Submitted:
30 August 2024
Posted:
02 September 2024
You are already at the latest version
Abstract
Keywords:
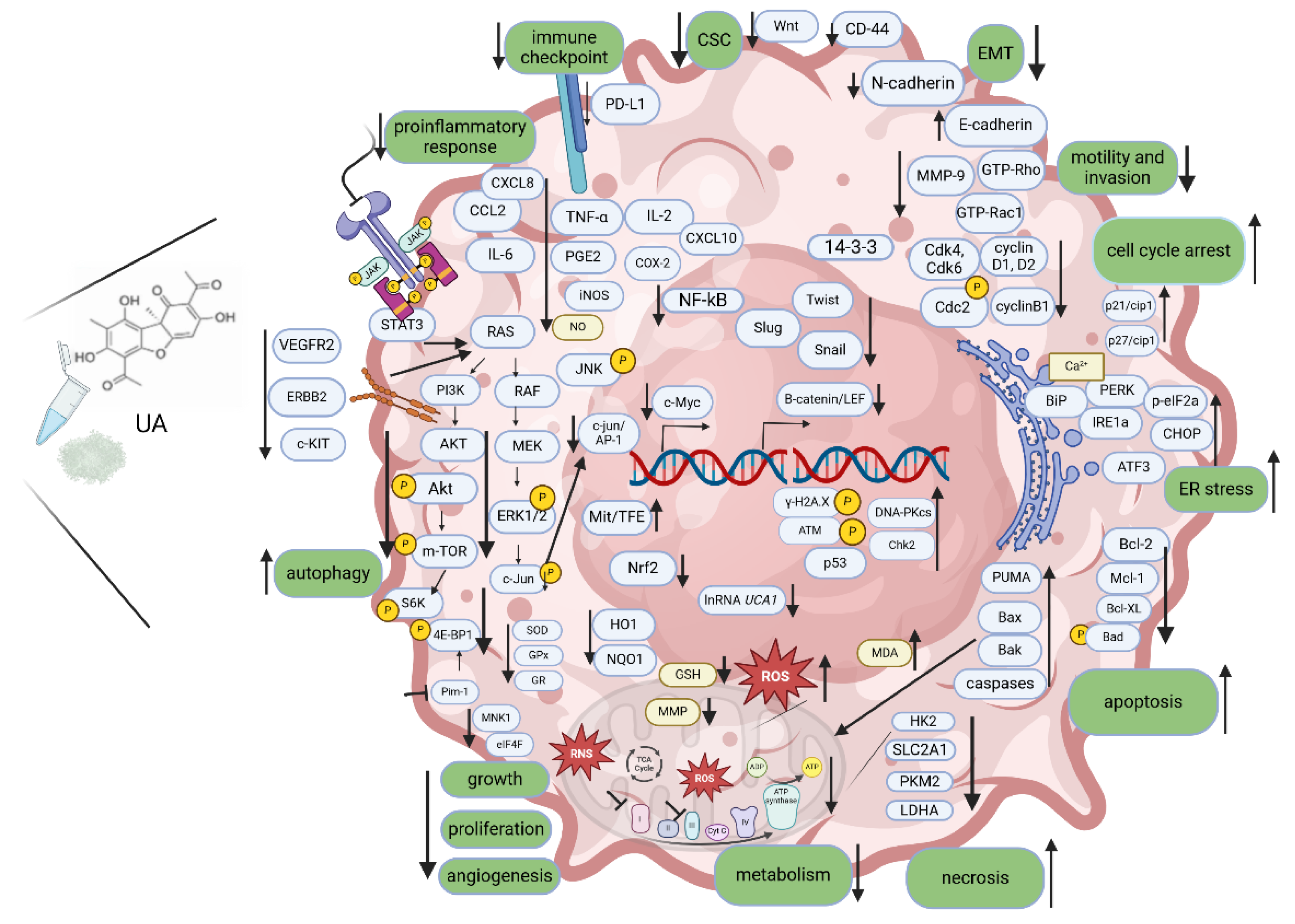
1. Introduction
2. UA Inhibits Cell Proliferation and Induces Apoptosis of Cancer Cells
3. UA Inhibits Angiogenesis, Cancer Cell Motility and Invasion
4. UA Facilitates the Immune Destruction of Cancer Cells
5. UA Acts against Tumor-Promoting Inflammation
6. UA Deregulates Energetics in Cancer Cells
7. Combination Therapies Using UA
8. Bioavailability and Pharmacokinetics
9. Perspectives for the Use of UA as an Anticancer Drug
10. Overall Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: the next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Huneck, S.; Yoshimura, Y. Identification of lichen substances, 1st ed.Springer, 1996. [Google Scholar]
- Galanty, A.P., P. Podolak, I. Enantioselective activity of usnic acid: a comprehensive review and future perspectives. Phytochem. Rev. 2019, 18, 527–548. [Google Scholar] [CrossRef]
- Galanty, A.; Zagrodzki, P.; Gdula-Argasinska, J.; Grabowska, K.; Koczurkiewicz-Adamczyk, P.; Wrobel-Biedrawa, D.; Podolak, I.; Pekala, E.; Pasko, P. A Comparative survey of anti-melanoma and anti-inflammatory potential of usnic acid enantiomers-a comprehensive in vitro approach. Pharmaceuticals 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shi, Q.; Fang, J.L.; Mei, N.; Ali, A.A.; Lewis, S.M.; Leakey, J.E.; Frankos, V.H. Review of usnic acid and Usnea barbata toxicity. J. Environ. Sci. Health. C Environ. Carcinog. Ecotoxicol. Rev. 2008, 26, 317–338. [Google Scholar] [CrossRef]
- Luzina, O.A.; Salakhutdinov, N.F. Usnic acid and its derivatives for pharmaceutical use: a patent review (2000-2017). Expert Opin. Ther. Pat. 2018, 28, 477–491. [Google Scholar] [CrossRef]
- Wang, H.; Xuan, M.; Huang, C.; Wang, C. Advances in research on bioactivity, toxicity, metabolism, and pharmacokinetics of usnic acid in vitro and in vivo. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Kupchan, S.M.; Kopperman, H.L. l-usnic acid: tumor inhibitor isolated from lichens. Experientia 1975, 31, 625. [Google Scholar] [CrossRef]
- Yang, Y.; Nguyen, T.T.; Jeong, M.H.; Crisan, F.; Yu, Y.H.; Ha, H.H.; Choi, K.H.; Jeong, H.G.; Jeong, T.C.; Lee, K.Y.; et al. Inhibitory activity of (+)-usnic acid against non-small cell lung cancer cell motility. PLoS One 2016, 11, e0146575. [Google Scholar] [CrossRef]
- Backorova, M.; Backor, M.; Mikes, J.; Jendzelovsky, R.; Fedorocko, P. Variable responses of different human cancer cells to the lichen compounds parietin, atranorin, usnic acid and gyrophoric acid. Toxicol. In Vitro 2011, 25, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Backorova, M.; Jendzelovsky, R.; Kello, M.; Backor, M.; Mikes, J.; Fedorocko, P. Lichen secondary metabolites are responsible for induction of apoptosis in HT-29 and A2780 human cancer cell lines. Toxicol. In Vitro 2012, 26, 462–468. [Google Scholar] [CrossRef] [PubMed]
- O'Neill, M.A.; Mayer, M.; Murray, K.E.; Rolim-Santos, H.M.; Santos-Magalhaes, N.S.; Thompson, A.M.; Appleyard, V.C. Does usnic acid affect microtubules in human cancer cells? Braz. J. Biol. 2010, 70, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Dobrovolsky, V.N.; Liu, F.; Wu, Y.; Zhang, Z.; Mei, N.; Guo, L. The role of autophagy in usnic acid-induced toxicity in hepatic cells. Toxicol. Sci. 2014, 142, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Yurdacan, B.; Egeli, U.; Eskiler, G.G.; Eryilmaz, I.E.; Cecener, G.; Tunca, B. The role of usnic acid-induced apoptosis and autophagy in hepatocellular carcinoma. Hum. Exp. Toxicol. 2019, 38, 201–215. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Yoon, S.; Yang, Y.; Lee, H.-B.; OH, S.; Jeong, M.-H.; Kim, J.-J.; Yee, S.-T.; Cris¸an, F.; Moon, C.; et al. Lichen secondary metabolites in Flavocetraria cucullata exhibit anti-cancer effects on human cancer cells through the induction of apoptosis and suppression of tumorigenic potentials. PLoS One 2014, 9, 1–14. [Google Scholar] [CrossRef]
- Ebrahim, H.Y.; Akl, M.R.; Elsayed, H.E.; Hill, R.A.; El Sayed, K.A. Usnic acid benzylidene analogues as potent mechanistic target of rapamycin inhibitors for the control of breast malignancies. J. Nat. Prod. 2017, 80, 932–952. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, X.; Zhou, B.; Zhang, C.; Tu, J.; Chen, X.; Wang, J.; Gao, H.; Qin, G.; Pan, W. Usnic acid induces cycle arrest, apoptosis, and autophagy in gastric cancer cells in vitro and in vivo. Med. Sci. Monit. 2018, 24, 556–566. [Google Scholar] [CrossRef]
