Submitted:
02 September 2024
Posted:
03 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometrics
2.3. Body Composition
2.4. Perometry
2.5. Forearm Circumference
2.6. Handgrip Strength
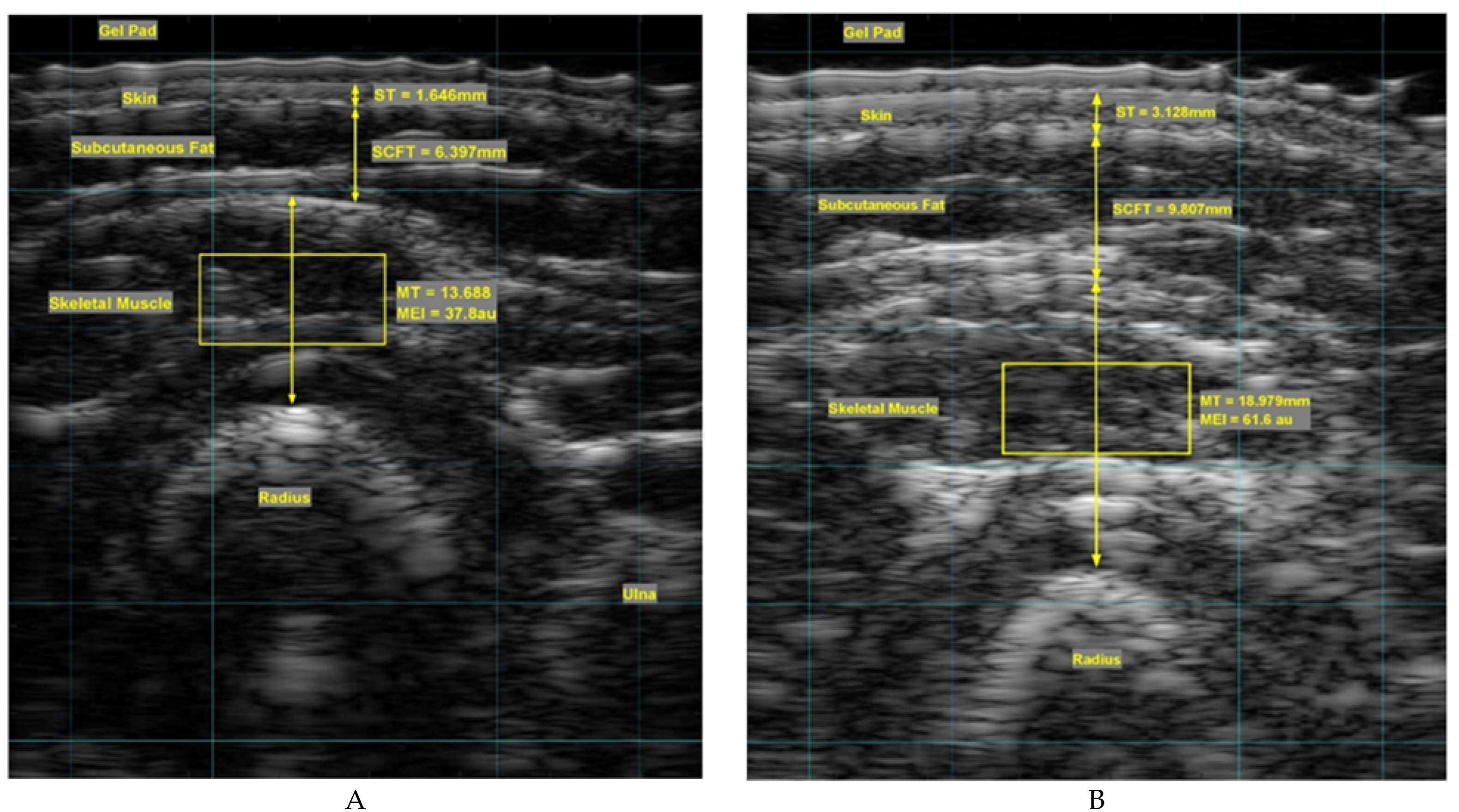
2.7. Ultrasound Data Recording
2.8. Data Conversion
2.9. Statistical Analyses
3. Results
3.1. Lymphedema and Non-Lymphedema Group Demographics and Clinical Data (Table 1):
3.2. Total Limb Volumes and Forearm Circumferences (Table 2):
3.3. Skin, Subcutaneous Fat, and Skeletal Muscle Thickness (Table 3):
3.4. Handgrip Strength (HGS), Muscle Echo-Intensity (MEI), and Muscle Quality (HGS/MT (Table 4)
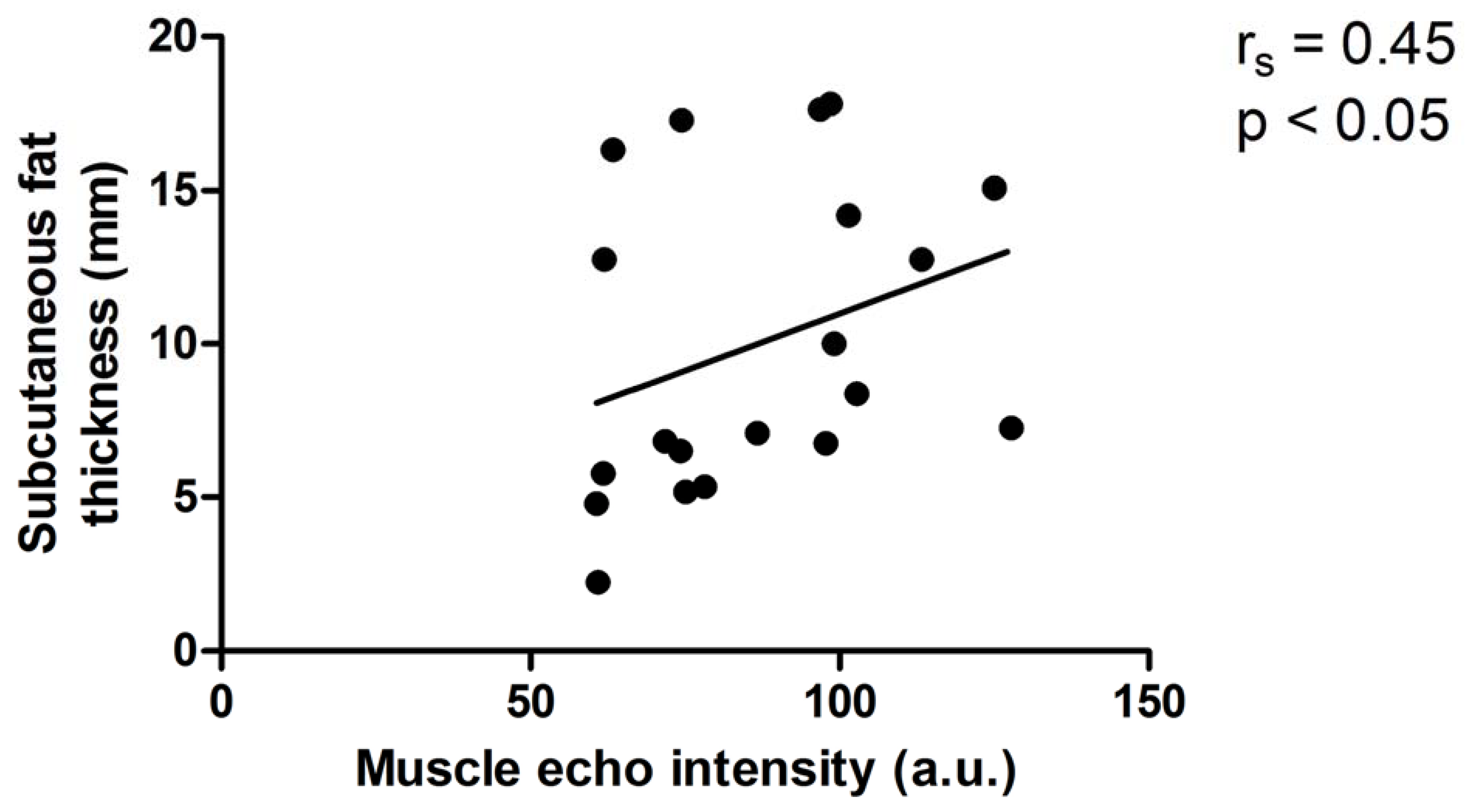
3.5. Relationships between 1) Subcutaneous Fat and Muscle Echo Intensity and 2) Forearm Circumference and Muscle Echo-Intensity in the Affected Arm (Figures 1&2)
3.6. Reliability of MT and MEI ultrasound analyses of the forearm images of the affected and unaffected arms. (Table 5)
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baran, E.; Ozcakar, L.; Ozgul, S.; Aksoy, S.; Akbayrak, T. Upper limb sensory evaluations and ultrasonic skin measurements in breast cancer-related lymphedema receiving complex decongestive physiotherapy. Supportive Care in Cancer 2021, 29, 6545–6553. [Google Scholar] [CrossRef] [PubMed]
- Mellor, R.; Bush, N.L.; Stanton, A.W.B.; Bamber, J.C.; Levick, J.R.; Mortimer, P.S. Dual-frequency ultrasound examination of skin and subcutis thickness in breast cancer-related lymphedema. Breast J 2004, 10, 496–503. [Google Scholar] [CrossRef]
