Submitted:
02 September 2024
Posted:
02 September 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Methods
Study Design
Blood Sample Collection
Measurement of Anti SARS-CoV-2 Antibodies
Detection of SARS-CoV-2 RNA Using RT-PCR
COVID-19 Antigen Testing of Respiratory Samples
Sample Size Estimation and Statistical Analysis
Results
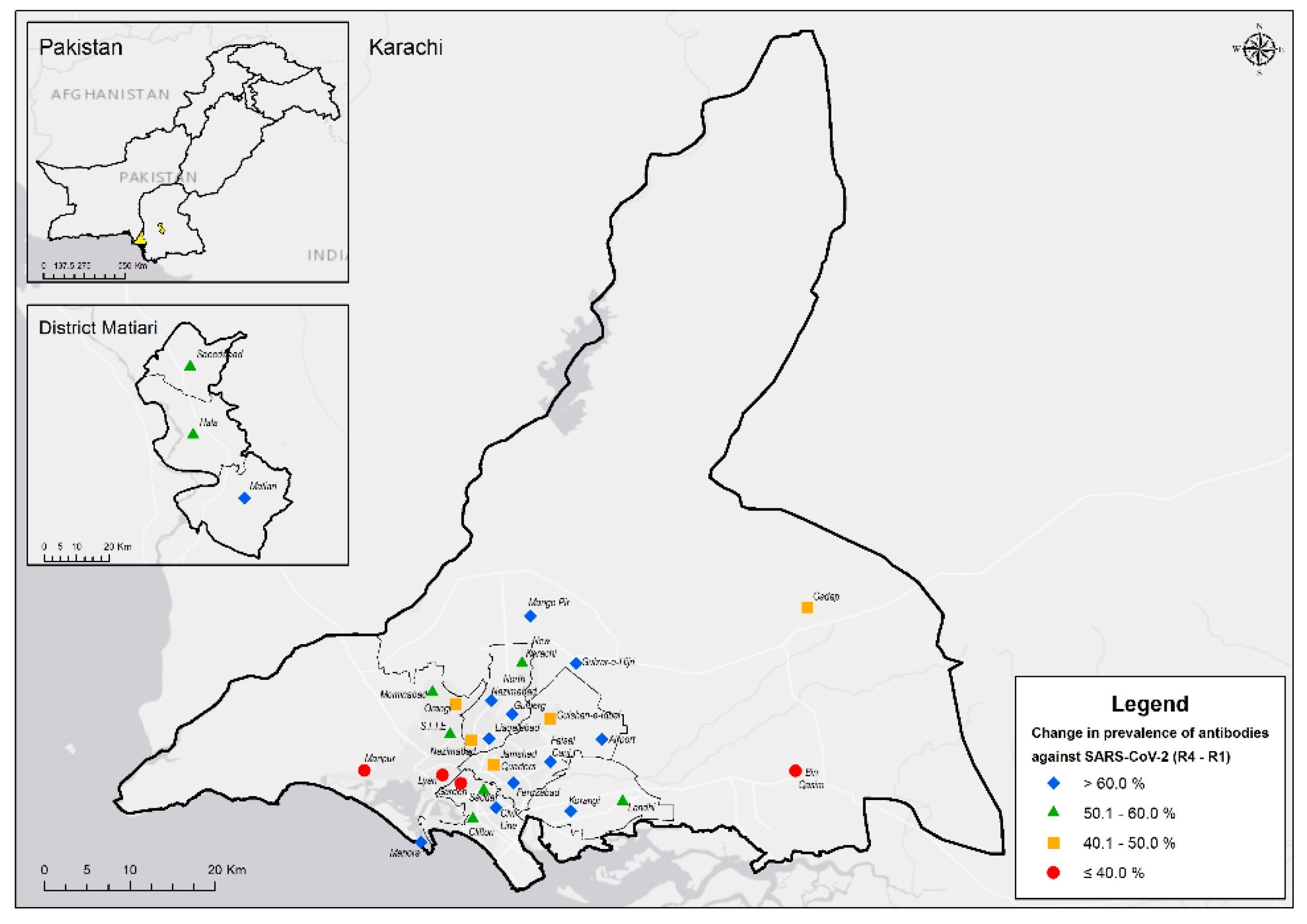
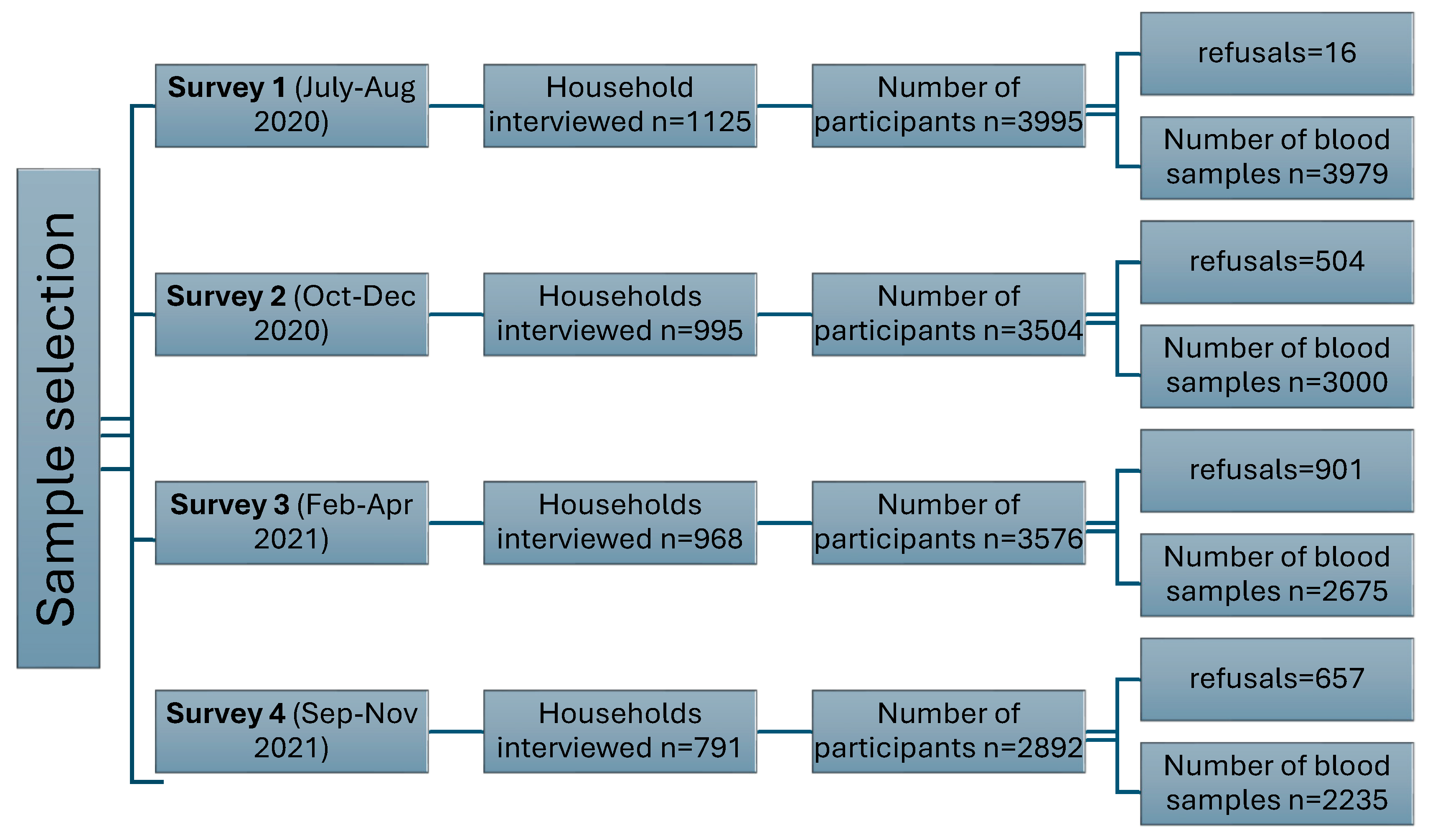
Description of Serosurveys
Demographic and Socio-Economic Description of Study Subjects
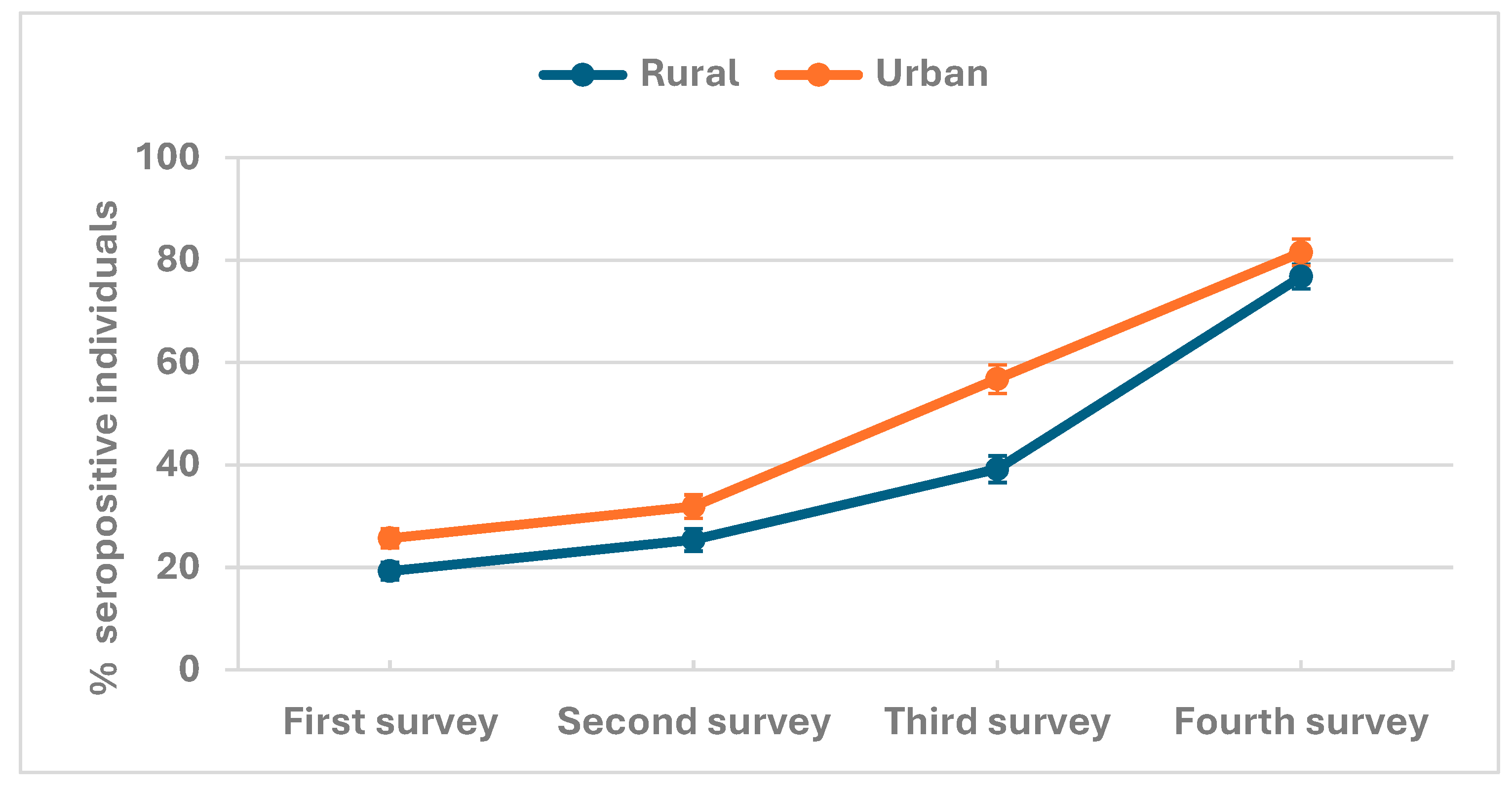
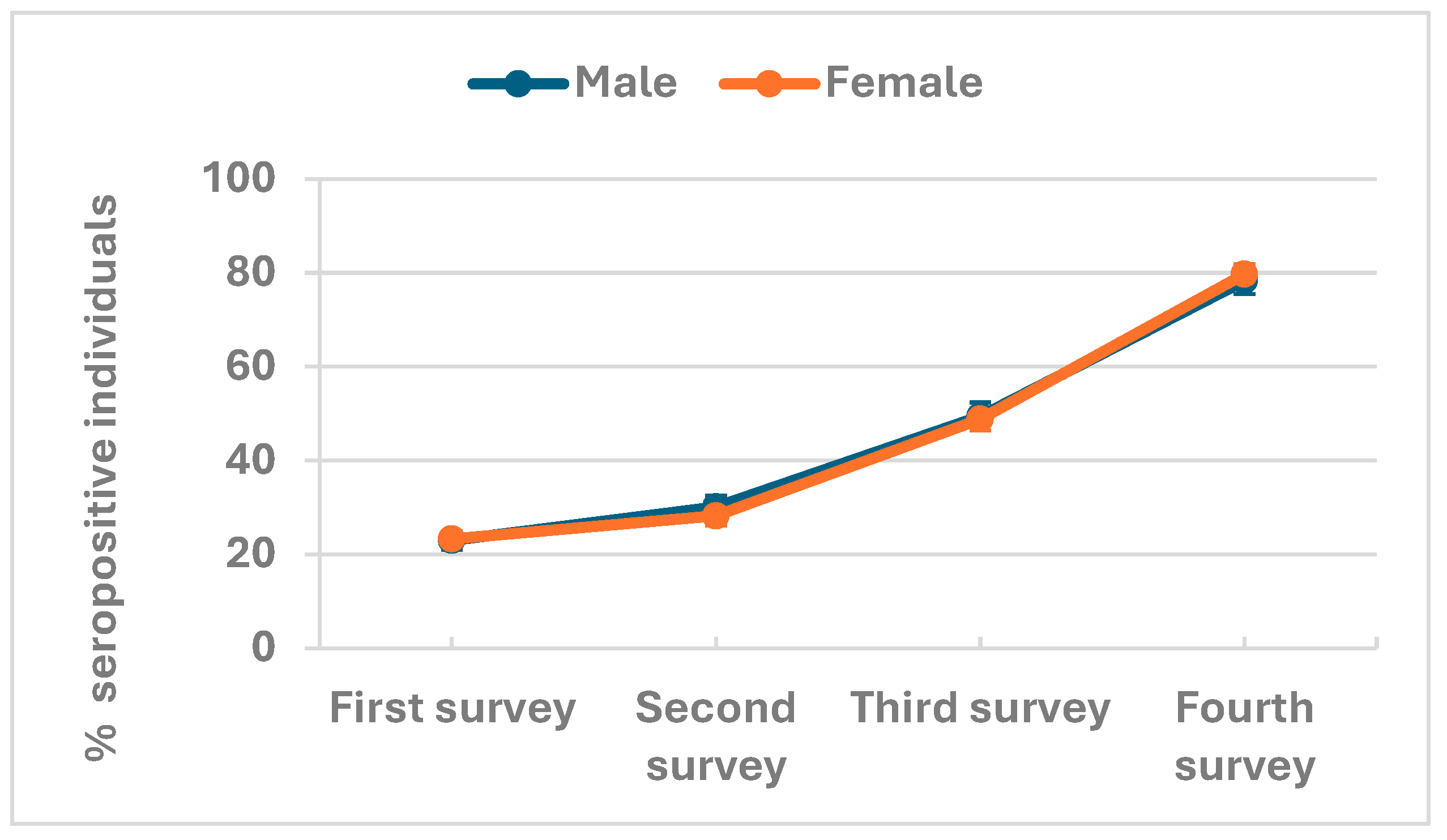
Antibody seroprevalence in rural and urban populations
Factors Associated with SARS-CoV-2 Seropositivity
Assessing Risk of COVID-19 Seroprevalence
Investigation of COVID-19 in Study Subjects
Discussion
Conclusions
Author Contributions
Funding Sources
Declaration of Interests
Acknowledgments
References
- CDC, C.f.D.C.a.P. Centers for Disease Control and Prevention CDC. Novel Coronavirus Wuhan, China. CDC 2019 [cited 2022 February 10,]; Available from: https://www.cdc.gov/coronavirus/2019-ncov/about/index.html.
- Lai, N.H., et al., Comparison of the predictive outcomes for anti-tuberculosis drug-induced hepatotoxicity by different machine learning techniques. Comput Methods Programs Biomed, 2019. 188: p. 105307. [CrossRef]
- WHO. World Health Organization Severe Acute Respiratory Syndrome 2022 [cited 2022 February 19]; Available from: https://www.who.int/health-topics/severe-acute-respiratory-syndrome.
- Abid, K., et al., Progress of COVID-19 Epidemic in Pakistan. Asia Pac J Public Health, 2020. 32(4): p. 154-156. [CrossRef]