- Kumari, M.; Kamat, S.; Jayabaskaran, C. Usnic acid induced changes in biomolecules and their association with apoptosis in squamous carcinoma (A-431) cells: A flow cytometry, FTIR and DLS spectroscopic study. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2022, 274, 121098. [Google Scholar] [CrossRef]
- Singh, N.; Nambiar, D.; Kale, R.K.; Singh, R.P. Usnic acid inhibits growth and induces cell cycle arrest and apoptosis in human lung carcinoma A549 cells. Nutr. Cancer 2013, 65 Suppl 1, 36–43. [Google Scholar] [CrossRef]
- Sun, T.X.; Li, M.Y.; Zhang, Z.H.; Wang, J.Y.; Xing, Y.; Ri, M.; Jin, C.H.; Xu, G.H.; Piao, L.X.; Jin, H.L.; et al. Usnic acid suppresses cervical cancer cell proliferation by inhibiting PD-L1 expression and enhancing T-lymphocyte tumor-killing activity. Phytother. Res. 2021, 35, 3916–3935. [Google Scholar] [CrossRef] [PubMed]
- Varli, M.; Bhosle, S.R.; Kim, E.; Yang, Y.; Tas, I.; Zhou, R.; Pulat, S.; Gamage, C.D.B.; Park, S.Y.; Ha, H.H.; et al. Usnic acid targets 14-3-3 proteins and suppresses cancer progression by blocking substrate interaction. JACS Au 2024, 4, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.H.; Kim, D.H.; Oh, S.; Park, M.S.; Kim, H.; Ha, H.H.; Cho, S.H.; Chung, I.J.; Bae, W.K. Transcriptome analysis upon potassium usnate exposure reveals ATF3-induced apoptosis in human gastric and colon cancer cells. Phytomedicine 2021, 91, 153655. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-B.; Huang, M.; Cao, Y.; Gong, P.; Liu, W.-B.; Jin, S.-Y.; Wen, J.-C.; Jing, Y.-K.; Liu, D.; Zhao, L.-X. Usnic acid is a novel Pim-1 inhibitor with the abilities of inhibiting growth and inducing apoptosis in human myeloid leukemia cells. RSC Advances 2016, 6, 24091–24096. [Google Scholar] [CrossRef]
- Galanty, A.; Koczurkiewicz, P.; Wnuk, D.; Paw, M.; Karnas, E.; Podolak, I.; Wegrzyn, M.; Borusiewicz, M.; Madeja, Z.; Czyz, J.; et al. Usnic acid and atranorin exert selective cytostatic and anti-invasive effects on human prostate and melanoma cancer cells. Toxicol. In Vitro 2017, 40, 161–169. [Google Scholar] [CrossRef]
- Erdogan, O.A.; B.I. Cevik, O. Usnic acid exerts antiproliferative and apoptotic effects by suppressing NF-κB p50 in DU145 cells. Eur. J. Biol. 2023, 82, 251–257. [Google Scholar]
- Eryilmaz, I.E.; Guney Eskiler, G.; Egeli, U.; Yurdacan, B.; Cecener, G.; Tunca, B. In vitro cytotoxic and antiproliferative effects of usnic acid on hormone-dependent breast and prostate cancer cells. J. Biochem. Mol. Toxicol. 2018, 32, e22208. [Google Scholar] [CrossRef]
- Einarsdottir, E.; Groeneweg, J.; Bjornsdottir, G.G.; Harethardottir, G.; Omarsdottir, S.; Ingolfsdottir, K.; Ogmundsdottir, H.M. Cellular mechanisms of the anticancer effects of the lichen compound usnic acid. Planta Med. 2010, 76, 969–974. [Google Scholar] [CrossRef]
- Çolak, B.; Cansaran-Duman, D.; Guney Eskiler, G.; Földes, K.; Yangın, S. Usnic acid-induced programmed cell death in ovarian cancer cells. Rendiconti Lincei. Scienze Fisiche e Naturali 2022, 33, 143–152. [Google Scholar] [CrossRef]
- Ozben, R.S.; Cansaran-Duman, D. The expression profiles of apoptosis-related genes induced usnic acid in SK-BR-3 breast cancer cell. Hum. Exp. Toxicol. 2020, 39, 1497–1506. [Google Scholar] [CrossRef]
- Kilic, N.; Islakoglu, Y.O.; Buyuk, I.; Gur-Dedeoglu, B.; Cansaran-Duman, D. Determination of usnic acid responsive miRNAs in breast cancer cell lines. Anticancer Agents Med. Chem. 2019, 19, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Degerli, E.; Torun, V.; Cansaran-Duman, D. miR-185-5p response to usnic acid suppresses proliferation and regulating apoptosis in breast cancer cell by targeting Bcl2. Biol. Res. 2020, 53, 19. [Google Scholar] [CrossRef] [PubMed]
- Seçme, M.; Dodurga, Y. Usnic acid inhibits cell proliferation and downregulates lncRNA UCA1 expression in Ishikawa endometrial cancer cells. Nat. Pro. Biotech. 2021, 1, 28–37. [Google Scholar]
- Zuo, S.-T.; Wang, L.-P.; Zhang, Y.; Zhao, D.-N.; Li, Q.-S.; Shao, D.; Fang, X.-D. Usnic acid induces apoptosis via an ROS-dependent mitochondrial pathway in human breast cancer cells in vitro and in vivo. RSC Advances 2015, 5, 153–162. [Google Scholar] [CrossRef]