- Suehiro, K.; Morikage, N.; Yamashita, O.; Harada, T.; Samura, M.; Takeuchi, Y.; Mizoguchi, T.; Nakamura, K.; Hamano, K. Skin and subcutaneous tissue ultrasonography features in breast cancer-related lymphedema. Ann. Vasc. Dis. 2016, 9, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Young, H-J. ; Jenkins, N.T.; Zhao, O.; McCully, K.K. Measurement of Intramuscular Fat by Muscle Echo Intensity. Muscle Nerve 2015, 52, 963–971. [Google Scholar] [CrossRef]
- Yusof, K.M.; Avery-Kiejda, K.A.; Ahmad Suhaimi, S.; Ahmad Zamri, N.; Rusli, M.E.F.; Mahmud, R.; Saini, S.M.; AbdulWahhab Ibraheem, S.; Abdullah, M.; Rosli, R. ; Assessment of potential risk factors and skin ultrasound presentation associated with breast cancer-related lymphedema in long-term breast cancer survivors. Diagnostics 2021, 11, 1303. [Google Scholar] [CrossRef]
- Choi, Y-H. ; Seo, K-S. Correlation among bioimpedance analysis, sonographic and circumferential measurement in assessment of breast cancer-related arm lymphedema. Lymphology 2014, 47, 123–133. [Google Scholar]
- Devoogdt, N.; Pans, S.; De Groef, A.; Geraerts, I.; Christiaens, M.R.; Neven, P.; Vergote, I.; Van Kampen, M. Postoperative evolution of thickness and echogenicity of cutis and subcutis of patients with and without breast cancer-related lymphedema. Lymphat. Res. Biol. 2014, 12, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Tassenoy, A.; Vermeiren, K.; Veen, P.; van der Stadnik, T.; Ridder, F.D.; Peters, E.; Van Schuerbeek, P.; Lamote, J.; Lievens, P. . Demonstration of tissue alterations by ultrasonography, magnetic resonance imaging and spectroscopy, and histology in breast cancer patients without lymphedema after axillary node dissection. Lymphology 2006, 39, 118–126. [Google Scholar]