- Achonu, C., et al. A seroprevalence study of pandemic influenza A H1N1 among Ontarians. in European Scientific Conference on Applied Infectious Disease Epidemiology Abstract Book. Lisbon. 2010.
- Organization, W.H., Coronavirus disease 2019 (COVID-19) Situation Report – 73. 2020.
- Organization, W.H., When capacity is limited, who should be tested for SARS-CoV-2? 2022, World Health Organization.
- GoP, Guidelines Clinical Management Guidelines for COVID-19 Infections, M.o.N.H.S. Government of Pakistan, Regulations & Coordination, Editor. 2020: Pakistan.
- NEWS, D., Provinces announce easing lockdown even as Pakistan witnesses record rise in coronavirus cases, in DAWN TODAY’S PAPER. 2020, DAWN.
- Emmanuel, F., et al., Pakistan’s COVID-19 Prevention and control response using the world health organization’s guidelines for epidemic response interventions. Cureus, 2023. 15(1). [CrossRef]
- Bhutta, Z.A., et al., Balancing science and public policy in Pakistan’s COVID-19 response. East Mediterr Health J, 2021. 27(8): p. 798-805. [CrossRef]
- Usman, S., et al., Descriptive analysis of health screening for COVID-19 at points of entry in pakistan according to the centers for disease control and prevention guidelines. International Journal of Travel Medicine and Global Health, 2022. 10(3): p. 108-113. [CrossRef]
- Szylovec, A., et al., Brazil’s Actions and Reactions in the Fight Against COVID-19 from January to March 2020. Int J Environ Res Public Health, 2021. 18(2). [CrossRef]
