1. Introduction
An immune response is a compulsory consequence of the activity of the human surveillance system upon the implantation of any biomaterial. Besides the implantation procedure, tissue damage is always associated with any surgical technique involving bone implant placement, affecting the local host environment and eliciting foreign body reactions [
1,
2]. This reaction is a reflex response from innate immune cells, identifying implantable biomaterials as foreign objects due to their physicochemical mismatch with native host tissues. Upon implantation, local injury to vascularized connective tissues results in platelet activation, damage-associated molecular patterns (DAMPs), and the secretion of different arrays of cytokines[
3,
4]. These chemoattractants are potent signals recognized by innate immune cells such as neutrophils and mastocytes, which migrate to the implant site [
5].
Neutrophils are one of the first members of the innate system to arrive at the implantation site of biomaterials [
6,
7]. This group of cells is the first lineage of circulating immune cells to respond to tissue injuries or against infections [
8]. They are generally associated with the acute inflammatory response, and depending on the extent of the injury on-site, the initial acute inflammation is turned into a chronic stage that can remain for weeks, depending on the arrival of mononuclear cells such as monocytes and lymphocytes [
3]. Monocytes usually arrive at the implantation site and differentiate into macrophages, which has been exhaustively investigated in the literature [
3,
6,
9]. However, macrophages are a ‘second line’ of defence of the body. Surprisingly, the influence of the neutrophils in the immune response and their ability to potentially mitigate early stages of inflammation and wound healing at implant sites remains to be fully delineated.
Neutrophils when activated rapidly achieve a tenfold increase in circulating numbers. Their mechanism of action can subsequently involve the internalization of pathogens or cell debris, recruiting other immune cells, releasing granular enzymes, reactive oxygen species or nuclear extracellular traps (NETs) [
5,
10]. It is postulated that neutrophils follow phagocytosis or NET formation based on distinguishing the size of pathogens, which are small microbes or particles internalized. In contrast, the NETosis pathway neutralises large ones [
5,
9,
11]. Thus, we hypothesized that this ability of neutrophils to sense the environment may also be responsive to differences across the topography of an implant surface. This would provide a further means to regulate the immune response by modulating neutrophil enzyme secretion in the very early stages of the healing period following implantation procedures. This experiment aims to validate if neutrophils are sensitive to differences in the degree of surface roughness of titanium and zirconia substrates and if this stimulus can affect neutrophil degranulation and NETosis.
2. Materials and Methods
Titanium grade 5 (Ti6Al4V) and Zirconium oxide (ZrO) discs (98.5mm in diameter and 14-20mm in height) for the Colado CAD milling system (Ivoclar Vivadent, Schaan, Lichtenstein) were used as sources to design sample discs with cylindrical shape (10mm in diameter and 1.5mm in height). Our samples were designed using an open-source computer design software (Meshmixer, Autocad) and milled using a 5-axis milling machine (Programat 7 Ivoclar Vivadent (Schaan Lichtenstein). Zirconium oxide discs were sintered at 1600
o C for 2 hours. Different grades of roughness were prepared by sequential polishing routes, including diamond-impregnated burs and polishing with diamond paste. Three levels of roughness were created for each Ti and Zr oxide sample: rough (R) with Ra= > 3µm, smooth (S) with Ra= ≥1 to 1.5µm and very smooth (VS) with Ra= <0.1µm[
12].The morphology of the surfaces was analyzed using a scanning electron microscope JEOL IT-300 (Tokyo, Japan) at an accelerating voltage of 15 kV with a stage at perpendicular orientation. Samples were submitted to gold sputtering. A profile from each surface topography was registered using a 3D optical profilometer (Zeta 300 3D, KLA, USA). Different roughness descriptors were measured using an x10 lens under a scanning area of 100 x 100 um and a Z range of 50 um. Neutrophils were isolated from five 12-week-old male Wistar rats (Animal Resource Centre, WA, Australia) in accordance with ethics committee approval protocol by the Griffith University Ethics Committee (DOH/02/20/AEC) and also with ARRIVE guidelines 2.0. The institutional and national guidelines have been followed for the care and use of the animals in the study. Rats were euthanized by isoflurane followed by cervical dislocation. 5ml of blood was collected by cardiac puncture, neutrophils were isolated by density centrifugation, and a purity of 90% was obtained for each experiment. Neutrophils were seeded on sterile Ti and Zr oxide surfaces at a density of 2 x 10
5 cells per 25 mm
2. Conditioned culture media was harvested after 1h, 2h, and 4h to measure the amount of neutrophil elastase (NE) and matrix metallopeptidases (MMP-8 and 9) by Enzyme-Linked Immunosorbent Assay – ELISA (R&D invitro Technologies, Australia).
