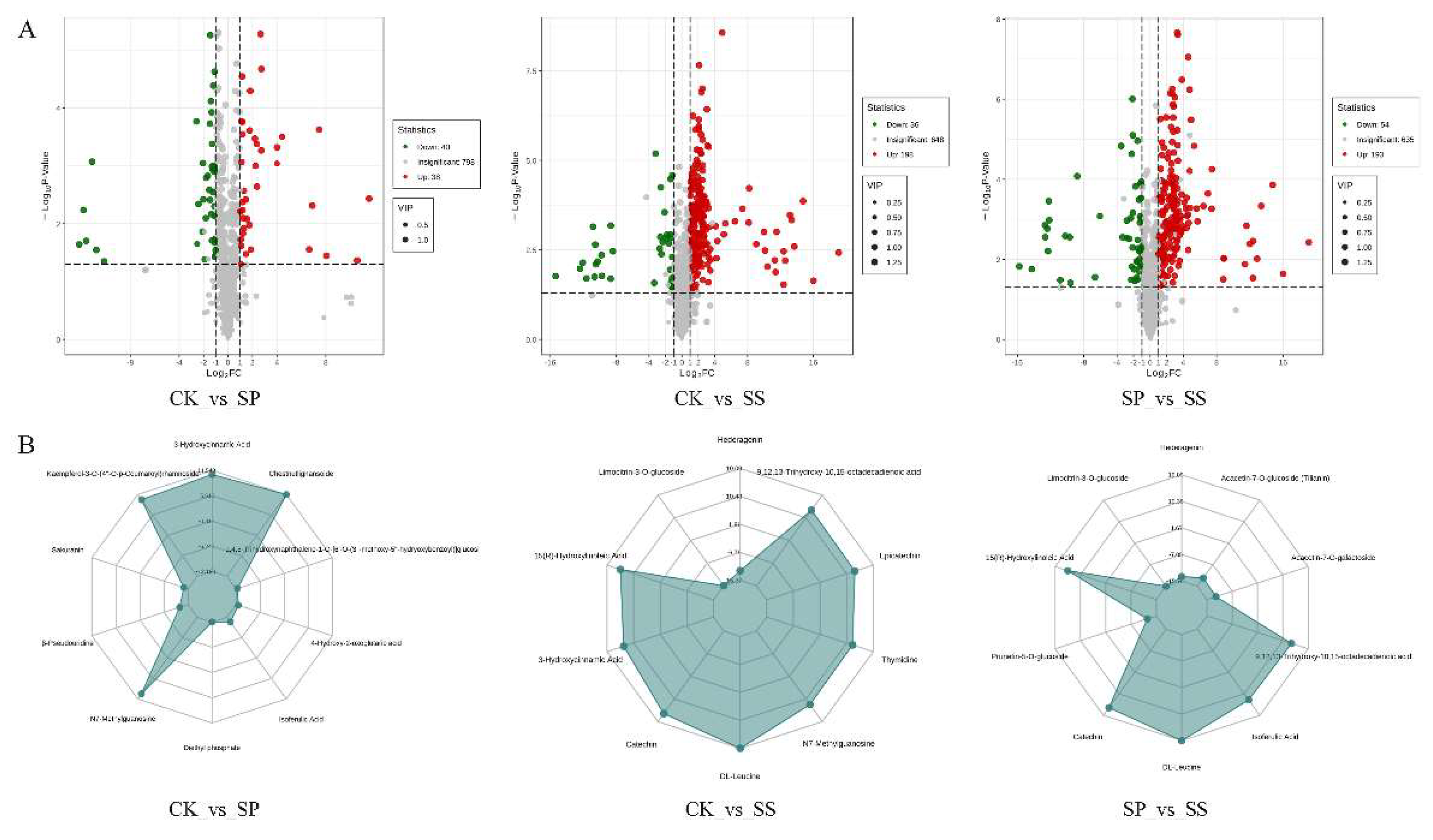
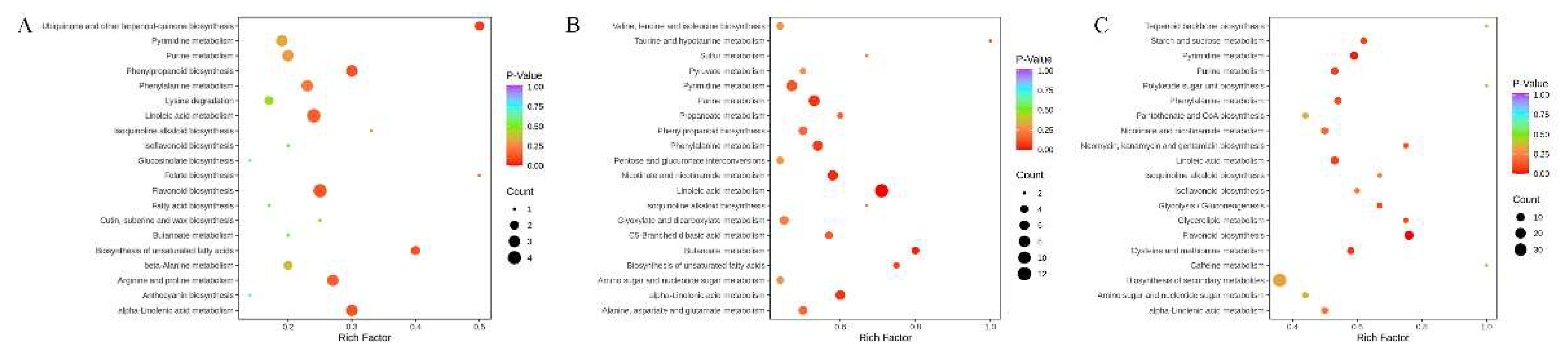
3.2. Metabolic Changes in Saffron under Three Cropping Patterns