- Ahmad, A.M., et al., COVID-19 seroprevalence in Pakistan: a cross-sectional study. BMJ open, 2022. 12(4): p. e055381. [CrossRef]
- Javed, W., et al., Sero-prevalence findings from metropoles in Pakistan: implications for assessing COVID-19 prevalence and case-fatality within a dense, urban working population. medRxiv, 2020: p. 2020.08. 13.20173914. [CrossRef]
- Nisar, M.I., et al., Serial population-based serosurveys for COVID-19 in two neighbourhoods of Karachi, Pakistan. Int J Infect Dis, 2021. 106: p. 176-182. [CrossRef]
- Haq, M., et al., SARS-CoV-2: big seroprevalence data from Pakistan-is herd immunity at hand? Infection, 2021.
- Ullah, I., Re-identifying the Rural/Urban: a case Study of Pakistan. Espaço e Economia. Revista brasileira de geografia econômica, 2022(23).
- macrotrends, Karachi, Pakistan Metro Area Population 1950-2024. 2024, macrotrends.
- Brinkhoff, T., MATIARI District in Pakistan, Pakistan Bureau of Statistics, U.S. Bureau of Census: Demobase Pakistan. .
- Zaidi, S., et al., Seroprevalence of anti-SARS-CoV-2 antibodies in residents of Karachi-challenges in acquiring herd immunity for COVID 19. J Public Health (Oxf), 2021. 43(1): p. 3-8. [CrossRef]
- Geanes, E.S., et al., Cross-reactive antibodies elicited to conserved epitopes on SARS-CoV-2 spike protein after infection and vaccination. Sci Rep, 2022. 12(1): p. 6496.
- Edouard Mathieu, H.R., Lucas Rodés-Guirao, Cameron Appel, Daniel Gavrilov, Charlie Giattino, Joe Hasell, Bobbie Macdonald, Saloni Dattani, Diana Beltekian, Esteban Ortiz-Ospina, and Max Roser, Coronavirus Pandemic (COVID-19). Our World in Data, 2020.
