1. Introduction
A carnivore diet consists of only animal-based products: meat, fish, eggs, and animal fats. It has been anecdotally attributed to a number of health benefits, including improved digestion, weight loss, and reduced inflammation. Preliminary evidence and personal testimony show that it may be effective in managing the symptoms of chronic IBDs. It is hypothesized that the carnivorous diet might have regenerative immunotherapeutic effects with the potential of inducing the resolution of IBD, having modes of action shared with, but not being exclusive to, the ketogenic diets. Our goal is to present best available evidence, formulate a hypothesis and provide an experimental framework for its clinical validation.
1.1. Ketogenic diet as regenerative immunotherapy
The ketogenic diet (KD) is a high-fat, very low-carbohydrate diet (VLCKD) that has been shown to induce a metabolic state similar to that of fasting, which stimulates the synthesis of ketone bodies in the liver. It has shown clinical efficacy in drug-resistant epilepsy [
1,
2,
3] and displays promising potential in the treatment of psychiatric [
4] and neurodegenerative diseases [
5]; it is now being studied with respect to metabolic and inflammatory conditions [
6]. Interestingly, KD regulates the immune response by virtue of lowered pro-inflammatory cytokine levels, Th1/Th2 balance, and pathways like the NLRP3 inflammasome [
7].
In an interesting manner, KD promotes the growth of colonic short-chain fatty acid-producing bacteria with an eventual effect on modulating gene expressions that regulate tissue inflammation. Of particular importance is this finding in the context of dysbiosis, which is highly prevalent in IBD patients [
8]. Ketone bodies were even able to directly regulate stem cell activity and control gene expression post-transcriptionally leading to improved intestinal regenerative capacity (See
Figure 1) [
9,
10].
1.2. Carnivore Diet as a Regenerative Immunotherapy
The carnivore diet has recently gained recognition as a potential paradigm-shifting therapeutic modality for complex chronic diseases. Possibly, the effects are mediated by this diet being closest to the evolutionary origins of our species, as homo sapiens were most likely an apex predator with a carnivorous diet in higher, rather rigid trophic levels up to the late pleistocene [
11].
The carnivore diet expands on principles of the VLCKD by excluding all plant-based foods and relying only on animal-derived nutrients. Advocates claim that it enhances gut health by removing plant toxins and anti-nutrients such as solanines, saponins, and lectins [
12,
13,
14,
15].
Furthermore, increased micronutrient availability in the carnivore diet due to lower phytate content and increased mineral density of animal based foods (see
Figure 2) may contribute to higher regenerative capacity and better immune regulation during carnivore diets [
16,
17].
The relatively low dietary fiber content is a promoted benefit for some conditions of the gastrointestinal tract since soluble dietary fiber may interfere with the activities of pancreatic enzymes and digestion of protein, whereas insoluble fiber could provoke bloating and distension [
18,
19]. Casual observations and self-claimed benefits are weight loss, improved glucose control, reduced medication needs, and improvements in gut health [
20]. However, scientific confirmation under tight regulation is still needed.
2. Hypothesized Mechanisms of the Carnivore Diet
The carnivore diet may offer some advantages over the VLCKD, particularly for patients with IBD. These are:
- 1.
Decreased Plant Toxins: Plants contain various toxins. Lectins, solanines, and saponins are associated with autoimmunity and inflammation [
12,
13,
15];
- 2.
Direct SCFA Supply: With the direct provision of SCFAs and the growth stimulation of SCFA-producing bacteria (see
Figure 3), the carnivore diet may bypass pre-existing dysbiosis, which is highly prevalent in the IBD population. [
8,
21,
22];
- 3.
Reduced Omega-6 (Linoleic Acid) consumption: Linoleic Acid may directly induce inflammation in the intestinal epithelium via formation of oxidative linoleic acid metabolites (OXLAMs) and consequent dysregulation of the Endocannabinoid System [
23]. Carnivore diets more closely resemble the pre-modern consumption of <2g/day linoleic acid vs. the modern consumption of 29g/day [
24];
- 4.
Higher Micronutrient Density: Animal Foods are more dense in most micronutrients (vitamins and minerals) relevant to ATP synthesis (see
Figure 4) compared with plant foods and lack anti-nutrients such as phytates [
16,
17], which may improve immune regulation and regenerative capacity of intestinal epithelial cells;
- 5.
Reduced Dietary Fiber: Soluble fiber inhibits activity of pancreatic enzymes and protein sequestration while insoluble fiber increases bloating and tension (see
Figure 5) possibly contributing to intestinal pathologies [
18,
19].
3. Possible Concerns
A carnivore diet has been subject to some stringent criticisms in terms of potential individual health risks:
- 1.
Gout Risks: The supersaturation of uric acid can -under the wrong circumstances- lead to the deposition of monosodium urate monohydrate crystals in the tissues, with resultant gout arthritis. Gout can be manifested by the elevation of serum urate, acute gouty arthritic attacks, the formation of tophi, gouty nephropathy, and uric acid stones. Meat itself has not been established as a causative agent, but the high amount of purine within it can serve as a triggering factor in causing episodes of gout arthritis in a pre-existing metabolic dysregulation. Our own clinical experience shows that a ketogenic/carnivore diet can even alleviate gout medium term. Hypothetically, this could be due to reduced oxidative stress since uric acid acts as an antioxidant, reduced availability of dietary monosodium (glutamate), or perhaps increased exercise in our patient population since muscle activity induces myokine secretion, hence helping in the conversion of uric acid to allantoin for excretion through the kidneys [
25]. Indeed, recent reviews have confirmed our observation of reduced uric acid in very low carbohydrate ketogenic diets [
26].
- 2.