- Qi, W.; Lu, C.; Huang, H.; Zhang, W.; Song, S.; Liu, B. (+)-Usnic acid induces ROS-dependent apoptosis via inhibition of mitochondria respiratory chain complexes and Nrf2 expression in lung squamous cell carcinoma. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Studzinska-Sroka, E.; Majchrzak-Celinska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wojcik, E.; Kapron, B.; Plech, T.; Zarowski, M.; Cielecka-Piontek, J. Lichen-derived compounds and extracts as biologically active substances with anticancer and neuroprotective properties. Pharmaceuticals 2021, 14. [Google Scholar] [CrossRef]
- Kumari, M.; Kamat, S.; Singh, S.K.; Kumar, A.; Jayabaskaran, C. Inhibition of autophagy increases cell death in HeLa cells through usnic acid isolated from lichens. Plants 2023, 12. [Google Scholar] [CrossRef]
- Mayer, M.; O'Neill, M.A.; Murray, K.E.; Santos-Magalhaes, N.S.; Carneiro-Leao, A.M.; Thompson, A.M.; Appleyard, V.C. Usnic acid: a non-genotoxic compound with anti-cancer properties. Anticancer Drugs 2005, 16, 805–809. [Google Scholar] [CrossRef]
- Emsen, B.; Aslan, A.; Turkez, H.; Joughi, A.; Kaya, A. The anti-cancer efficacies of diffractaic, lobaric, and usnic acid: In vitro inhibition of glioma. J. Cancer Res. Ther. 2018, 14, 941–951. [Google Scholar] [CrossRef]
- Kumar, K.; Mishra, J.P.N.; Singh, R.P. Usnic acid induces apoptosis in human gastric cancer cells through ROS generation and DNA damage and causes up-regulation of DNA-PKcs and gamma-H2A.X phosphorylation. Chem. Biol. Interact. 2020, 315, 108898. [Google Scholar] [CrossRef]
- Wu, W.; Gou, H.; Dong, J.; Yang, X.; Zhao, Y.; Peng, H.; Chen, D.; Geng, R.; Chen, L.; Liu, J. Usnic acid inhibits proliferation and migration through ATM mediated DNA damage response in RKO colorectal cancer cell. Curr. Pharm. Biotechnol. 2021, 22, 1129–1138. [Google Scholar] [CrossRef]
- Prokopiev, I.; Filippova, G.; Filippov, E.; Voronov, I.; Sleptsov, I.; Zhanataev, A. Genotoxicity of (+)- and (-)-usnic acid in mice. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 839, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Azhamuthu, T.; Kathiresan, S.; Senkuttuvan, I.; Asath, N.A.A.; Ravichandran, P.; Vasu, R. Usnic acid alleviates inflammatory responses and induces apoptotic signaling through inhibiting NF-kB expressions in human oral carcinoma cells. Cell Biochem. Funct. 2024, 42, e4074. [Google Scholar] [CrossRef] [PubMed]
- Zakharenko, A.; Sokolov, D.; Luzina, O.; Sukhanova, M.; Khodyreva, S.; Zakharova, O.; Salakhutdinov, N.; Lavrik, O. Influence of usnic acid and its derivatives on the activity of mammalian poly(ADP-ribose)polymerase 1 and DNA polymerase beta. Med. Chem. 2012, 8, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Dai, F.; Zhai, D.; Dong, Y.; Zhang, J.; Lu, B.; Luo, J.; Liu, M.; Yi, Z. Usnic acid inhibits breast tumor angiogenesis and growth by suppressing VEGFR2-mediated AKT and ERK1/2 signaling pathways. Angiogenesis 2012, 15, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Koparal, A.T. Anti-angiogenic and antiproliferative properties of the lichen substances (-)-usnic acid and vulpinic acid. Z. Naturforsch. C J. Biosci. 2015, 70, 159–164. [Google Scholar] [CrossRef]
- Yang, Y.; Bae, W.K.; Lee, J.Y.; Choi, Y.J.; Lee, K.H.; Park, M.S.; Yu, Y.H.; Park, S.Y.; Zhou, R.; Tas, I.; et al. Potassium usnate, a water-soluble usnic acid salt, shows enhanced bioavailability and inhibits invasion and metastasis in colorectal cancer. Sci. Rep. 2018, 8, 16234. [Google Scholar] [CrossRef]
- Wu, W.; Hou, B.; Tang, C.; Liu, F.; Yang, J.; Pan, T.; Si, K.; Lu, D.; Wang, X.; Wang, J.; et al. (+)-Usnic acid inhibits migration of c-KIT positive cells in human colorectal cancer. Evid. Based Complement. Alternat. Med. 2018, 2018, 5149436. [Google Scholar] [CrossRef]
- Zhai, L.; Ladomersky, E.; Lenzen, A.; Nguyen, B.; Patel, R.; Lauing, K.L.; Wu, M.; Wainwright, D.A. IDO1 in cancer: a Gemini of immune checkpoints. Cell. Mol. Immunol. 2018, 15, 447–457. [Google Scholar] [CrossRef]
- Meireson, A.; Devos, M.; Brochez, L. IDO expression in cancer: Different compartment, different functionality? Front. Immunol. 2020, 11, 531491. [Google Scholar] [CrossRef]
- Pazdziora, W.; Podolak, I.; Grudzinska, M.; Pasko, P.; Grabowska, K.; Galanty, A. Critical assessment of the anti-Inflammatory potential of usnic acid and its derivatives - a review. Life (Basel) 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Donelan, W.; Dominguez-Gutierrez, P.R.; Kusmartsev, S. Deregulated hyaluronan metabolism in the tumor microenvironment drives cancer inflammation and tumor-associated immune suppression. Front. Immunol. 2022, 13, 971278. [Google Scholar] [CrossRef]