- Borri, M.; Gordon, K.D.; Hughes, J.C.; Scurr, E.D. ; Koh, D-M.; Leach, M.O.; Mortimer, P.S.; Schmidt, M.A. Magnetic resonance imaging-based assessment of breast cancer-related lymphoedema tissue composition. Invest. Radiol.
- Crescenzi, R.; Donahue, P.M.C.; Garza, M.; Lee, C.A.; Patel, N.J.; Gonzalez, V.; Sky Jones, R.; Donahue, M.J. Elevated magnetic resonance imaging measures of adipose tissue deposition in women with breast cancer treatment-related lymphedema. Breast Cancer Res. Treat. 2022, 191, 115–124. [Google Scholar] [CrossRef]
- Brorson, H.; Ohlin, K.; Olsson, G.; Karlsson, M.K. Breast cancer-related chronic arm lymphedema is associated with excess adipose and muscle tissue. Lymphat. Res. Biol. 2009, 7, 3–10. [Google Scholar] [CrossRef]
- Jeon, Y.; Beom, J.; Ahn, S.; Bok, S.K. Ultrasonographic Evaluation of Breast Cancer-related Lymphedema. J. Vis. Exp. 2017, 119. [Google Scholar]
- Abe, T.; Loenneke, J.P.; Thiebaud,R. S.; Loftin, M. Morphological and functional relationships with ultrasound measured muscle thickness of the upper extremity and trunk. Ultrasound 2014, 22, 229–235. [Google Scholar] [CrossRef]
- Aruna, R. ; Sivarajan,; A.A,; Madhumitha, M.; Vasanth, C.J. Association of hand grip strength with Ultrasound-derived forearm muscle thickness and echo intensity in young Indian adults. J. Med. Ultrasound.