Carcinogenicity: The World Healths Organizations (WHO) International Agency for the Research of Cancer (IARC) has classified Processed Meat as carcinogenic (Class I), and unprocessed Red Meat as possibly carcinogenic (Class IIa). No causal relationships have been established and no causal agents in red meat have been identified to date. By the classification standards of IARC, the classification is to be based on an associative relationship and does not establish the magnitude of risk. Recent systematic reviews have argued that evidence even for the proposed associative relationship between unprocessed red meat and negative health outcomes (including cancer) is lacking, and recommendations for reduced consumption of unprocessed red meat are currently not backed by scientific data [
14,
27].
- 3.
Dyslipidemia and Cardiovascular Risks: For a given population on a standard diet, increased serum total LDL Lipoprotein molecular mass (measured in mg/dl) has been considered causal in the progression of atherosclerosis (Libby 2021). On ketogenic diets, total serum LDL lipoprotein mass can and most likely will increase; however, the size of the LDL particle becomes larger, thereby reducing the number of atherogenic particles [
28,
29,
30,
31]. A reduction in the number of the atherogenic small and dense LDL lipoproteins and concurrent increase in the lipoprotein size is associated with improved cardiovascular risk markers such as reduced BMI, body weight, inflammatory markers, sdLDL, Triglycerides, Lipoprotein A, Apolipoprotein B, Blood Glucose, HbA1c, Insulin, and Blood Pressure, and increased HDL. During ketogenic diets LDL Lipoproteins serve other functions as in standard diets and are not to be interpreted as signs of metabolic dysfunction [
32]. Therefore, increased serum LDL on a ketogenic diet has to be evaluated differently than increased serum LDL on a standard diet and statin therapy is usually not warranted in a low-carbohydrate ketogenic diet [
33].
None of the proposed risks have so far been conclusively confirmed nor denied. Small associations have been spotted between intake of meat and gout, cancers, and cardiovascular events absent identification of causal relationships or conclusive causal agents. On the other hand, there are also no conclusive trials to indicate the long-term safety of a carnivore or meat-intensive diet. Hence further research is warranted.
4. Testing the Hypothesis – Study Design and Methodology
The hypothesis can be tested in a 12-week clinical study, investigating safety, feasibility, and clinical efficacy of a carnivore diet in a cohort of IBD patients.
4.1. Study Objectives
The primary objective is to establish the feasibility and safety of a carnivore diet in a cohort of IBD patients. Secondary objectives will assess clinical and functional outcomes at 1, 6, and 12 weeks with the IBDQ-32, SCCI, and CDAI. Other measures will include heart rate variability, bioimpedance, blood pressure, body measurements, body temperature, breath analysis, gut microbiota, inflammation markers, urea and electrolyte levels, liver function, fasting insulin, HbA1c, vitamin and mineral status, and lipid profile. Appendix A provides a detailed listing of the lab parameters to be measured, which include, but are not limited to, inflammation markers, lipid profile, vitamins, mineral levels, and other biomarkers as needed. Participants will keep a food diary daily and monitor their blood ketone levels.
4.2. Study Design
This prospective study should enroll a minimum of 12 IBD patients in a 12-week non-randomized, single-arm pilot study. All participants will be taken through written informed consent.
4.3. Participant Criteria
4.3.1. Inclusion Criteria
4.3.2. Exclusion Criteria: General
Pregnant or intending to become pregnant within the next 3 months,
Currently abusing substances,
On ketogenic or carnivore diet in last 6 months,
Currently Vegan or vegetarian diet and unwilling to switch to carnivore diet,
Hospitalization during the last 3 months,
Participation in another research project,
Inability to fill out the initial questionnaires,
Active liver, kidney, or cardiovascular diseases, kidney stones, severe hyperlipidemia.
4.3.3. Exclusion Criteria: Metabolic Disorders
Glycogen storage disease type 1 (von Gierke disease),
Carnitine palmitoyltransferase deficiencies (CPT I/II),
Primary carnitine deficiency,
Carnitine-acylcarnitine translocase deficiency,
Pyruvate carboxylase deficiency,
Succinyl-CoA acetoacetate transferase deficiency,
Various fatty acid oxidation disorders,
Acute intermittent porphyria.
4.4. Implementation and Follow-Up
4.4.1. Participant Training
The participants will be trained once in an educational workshop, given at the Kick-off event of the study, about:
Nutritional science behind the ketogenic and carnivore diets,
Appropriate foods and sample recipes,
Targeted ketone and glucose levels,
Food measurement-grams,
Preparation for diet initiation across environments,
Overcoming obstacles-quality, procurement, and preparation,
Dining out, traveling, and illness guidelines,
Medication guidelines,
Prevention/management of potential side effects; for example hypoglycemia or hyperketosis,
Why diets may fail,
Modifications for illness-more water, no concern for ketone level,
Fitting the diet into larger ecological, spiritual, and economic contexts.
4.4.2. Ketone Monitoring
The nutritional and socio-medical history will be obtained through digital intake interviews with the participants. Continuous monitoring of glucose/ketones will be done with monitoring devices, the data of which is managed daily by the specialist. The participants will have digital access to a community platform, where they can find recipes and instructions. Group exchanges in this online format should also be possible. Once a week, video calls with the specialist and mentor will be used to discuss practical and emotional problems arising from dietary adherence.
5. Discussion
Chronic complex illnesses such as IBD can be frustrating for both patients and therapists, because of a lack of promising treatment options that resemble full reconstitution/regeneration. The need to advance medical knowledge and care for patients afflicted by these diseases is rising. The carnivore diet -a possible new regenerative immunotherapy- seems promising. Yet, for patients with IBD, being put on a radical carnivorous diet would require multifaceted consideration of ethical, ecological, economic concerns and individual health needs.
Fully informed consent -as in all medical therapies- is needed, where all possible benefits and risks should be presented. Ecologically, a diet with a high intake of animal products has sustainability concerns, which are yet unclear; hence, this too will require discussion in light of finding ethical and sustainable ways of sourcing animal products. Economically, dietary cost and food access need consideration to ensure that it will place no undue burden on the patients or that it will not further exacerbate health disparities.