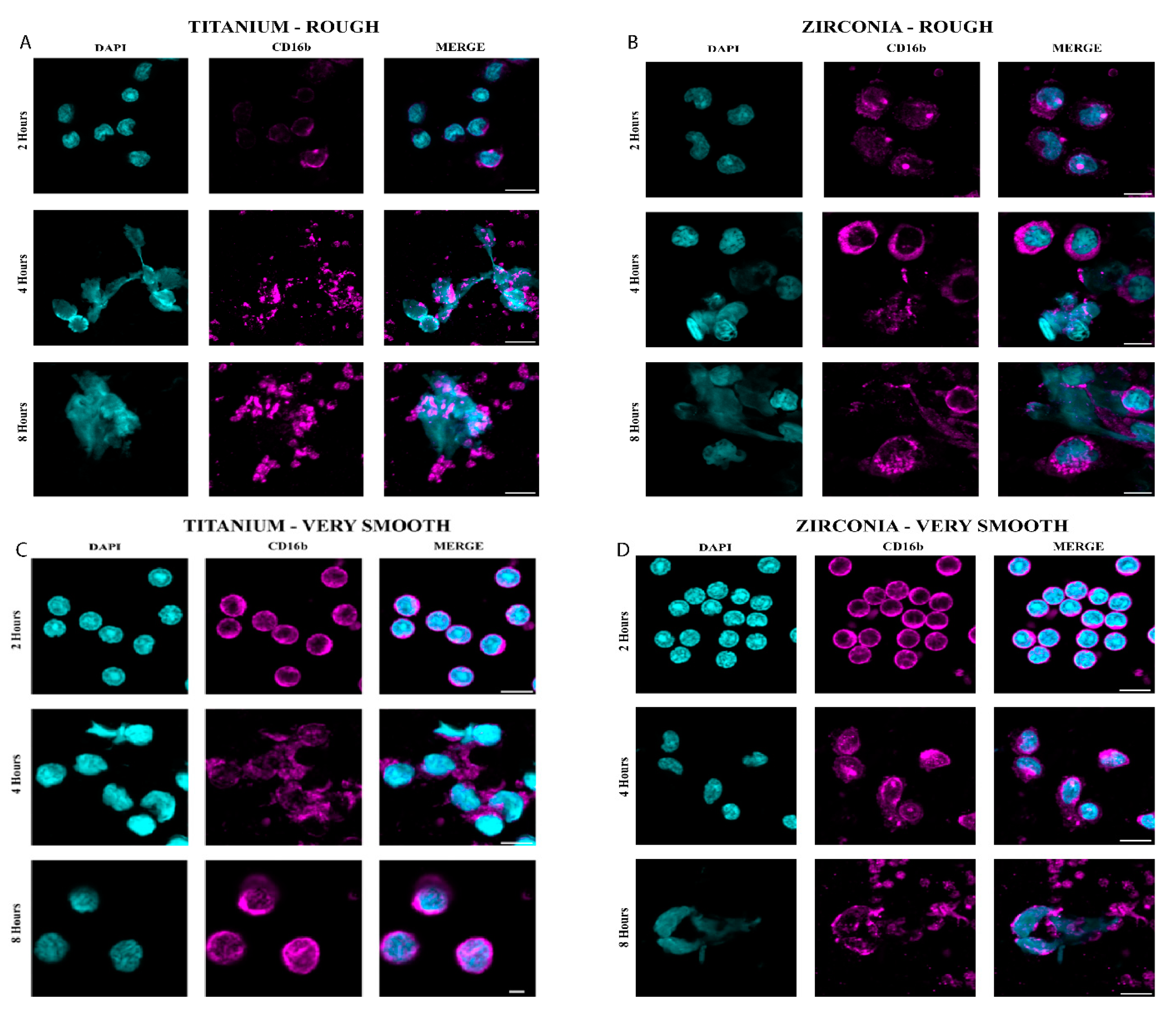
Morphologic analysis of neutrophil by NETosis was taken following 2h, 4h and 8h of culture on the surface of rough and very smooth Ti and Zr oxide disks at a density of 105 cells per 25 mm2. Adequate representation of the central and peripheral zones of the biomaterial surfaces was taken using a Nikon Ti2 widefield microscope (Nikon, NY, USA) at 20X magnification. Quantification of NET zones was performed by measurements of the extracellular stained DNA zones using an Olympus FV3000 confocal laser scanning microscope (Olympus, Tokyo, Japan) and cell profiler software (Broad Institute-MIT, Cambridge, MA, USA), where extracellular DNA was stained blue with 4′,6-diamidino-2-phenylindole (DAPI, ThermoFisher Scientific, MA, USA) and neutrophils surface pink with CD16b (Thermofisher Scientific, USA). The area covered by the extracellular DNA (blue) surrounded by CD16b (pink) was traced, and the total area per stack was measured in μm2. The software also generated the neutrophil DNA’s circularity (value=1 represents a perfectly circular object) on each sample.