- Muraki, S.; Fukumoto, K.; Fukuda, O. Prediction of the muscle strength by the muscle thickness and hardness using ultrasound muscle hardness meter. Springer 2013, 2, 7. [Google Scholar] [CrossRef]
- Baklaci, M.; Eyigör, S.; Tanıgör, G.; Özgür İnbat, M.; Çalışkan Kabayel, S. Assessment of muscle strength and volume changes in patients with breast cancer-related lymphedema. Oncol. Res. Treat. 2020, 43, 584–591. [Google Scholar] [CrossRef] [PubMed]
- De Groef, A.; Van Kampen, M.; Tieto, E.; Schonweger, P.; Christiaens, M.-R.; Neven, P.; Geraerts, I.; Gebruers, N.; Devoogdt, N. Arm lymphedema and upper limb impairments in sentinel node-negative breast cancer patients: A one year follow-up study. Breast 2016, 29, 102–108. [Google Scholar] [CrossRef]
- Rietman, J.S.; Geertzen, J.H.; Hoekstra, H.J.; Baas, P.; Dolsma, W.V.; de Vries, J.; Groothoff, J.W.; Eisma, W.H.; Dijkstra, PU.; et al. Long term treatment related upper limb morbidity and quality of life after sentinel lymph node biopsy or stage I or II breast cancer. Eur. J. Surg. Oncol. 2006, 32, 148–52. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.R.L.; Freitas, I.F.Jr.; da Silva, C.B.; Gomes, I.C.; Rocha, A.P.R.; Salgado, A.S.I.; do Carmo, E.M. Short-term changes in handgrip strength, body composition, and lymphedema induced by breast cancer surgery. Rev. Bras. Ginecol. Obstet. 2014, 36, 36,244–50. [Google Scholar] [CrossRef] [PubMed]
- Giray, E.; Akyüz, G. Assessment of Family Caregiver Burden and Its Relationships Between Quality of Life, Arm Disability, Grip Strength, and Lymphedema Symptoms in Women with Postmastectomy Lymphedema: A Prospective Cross-Sectional Study. Eur. J. Breast Health 2019, 15, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Merchant, C.R.; Chapman, T.; Kilbreath, S.L.; Refshauge, K.M.; Krupa, K. Decreased muscle strength following management of breast cancer. Disability and Rehabilitation 2008, 30, 1098–1105. [Google Scholar] [CrossRef]
- Lee, D.; Hwang, J.H.; Chu, I.; Chang, H.J.; Shim, Y.H.; Kim, J.H. Analysis of factors related to arm weakness in patients with breast cancer-related lymphedema. Support Care Cancer 2015, 23, 2297–2304. [Google Scholar] [CrossRef]
- Pillen, S.; Arts, I.M.P.; Zwarts, M.J. Muscle ultrasound in neuromuscular disorders. Muscle Nerve 2008, 37, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Pillen, S.; Tak, R.O.; Zwarts, M.J.; Lammens, M.M.; Verrijp, K.N.; Arts, I.M.; van der Laak, J.; Hoogerbrugge, P.M.; van Engelen, B.G.; Verrips, A. Skeletal muscle ultrasound: Correlation between fibrous tissue and echo intensity. Ultrasound Med. Biol. 2009, 35, 443–446. [Google Scholar] [CrossRef]
- Stock, M.J.; Thompson, B.J. Echo intensity as an indicator of skeletal muscle quality: applications, methodology, and future directions. Eur. J. Appl. Physiol. 2021, 121, 121,369–380. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Fujita, E.; Thiebaud, R.S.; Loenneke, J.P.; Akamine, T. Ultrasound-Derived Forearm Muscle Thickness Is a Powerful Predictor for Estimating DXA-Derived Appendicular Lean Mass in Japanese Older Adults. Ultrasound Med. Biol. 2016, 42, 2341–2344. [Google Scholar] [CrossRef]