From the point of view of individual health, even if there is plenty of anecdotal evidence, in particular, as to the benefits, the adverse effects should be carefully and intensively investigated. High animal product consumption entails risks for diseases such as gout, cardiovascular diseases, and particular types of cancer. These risks should be assessed and followed closely for therapeutic carnivore diets in order to attend to the subjects’ long-term safety and vitality.
The following monitoring protocols are necessary:
for gout risk, check uric acid levels on a regular basis.
for cancer risk, perform long-term monitoring of biomarkers for cancer.
for cardiovascular risk, evaluate lipid profiles including Apolipoprotein B as a measure of particle number, blood pressure, visceral body fat and markers of systemic inflammation such as hsCRP.
6. Conclusions
Academia and clinical medicine needs a multi-disciplinary approach, which has to integrate nutrition, behavioral psychology, immunology, gastroenterology, and bioethics. By balancing and reviewing all aspects, we want to explore the healing potential of a carnivore diet while ensuring the safety of the patient and also regarding the possible ethical, ecological as well as economic issues.
We hereby summarize the current best available evidence for a therapeutic carnivore diet as a regenerative immunotherapy and hypothesize that it could make a significant difference in the management of IBD and perhaps bring relief to patients unresponsive to current treatments. Such a therapeutic carnivore diet requires very considered planning, transparent reporting, and dedication to responsible and comprehensive patient care. An experimental design to test our hypothesis was proposed. This review, hypothesis, and experimental design guarantee a comprehensive framework for investigating the carnivore diet as a novel regenerative immunotherapy in IBD, which can have a clinical application based on such findings. Insights from this would extend beyond the specific focus to wider applications in other chronic inflammatory diseases.
Funding
`This research received no external funding.
Acknowledgments
Kind thanks to Christoph Wesseling for graphical conceptualization and realization.
Appendix A. Suggested Lab Analysis
Appendix A.1. Standard Labs
Alkaline Phosphatase, Bilirubin (Serum), Calcium (Serum), Chloride (Serum), Cholesterol (Serum), HDL (Serum), LDL (Serum), CK (Serum), CK-MB (Serum), Iron (Serum), Protein Electrophoresis (Serum), Total Protein (Serum), GOT (Serum), GPT (Serum), Uric Acid (Serum), Urea (Serum), HbA1 (EDTA), Potassium (Serum), Creatinine (Serum), LDH (Serum), Sodium (Serum), Inorganic Phosphate (Serum), Transferrin (Serum), Triglycerides (Serum), Full Blood Count (EDTA), Reticulocytes (EDTA), Quick/INR (Citrate), PTT (Citrate), Thrombin Time (Citrate), Indirect Bilirubin (Serum), hsCRP (Serum), Ferritin (Serum), TSH Basal (Serum)
Appendix A.2. Specialized Labs
Whole Blood Minerals 11+4 (Heparin), TNF-alpha (Serum), Vitamin B1 bioactive (Serum), Vitamin B2 bioactive (Serum), Vitamin B6 bioactive (Serum), Vitamin B9 bioactive (EDTA), Vitamin B12 bioactive (Serum), 25-OH-Vitamin D (Serum), Amino Acids Metabolism (EDTA Plasma), Amino Acids Neuro (EDTA Plasma), Lactate/Pyruvate (Fluoride 3x), Nitrotyrosine (Serum), Carnitine (Serum), Fatty Acids of Erythrocyte Membrane (EDTA), Lipoprotein (a) (Serum), Apo-Lipoprotein B (Serum), Homocysteine (Serum centrifuged), SCFA (Short-Chain Fatty Acids) (Serum), MDA-LDL (Serum), AGE (Serum), IL-6 (Serum), BDNF (Serum), Lipopolysaccharide Binding Protein (LBP) (Serum centrifuged), IFABP (Serum)
Appendix A.3. Stool and Saliva Labs
Cortisol Awake Response (Saliva), Molecular Genetic Profile Microbiota (Stool) SCFA (Short-Chain Fatty Acids) (Stool), Pancreatic Elastase (Stool), Bile Acids (Stool), Alpha-1-Antitrypsin (Stool), Zonulin (Stool), Calprotectin (Stool)
References
- Martin-McGill, K.J.; Bresnahan, R.; Levy, R.G.; Cooper, P.N. Ketogenic diets for drug-resistant epilepsy. Cochrane Database of Systematic Reviews 2020, 2020, Not available. [Google Scholar] [CrossRef]