- Ahmad, T., et al., COVID-19 in Pakistan: A national analysis of five pandemic waves. PLoS One, 2023. 18(12): p. e0281326. [CrossRef]
- Chughtai, O.R., et al., Frequency of COVID-19 IgG Antibodies among Special Police Squad Lahore, Pakistan. J Coll Physicians Surg Pak, 2020. 30(7): p. 735-739. [CrossRef]
- Hasan, M., et al., IgG antibodies to SARS-CoV-2 in asymptomatic blood donors at two time points in Karachi. PLoS One, 2022. 17(8): p. e0271259. [CrossRef]
- Rashid, M. and M. Piracha, SECOND WAVE OF COVID-19 IN PAKISTAN; HOW WORSE IT CAN GET?
- Mueed, A., et al., Impact of school closures and reopening on COVID-19 caseload in 6 cities of Pakistan: An Interrupted Time Series Analysis. medRxiv, 2022. [CrossRef]
- Ghanchi, N.K., et al., Higher entropy observed in SAR-CoV-2 genomes from the first COVID-19 wave in Pakistan PLoS ONE, 2021. 16(8)(e0256451): p. [CrossRef]
- Umair, M., et al., Whole-genome sequencing of SARS-CoV-2 reveals the detection of G614 variant in Pakistan. PLoS One, 2021. 16(3): p. e0248371. [CrossRef]
- Nasir, A., et al., Tracking SARS-CoV-2 variants through pandemic waves using RT-PCR testing in low-resource settings. PLOS Glob Public Health, 2023. 3(6): p. e0001896. [CrossRef]
- Siddiqui, A., et al., An overview of procurement, pricing, and uptake of COVID-19 vaccines in Pakistan. Vaccine, 2021. 39(37): p. 5251-5253. [CrossRef]
- Kumar, D., A. Burma, and A.K. Mandal, A seroprevalence study of Covid 19 antibody after 1st wave of the pandemic in South Andaman district, India. Clinical epidemiology and global health, 2021. 12: p. 100901. [CrossRef]
- Pierce, M., et al., Mental health before and during the COVID-19 pandemic: a longitudinal probability sample survey of the UK population. The Lancet Psychiatry, 2020. 7(10): p. 883-892. [CrossRef]
- Shervani, Z., D. Bhardwaj, and R. Nikhat, COVID-19 Infection in India: Seropositivity versus the Dynamics of the Spread. European Journal of Medical and Health Sciences, 2021. 3(4): p. 27-31. [CrossRef]
- Inbaraj, L.R., C.E. George, and S. Chandrasingh, Seroprevalence of COVID-19 infection in a rural district of South India: A population-based seroepidemiological study. PloS one, 2021. 16(3): p. e0249247. [CrossRef]
- Nisar, M.I., et al., Serial population-based serosurveys for COVID-19 in two neighbourhoods of Karachi, Pakistan. International Journal of Infectious Diseases, 2021. 106: p. 176-182. [CrossRef]
- Montenegro, P., et al., Community seroprevalence of COVID-19 in probable and possible cases at primary health care centres in Spain. Family Practice, 2021. 38(2): p. 153-158. [CrossRef]
- Shakiba, M., et al., Seroprevalence of COVID-19 virus infection in Guilan province, Iran. MedRxiv, 2020.
- Shaw, J.A., et al., Higher SARS-CoV-2 seroprevalence in workers with lower socioeconomic status in Cape Town, South Africa. PLoS One, 2021. 16(2): p. e0247852. [CrossRef]
- Reyes-Vega, M.F., et al., SARS-CoV-2 prevalence associated to low socioeconomic status and overcrowding in an LMIC megacity: A population-based seroepidemiological survey in Lima, Peru. EClinicalMedicine, 2021. 34: p. 100801. [CrossRef]
- Hassan, S., et al., An audit of clinical laboratory data of 25 [OH]D at Aga Khan University as reflecting vitamin D deficiency in Pakistan. J Pak Med Assoc, 2015. 65(11): p. 1247-50.
- Asghar, M.S., et al., Evaluation of Vitamin-D Status and Its Association with Clinical Outcomes Among COVID-19 Patients in Pakistan. The American journal of tropical medicine and hygiene, 2022. 106(1): p. 150. [CrossRef]
- Masood, K.I., et al., Humoral and T cell responses to SARS-CoV-2 reveal insights into immunity during the early pandemic period in Pakistan. BMC Infect Dis, 2023. 23(1): p. 846.
- Fenwick, C., et al., Changes in SARS-CoV-2 Spike versus Nucleoprotein Antibody Responses Impact the Estimates of Infections in Population-Based Seroprevalence Studies. J Virol, 2021. 95(3). [CrossRef]
- Dogan, M., et al., SARS-CoV-2 specific antibody and neutralization assays reveal the wide range of the humoral immune response to virus. Commun Biol, 2021. 4(1): p. 129.
- Shrwani, K., et al., Detection of Serum Cross-Reactive Antibodies and Memory Response to SARS-CoV-2 in Prepandemic and Post-COVID-19 Convalescent Samples. J Infect Dis, 2021. 224(8): p. 1305-1315. [CrossRef]
- Yuen, R.R., et al., Novel ELISA Protocol Links Pre-Existing SARS-CoV-2 Reactive Antibodies With Endemic Coronavirus Immunity and Age and Reveals Improved Serologic Identification of Acute COVID-19 via Multi-Parameter Detection. Front Immunol, 2021. 12: p. 614676. [CrossRef]
- Diez, J.M., C. Romero, and R. Gajardo, Effective presence of antibodies against common human coronaviruses in immunoglobulin medicinal products. Int J Infect Dis, 2021. 116: p. 68-73. [CrossRef]
- Zedan, H.T. and G.K. Nasrallah, Is preexisting immunity to seasonal coronaviruses limited to cross-reactivity with SARS-CoV-2? A seroprevalence cross-sectional study in north-eastern France. EBioMedicine, 2021. 71: p. 103580.