- Yildirim, M.; Degirmenci, U.; Akkapulu, M.; Gungor, M.; Oztornacı, R.O.; Berkoz, M.; Comelekoglu, U.; Yalın, A.E.; Yalın, S. Anti-inflammatory effects of usnic acid in breast cancer. Russ. J. Bioorg. Chem. 2022, 48, S110–S114. [Google Scholar] [CrossRef]
- Azhamuthu, T.; Kathiresan, S.; Senkuttuvan, I.; Abulkalam Asath, N.A.; Ravichandran, P. Usnic acid attenuates 7,12-dimethylbenz[a] anthracene (DMBA) induced oral carcinogenesis through inhibiting oxidative stress, inflammation, and cell proliferation in male golden Syrian hamster model. J. Biochem. Mol.Toxicol. 2024, 38, e23553. [Google Scholar] [CrossRef] [PubMed]
- Kwong, S.P.; Wang, C. Review: Usnic acid-induced hepatotoxicity and cell death. Environ. Toxicol. Pharmacol. 2020, 80, 103493. [Google Scholar] [CrossRef]
- Croce, N.; Pitaro, M.; Gallo, V.; Antonini, G. Toxicity of usnic acid: A narrative review. J. Toxicol. 2022, 2022, 8244340. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Khailova, L.S.; Rokitskaya, T.I.; Nosikova, E.S.; Nazarov, P.A.; Luzina, O.A.; Salakhutdinov, N.F.; Kotova, E.A. Mechanism of action of an old antibiotic revisited: Role of calcium ions in protonophoric activity of usnic acid. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 310–316. [Google Scholar] [CrossRef]
- Fujimoto, K.; Kishino, H.; Hashimoto, K.; Watanabe, K.; Yamoto, T.; Mori, K. Biochemical profiles of rat primary cultured hepatocytes following treatment with rotenone, FCCP, or (+)-usnic acid. J. Toxicol. Sci. 2020, 45, 339–347. [Google Scholar] [CrossRef]
- Chelombitko, M.A.; Firsov, A.M.; Kotova, E.A.; Rokitskaya, T.I.; Khailova, L.S.; Popova, L.B.; Chernyak, B.V.; Antonenko, Y.N. Usnic acid as calcium ionophore and mast cells stimulator. Biochim. Biophys. Acta. Biomembr. 2020, 1862, 183303. [Google Scholar] [CrossRef]
- Bessadottir, M.; Egilsson, M.; Einarsdottir, E.; Magnusdottir, I.H.; Ogmundsdottir, M.H.; Omarsdottir, S.; Ogmundsdottir, H.M. Proton-shuttling lichen compound usnic acid affects mitochondrial and lysosomal function in cancer cells. PLoS One 2012, 7, e51296. [Google Scholar] [CrossRef]
- Farzan, M.V.; Mirian, M.; Minaiyan, M.; Pezeshki, A. Hyaluronic acid-conjugated gliadin nanoparticles for targeted delivery of usnic acid in breast cancer: An in vitro / in vivo translational study. J. Drug Deliv. Sci. Technol. 2023, 84. [Google Scholar] [CrossRef]
- Guney Eskiler, G.; Eryilmaz, I. E.; Yurdacan, B.; Egeli, U.; Cecener, G.; Tunca, B. Synergistic effects of hormone therapy drugs and usnic acid on hormone receptor-positive breast and prostate cancer cells. J. Biochem. Mol. Toxicol. 2019, 33, 1–13. [Google Scholar] [CrossRef]
- Yurdacan, B.; Egeli, U.; Guney Eskiler, G.; Eryilmaz, I.E.; Cecener, G.; Tunca, B. Investigation of new treatment option for hepatocellular carcinoma: a combination of sorafenib with usnic acid. J Pharm. Pharmacol. 2019, 71, 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.Q.; Liu, Y.H.; Guo, H.Z.; Sun, C.Y.; Xie, J.H.; Li, Y.C.; Chen, J.N.; Lai, X.P.; Su, Z.R.; Chen, H.M. Effect-enhancing and toxicity-reducing activity of usnic acid in ascitic tumor-bearing mice treated with bleomycin. Int. Immunopharmacol. 2017, 46, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Krishna, D.R.; Venkataramana, D. Pharmacokinetics of D(+)-usnic acid in rabbits after intravenous and oral administration. Drug Metab. Dispos. 1992, 20, 909–911. [Google Scholar] [PubMed]
- Krishna, D.R.; Ramana, D.V.; Mamidi, N.V. In vitro protein binding and tissue distribution of D(+) usnic acid. Drug Metabol. Drug Interact. 1995, 12, 53–63. [Google Scholar] [CrossRef]
- Foti, R.S.; Dickmann, L.J.; Davis, J.A.; Greene, R.J.; Hill, J.J.; Howard, M.L.; Pearson, J.T.; Rock, D.A.; Tay, J.C.; Wahlstrom, J.L.; et al. Metabolism and related human risk factors for hepatic damage by usnic acid containing nutritional supplements. Xenobiotica 2008, 38, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Piska, K.; Galanty, A.; Koczurkiewicz, P.; Zmudzki, P.; Potaczek, J.; Podolak, I.; Pekala, E. Usnic acid reactive metabolites formation in human, rat, and mice microsomes. Implication for hepatotoxicity. Food Chem. Toxicol. 2018, 120, 112–118. [Google Scholar] [CrossRef]
- Wang, H.; Xuan, M.; Diao, J.; Xu, N.; Li, M.; Huang, C.; Wang, C. Metabolism and toxicity of usnic acid and barbatic acid based on microsomes, S9 fraction, and 3T3 fibroblasts in vitro combined with a UPLC-Q-TOF-MS method. Front. Pharmacol. 2023, 14, 1207928. [Google Scholar] [CrossRef]
- Francos, V. NTP nomiation for usnic acid and Usnea barbata Herb. (accessed on 21 August 2024).