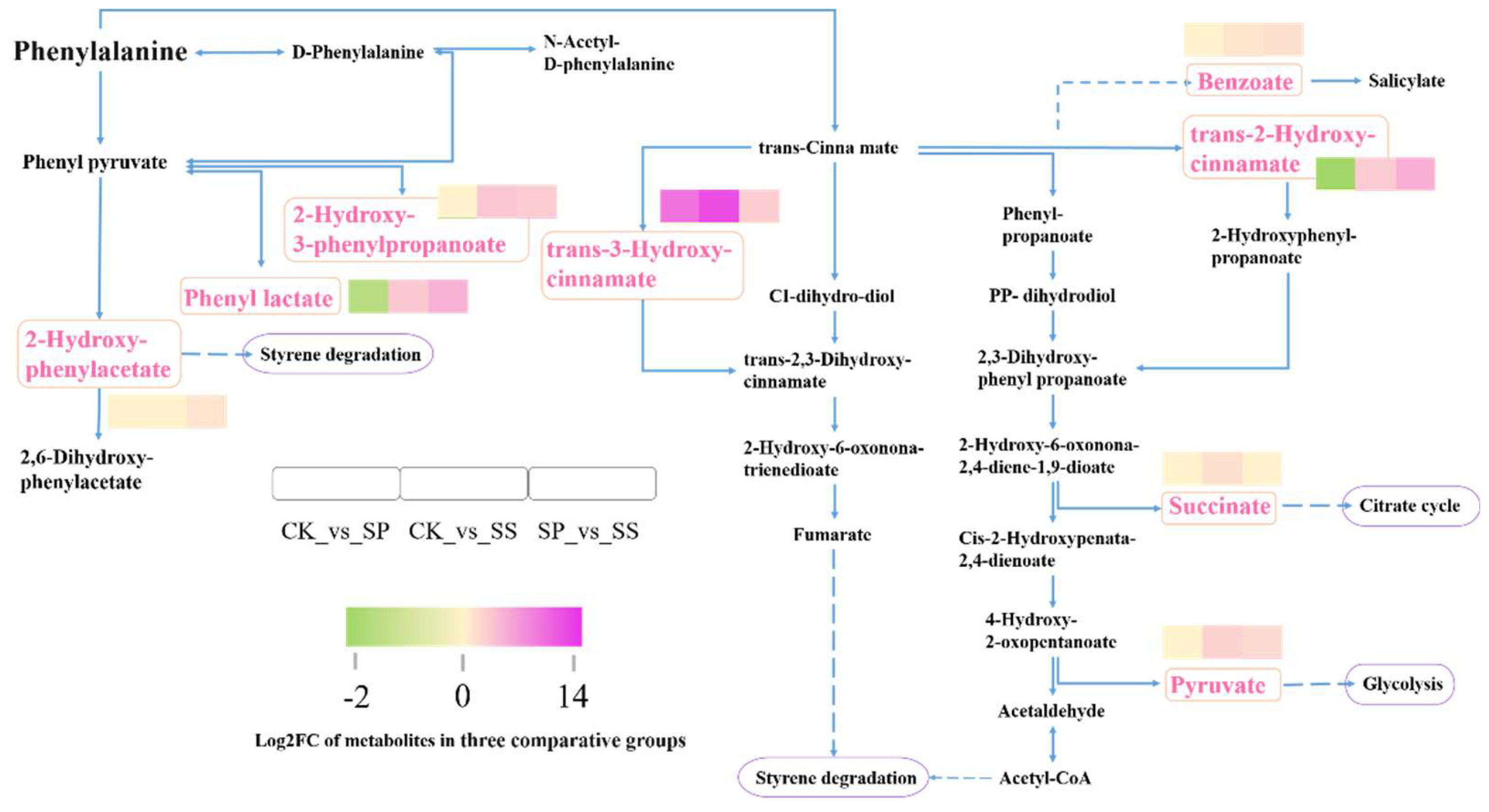
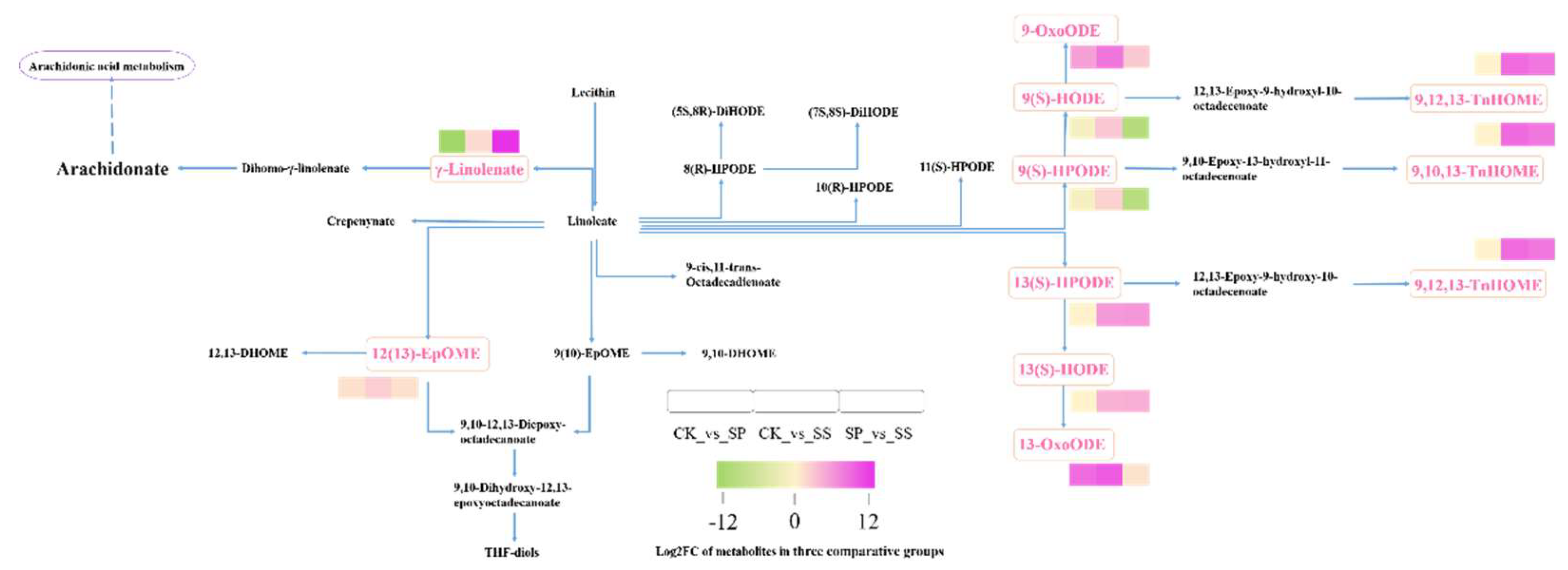
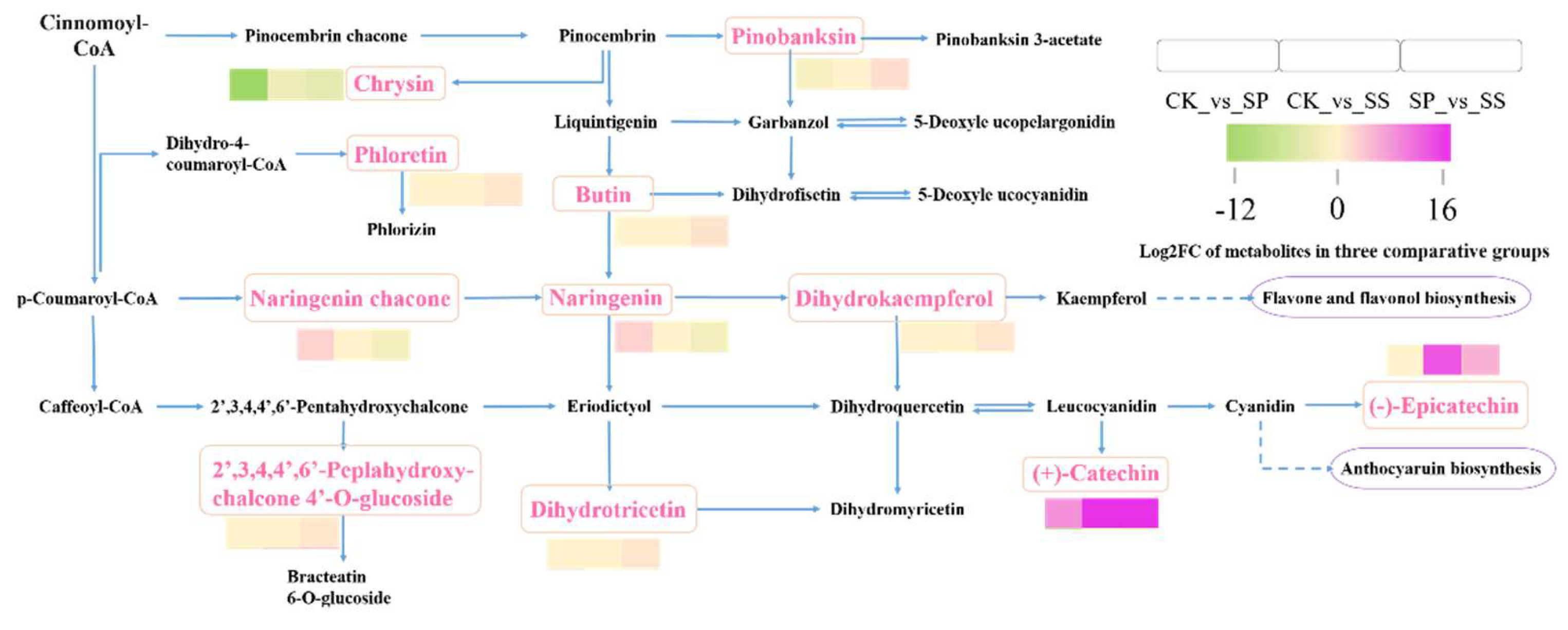