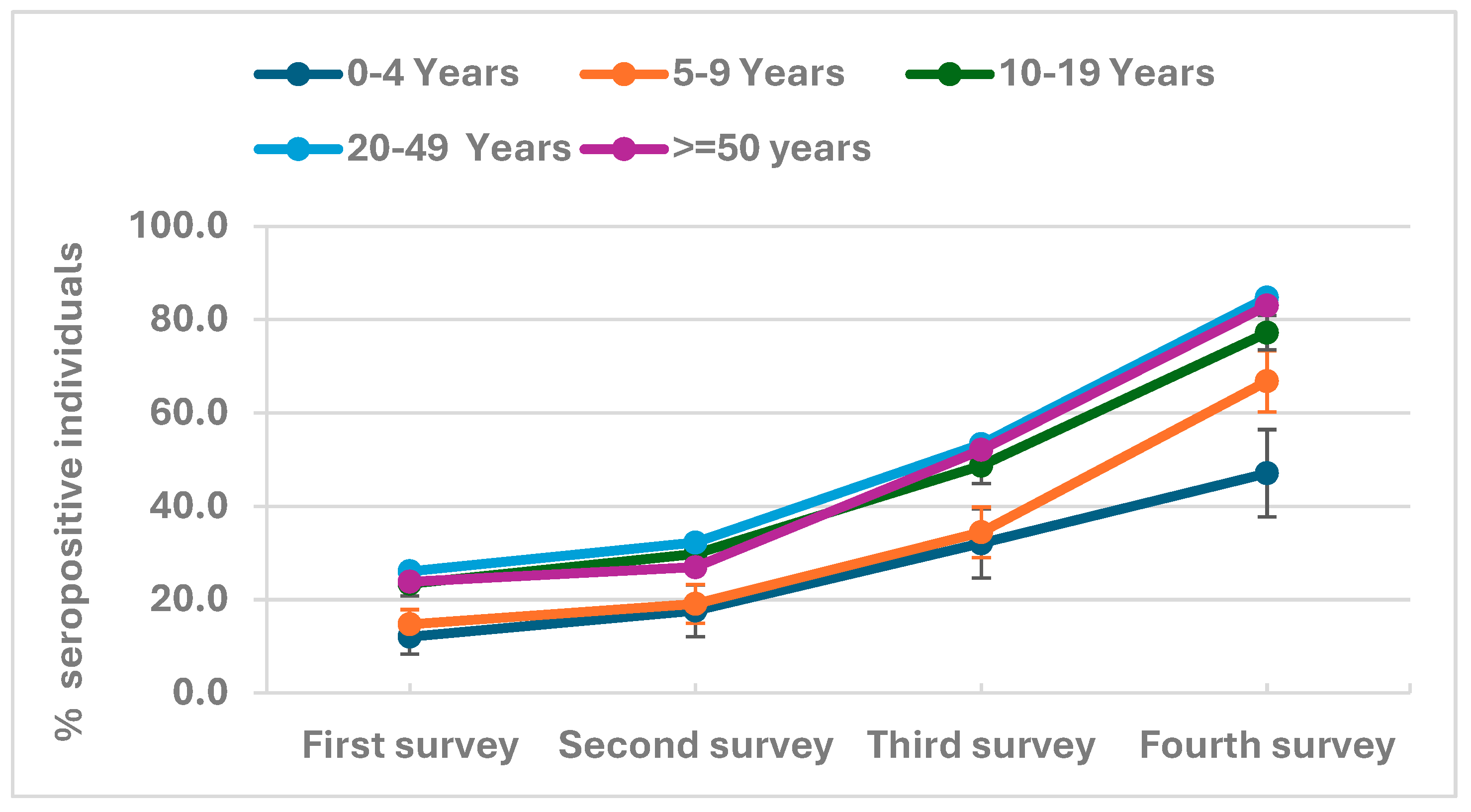
| Overall | First survey (July-Aug 2020) |
Second survey (Oct-Dec 2020) |
Third survey (Feb-April 2021) |
Fourth survey (Sep-Nov 2021) |
P-value | |
|---|---|---|---|---|---|---|
| N=11889 | N=3979 | N=3000 | N=2675 | N=2235 | ||
| Location | ||||||
| Rural | 5,187 (43.6%) | 1,550 (39.0%) | 1,340 (44.7%) | 1,176 (44.0%) | 1,121 (50.2%) | <0.001 |
| Urban | 6,702 (56.4%) | 2,429 (61.0%) | 1,660 (55.3%) | 1,499 (56.0%) | 1,114 (49.8%) | |
| Gender | ||||||
| Male | 4,711 (39.6%) | 1,629 (40.9%) | 1,158 (38.6%) | 1,048 (39.2%) | 876 (39.2%) | 0.200 |
| Female | 7,178 (60.4%) | 2,350 (59.1%) | 1,842 (61.4%) | 1,627 (60.8%) | 1,359 (60.8%) | |
| Age (Years) | ||||||
| 0–4 | 595 (5.0%) | 224 (5.6%) | 136 (4.5%) | 131 (4.9%) | 104 (4.7%) | 0.930 |
| 5-9 | 1,171 (9.8%) | 401 (10.1%) | 283 (9.4%) | 273 (10.2%) | 214 (9.6%) | |
| 10-19 | 2,876 (24.2%) | 932 (23.4%) | 729 (24.3%) | 657 (24.6%) | 558 (25.0%) | |
| 20-29 | 2,159 (18.2%) | 739 (18.6%) | 562 (18.7%) | 463 (17.3%) | 395 (17.7%) | |
| 30-39 | 1,899 (16.0%) | 619 (15.6%) | 472 (15.7%) | 437 (16.3%) | 371 (16.6%) | |
| 40-49 | 1,474 (12.4%) | 481 (12.1%) | 384 (12.8%) | 334 (12.5%) | 275 (12.3%) | |
| 50-59 | 876 (7.4%) | 298 (7.5%) | 224 (7.5%) | 184 (6.9%) | 170 (7.6%) | |
| 60-69 | 539 (4.5%) | 179 (4.5%) | 132 (4.4%) | 126 (4.7%) | 102 (4.6%) | |
| 70-79 | 245 (2.1%) | 84 (2.1%) | 64 (2.1%) | 58 (2.2%) | 39 (1.7%) | |
| 80 and above | 55 (0.5%) | 22 (0.6%) | 14 (0.5%) | 12 (0.4%) | 7 (0.3%) | |
| Household size | ||||||
| 1 | 28 (0.2%) | 10 (0.3%) | 5 (0.2%) | 6 (0.2%) | 7 (0.3%) | 0.270 |
| 2 | 439 (3.7%) | 143 (3.6%) | 114 (3.8%) | 93 (3.5%) | 89 (4.0%) | |
| 3 | 228 (1.9%) | 81 (2.0%) | 54 (1.8%) | 53 (2.0%) | 40 (1.8%) | |
| 4-5 | 1,485 (12.5%) | 542 (13.6%) | 386 (12.9%) | 311 (11.6%) | 246 (11.0%) | |
| 6 and above | 9,709 (81.7%) | 3,203 (80.5%) | 2,441 (81.4%) | 2,212 (82.7%) | 1,853 (82.9%) | |
| Wealth Index(quintiles) | ||||||
| Poorest | 2,179 (18.3%) | 756 (19.0%) | 545 (18.2%) | 452 (16.9%) | 426 (19.1%) | 0.053 |
| Poor | 2,468 (20.8%) | 830 (20.9%) | 645 (21.5%) | 559 (20.9%) | 434 (19.4%) | |
| Middle | 2,230 (18.8%) | 771 (19.4%) | 567 (18.9%) | 469 (17.5%) | 423 (18.9%) | |
| Rich | 2,313 (19.5%) | 761 (19.1%) | 595 (19.8%) | 534 (20.0%) | 423 (18.9%) | |
| Richest | 2,699 (22.7%) | 861 (21.6%) | 648 (21.6%) | 661 (24.7%) | 529 (23.7%) | |
| Mother tongue | ||||||
| Urdu | 2,209 (18.6%) | 716 (18.0%) | 538 (17.9%) | 530 (19.8%) | 425 (19.0%) | <0.001 |
| Punjabi | 840 (7.1%) | 376 (9.4%) | 223 (7.4%) | 146 (5.5%) | 95 (4.3%) | |
| Sindhi | 6,271 (52.7%) | 1,936 (48.7%) | 1,596 (53.2%) | 1,462 (54.7%) | 1,277 (57.1%) | |
| Pashto | 790 (6.6%) | 287 (7.2%) | 206 (6.9%) | 144 (5.4%) | 153 (6.8%) | |
| Balochi | 453 (3.8%) | 166 (4.2%) | 111 (3.7%) | 97 (3.6%) | 79 (3.5%) | |
| Saraiki | 90 (0.8%) | 41 (1.0%) | 17 (0.6%) | 24 (0.9%) | 8 (0.4%) | |
| Hindko | 90 (0.8%) | 36 (0.9%) | 18 (0.6%) | 19 (0.7%) | 17 (0.8%) | |
| Others | 1,146 (9.6%) | 421 (10.6%) | 291 (9.7%) | 253 (9.5%) | 181 (8.1%) | |
| Vaccinated against COVID-19 | 1113 (49.8%) | <0.001 |