- Sourbron, J.; Klinkenberg, S.; van Kuijk, S.M.J.; Lagae, L.; Lambrechts, D.; Braakman, H.M.H.; Majoie, M. Ketogenic diet for the treatment of pediatric epilepsy: Rview and meta-analysis. Child’s Nervous System 2020, 36, 1099–1109. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Turner, Z.; Cervenka, M.C.; Barron, B.J. Ketogenic Diet Therapies For Epilepsy and Other Conditions. Not available 2020, Not available, Not available. [Google Scholar] [CrossRef]
- Needham, N.; Campbell, I.; Grossi, H.; Kamenska, I.; Rigby, B.; Simpson, S.; McIntosh, E.; Bahuguna, P.; Meadowcroft, B.; Creasy, F.; Mitchell-Grigorjeva, M.; Norrie, J.; Thompson, G.; Gibbs, M.; McLellan, A.; Fisher, C.; Moses, T.; Burgess, K.; Brown, R.; Smith, D. Pilot study of a ketogenic diet in bipolar disorder. BJPsych Open 2023, 9. [Google Scholar] [CrossRef]
- Bohnen, J.L.B.; Albin, R.L.; Bohnen, N.I. Ketogenic interventions in mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease: A systematic review and critical appraisal. Frontiers in Neurology 2023, 14, Not available. [Google Scholar] [CrossRef]
- Tóth, C.; Dabóczi, A.; Howard, M.; Miller, N.J.; Clemens, Z. Crohn’s disease successfully treated with the paleolithic ketogenic diet. International Journal of Case Reports and Images 2016, 7, 570. [Google Scholar] [CrossRef]
- Srivastava, S.; Pawar, V.; Tyagi, A.; Sharma, K.; Kumar, V.; Shukla, S. Immune Modulatory Effects of Ketogenic Diet in Different Disease Conditions. Immuno 2022, 3, 1–15. [Google Scholar] [CrossRef]
- Kaur, N.; Chen, C.C.; Luther, J.; Kao, J.Y. Intestinal dysbiosis in inflammatory bowel disease. Gut Microbes 2011, 2, 211–216. [Google Scholar] [CrossRef]
- Andersen, O.E.; Poulsen, J.V.; Farup, J.; de Morree, A. Regulation of adult stem cell function by ketone bodies. Frontiers in Cell and Developmental Biology 2023, 11, Not available. [Google Scholar] [CrossRef]
- Cheng, C.W.; Biton, M.; Haber, A.L.; Gunduz, N.; Eng, G.; Gaynor, L.T.; Tripathi, S.; Calibasi-Kocal, G.; Rickelt, S.; Butty, V.L.; Moreno-Serrano, M.; Iqbal, A.M.; Bauer-Rowe, K.E.; Imada, S.; Ulutas, M.S.; Mylonas, C.; Whary, M.T.; Levine, S.S.; Basbinar, Y.; Hynes, R.O.; Mino-Kenudson, M.; Deshpande, V.; Boyer, L.A.; Fox, J.G.; Terranova, C.; Rai, K.; Piwnica-Worms, H.; Mihaylova, M.M.; Regev, A.; Ömer, H. Yilmaz. Ketone Body Signaling Mediates Intestinal Stem Cell Homeostasis and Adaptation to Diet. Cell 2019, 178, 1115–1131.e15. [Google Scholar] [CrossRef]
- Ben-Dor, M.; Sirtoli, R.; Barkai, R. The evolution of the human trophic level during the Pleistocene. American Journal of Physical Anthropology 2021, 175, 27–56. [Google Scholar] [CrossRef]
- Kuang, R.; Levinthal, D.J.; Ghaffari, A.A.; del Aguila de Rivers, C.R.; Tansel, A.; Binion, D.G. Nightshade Vegetables: A Dietary Trigger for Worsening Inflammatory Bowel Disease and Irritable Bowel Syndrome? Digestive Diseases and Sciences 2023, 68, 2853–2860. [Google Scholar] [CrossRef]
- Konijeti, G.G.; Kim, N.; Lewis, J.D.; Groven, S.; Chandrasekaran, A.; Grandhe, S.; Diamant, C.; Singh, E.; Oliveira, G.; Wang, X.; Molparia, B.; Torkamani, A. Efficacy of the Autoimmune Protocol Diet for Inflammatory Bowel Disease. Inflammatory Bowel Diseases 2017, 23, 2054–2060. [Google Scholar] [CrossRef]
- Lescinsky, H.; Afshin, A.; Ashbaugh, C.; Bisignano, C.; Brauer, M.; Ferrara, G.; Hay, S.; He, J.; Iannucci, V.; Marczak, L.; McLaughlin, S.; Mullany, E.; Parent, M.; Serfes, A.; Sorensen, R.; Aravkin, A.; Zheng, P.; Murray, C. Health effects associated with consumption of unprocessed red meat: A Burden of Proof study. Nature Medicine 2022, 28, 1–8. [Google Scholar] [CrossRef]
- Iablokov, V.; Sydora, B.C.; Foshaug, R.; Meddings, J.; Driedger, D.; Churchill, T.; Fedorak, R.N. Naturally Occurring Glycoalkaloids in Potatoes Aggravate Intestinal Inflammation in Two Mouse Models of Inflammatory Bowel Disease. Digestive Diseases and Sciences 2010, 55, 3078–3085. [Google Scholar] [CrossRef]
- O’Hearn, A. Can a carnivore diet provide all essential nutrients? Current Opinion in Endocrinology, Diabetes and Obesity 2020, 27, 312–316. [Google Scholar] [CrossRef]