- Abe, T.; Thiebaud, R.S.; Loenneke, J.P. Age-related change in handgrip strength in men and women: is muscle quality a contributing factor? Age (Dordr) 2016, 38, 28. [Google Scholar] [CrossRef] [PubMed]
- Wong, S. Grip strength reference values for Canadians aged 6 to 79: Canadian Health Measures Survey, 2007 to 2013. Statistics Canada - Health Reports 2016, 27, 8. [Google Scholar]
- McSharry, V.; Glennon, K.; Mullee, A.; Brennan, D. The impact of body composition on treatment in ovarian cancer: a current insight. Expert Rev. Clin. Pharmacol. 2021, 14, 14,1065–1074. [Google Scholar] [CrossRef]
- Sanada, K.; Kearns, C.F.; Midorikawa, T.; Abe, T. Prediction and Validation of Total and Regional Skeletal Muscle Mass by Ultrasound in Japanese Adults. Eur. J. Appl. Physiol. 2006, 96, 24–31. [Google Scholar] [CrossRef]
- Reeves, N.D.; Maganaris, C.N.; Narici, M.V. Ultrasonographic Assessment of Human Skeletal Muscle Size. Eur. J. Appl. Physiol. 2004, 91, 116–118. [Google Scholar] [CrossRef]
- Hashemi, H.S.; Fallone, S.; Boily, M.; Towers, A.; Kilgour, R.D.; Rivaz, H. Assessment of Mechanical Properties of Tissue in Breast Cancer-Related Lymphedema Using Ultrasound Elastography. IEEE, TUFFC, 2019, 66, 541–550. [Google Scholar] [CrossRef]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A Review of the Measurement of Grip Strength in Clinical and Epidemiological Studies: Towards a Standardised Approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Colley, R.C.; Doyon, C.; Wong, S.L.; Tomkinson, G.R.; Lang, J.J. Normative-referenced percentile values for physical fitness among Canadians. Statistics Canada Health Reports, 2019, 30, 14–22. [Google Scholar]
- Bohannon, R.W. ; Wang, Y-C.; Yen, S-C., Ed.; Grogan, K.A. Handgrip Strength: A Comparison of Values Obtained From the NHANES and NIH Toolbox Studies. American Journal of Occupational Therapy, 2019. [Google Scholar]
- Peters, M.J.H.; van Nes, S.I.; Vanhoutte, E.K.; Bakkers, M.; van Doorn, P.A.; Merkies, I.S.J.; Faber, C.G. ; on behalf of the PeriNomS Study group. Journal of the Peripheral Nervous System 2011, 16, 16,47–50. [Google Scholar]
- Jorgensen, M.G.; Toyserkani, N.M.; Hansen, F.G.; Bygum, A.; Sorensen, J.A. Impact of lymphedema on health-related quality of life up to 10 years after breast cancer treatment. NPJ Breast Cancer 2021, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Heins, M.J.; de Ligt, K.M.; Verloop, J.; Siesling, S.; Korevaar, J.C. on behalf of the PSCCR group. Adverse health effects after breast cancer up to 14 years after diagnosis. Breast 2022, 61, 22e28. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.; Siqueira, A.F.; Ferreira-Junior, J.B.; Pereira, P.; Wagner, D.; Bottaro, M. Ultrasound imaging in women’s arm flexor muscles: intra-rater reliability of muscle thickness and echo intensity. Braz. J. Phys. Ther. 2016, 20, 20,535–542. [Google Scholar] [CrossRef]
- Bystrim, S.; Kilbom, A. Physiological response in the forearm during and after isometric intermittent handgrip. Eur. J. Appl. Physiol. 1990, 60, 457–466. [Google Scholar] [CrossRef]
- Hagg, G.; Milerad, E. Forearm extensor and flexor muscle exertion during simulated gripping work-An electromyographic study. Clinical Biomechanics 1997, 12, 39–43. [Google Scholar] [CrossRef]