Statistical analysis was conducted using IBM SPSS 20 software (IBM, NY, USA), and data was compiled as mean ± SD. Single-factor ANOVA was used to attest the difference between groups at α = 0.05. Multiple comparisons were performed using Turkey’s HSD test.
3. Results
The biomaterial surface arithmetical mean height descriptor ‘Ra’ revealed higher values for titanium samples than for Zr oxide sources. Ti-R (3.5 ± 0.06 µm) and Zr oxide-R (3.2 ± 0.07 µm) surfaces revealed higher values than Ti-S (1.5 ± 0.04 µm) and Zr oxide-S (1.1 ± 006 µm) surfaces, which were higher than Ti-VS (0.05 ± 0.002 µm) and Zr oxide-VS (0.02 ± 0.005 µm) sources. Rq values were also higher with rougher substrates and generally higher with titanium than the Zr oxide substrates. Conversely, Rz values were lower in the Titanium substrates. However, rough surfaces demonstrated higher values than smooth and very smooth surfaces. All three roughness descriptors are disclosed in
Table 1.
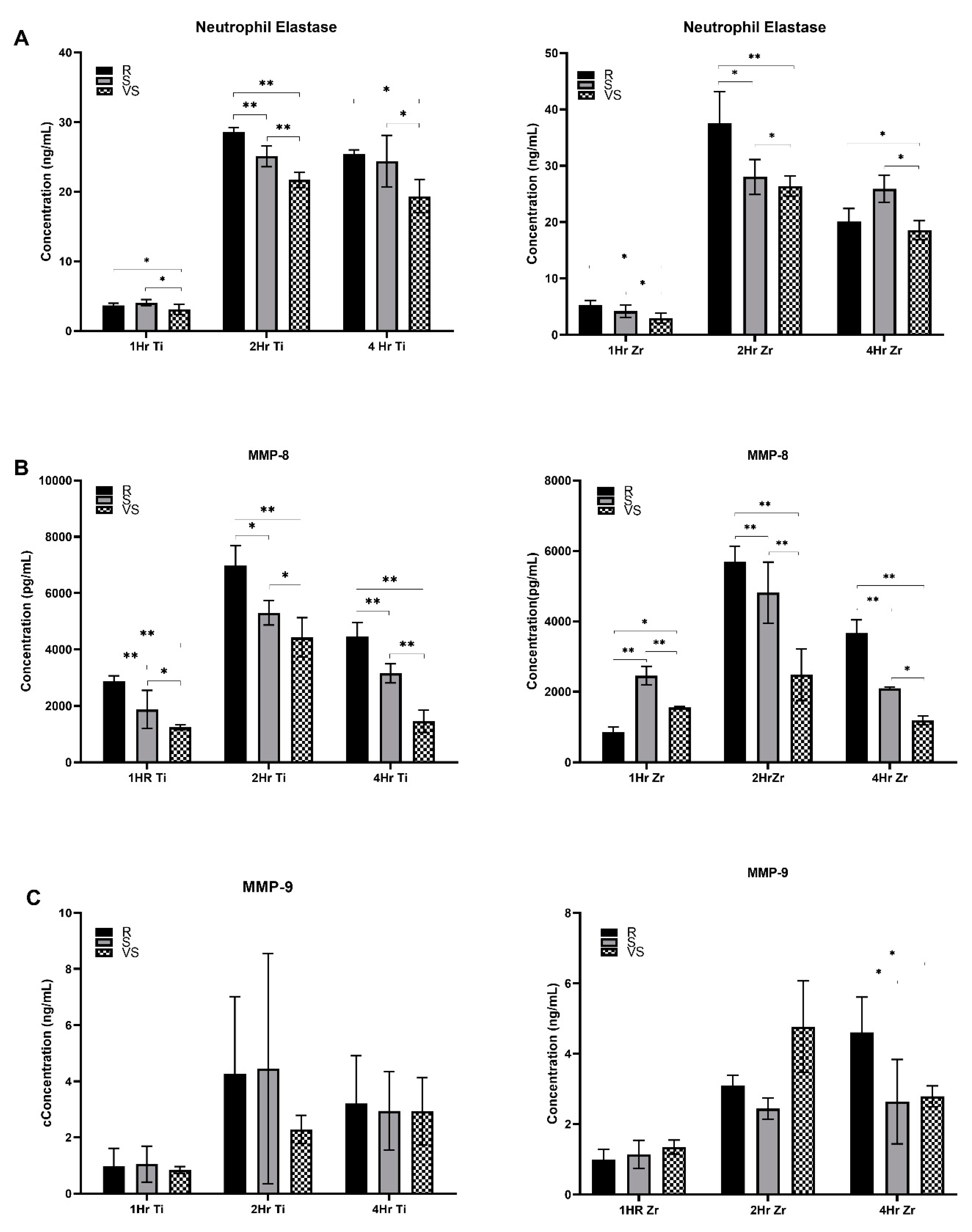
The concentration of neutrophil elastase in the culture supernatant was very low (