- Sanchez, W.; Maple, J.T.; Burgart, L.J.; Kamath, P.S. Severe hepatotoxicity associated with use of a dietary supplement containing usnic acid. Mayo Clin. Proc. 2006, 81, 541–544. [Google Scholar] [CrossRef]
- Guzow-Krzeminska, B.G.; Herman-Antosiewicz, A. Usnic acid derivatives as cytotoxic agents against cancer cells and the mechanisms of their activity. Curr. Pharm. Rep. 2019, 5, 429–439. [Google Scholar] [CrossRef]
- Gimla, M.; Pyrczak-Felczykowska, A.; Malinowska, M.; Hac, A.; Narajczyk, M.; Bylinska, I.; Reekie, T.A.; Herman-Antosiewicz, A. The pyrazole derivative of usnic acid inhibits the proliferation of pancreatic cancer cells in vitro and in vivo. Cancer Cell Int. 2023, 23, 210. [Google Scholar] [CrossRef] [PubMed]
- Araujo, H.D.A.; Silva, H.; Silva Junior, J.G.D.; Albuquerque, M.; Coelho, L.; Aires, A.L. The natural compound hydrophobic usnic acid and hydrophilic potassium usnate derivative: Applications and comparisons. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Araujo, H.D.A.; Silva Junior, J.G.; Saturnino Oliveira, J.R.; Helena, M.L.R.M.; M, C.B.M.; Cavalcanti Bezerra, M.A.; Lima Aires, A.; M, C.P.A.A.; Melo-Junior, M.R.; Pontes Filho, N.T.; et al. Usnic acid potassium salt: Evaluation of the acute Toxicity and antinociceptive effect in murine model. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Zugic, A.; Tadic, V.; Savic, S. Nano- and microcarriers as drug delivery systems for usnic acid: Review of literature. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef] [PubMed]
| Organ/tissue | Cell lines | Compound concentrations tested/IC50 | Effects in vitro | Effects in vivo | References |
|---|---|---|---|---|---|
| Head | human glioblastoma cell lines: A172 T98G |
(-)-UA extracted from Cladonia uncialis IC50 = 91.4 ± 2.0 µM IC50 = 37.8 ± 3.8 µM (48 h, MTT) |
UA dose-dependently decreased the viability of cancer cells. It inhibited the activities of IDO1, COX2, hyaluronidase, SOD, GR and GPx in in vitro assays. Results of the Parallel Artificial Membrane Permeability Assay indicated that UA can cross the blood-brain barrier. |
- | [37] |
| human glioblastoma cells U87MG primary rat cerebral cortex cells PRCC |
UA IC50 = 41.55 µg/ml IC50 =132.69 μg/ml (48 h, MTT) |
UA in a dose-dependent manner lowered viability and increased LDH release, especially in cancer cells. It revealed high antioxidant capacity in healthy cells (max at 10 µg/ml). UA non-significantly increased 8-OH-2’-deoxyguanosine levels in cancer cells. |
- | [40] | |
| human oral carcinoma cells KB Normal fibroblasts HGF-1 |
UA IC50 = 30 µM (24 h, MTT) |
UA in dose-dependent manner reduced viability of KB cancer cells and normal fibroblasts (HGF-1) were significantly more resistant. UA at concentration 10, 20 or 30 µM elevated ROS level, lipid peroxidation, decreased SOD, CAT, GPx activities and reduced GSH level, MMP. It induced DNA damage, apoptosis with downregulation of Bcl-2 and upregulation of p53, Bax, caspases 9 and 3. It decreased NF-κB, TNF-α and IL-6 levels | - | [44] | |
| Lung | non-small cell lung cancer cells A549 |
UA 25, 50, 100 μM (24 and 48 h trypan blue) |
UA decreased viability in a dose- and time-dependent manner, induced G0/G1 cell cycle arrest with a drop in CDK4, CDK6, cyclin D1 and an increase in p21 levels, mitochondrial membrane depolarization (at 100 μM), induced apoptosis. | - | [21] |
| non-small cell lung cancer cells A549 (and a panel of other cancer and noncancerous cells) |
UA 12.5, 25, 50, 100 µM |
UA induced S or G0/G1 arrest dependent on concentration, apoptosis, decreased Bcl-xL:Bax ratio, reduced clonogenic potential (10 µM) and anchorage-independent growth, motility (5 and 10 µM) and invasion (10 µM). UA elevated E-cadherin (at mRNA ad protein level), reduced mRNA for N-cadherin, Twist and Snail (10 µM); reduced p-c-jun, p-Akt and p-ERK1/2. | Tumor-free survival of BALB/c nude mice with subcutaneously injected A549 was longer if cells were pretreated with the sublethal concentration of UA (10 µM). | [17] | |
| non-small cell lung cancer cells: A549 H460 H1650 H1975 |
(+)-UA IC50 = 65.3 ± 0.65 μM n/a n/a n/a |
5 µM UA reduced transcriptional activity of β-catenin/LEF and AP-1; reduced mRNA for CD44, cyclin D, c-myc; decreased GTP-Rac1 and GTP-RhoA levels, inhibited motility and invasion of lung cancer cells; potentiated activity of cetuximab in inhibiting invasive potential | - | [11] | |
| lung squamous carcinoma cells H520 Calu-1 |
(+)-UA IC50 = 32.51 ± 0.44 µM IC50 = 34.25 ± 0.05 µM (48 h, MTT) |
UA induced dose-dependently ROS (10, 20 40 µM) and ROS-dependent apoptosis, inhibited mitochondria respiratory chain complexes I and III, decreased Nrf2 protein level and its transcriptional activity (drop in expression of its target genes, HO1 and NQO1) which was mediated by PI3K/Akt pathway inhibition. UA at 15 µM enhanced the cytotoxic activity of paclitaxel (at 0.1 µM) in vitro | Tumor growth in athymic nude mice inoculated with H520 cells was significantly retarded by UA (50 mg/kg, thrice weekly i.p.) compared with controls. UA at such a dose enhanced the effect of paclitaxel (10 mg/kg, thrice-weekly i.p.) |
[36] | |
| Breast | human breast cancer cell lines MCF-7 MDA MB 231 |
UA IC50 = 18.9 μM IC50 = 22.3 μM |
The antiproliferative activity of UA did not involve DNA damage or p53 activation. Although there was an accumulation of p53 and p21 proteins in UA-treated MCF-7 cells, the transcriptional activity of p53 remained unaffected and there was no phosphorylation of p53 at Ser15. | - | [39] |
| human breast cancer cells T47D |