| First survey (July-Aug 2020) | Second survey (Oct-Dec 2020) | Third survey (Feb-April 2021) | Fourth survey (Sep-Nov 2021) | |||||
|---|---|---|---|---|---|---|---|---|
| Positive tests | estimate [95% CI] | Positive tests | estimate [95% CI] | Positive tests | estimate [95% CI] | Positive tests | estimate [95% CI] | |
| Overall seroprevalence | 923 /3979 | 23.2% (21.9-24.5) | 869 /3000 | 29.0% (27.4-30.6) | 1312 /2675 | 49.0% (47.2-50.9) | 1769 /2235 | 79.1% (77.4-80.8) |
| Location | ||||||||
| Rural | 299 /1550 | 19.3% (17.4 - 21.3) | 340 /1340 | 25.4% (23.1 - 27.8) | 461 /1176 | 39.2% (36.4 - 42.0) | 861 /1121 | 76.8% (74.2 -79.2) |
| Urban | 624 /2429 | 25.7% (24.0 - 27.5) | 529 /1660 | 31.9% (29.7 - 34.2) | 851 /1499 | 56.8% (54.2 - 59.3) | 908 /1114 | 81.5% (79.1 -83.7) |
| Gender | ||||||||
| Male | 375 /1629 | 23.0% (21.0 - 25.1) | 349 /1158 | 30.1% (27.6 - 32.8) | 518 /1048 | 49.4% (46.4 - 52.5) | 686 /876 | 78.3% (75.5 -80.9) |
| Female | 548 /2350 | 23.3% (21.7 - 25.1) | 520 /1842 | 28.2% (26.2 - 30.3) | 794 /1627 | 48.8% (46.4 - 51.2) | 1083 /1359 | 79.7% (77.5 -81.7) |
| Age (Years) | ||||||||
| 0–4 | 27 /224 | 12.1% (8.4 - 17.0) | 24 /136 | 17.6% (12.1 - 25.0) | 42 /131 | 32.1% (24.6 - 40.5) | 49 /104 | 47.1% (37.7 -56.7) |
| 5-9 | 59 /401 | 14.7% (11.6 - 18.5) | 54 /283 | 19.1% (14.9 - 24.1) | 94 /273 | 34.4% (29.0 - 40.3) | 143/214 | 66.8% (60.2 -72.8) |
| 10-19 | 218 /932 | 23.4% (20.8 - 26.2) | 217 /729 | 29.8% (26.6 - 33.2) | 320 /657 | 48.7% (44.9 - 52.5) | 431/558 | 77.2% (73.6 -80.5) |
| 20-29 | 174 /739 | 23.5% (20.6 - 26.7) | 169 /562 | 30.1% (26.4 - 34.0) | 233 /463 | 50.3% (45.8 - 54.9) | 322 /395 | 81.5% (77.4 -85.0) |
| 30-39 | 171 /619 | 27.6% (24.2 - 31.3) | 148 /472 | 31.4% (27.3 - 35.7) | 237 /437 | 54.2% (49.5 - 58.9) | 318 /371 | 85.7% (81.8 -88.9) |
| 40-49 | 135 /481 | 28.1% (24.2 - 32.3) | 140 /384 | 36.5% (31.8 - 41.4) | 188 /334 | 56.3% (50.9 - 61.5) | 242 /275 | 88.0% (83.6 -91.3) |
| 50-59 | 73 /298 | 24.5% (19.9 - 29.7) | 58 /224 | 25.9% (20.6 - 32.0) | 96 /184 | 52.2% (45.0 - 59.3) | 149 /170 | 87.6% (81.8 -91.8) |
| 60-69 | 45 /179 | 25.1% (19.3 - 32.0) | 40 /132 | 30.3% (23.1 - 38.7) | 67 /126 | 53.2% (44.4 - 61.7) | 77 /102 | 75.5% (66.2 -82.9) |
| 70-79 | 17 /84 | 20.2% (13.0 - 30.2) | 16 /64 | 25.0% (15.9 - 37.0) | 31 /58 | 53.4% (40.7 - 65.8) | 32 /39 | 82.1% (66.9 -91.2) |
| 80 and above | 4 /22 | 18.2% (7.0 - 39.6) | 3 /14 | 21.4% (7.1 - 49.4) | 4 /12 | 33.3% (13.1 - 62.4) | 6 /7 | 85.7% (41.9 -98.0) |
| District | ||||||||
| Matiari | 299 /1550 | 19.3% (17.4 - 21.3) | 340 /1340 | 25.4% (23.1 - 27.8) | 461 /1176 | 39.2% (36.4 - 42.0) | 861 /1121 | 76.8% (74.2 -79.2) |
| Karachi Central | 113 /498 | 22.7% (19.2 - 26.6) | 94 /345 | 27.2% (22.8 - 32.2) | 196 /306 | 64.1% (58.5 - 69.2) | 236 /281 | 84.0% (79.2 -87.8) |
| Karachi East | 133 /529 | 25.1% (21.6 - 29.0) | 92 /324 | 28.4% (23.7 - 33.6) | 152 /280 | 54.3% (48.4 - 60.0) | 175 /220 | 79.5% (73.7 -84.4) |
| Karachi South | 85 /323 | 26.3% (21.8 - 31.4) | 62 /244 | 25.4% (20.3 - 31.2) | 116 /221 | 52.5% (45.9 - 59.0) | 89 /124 | 71.8% (63.2 -79.0) |