Figure 1) after 1 hour of neutrophil exposure to the Titanium and Zr oxide surfaces for all three roughness levels under analysis. After 2 hours, secretion of elastase was higher when neutrophils were exposed to rough Titanium and Zr oxide surfaces in comparison with smooth (Ti-S: p<0.001 and ZrO-S: p≤0.001) and very smooth surfaces (p≤0.001). At 4 hours, Ti (p≤0.007) and Zr oxide (p=0.056) rough surfaces again induced higher levels of secretion than very smooth surfaces. However, no significant differences were detected when compared with Ti and Zr oxide smooth surfaces (p=0.05). Zr oxide rough surfaces induced higher levels of elastase secretion after 1 hour (p=0.011) and 2 hours (p=0.024). However, after 4 hours, Titanium-based surfaces induced higher levels of elastase (p=0.035).
More MMP-8 was released by neutrophils exposed for 1 hour to Ti-R surfaces than Ti-S (p≤0.001) and Ti-VS (p≤0.001). The same trend was observed for 2 and 4 hours, as seen in
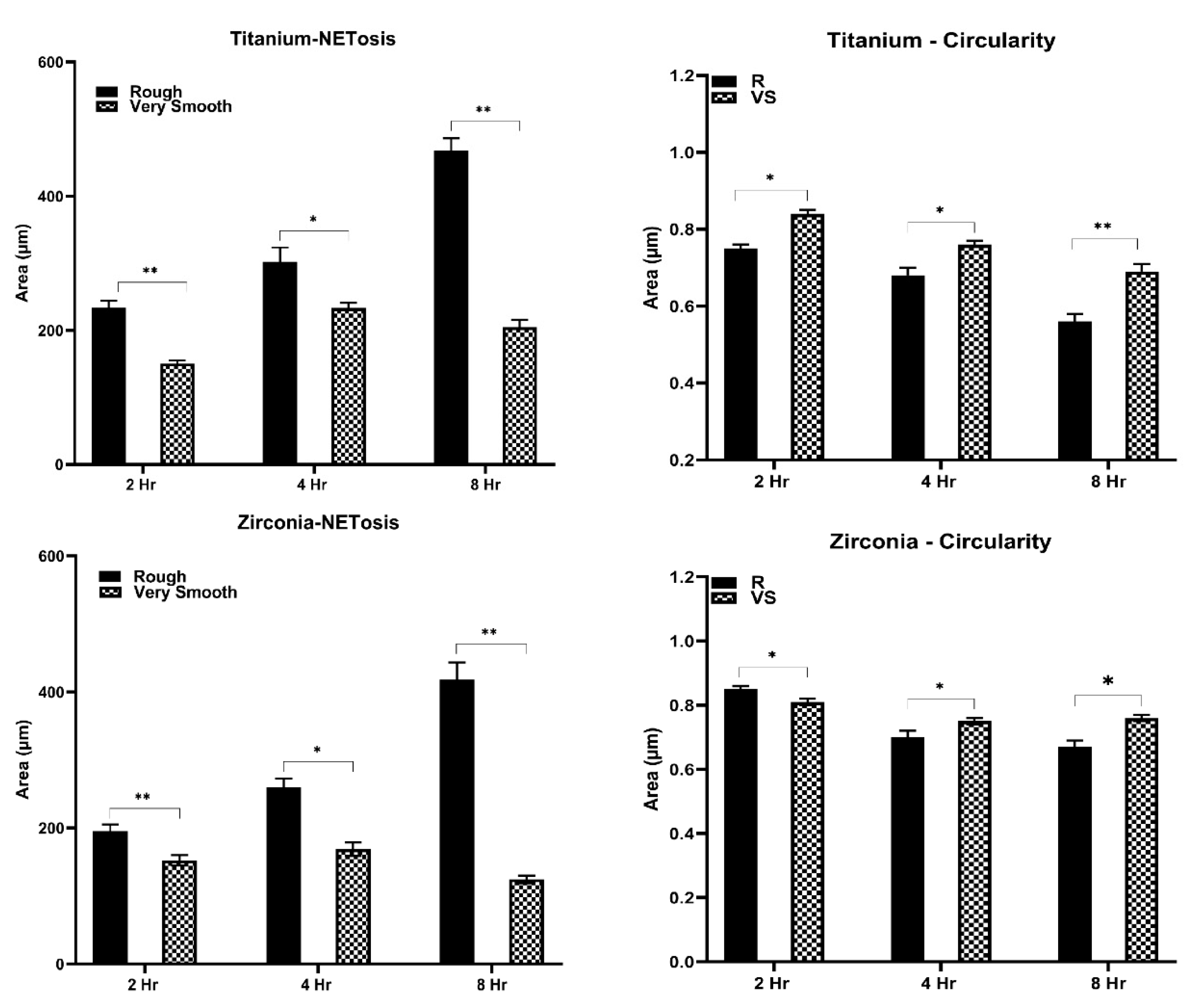
Table 2. Neutrophils exposed to Zr oxide surface after 1 hour released more MMP-8 on the smooth surface in comparison with rough (p≤0.001) and very smooth (p=0.005). After 2 and 4 hours, higher levels of MMP-8 were released in Zr oxide-R surfaces than with S (p≤0.001) and VS (p≤0.001) surfaces. Generally, Titanium surfaces induced higher levels of MMP-8 than Zr oxide counterparts after 1 hour (p≤0.001 ZrO-R and p=0.017 ZrO-S), 2 hours (p=0.011 