(+)-UA isolated from Cladonia arbuscula IC50 = 4.2 µg/ml (−)-UA from Alectoria ochroleuca IC50 = 4.0 µg/ml (24 h, [3H] thymidine incorporation) |
Both enantiomers were equally effective in inhibiting cell proliferation. (+)-UA induced G0/G1 cell cycle arrest, decreased MMP. No evidence of apoptosis of cells treated with 20 µg/ml after 24 h or necrosis of cells treated with 5 and 10 µg/ml UA for 24 or 48 h. | - | [29] | |
| human breast cancer cells T47D |
(+)-UA isolated from Cladonia arbuscula 5 and 10 µg/ml tested |
UA decreased ATP level after the 24-hour exposition. It resulted in activating phosphorylation of AMPK, decreased mTOR/S6K signaling, upregulation of p-eIF2α and induction of autophagy. Autophagy flux was impaired due to the disruption of lysosomal acidification. | - | [61] | |
| human medullary breast cancer cells Bcap-37 human umbilical vascular endothelial cells HUVEC |
UA 1-50 μM (48–72 h, MTS) |
UA dose-dependently inhibited the proliferation of Bcap-37 cancer cells and HUVEC endothelial cells. At 1, 10, 20 M concentrations UA inhibited migration and capillary structure formation by HUVEC cells, induced their apoptosis and inhibited activation of VEGFR2 and Akt/p70 S6K/S6 and MEK/ERK1/2 signaling pathways | In nude mice xenografted with Bcap-37 cancer cells and treated intralesionally with 60 mg/kg/day (22 days) of UA tumor growth and angiogenesis were inhibited. |
[46] | |
| human breast cancer cells MCF-7 MDA MB 231 SKBR-3 normal human mammary epithelial cells MCF-10A |
UA IC50=34.12 ± 1.25 µM IC50=38.41 ± 1.64 µM IC50=48.07 ± 1.52 µM (24 h, MTT) |
UA decreased the viability of cancer cells in a dose- and time-dependent manner, while up to 25 µM did not affect normal MCF-10A cells. UA induced apoptosis in MCF-7 cells through the mitochondrial pathway, increased Bax:Bcl-2 ratio, reduced MMP, increased ROS (25 µM for 24 h) and activated JNK. | UA dose-dependently suppressed tumor growth in nude mice xenografted with MCF-7 cells (i.p. at 25, 50 or 100 mg/kg every 2 days for 21 days). The highest dose (100 mg/kg) was more effective for cancer cells and much less toxic than cyclophosphamide CTX (25 mg/kg). |
[35] | |
| human breast cancer cell lines MCF-7 T47D MDA MB 231 MDA MB 468 SKBR-3 BT-474 |
UA IC50=11.2 μM IC50=15.9 μM IC50=13.1 μM IC50=13.7 μM IC50=14.4 μM IC50=15.1 μM (72 h, MTT) |
UA at 15 and 25 μM induced autophagy in MCF-7 and MDA MB 231 cells, which correlated with a drop in p-Akt, p-4E-PB1, and p-S6K. It inhibited migration and invasion (5-30 μM) of MDA MB 231 cells. Its benzylidene derivative 52 was much more potent in vitro and in vivo. | UA benzylidene derivative 52 was tested in vivo. It inhibited MDA MB 231 and MCF-7 cells xenografted to nude mice (10 mg/kg bw, i.p. 3 times per week). | [18] | |
| human breast cancer cell lines MCF-7 MDA MB 231 BT-474 |
UA IC50=13.11± 0.01 µM IC50=12.84 ± 0.01 µM IC50=12.65 ± 1.00 µM (48 h, MTT) |
Differentially expressed UA-responsive miRNAs were identified and they appeared almost unique to each cell line. The targets are enriched in basal cell carcinoma, MAPK and Hedgehog signaling pathways (MCF-7 cells), ErbB and mTOR signaling, focal adhesion and gap junctions (in BT474 cells), MAPK, ErbB2, PI3K-Akt and p53 signaling pathways (MDA MB 231). Among the upregulated miRNA was tumor suppressor, has-miR-185-5p. | - | [32] | |
| human breast cancer cells SK-BR-3 normal breast epithelial cells MCF-12A |
UA IC50=7.21 µM (48 h, MTT) |
UA dose-dependently decreased cancer cell viability with no effect on normal cells. It modulated the expression of apoptosis-related genes, such as these coding for caspases, BCL-, TRAF- and TNF-family members, increased Bax, caspase 3 and 9 protein levels, when applied at 7.21 µM for 48 h. | - | [31] | |
| human breast cancer cells MCF-7 |
UA LD50 = 13.11 μM |
UA decreased NO, VEGF, PGE2 levels, gene expression levels of COX-2 and iNOS, and cytokines (IL 2, CXCL10, CXCL8, CCL2, TNF-α, IL-6). It decreased glutathione levels and increased MDA levels in a dose-dependent manner. | - | [54] | |
| mouse mammary cancer cells 4T1 |
UA, HA-UA-GNPs and UA-GNPs IC50 = 120.04 ± 4.8 μM IC50 = 0.56 ± 2.8 μM IC50 = 92.64 ± 3.6 μM, respectively (24 h, MTT) |
The cytotoxicity and cellular uptake of UA loaded into gliadin nanoparticles (GNPs) functionalized with hyaluronic acid (HA, targeting DC44 receptor) was higher than UA or UA-GNPs |
Tumor (4T1 cells) growth in BALB/C female mice was efficiently reduced by HA-UA-GNPs, compared with an equal dose (100 mg/kg of UA as an i.p. injection every two days for 21 days) of non-targeted UA-GNPs and free UA |
[62] | |
| Liver | human hepatocellular carcinoma cells HepG-2 |
UA 1.56-50 μM |
UA in time- and dose-dependent manner decreased cell viability, at 24 and 48 h it induced LDH release, and after 24 h induced S phase arrest and apoptosis. It decreased antiapoptotic proteins (Bcl-2, Mcl-1), and reduced activating phosphorylation of Akt, PDK1, mTOR and its substrates (S6K and 4E-BP1). UA elevated autophagy (induction and flux) which was a protective mechanism. UA elevated phosphorylation of ERK1/2, p38 and JNK. The latter kinase was involved in autophagy and apoptosis regulation. | - | [15] |
| human hepatocellular carcinoma cells HepG-2 (HBV(-)) SNU-449 (HBV(+)) human umbilical vascular endothelial cells HUVEC |
UA 6.25,12.5, 25, 50, 75 and 100 μM |
UA at lower concentrations, 6.25 and 12.5 μM for HepG2 and 6.25 μM for SNU-449, increased viability measured after 24 h. Longer treatment (48 h) was connected with dose-dependent viability drop. UA induced G0/G1 cell cycle arrest in HepG2, S and G2/M arrest in SNU-449, apoptosis and autophagy in both cancer cell lines with limited effect on normal control cells (HUVEC). | - | [16] | |