- Hoozemans, M.J.M.; van Dieën, J.H. Prediction of handgrip forces using surfacEMG of forearm muscles. J. Electromyogr. Kinesiol. 2005, 15, 358–366. [Google Scholar] [CrossRef]
- Dahlqvist, C.; Nordander, C.; Granqvist, L.; Forsman, M.; Hanson, G.-A. Comparing two methods to record maximal voluntary contractions and different electrode positions in recordings of forearm extensor muscle activity: Refining risk assessments for work-related wrist disorders. Work 2018, 59, 231–242. [Google Scholar] [CrossRef]
- Mander, A.; Venosi, S.; Menegatti, E.; Byung-Boong, L.; Neuhardt, D.; Maietti, E.; Gianesini, S. Upper limb secondary lymphedema ultrasound mapping and characterization. Int. Angiol. 2019, 38, 334–42. [Google Scholar] [CrossRef] [PubMed]
| Variable | Lymphedema (n=20) | Non-lymphedema (n=20) | P-Value |
|---|---|---|---|
| Age (yrs) | 56 ± 14.8 | 48 ± 20.8 | 0.184 |
| Height (m) | 1.62 ± 0.07 | 1.59 ± 0.06 | 0.198 |
| Weight (kg) | 84.6 ± 18.4 | 73.9 ± 11.1 | 0.030* |
| BMI (kg/m2) | 32.1 ± 7.2 | 29.0 ± 4.2 | 0.101 |
| Body Fat (%) | 47.5 ± 6.9 | 42.4 ± 6.4 | 0.023* |
| Total Fat (kg) | 39.1 ± 13.1 | 30.5 ± 8.1 | 0.021* |
| Total Lean (kg) | 41.6 ± 5.8 | 40.1 ± 4.5 | 0.363 |
| BMD (g/cm2) | -0.673 ± 1.077 | -0.110 ± 1.411 | 0.200 |
| BCRL duration (yrs) | 10.51 ± 6.21 | n/a |
| Variables | Medians (IQR) | P-values | ||||
|---|---|---|---|---|---|---|
| AA | UA | CA | AA vs UA | AA vs CA | UA vs CA | |
| Forearm circumference (cm) | 25.2 (22.3-27.3) |
21.6 (20.2-24.9) |
21.4 (20.3-22.4) |
0.022 | 0.002 | 0.579 |
| Total limb volume (ml) | 3297 (3052-3839) |
2809 (2652-3297) |
2993 (2345-3651) |
0.05 | 0.05 | 0.745 |
| Tissue Thickness (mm) | Medians (IQR) | P-values | ||||
|---|---|---|---|---|---|---|
| AA | UA | CA | AA vs UA | AA vs CA | UA vs CA | |
| Skin | 2.37 (1.73-3.07) |
1.66 (1.58-1.85) |
1.73 (1.61-1.90) |
0.001 | 0.004 | 0.597 |
| Subcutaneous fat | 7.81 (6.00-14.85) |
5.55 (3.98-8.32) |
5.12 (3.55-6.42) |
0.001 | 0.001 | 0.364 |
| Skeletal muscle | 11.54 (10.36-12.74) |
11.76 (10.27-13.67) |
11.07 (9.57-13.19) |
0.401 | 0.410 | 0.490 |
| Variables | Median (IQR) | P-values | ||||
|---|---|---|---|---|---|---|
| AA | UA | CA | AA vs UA | AA vs CA | UA vs CA | |
| HGS (kg) | 23.0 (15.9-28.4) |
23.3 (19.5-28.0) |
27.9 (23.5-31.9) |
0.524 | 0.048 | 0.061 |
| MEI (a.u.) | 82.5 (65.5-100.9) |
70.4 (58.0-81.1) |
64.2 (57.1-75.6) |
0.013 | 0.001 | 0.394 |
| HGS/MT (kg/mm) | 1.77 (1.39-2.46) |
1.98 (1.75-2.48) |
2.38 (1.86-3.06) |
0.588 | 0.025 | 0.132 |
| Ultrasound parameter | M1 | SD | M2 | SD | R | P-value | 95% CI |
|---|---|---|---|---|---|---|---|
| MT AA | 10.20 | 2.58 | 9.96 | 3.09 | 0.990 | 0.810 | 0.824-0.998 |
| MT UA | 10.85 | 3.32 | 11.24 | 3.49 | 0.993 | 0.109 | 0.881-0.999 |
| MT CA (right) | 10.34 | 1.61 | 10.28 | 1.85 | 0.992 | 0.671 | 0.859-0.999 |
| MT CA (left) | 11.42 | 2.10 | 11.39 | 2.03 | 0.995 | 0.764 | 0.852-0.998 |
| MEI AA | 69.90 | 3.92 | 73.82 | 8.55 | 0.839 | 0.197 | -0.125-0.997 |
| MEI UA | 59.73 | 13.32 | 60.17 | 13.27 | 0.996 | 0.414 | 0.938-0.991 |
| MEI CA (right) | 56.97 | 8.83 | 58.41 | 9.07 | 0.987 | 0.085 | 0.789-0.998 |
| MEI CA (left) | 54.16 | 10.55 | 54.24 | 10.57 | 0.991 | 0.901 | 0.906-0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