ZrO-R, p=0.004 ZrO-S and p=0.005 ZrO-VS) and 4 hours (p=0.004 ZrO-R and p≤0.001 ZrO-S). MMP-9 levels were higher following culture on Zr oxide-R surfaces than on S (p=0.01) and VS (p=0.017) surfaces after 4 hours. No significant difference between Ti and Zr oxide substrates was observed in neutrophil MMP-9 secretion levels (p>0.05).Neutrophils exposed for 2 hours to Ti-R and Zr oxide-R surfaces revealed swollen and elongated nuclei with larger DAPI-stained areas than observed in Ti-VS (p≤0.001) and Zr oxide-VS (p≤0.001) surfaces. No signs of NETosis were seen on VS surfaces, where neutrophil nuclei were compact and round. Ti-R (p<0.003) and Zr oxide-R (p<0.018) surfaces induced larger areas of NETs than VS surfaces after 4 hours (
Figure 2). Enlarged nuclei oozing out of the neutrophils were observed with R surfaces, whilst elongated neutrophils were observed when in contact with VS surfaces (
Figure 3). Significant NET zones and vast web-like structures were seen in Ti-R (p≤0.001) and Zr oxide-R (p≤0.001) surfaces after 8 hours (
Figure 2 and 3). VS surfaces revealed smaller zones of NETs. Ti-R (p<0.038) and Ti-VS (p≤0.001) induced larger areas of NETs than Zr oxide counterparts. Ti-VS surfaces presented larger zones of NETs than Zr oxide-VS (p≤0.001).