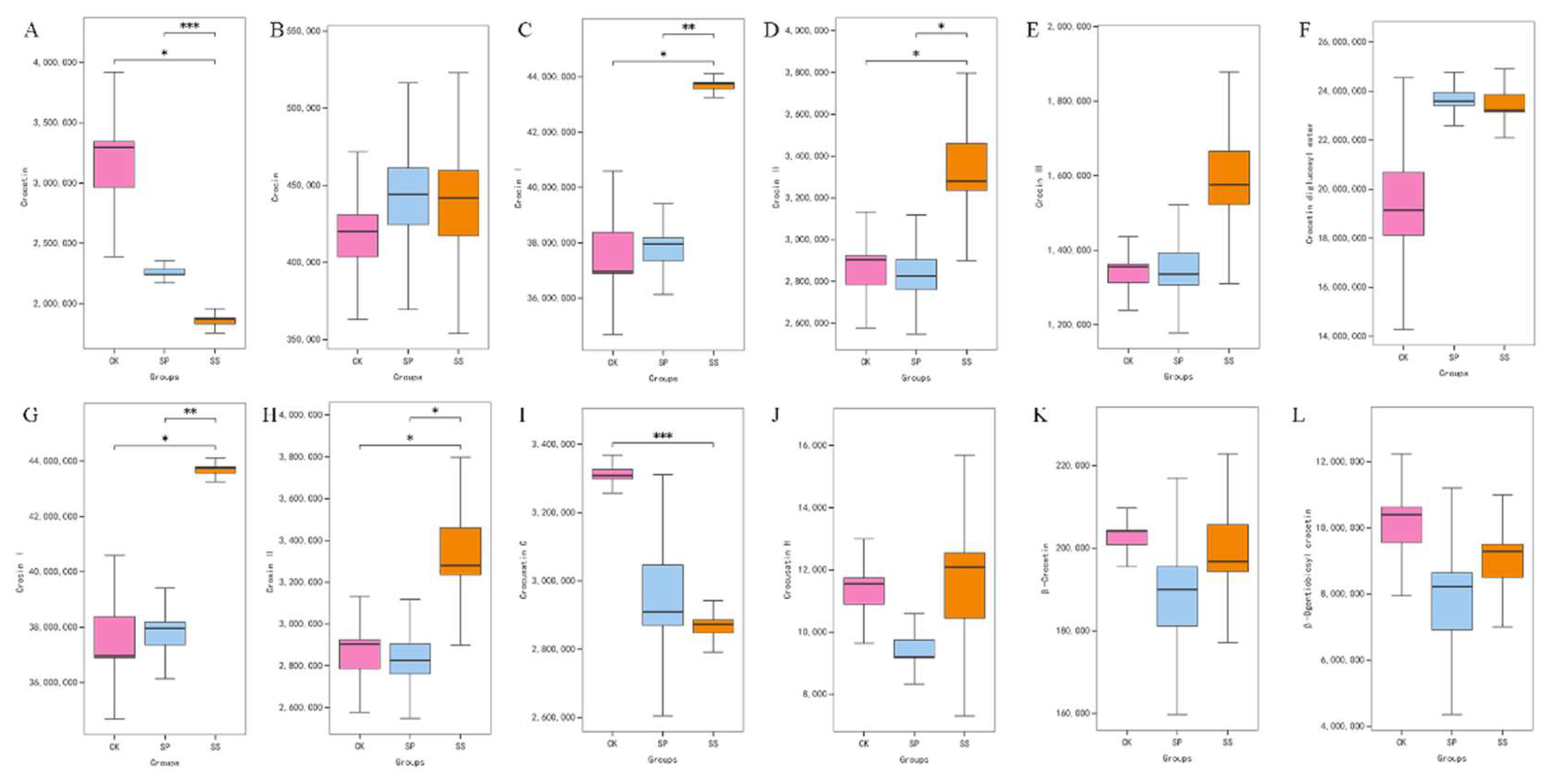
3.3. Metabolites Related to Safranal and Picrocrocin Change under Three Cropping Patterns
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sepaskhah, A.R.; Kamgar-Haghighi, A.A. Saffron irrigation regime. International Journal of Plant Production 2009. 3, 1735–8043. https://www.researchgate.net/publication/285805929_Saffron_Irrigation_Regime.