| Stomach | human gastric carcinoma cells BGC823 SGC7901 |
(+)-UA IC50 = 236.55 ± 11.12 μM IC50 = 618.82 ± 1.77 μM (24 h, CCK-8) |
UA induced G0/G1 cell cycle arrest in BCG823 (100, 200, 400 μM, 24 h) and G2/M arrest in SGC7901 (300, 600, 1200 μM), apoptosis with the rise in Bax, cleaved PARP and caspase 3 and a decrease in Bcl-2 levels. UA induced autophagy (elevated LC3-II and decreased p62 levels). | BGC823-bearing nude mice were treated with 100 mg/kg UA i.p. for 11 days (every 2 days), tumor volume and mass were 2-fold lower than control, PBS-treated mice. UA was more effective than 5-FU (25 mg/kg). | [19] |
| human gastric adenocarcinoma cells AGS SNU-1 |
UA 10–50 μM |
UA in a dose- and time-dependent manner decreased cell viability, clonogenicity and elevated apoptosis. It reduced MMP and increased Bax:Bcl-2 ratio. In AGS cells UA increased ROS generation in a time-dependent manner and DNA damage was detected by alkaline comet assay after 48-h treatment. UA (15 or 25 μM) in ROS-dependent manner up-regulated p-ATM, γ-H2A.X, DNA-PKcs, p53, Chk-2 levels. NAC protected against these effects. | - | [41] | |
| Pancreas | human pancreatic adenocarcinoma cells Capan-2 |
(+)-UA isolated from Cladonia arbuscula IC50 =5.3 µg/ml (−)-UA from Alectoria ochroleuca IC50 = 5.0 µg/ml (24 h, [3H] thymidine incorporation) |
Both enantiomers were equally effective in inhibiting cell proliferation. (+)-UA induced G0/G1 cell cycle arrest, decreased MMP. No evidence of apoptosis of cells treated with 20 µg/ml after 24 h, and necrosis was detected in cells treated with 5 or 10 µg/ml UA for 48 h. | - | [29] |
| Colon |
human colon adenocarcinoma cells HT-29 |
(+) UA, IC50 = 99.7 ± 18.8 µM (72 h, MTT) |
UA at 50 or 100 µM in a time-dependent (24, 48 and 72 h) manner decreased MMP and induced apoptosis. It was preceded by ROS elevation (observed after 1, 3 or 6 h post-treatment). | - | [13] |
| human colorectal cancer cell lines HCT116 LS174 |
(+)-UA 2, 4, 8 μM tested |
8 µM UA for 24 or 48 h inhibited SCF-induced cell proliferation and migration; decreased cellular ATP content, and increased LDH release. It inhibited mTOR signaling (drop in p-S6K, p-4E-BP) and PKC-A. It elevated autophagy (LC3-II) which was responsible for UA-induced reduction of c-KIT receptor. | - | [49] | |
| human colorectal cancer cell lines HCT116 DLD1 SW480 HT29 SW620 Caco2 COLO320 Mouse colorectal cancer cells CT26 |
UA and potassium usnate (KU), a water-soluble usnic acid salt; 12.5–100 μM tested |
UA reduced viability of cells in a dose-dependent manner, at 5 μM concentration reduced invasion in vitro. KU was more effective in the majority of tested cells. Both, UA and KU decreased mRNA for N-cadherin, Snail, Twist, Slug and ZEB2, and protein levels of Twist, Snail and Slug EMT markers in Caco2 cells. KU decreased expression of genes related to motility (CAPN1, CDC42, CFL1, IGF1, WASF1, WASL) in Caco2 cells (UA only affected CFL1 and IGF1) | Firefly luciferase-expressing CT26 cells were inoculated via splenic injection to form multiple tumor foci in the livers of male BALB/c mice. UA at 5 or 10 mg/ml i.p. (6 or 10 times during 2 weeks) or KU at a dose of 5, 10 or 20 mg/kg/mouse i.p. (6 times during 2 weeks) were applied. KU exhibited more potent anticancer effects (PARP cleavage, caspase 3 activation, reduction in EMT markers) and at 20 mg/kg inhibited liver metastasis in an orthotopic murine colorectal cancer model. |
[48] | |
| human colorectal cancer cell lines Caco2, HCTT116 HT29 human gastric cancer cells AGS, MNK45, SNU638 |
KU- potassium usnate IC50 = 38.9 ± 1.76 µM IC50 = 56.5 ± 1.49 µM IC50 = 103.5 ± 0.76 µM IC50 = 41.3 ± 1.61 µM IC50 = 120.8 ± 0.51 µM IC50 = 46.4 ± 1,63 µM (24 h, MTT) |
The viability of a panel of cell lines was dose- and time-dependently reduced by KU. 24-h treatment with KU at IC50 concentrations induced cell cycle arrest in G0/G1 or S phase, depending on the cell line, reduced CDK4, cyclin D2 and transiently elevated p21 protein levels. It induced apoptosis by mitochondrial pathway. In SNU638 and HCT116 cells KU induced ER stress with the elevation of intracellular Ca2+, ROS and ER stress markers, such as BIP, PERK, IRE1α, p-eIF2a, CHOP and ATF3 proteins as well as ATF3-regulated genes. Downregulation of ATF3 by specific siRNA protected against KU-induced elevation of Bak, p-BAD, PUMA, activation of caspase 3 and cell death. |
KU applied (20 mg/kg i.p. injections for 16 days) to mice with CT26 metastatic colon cancer cells reduced the number of metastatic nodules in livers, elevated ATF3 and cancer cell apoptosis levels. | [24] | |
| human colon carcinoma cells RKO |
UA 0.5, 1, 5, 10 μM tested |
UA at 5 or 10 μM potentiated the inhibitory effect of H2O2 (400 mM) on the proliferation and migration of RKO cells. Combined treatment enhanced DNA damage, ATM, p-ATM and γ-H2AX elevation, G2/M cell cycle arrest, apoptosis and autophagy, and elevated ROS. ATM level was controlled by UA-upregulated mir18a-5p. | - | [42] | |
| human colorectal cancer cells Caco2 HCT116 |
UA 2.5, 5, 10, 20 μM tested |