4. Discussion
The mechanobiological modulation of immune cells has been explored in macrophages, the most representative cells from the innate system, due to its significant ability to orchestrate the inflammatory response and healing process. However, neutrophils are the first cells en masse recruited to arrive at implantation sites, and their ability to be physically modulated is has not been fully delineated. In this study, we used a range of surface roughness in two common implant biomaterials to analyze its influence upon the enzyme secretory ability of neutrophils. We quantified one representative enzyme from each of the three main granule-classes released by neutrophils during degranulation events. Elastase secretion suggests a direct response from neutrophils when faced with rougher surfaces of Titanium or Zirconium oxide substrates. NE values indicate that higher amounts of the enzyme were released after 2 and 4 hours of exposure to the Ti-R and after 2 hours of the ZrO-R surfaces. Hence, neutrophils were less responsive regarding elastase secretion when interacting with smooth and very smooth topographies regardless of the surface substrate. An exemption was observed after 4 hours of exposure of neutrophils to the ZrO-S surface, where its secretion of elastase was higher than observed on ZrO-R sources. A possible explanation for this finding could be the significant variability of the peaks and valleys pointed out by the Rq and Rz vertical descriptors across the roughness profile of ZrO surfaces, as seen in
Table 1. Larger peaks and valleys are expected along ZrO-R and ZrO-S compared to Titanium counterparts. Although the Rz descriptor provides an insight regarding the difference between the highest peak and deepest valley from the whole profile, Rz values from ZrO-R and ZrO-S profiles confirm a higher variability and the presence of higher peaks and more profound valleys in ZrO surfaces in comparison with Ti ones. Although the response of neutrophils against roughness is not well documented in ZrO-based surfaces, their response against Titanium substrates has already been presented by different authors[
4,
6,
7,
13,
14,
15]. Some studies revealed higher recruitment and secretion of elastase and MMP when neutrophils were exposed to smooth surfaces (Sa between 0.5 and 1.5 um), which is in agreement with our results until the first hour of incubation regarding elastase and MMP-8 and MMP-9 [
4,
13]. Over time, neutrophils became more responsive in a time-dependent manner to roughness in terms of elastase and metalloproteinase secretion, and higher levels of elastase and MMP-8 were secreted at 4 hours of incubation and MMP-9 after 1 and 2 hours of incubation.
Regarding ZrO substrates, neutrophils exposed to ZrO-R surfaces secreted higher levels of elastase for an hour and at 2 hours of incubation, higher amounts of MMP-8 after 2 hours and at 4 hours and released more MMP-9 at 4 hours of incubation. As the response of neutrophils exposed to ZrO surfaces has never been presented before, we established a comparison with Titanium surfaces at similar levels of roughness[
4,
13]. Hence, neutrophils showed reactiveness and higher levels of elastase were secreted on Zr-S surfaces than on Zr-R surfaces at 4 hours. Although this behaviour contrasts with our general findings, other studies have already stated that neutrophil expression was modulated by Titanium sources with similar patterns of roughness [
4,
13]. Regardless, the reason for such discrepancy is still unknown. It could result from the hydrophobic nature of our surfaces, as they were manufactured by milling process instead of acid etching or nitrogen-controlled atmosphere storage protocols[
4,
13]. Lower levels of elastase were also identified after one hour on both Ti and ZrO surfaces at the different roughness levels under evaluation. It is coherent with the complexity of the NETosis pathway, which usually demands around 4 hours from the decondensation of chromatin until the release of large extracellular web-like structures[
9].
Neutrophils usually are activated towards phagocytosis or NET’s release pathways. Their decision directly influences the resolution of chronic inflammation and the prevention of any unnecessary immune response that could lead to aberrant NETosis [
9]. Our results demonstrated an evolution of the neutrophils morphology and activation of the NETosis pathway when exposed to different roughness patterns over time. Although at 2 hours, no signs of NETosis were expected, elongated and swollen nuclei were observed in Ti-R and ZrO-R surfaces when compared to Ti-VS and ZrO-VS substrates where neutrophils were still shown rounded and compacted morphology. As time increased to 4 hours, neutrophils exposed to Ti-R and ZrO-R surfaces revealed enlarged nuclei oozing out of cells and larger areas usually associated with NET’s formation, whilst cells in touch with Ti-VS and ZrO-VS revealed a transition to elongated morphology. At the end of 8 hours, neutrophils in contact with Ti-R and ZrO-R substrates showed mature NETs structures extruded as fibrillary networks, while even Ti-VS and ZrO-VS presented traces of very tiny zones of NETs formation.