- Beal, T.; Ortenzi, F. Priority Micronutrient Density in Foods. Frontiers in Nutrition 2022, 9, Not available. [Google Scholar] [CrossRef]
- Tan, K.Y. Fiber and colorectal diseases: Separating fact from fiction. World Journal of Gastroenterology 2007, 13, 4161. [Google Scholar] [CrossRef]
- Ho, K.S. Stopping or reducing dietary fiber intake reduces constipation and its associated symptoms. World Journal of Gastroenterology 2012, 18, 4593. [Google Scholar] [CrossRef]
- Lennerz, B.S.; Mey, J.T.; Henn, O.H.; Ludwig, D.S. Behavioral Characteristics and Self-Reported Health Status among 2029 Adults Consuming a “Carnivore Diet”. Current Developments in Nutrition 2021, 5, nzab133. [Google Scholar] [CrossRef]
- Alsharairi, N.A. The Role of Short-Chain Fatty Acids in Mediating Very Low-Calorie Ketogenic Diet-Infant Gut Microbiota Relationships and Its Therapeutic Potential in Obesity. Nutrients 2021, 13, 3702. [Google Scholar] [CrossRef]
- Venegas, D.P.; la Fuente, M.K.D.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Frontiers in Immunology 2019, 10, Not available. [Google Scholar] [CrossRef]
- Deol, P.; Ruegger, P.; Logan, G.D.; Shawki, A.; Li, J.; Mitchell, J.D.; Yu, J.; Piamthai, V.; Radi, S.H.; Hasnain, S.; Borkowski, K.; Newman, J.W.; McCole, D.F.; Nair, M.G.; Hsiao, A.; Borneman, J.; Sladek, F.M. Diet high in linoleic acid dysregulates the intestinal endocannabinoid system and increases susceptibility to colitis in Mice. Gut Microbes 2023, 15, Not. [Google Scholar] [CrossRef]
- Mercola, J.; D’Adamo, C.R. Linoleic Acid: A Narrative Review of the Effects of Increased Intake in the Standard American Diet and Associations with Chronic Disease. Nutrients 2023, 15, 3129. [Google Scholar] [CrossRef]
- Roman, Y.M. The Role of Uric Acid in Human Health: Insights from the Uricase Gene. Journal of Personalized Medicine 2023, 13, 1409. [Google Scholar] [CrossRef]
- Gohari, S.; Ghobadi, S.; Jafari, A.; Ahangar, H.; Gohari, S.; Mahjani, M. The effect of dietary approaches to stop hypertension and ketogenic diets intervention on serum uric acid concentration: a systematic review and meta-analysis of randomized controlled trials. Scientific Reports 2023, 13, Not available. [Google Scholar] [CrossRef]
- Unprocessed Red Meat and Processed Meat Consumption: Dietary Guideline Recommendations From the Nutritional Recommendations (NutriRECS) Consortium. Annals of Internal Medicine 2019, 171, 756–764. [CrossRef] [PubMed]
- Westman, E.C.; Yancy, W.S.; Olsen, M.K.; Dudley, T.; Guyton, J.R. Effect of a low-carbohydrate, ketogenic diet program compared to a low-fat diet on fasting lipoprotein subclasses. International Journal of Cardiology 2006, 110, 212–216. [Google Scholar] [CrossRef]
- Falkenhain, K.; Roach, L.A.; McCreary, S.; McArthur, E.; Weiss, E.J.; Francois, M.E.; Little, J.P. Effect of carbohydrate-restricted dietary interventions on LDL particle size and number in adults in the context of weight loss or weight maintenance: A systematic review and meta-analysis. The American Journal of Clinical Nutrition 2021, 114, 1455–1466. [Google Scholar] [CrossRef]
- Froyen, E. The effects of fat consumption on low-density lipoprotein particle size in healthy individuals: a narrative review. Lipids in Health and Disease 2021, 20. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.N.; Zou, Y.L.; Guo, S.D. Low-density lipoprotein particles in atherosclerosis. Frontiers in Physiology 2022, 13, Not available. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Soto-Mota, A.; Kaplan, B.; Ludwig, D.S.; Budoff, M.; Kontush, A.; Feldman, D. The Lipid Energy Model: Reimagining Lipoprotein Function in the Context of Carbohydrate-Restricted Diets. Metabolites 2022, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Diamond, D.M.; Bikman, B.T.; Mason, P. Statin therapy is not warranted for a person with high LDL-cholesterol on a low-carbohydrate diet. Current Opinion in Endocrinology, Diabetes and Obesity 2022, 29, 497–511. [Google Scholar] [CrossRef] [PubMed]
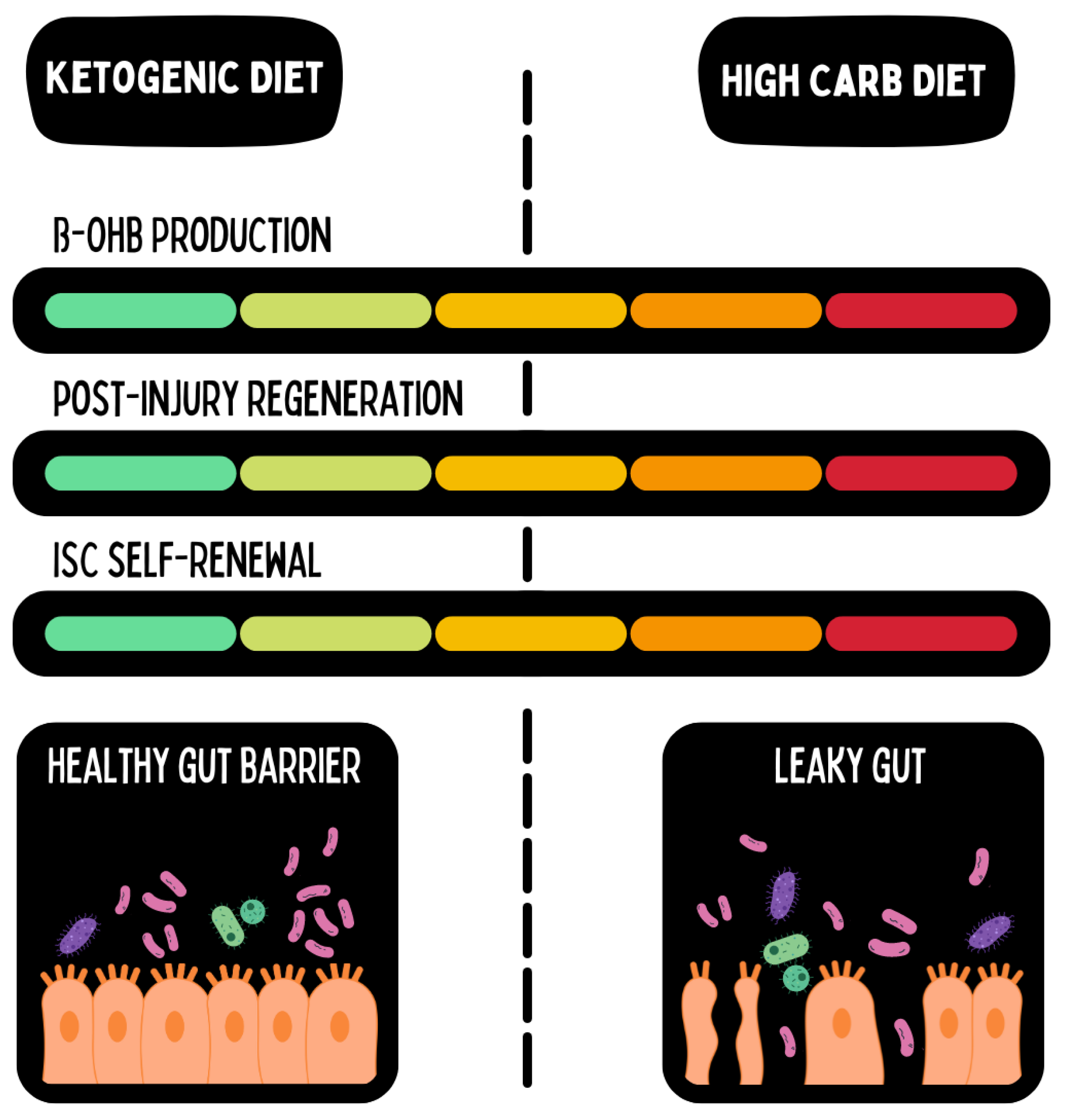
Figure 1.