UA time- and dose-dependently reduced 14-3-3 proteins which depended on proteasome and autophagy. It correlated with decreased p-cdc2 level and G0/G1 arrest. UA at 5 μM decreased invasion in cells expressing different isoforms of 14-3-3 as well as EMT markers, Snail, Twist, N-cadherin, β-catenin (at 10 μM). Among other downregulated proteins were cyclin D1, cyclin B1, p-mTOR, p-Akt, p-STAT3, and p-JNK. UA also reduced the activity of AP-1, STAT, and NF-kB transcription factors which were elevated by overexpression on 14-3-3 isoforms in HEK293T cells. UA inhibited glycolysis and mitochondrial respiration in CaCo2 and HCT116 which correlated with decreased expression of metabolic genes and drop in SLC2A1, HK2, PKM2, and LDHA proteins, even when these processes were elevated by the surplus of an isoform of 14-3-3. | - | [23] | |
| Prostate | human prostate cancer cell lines PC-3 DU-145 normal prostate epithelial cells PNT2 Skin fibroblasts HSF |
UA isolated from Cladonia arbuscula (Wallr.) EC50 = 2.67 μg/ml EC50 = 8.6 μg/ml EC50 = 18.2 μg/ml EC50 = 20.5 μg/ml (48 h, cell number) |
UA inhibited the proliferation of both prostate cancer cells, and induced apoptosis of PC-3 cells (cleavage PARP, caspase 7 and 9 elevations). UA induced actin cytoskeleton rearrangements in a dose-dependent manner in both cancer cell lines and reduced DU-145 cell motility. | - | [26] |
| human prostate cancer cells DU-145 |
UA IC50 = 42.15±3.76 μM (48 h, MTT) |
UA decreased cell viability in a dose- and time-dependent manner, reduced MMP, elevated Bax:Bcl-2 mRNA, activated apoptosis, and downregulated NF-κB p50. | - | [27] | |
| Ovaries | human ovarian adenocarcinoma cells A2780 |
(+)-UA IC50 = 75.9 ± 2 μM (72 h, MTT) |
At 50 or 100 μM in a time-dependently (48 and 72 h) increased S phase cell cycle arrest, decreased MMP, and induced apoptosis. It was preceded by RNS elevation (observed after 3 or 6 h post-treatment). | - | [12,13] |
| human ovarian adenocarcinoma cells lines OVCAR-3 A2780 mouse fibroblasts L929 |
(+)-UA IC50 = 20 µM xCELLingence system and MTT |
UA in a dose- and time-dependent manner reduced the viability of cancer cells while normal fibroblasts were more resistant (24 h). 20 µM UA induced G0/G1 cell cycle arrest and apoptosis, inhibited migration and invasion of OVCAR-3 cells. UA modulated transcriptome, particularly elevated expression of some genes connected with apoptosis (caspase 1 and 8, for instance). | - | [30] | |
| Uterus | human cervical cancer cell lines HeLa SiHa CaSKi (and other types of cancer cells) normal human cervical epithelial cells HcerEpic human umbilical vascular endothelial cells HUVEC |
UA 3, 10, 30, and 100 μM tested (24h, MTT) |
UA dose-dependently reduced PD-L1 levels in a panel of cancer cells, including HeLa. It inhibited PD-L1 protein synthesis and enhanced its degradation in HeLa cells which correlated with its lower level at the cell surface and enhanced T-lymphocyte killing activity toward cervical cancer cells. It inhibited mTOR which induced autophagy and autophagic degradation of PD-L1. UA inhibited Jak1/2-Src-STAT3 and Ras-MEK-ERK pathways leading to reduced PD-L1 expression, drop in c-myc and cyclin D1 levels, reduced clonogenic potential. UA diminished PD-L1- mediated angiogenic potential of HUVEC cells. | - | [22] |
| human cervical cancer cells HeLa |
UA isolated from Usnea cornuta IC50 = 48.65 µM (24 h, MTT) |
UA at 25 and 50 µM increased ROS levels, lipid peroxidation, decreased MMP, and GSH level, increased caspase3/7 activity and cell death. UA induced protective autophagy – its inhibition by chloroquine increased UA cytotoxicity. | - | [38] | |
| endometrial cancer cells Ishikawa |
UA IC50 = 51.76 µM (48 h, XTT) |
UA inhibited cell proliferation and downregulated the expression of oncogenic lncRNA UCA1. | - | [34] | |
| Blood | human acute myeloid leukemia cells HL-60 human chronic myelogenous leukemia cells K562 |
UA IC50 10.00 ± 1.03 μM IC50 = 10.39 ± 0.60 µM (3 days, cell number) |
UA induced apoptosis in human leukemia cells with HL-60 cells being more responsive. It correlated with caspase 3, 9 and 8 activation, PARP cleavage, and drop in Mcl-1. UA inhibited Mnk1/eIF4E and Pim1/4E-BP1 signaling, increased p27 and decreased cyclin D1, p-Bad, c-myc, Pim-1 levels. UA inhibited Pim-1 activity in vitro. | - | [25] |
| Skin | human melanoma cells HBT-140 Skin fibroblasts HSF |
UA isolated from Cladonia arbuscula (Wallr.) EC50 = 13.7 μg/ml EC50 = 19.3 μg/ml (72 h, cell number) |
UA exerted weak cytostatic effects and apoptosis induction. At 10 and 25 μg/ml, it induced rearrangements of the actin cytoskeleton in a dose-dependent manner, 10 g/ml UA inhibited cell motility. | - | [26] |
| human melanoma cell lines HTB140 A375 WM793 Murine macrophages RAW264.7 |
(+)-UA and (-)-UA IC50 = 14.7 and 20.6 µg/ml IC50 = 11.8 and 22.2 µg/ml IC50 = 30.1 and 52.1 µg/ml respectively (48 h, LDH) |
Both enantiomers decreased the viability and proliferation of cells in a dose- and time-dependent manner but their potency was enantiomers and cell-line specific. They inhibited cell migration (at 10 µg/ml), and acted synergistically with doxorubicin in A375 cells. They weakly decreased the release of pro-inflammatory TNF-a, IL-6 and NO and significantly reduced the synthesis of TLR4, cPLA2, COX-1 and COX-2 in LPS-stimulated macrophages (10 or 25 µg/ml concentrations tested). | - | [6] | |
| squamous cell carcinoma A-431 normal human embryonic kidney cells HEK293T |
UA IC50= 98.9 ± 6 μM (48 h, MTT) |
UA induced dose-dependent cytotoxicity (within the range 25-250 M) in cancer but not in normal cells. It induced LDH release and PI accumulation by cancer cells. It correlated with ROS elevation, lipid peroxidation, changes in surface lipids and proteins, drop in GSH level and MMP. UA induced G0/G1 cell cycle arrest and apoptosis. | - | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