- Fabio, G.; Carmelo, S.; Giovanni, A. Crop rotation as an effective strategy for Saffron (Crocus sativus L.) cultivation. Scientia Horticulturae 2016. 211, 34-39. [CrossRef]
- Souret, F. F.; Weathers, P. J. Cultivation, in vitro Culture, Secondary Metabolite Production, and Phytopharmacognosy of Saffron (Crocus sativus L.). Journal of Herbs, Spices & Medicinal Plants 2000. 6(4), 99–116. [CrossRef]
- Kafi, M., Koocheki, A., Molafilabi, M., Saffron Production and Processing. Ferdowsi University of Mashhad Press, Iran. 2002. http://refhub.elsevier.com/S0926-6690(19)30314-0/sbref0135.
- Tammaro, F. Saffron (Crocus sativus L.) in Italy. In Negbi. M. (ED.). Saffron: Crocus sativus L. Harwood Academic Publishers, Australia, 1999. 53–62.
- Kumar, A., Devi, M., Kumar, R. et al. Introduction of high-value Crocus sativus (Saffron) cultivation in non-traditional regions of India through ecological modelling. Sci Rep 2022. 12, 11925. [CrossRef]
- Sampathu, S.R.; Shivashankar, S.; Lewis, Y.S.; Wood, A.B. Saffron (Crocus Sativus Linn.)—Cultivation, processing, chemistry and standardization. CRC Critical Reviews in Food Science and Nutrition 2009. 20(2), 123–157. [CrossRef]
- Alireza, K.; Seyyed, M.S. Mother Corm origin and planting depth affect physiological responses in Saffron (Crocus sativus L.) under controlled freezing conditions. Industrial Crops and Products 2019. 138. 111468. [CrossRef]
- Wohor, O.Z., Rispail, N., Ojiewo, C.O., Rubiales, D. Pea breeding for resistance to rhizospheric pathogens. Plants (Basel) 2022. 11, 2664. [CrossRef]
- Zhu, Y.C.; Zhang, J.H.; Gao, X.Q.; Shen, Y.; Qin, L.P.; Zhu, B. Metabolites from a co-culture of Trichoderma yunnanense and Paenibacillus peoriae improve resistance to corm rot disease in Crocus sativus. Industrial Crops and Products 2024. 213, 118465. [CrossRef]
- Shah, A.A.; Tripathi, R.B. Improved technology of Saffron (crocus sativus l.) cultivation in kashmir. Asian Journal of Horticulture 2008. 3, 446-448.
- Seyyed, J.A.; Ali, S.; Jafar, N.; Ehsan, O. Relationship between fertilization and planting depths on antioxidant activity in Saffron (Crocus sativus L.). Industrial Crops and Products 2023. 191, 116004. [CrossRef]
- Imane, C.; Laila, S.; Jamal, A. Improved growth and quality of Saffron (Crocus sativus L.) in the field conditions through inoculation with selected native plant growth-promoting rhizobacteria (PGPR). Industrial Crops and Products 2023. 197, 116606. [CrossRef]
- Cid-P’erez, T.S.; Nev’arez-Moorillo’n, G.V.; Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Herna’ndez-Carranza, P.; Avila-Sosa, R. The relation between drying conditions and the development of volatile compounds in Saffron (Crocus sativus). Molecules 2021. 26, 6954. [CrossRef]
- Alonso, D.G.L.; Arghittu, A.; Astrka, K., et al. White Book Saffron in Europe Problems and Strategies for improving the quality and strengthen competitiveness [M]. 2006.