Ketone bodies directly interact with gene expression and post-translational modifications on the level of intestinal stem cells. This strengthens the regenerative capacity of the intestinal wall. Adapted from Cheng et al., 2019. Graphics with kind permission from the MOJO Institute.
Figure 1.
Ketone bodies directly interact with gene expression and post-translational modifications on the level of intestinal stem cells. This strengthens the regenerative capacity of the intestinal wall. Adapted from Cheng et al., 2019. Graphics with kind permission from the MOJO Institute.
Figure 2.
Mineral and vitamin density per calorie is significantly higher in animal-based foods compared to plant-based foods, and especially grains, which exhibit the lowest bioavailable nutrient density. Adapted from Beal et al., 2022, with permission from the MOJO Institute.
Figure 2.
Mineral and vitamin density per calorie is significantly higher in animal-based foods compared to plant-based foods, and especially grains, which exhibit the lowest bioavailable nutrient density. Adapted from Beal et al., 2022, with permission from the MOJO Institute.
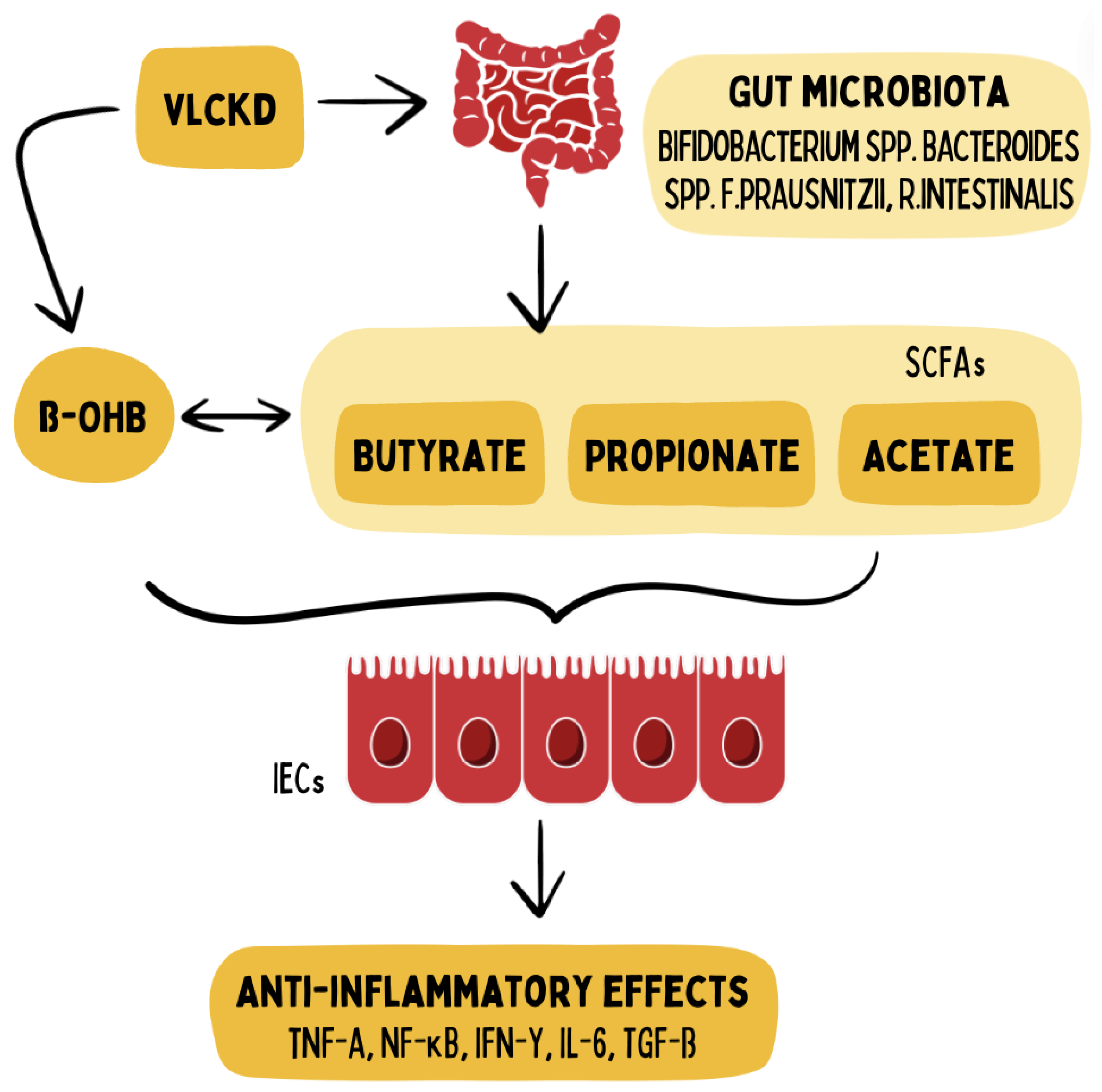
Figure 3.