| Karachi West | 182 /587 | 31.0% (27.4 - 34.9) | 179 /455 | 39.3% (35.0 - 43.9) | 224 /401 | 55.9% (51.0 - 60.7) | 275 /325 | 84.6% (80.3 -88.1) |
| Korangi | 57 /212 | 26.9% (21.3 - 33.3) | 59 /147 | 40.1% (32.5 - 48.3) | 79 /143 | 55.2% (47.0 - 63.2) | 82 /95 | 86.3% (77.8 -91.9) |
| Malir | 54 /280 | 19.3% (15.1 - 24.3) | 43 /145 | 29.7% (22.8 - 37.6) | 84 /148 | 56.8% (48.7 - 64.5) | 51 /69 | 73.9% (62.3 -82.9) |
| Household size | ||||||||
| 1 | 1 /10 | 10.0% (1.4 - 46.7) | 1 /5 | 20.0% (2.7 - 69.1) | 2 /6 | 33.3% (8.4 - 73.2) | 4 /7 | 57.1% (23.0 -85.6) |
| 2 | 38 /143 | 26.6% (20.0 - 34.4) | 38 /114 | 33.3% (25.3 - 42.5) | 43 /93 | 46.2% (36.4 - 56.4) | 73 /89 | 82.0% (72.6 -88.7) |
| 3 | 15 /81 | 18.5% (11.5 - 28.5) | 15 /54 | 27.8% (17.5 - 41.1) | 27 /53 | 50.9% (37.7 - 64.0) | 30 /40 | 75.0% (59.4 -86.0) |
| 4-5 | 135 /542 | 24.9% (21.4 - 28.7) | 109 /386 | 28.2% (24.0 - 32.9) | 158 /311 | 50.8% (45.3 - 56.3) | 200 /246 | 81.3% (75.9 -85.7) |
| 6 and above | 734 /3203 | 22.9% (21.5 - 24.4) | 706 /2441 | 28.9% (27.2 - 30.8) | 1082 /2212 | 48.9% (46.8 - 51.0) | 1462 /1853 | 78.9% (77.0 -80.7) |
| Wealth Quintile | ||||||||
| Poorest | 154 /756 | 20.4% (17.6 - 23.4) | 133 /545 | 24.4% (21.0 - 28.2) | 182 /452 | 40.3% (35.8 - 44.9) | 320 /426 | 75.1% (70.8 -79.0) |
| Poor | 172 /830 | 20.7% (18.1 - 23.6) | 167 /645 | 25.9% (22.7 - 29.4) | 251 /559 | 44.9% (40.8 - 49.1) | 340 /434 | 78.3% (74.2 -82.0) |
| Middle | 192 /771 | 24.9% (22.0 - 28.1) | 183 /567 | 32.3% (28.6 - 36.2) | 247 /469 | 52.7% (48.1 - 57.2) | 345 /423 | 81.6% (77.6 -85.0) |
| Rich | 203 /761 | 26.7% (23.7 - 29.9) | 188 /595 | 31.6% (28.0 - 35.4) | 291 /534 | 54.5% (50.2 - 58.7) | 349 /423 | 82.5% (78.6 -85.8) |
| Richest | 202 /861 | 23.5% (20.7 - 26.4) | 198 /648 | 30.6% (27.1 - 34.2) | 341 /661 | 51.6% (47.8 - 55.4) | 415 /529 | 78.4% (74.7 -81.7) |
| Mother tongue | ||||||||
| Urdu | 163 /716 | 22.8% (19.8 - 26.0) | 137 /538 | 25.5% (22.0 - 29.3) | 286 /530 | 54.0% (49.7 - 58.2) | 333 /425 | 78.4% (74.2 -82.0) |
| Punjabi | 105 /376 | 27.9% (23.6 - 32.7) | 83 /223 | 37.2% (31.1 - 43.8) | 83 /146 | 56.8% (48.7 - 64.6) | 76 /95 | 80.0% (70.8 -86.9) |
| Sindhi | 379 /1936 | 19.6% (17.9 - 21.4) | 432 /1596 | 27.1% (24.9 - 29.3) | 647 /1462 | 44.3% (41.7 - 46.8) | 994 /1277 | 77.8% (75.5 -80.0) |
| Pashto | 87 /287 | 30.3% (25.3 - 35.9) | 74 /206 | 35.9% (29.7 - 42.7) | 82 /144 | 56.9% (48.7 - 64.8) | 128 /153 | 83.7% (76.9 -88.7) |
| Balochi | 53 /166 | 31.9% (25.3 - 39.4) | 35 /111 | 31.5% (23.6 - 40.7) | 52 /97 | 53.6% (43.7 - 63.3) | 66 /79 | 83.5% (73.7 -90.2) |
| Saraiki | 10 /41 | 24.4% (13.7 - 39.7) | 7 /17 | 41.2% (21.0 - 64.8) | 9 /24 | 37.5% (20.8 - 57.8) | 5 /8 | 62.5% (28.5 -87.5) |
| Hindko | 10 /36 | 27.8% (15.6 - 44.4) | 6 /18 | 33.3% (15.8 - 57.1) | 8 /19 | 42.1% (22.6 - 64.4) | 11 /17 | 64.7% (40.4 -83.2) |
| Others | 116 /421 | 27.6% (23.5 - 32.0) | 95 /291 | 32.6% (27.5 - 38.2) | 145 /253 | 57.3% (51.1 - 63.3) | 156 /181 | 86.2% (80.4 -90.5) |
| COVID-19 vaccination status | ||||||||
| Vaccinated | 965/1113 | 86.7% (80.2-90.5) | ||||||
| Not vaccinated | 804/1122 | 71.7% (63.2 -79.0) | ||||||