- Yaser, E.; Mohammad, B.A.; Ehsan, N. High density planting and manure affect flower yield, corm characteristics, and volatile compounds of Saffron (Crocus sativus L.). Industrial Crops and Products 2006. 176. 114363. [CrossRef]
- Fallahi, H.R.; Aghhavani-Shajari, M.; Sahabi, H.; Behdani, M.A.; Sayyari-zohan, M.H.; Vatandoost, S. Influence of some pre and post-harvest practices on quality of Saffron stigmata. Sci. Hortic 2021. 278, 109846.
- Zhang, J.; Lu, J.; Zhu, Y.; Huang, Q.; Qin, L.; Zhu, B. Rhizosphere microorganisms of Crocus sativus as antagonists against pathogenic Fusarium oxysporum. Front. Plant Sci 2022. 13, 1045147. [CrossRef]
- Guan, B.B.; Xiong, J.E.; Li, K.Q. Research progress of influence of cultivation conditions and technology on yield and quality of Saffron. FUJIAN ANALYSIS & TESTING 2023. 32(03), 20-25.
- Wang, Z. Cultivation technique of Saffron rice rotation. Journal of Zhejiang Agricultural Sciences 2015. 56(06), 849-850. https://www.nstl.gov.cn/paper_detail.html?id=feefff99f2c1dfb8bcc76010fd8fe92a.
- Lin, L.H.; Zhu, M. Paddy-upland rotation of ‘rice-Saffron’. Shanghai Agricultural Science and Technology 2019. 04, 138-139. https://www.nstl.gov.cn/paper_detail.html?id=3b804bcf5b61ea9fdcd53a7f887b49ea.
- Deng, S.F.; Wang, X.R.; Zhang, A.C. Study on Saffron-rice rotation in the lower-middle reaches of Yangtze River basin. Jiangxi Agriculture 2019. 14, 7+10. https://www.cnki.com.cn/Article/CJFDTotal-JXNG201914006.htm.
- Yang, H.Q.; Li, C.M.; An, S.F. Study on Maize-Saffron rotation pattern in Henan Province. Tillage and Cultivation 2022. 42(02), 75-77. https://www.cnki.com.cn/Article/CJFDTotal-GZZP202202018.htm.
- Xu, J. Effects of intercropping of walnut-Saffron on yield, quality and soil microbial quantity [D]. Xinjiang Agricultural University 2022. https://cdmd.cnki.com.cn/Article/CDMD-10758-1023408373.htm.
- Oliver, S.G.; Winson, M.K.; Kell, D.B.; Baganz, F. Systematic functional analysis of the yeast genome. Trends in Biotechnology 1998, 16(9), 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kopka, J.; Fernie, A.; Weckwerth, W. Metabolite profiling in plant biology: platforms and destinations. Genome Biol 2004, 5, 109. [Google Scholar] [CrossRef]
- Fiehn, O.; Kopka, J.; Dörmann, P. Metabolite profiling for plant functional genomics. Nat Biotechnol 2000, 18, 1157–1161. [Google Scholar] [CrossRef]
- Jewett, M.C.; Oliveira, A.P.; Patil, K.R. The role of high-throughput transcriptome analysis in metabolic engineering. Biotechnol. Bioprocess Eng 2005, 10, 385. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Yuan, H.; Zeng, X.; Shi, J.; Xu, Q.; Wang, Y.; Jabu, D.; Sang, Z.; Nyima, T. Time-course comparative metabolite profiling under osmotic stress in tolerant and sensitive Tibetan hulless barley. Biomed. Res. Int 2018, 9415409. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.F.; Zander, A.; Harris, C.; Booker, T.; Lofton, J. Integrating Cover Crops into Soybean Systems in the Southern Great Plains: Impacts on Yield and Yield Components. Agronomy 2024, 14, 1356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