A ketogenic diet supports the proliferation of SCFA-producing bacteria and increases supply of ketone bodies like ß-hydroxybutyrate (ß-OHB), in turn modifying epigenetic control of gene expression in intestinal epithelial cells (IECs) and thus regulating inflammatory cytokines in IBD. Adapted from Alsharairi et al., 2021. Graphic provided with the permission of the MOJO Institute, Hennef, Germany.
Figure 3.
A ketogenic diet supports the proliferation of SCFA-producing bacteria and increases supply of ketone bodies like ß-hydroxybutyrate (ß-OHB), in turn modifying epigenetic control of gene expression in intestinal epithelial cells (IECs) and thus regulating inflammatory cytokines in IBD. Adapted from Alsharairi et al., 2021. Graphic provided with the permission of the MOJO Institute, Hennef, Germany.
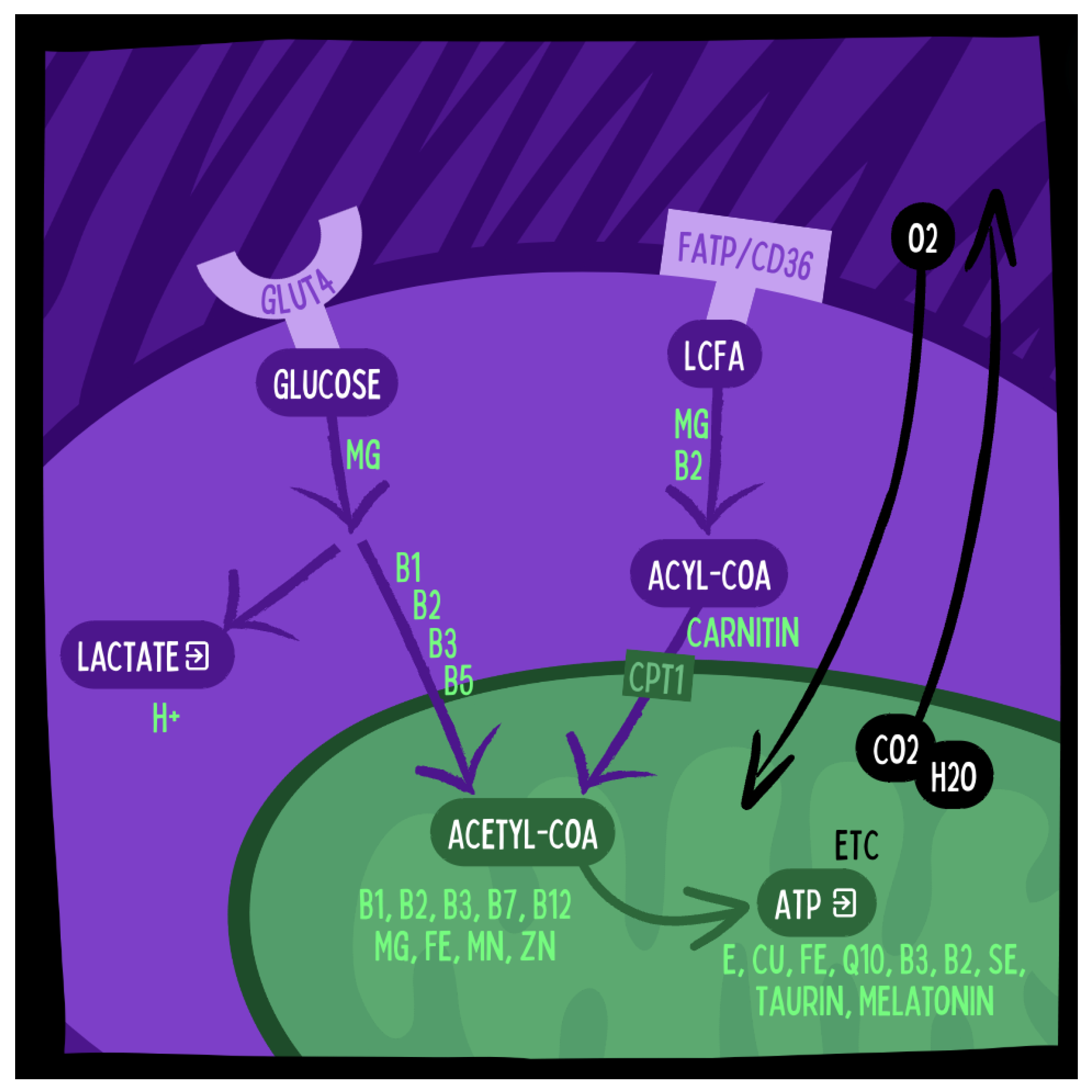
Figure 4.
The Oxidative Metabolization of Glucose and Fatty Acids into ATP in Mitochondria and the Electron Transport Chain (ETC) Requires Multiple Micronutrients Including Vitamins and Minerals. These micronutrients are more concentrated in animal products than in plant based foods. Illustration by MOJO Institute, Hennef/Germany.
Figure 4.
The Oxidative Metabolization of Glucose and Fatty Acids into ATP in Mitochondria and the Electron Transport Chain (ETC) Requires Multiple Micronutrients Including Vitamins and Minerals. These micronutrients are more concentrated in animal products than in plant based foods. Illustration by MOJO Institute, Hennef/Germany.