| Variables | Adjusted OR (95% CI) | P-values |
|---|---|---|
| Round | ||
| 1 | Ref. | |
| 2 | 1.8 (1.5,2.1) | <0.001 |
| 3 | 10 (8.3,11.9) | <0.001 |
| 4 | 128.6 (99,167) | <0.001 |
| Area | ||
| Rural | Ref. | |
| Urban | 2.6 (1.9,3.6) | <0.001 |
| Age | ||
| 0-4 Years | Ref. | |
| 5-9 Years | 1.8 (1.0,3.1) | 0.052 |
| 10-19 Years | 5.1 (3.1,8.6) | <0.001 |
| 20-49 Years | 7.5 (4.6,12.4) | <0.001 |
| >=50 years | 6.4 (3.7,10.9) | <0.001 |
| Mother tongue | ||
| Urdu | Ref. | |
| Punjabi | 1.8 (1.2,2.7) | 0.007 |
| Sindhi | 1.5 (1.0,2.2) | 0.028 |
| Pashto | 2.1 (1.3,3.3) | 0.001 |
| Balochi | 1.9 (1.1,3.3) | 0.022 |
| Saraiki | 1.4 (0.5,4.2) | 0.508 |
| Hindko | 0.8 (0.3,2.3) | 0.615 |
| Others | 1.5 (1.0,2.2) | 0.054 |
| Wealth Index(quintiles) | ||
| Poorest | Ref. | |
| Poor | 1.3 (1.0,1.8) | 0.07 |
| Middle | 1.8 (1.3,2.4) | <0.001 |
| Rich | 1.9 (1.4,2.7) | <0.001 |
| Richest | 1.5 (1.1,2.0) | 0.018 |
| Conjunctivitis | ||
| Yes | 1.6 (1.1,2.2) | 0.007 |
| No | Ref. | |
| Contact with a confirmed COVID-19 case in last 2 weeks | ||
| Yes | 3.2 (1.5,6.8) | 0.003 |
| No | Ref. | |
| Vitamin D Level (µmol/L) | ||
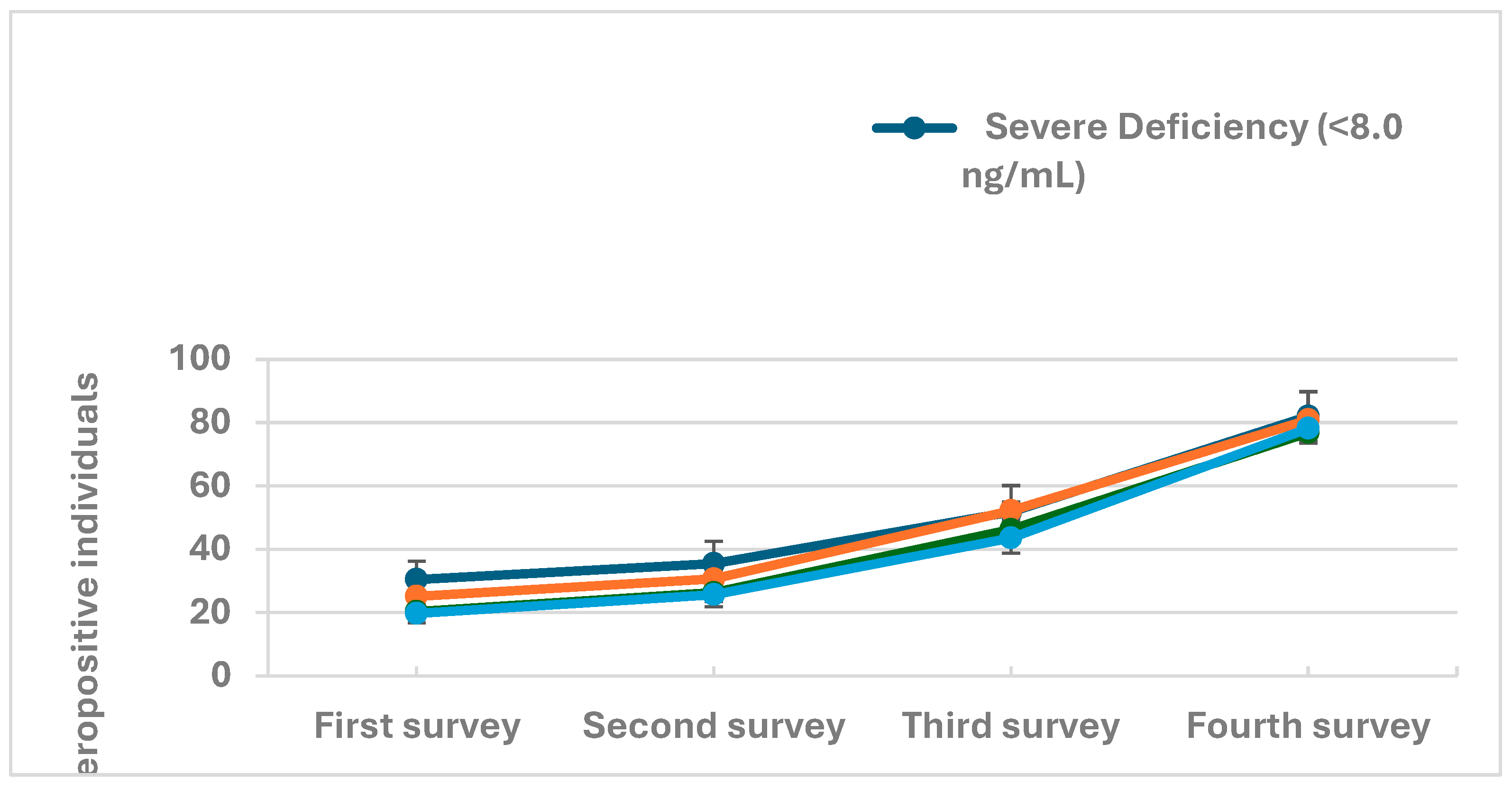
| Severe Deficiency (<8.0 ng/mL) | 2.1 (1.3,3.5) | 0.003 |
| Deficiency (8.0 - 20.0 ng/mL) | 1.7 (1.3,2.3) | 0.001 |
| Desirable (>20.0 - 30.0 ng/mL) | 1.2 (0.9,1.7) | 0.211 |
| Sufficient (>30.0 ng/mL) | Ref. |
| Variables | Adjusted (HR 95% CI) | P-values |
|---|---|---|
| Age | ||
| 0-4 Years | Ref. | |
| 5-9 Years | 1.3 (1.0,1.7) | 0.035 |
| 10-19 Years | 1.9 (1.5,2.5) | <0.001 |
| 20-49 Years | 2.2 (1.7,2.7) | <0.001 |
| >=50 years | 2.2 (1.7,2.8) | <0.001 |
| Mother tongue | ||
| Urdu | Ref. | |
| Punjabi | 1.4 (1.2,1.6) | <0.001 |
| Sindhi | 1.2 (1.1,1.3) | 0.002 |
| Pashto | 1.0 (0.8,1.2) | 0.835 |
| Balochi | 1.3 (1.0,1.6) | 0.035 |
| Saraiki | 1.5 (0.9,2.3) | 0.105 |
| Hindko | 0.8 (0.5,1.3) | 0.399 |
| Others | 1.2 (1.1,1.5) | 0.006 |
| Wealth Index(quintiles) | ||
| Poorest | Ref. | |
| Poor | 1.2 (1.1,1.4) | 0.003 |
| Middle | 1.2 (1.1,1.4) | 0.002 |
| Rich | 1.3 (1.1,1.5) | <0.001 |
| Richest | 1.1 (1.0,1.3) | 0.048 |
| Vitamin D Level (µmol/L) | ||
| Severe Deficiency (<8.0 ng/mL) | 1.4 (1.1,1.7) | 0.001 |
| Deficiency (8.0 - 20.0 ng/mL) | 1.2 (1.1,1.4) | <0.001 |
| Desirable (>20.0 - 30.0 ng/mL) | 1.1 (1.0,1.2) | 0.12 |
| Sufficient (>30.0 ng/mL) | Ref. |
| Survey round | tested respiratory swabs tested (PCR + antigen, n) | COVID-19 positive specimens | % of COVID-19 positive specimens | PCR tests conducted (n) | Number of PCR Positive specimens | % of PCR-positive specimens | Antigen tests conducted (n) | Number of Antigen Positive specimens | % of Antigen positive specimens | |
|---|---|---|---|---|---|---|---|---|---|---|
| Round- 1 | 606 | 10 | 1.7 | 606 | 10 | 1.7 | - | - | - | |
| Round- 2 | 1460 | 50 | 3.4 | 742 | 26 | 3.5 | 718 | 24 | 3.3 | |
| Round- 3 | 631 | 1 | 0.2 | 316 | 0 | 0.0 | 315 | 1 | 0.3 | |
| Round- 4 | 765 | 2 | 0.3 | 383 | 1 | 0.3 | 382 | 1 | 0.3 | |
| Total | 3462 | 63 | 1.8 | 2047 | 37 | 1.8 | 1415 | 26 | 1.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