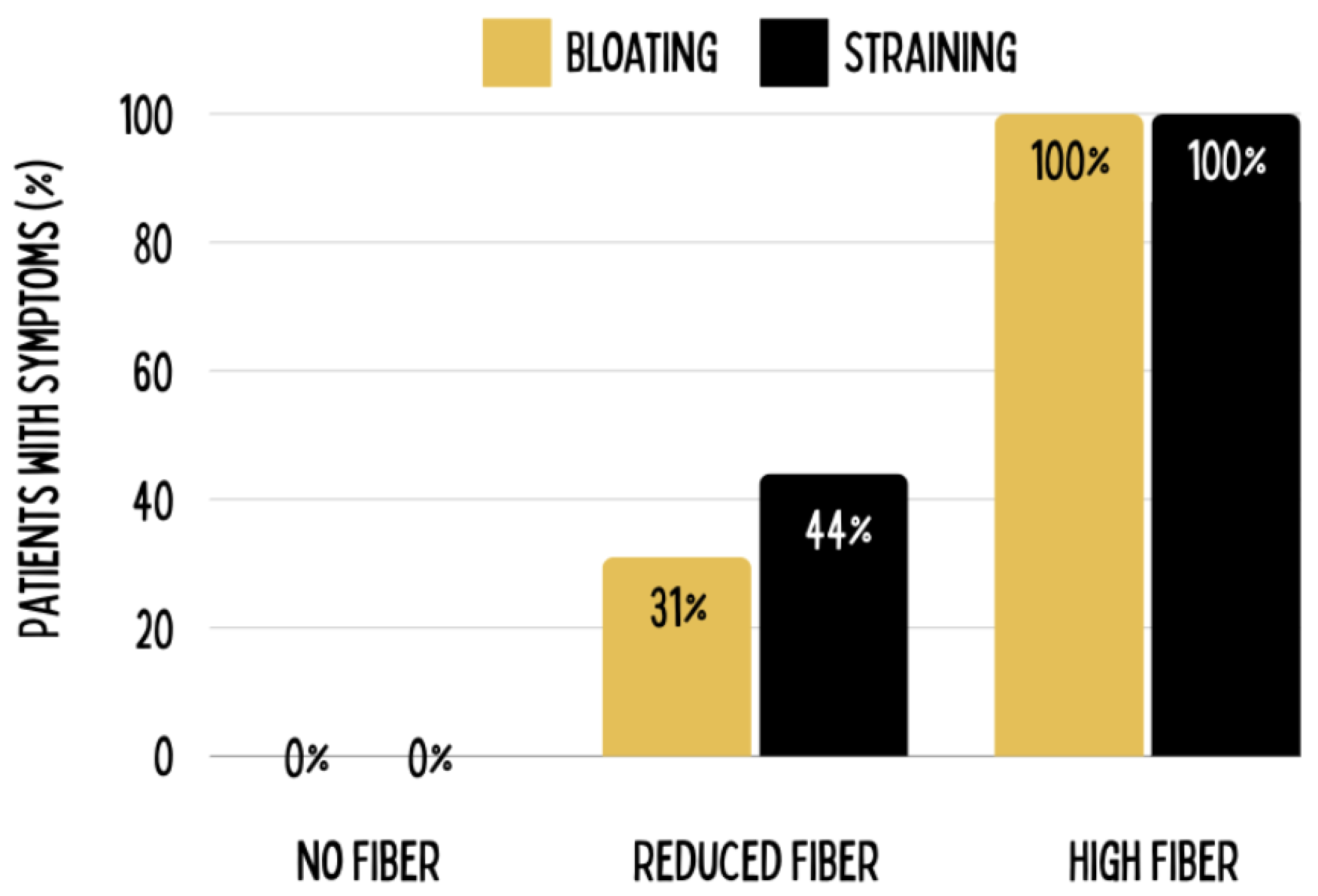
Figure 5.
Dietary fiber can contribute to bloating and straining, as was revealed by an experimental study in which 63 patients were subjected to three levels of fiber intake: no fiber restriction, medium fiber restriction, and complete fiber restriction over six months. Results indicated that while all patients on the high-fiber diet remained symptomatic, none of the patients on the zero-fiber diet exhibited symptoms after six months. Figure adapted from Tan et al., 2012, courtesy of the MOJO Institute, Hennef, Germany.
Figure 5.
Dietary fiber can contribute to bloating and straining, as was revealed by an experimental study in which 63 patients were subjected to three levels of fiber intake: no fiber restriction, medium fiber restriction, and complete fiber restriction over six months. Results indicated that while all patients on the high-fiber diet remained symptomatic, none of the patients on the zero-fiber diet exhibited symptoms after six months. Figure adapted from Tan et al., 2012, courtesy of the MOJO Institute, Hennef, Germany.
Figure 6.
Low Density Lipoproteins (LDL) vary by size. Smaller Lipoproteins have a higher propensity to oxidize and paracellularly pass the endothelium to assist in the progression of atherosclerosis. Under ketogenic diets lipoproteins tend to increase in size without increasing the particle number, resulting in higher serum weights (measured in mg/dl). According to best available evidence this increase in mass, while at the same time increasing size with stable or even decreased particle numbers (as measured by reduced apolipoprotein B unter ketogenic conditions) does not constitute an increased cardiovascular risk and does not warrant statin therapy. Adapted from Qiao et al., 2022, courtesy of the MOJO Institute, Hennef, Germany.
Figure 6.
Low Density Lipoproteins (LDL) vary by size. Smaller Lipoproteins have a higher propensity to oxidize and paracellularly pass the endothelium to assist in the progression of atherosclerosis. Under ketogenic diets lipoproteins tend to increase in size without increasing the particle number, resulting in higher serum weights (measured in mg/dl). According to best available evidence this increase in mass, while at the same time increasing size with stable or even decreased particle numbers (as measured by reduced apolipoprotein B unter ketogenic conditions) does not constitute an increased cardiovascular risk and does not warrant statin therapy. Adapted from Qiao et al., 2022, courtesy of the MOJO Institute, Hennef, Germany.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).