Herein, rough Ti and Zr surfaces stimulated higher NETs expression. Similar to our findings, [
14] found that sandblasted large-grit acid-etched (SLA) triggered histone citrullination and NET release. In contrast to our findings, [
13] showed a small area of NETs formation on rough Ti surfaces compared to smooth ones. The reason for such discrepancy is unknown. We believe that the strong activation of the azurophilic and specific granules is related to a high ROS production and, consequently, might have induced a higher NET formation.
5. Conclusions
Our findings suggested that rough Ti and ZrO surfaces triggered an increased secretion of NE, MMP-8, and NETs formation from neutrophils compared to smooth surfaces. Also, rough Ti surfaces seem to induce a higher neutrophil elastase expression and enhance NETs formation than ZrO surfaces.
Author Contributions
Conceptualization: Carlos Marcelo S. Figueredo. Methodology: Gayathiri Elangovan, A. Cameron, Joao M. Mello-Neto, Souptik Basu and Carlos Marcelo S. Figueredo. Validation: Gayathiri Elangovan and Andrew Cameron, and Formal analysis: Gayathiri Elangovan and Andrew Cameron. Investigation: Gayathiri Elangovan, Souptik Basu and Andrew Cameron. Resources: Gayathiri Elangovan and Joao M. Mello-Neto. Data curation: Gayathiri Elangovan and Anna Peishan Jiang1. Writing—original draft preparation: Daniel J Fernandes, Gayathiri Elangovan and Anna Peishan Jiang1. Writing, review, and editing: Carlos Marcelo S. Figueredo, Joao M. Mello-Neto, Peter Reher, and Stephen Hamlet. Visualization: Carlos Marcelo S. Figueredo, Gayathiri Elangovan, Andrew Cameron, Joao M. Mello-Neto, Peter Reher, Anna Peishan Jiang1, Stephen Hamlet. Supervision: Carlos Marcelo S. Figueredo. Project administration: Carlos Marcelo S. Figueredo.
Funding
Not applicable. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
no conflict of interest.
References
- Welch, N.G., D.A. Winkler, and H. Thissen, Antifibrotic strategies for medical devices. Adv Drug Deliv Rev, 2020. 167: p. 109-120. [CrossRef]
- Chandorkar, Y., R. K, and B. Basu, The Foreign Body Response Demystified. ACS Biomater Sci Eng, 2019. 5(1): p. 19-44. [CrossRef]
- Jhunjhunwala, S., Neutrophils at the Biological-Material Interface. ACS Biomater Sci Eng, 2018. 4(4): p. 1128-1136. [CrossRef]
- Morandini, L., et al., Reduction of neutrophil extracellular traps accelerates inflammatory resolution and increases bone formation on titanium implants. Acta Biomater, 2023. 166: p. 670-684. [CrossRef]
- Selders, G.S., et al., An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen Biomater, 2017. 4(1): p. 55-68. [CrossRef]
- Abaricia, J.O., A.H. Shah, and R. Olivares-Navarrete, Substrate stiffness induces neutrophil extracellular trap (NET) formation through focal adhesion kinase activation. Biomaterials, 2021. 271: p. 120715. [CrossRef]
- El Kholy, K., et al., Investigating the Response of Human Neutrophils to Hydrophilic and Hydrophobic Micro-Rough Titanium Surfaces. Materials (Basel), 2020. 13(15). [CrossRef]
- Bouvain, P., et al., Non-invasive mapping of systemic neutrophil dynamics upon cardiovascular injury. Nature Cardiovascular Research, 2023. 2(2): p. 126-143. [CrossRef]
- Branzk, N., et al., Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol, 2014. 15(11): p. 1017-25. [CrossRef]
- Cruz, M.A., et al., Nanomedicine platform for targeting activated neutrophils and neutrophil-platelet complexes using an alpha(1)-antitrypsin-derived peptide motif. Nat Nanotechnol, 2022. 17(9): p. 1004-1014. [CrossRef]
- Veiseh, O., et al., size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat Mater, 2015. 14(6): p. 643-51. [CrossRef]
- Elangovan, G., et al., A Novel Apparatus to Standardize the Polishing Protocol to Achieve Different Roughness of Titanium and Zirconia Disc Surfaces. Int J Prosthodont, 2023: p. 1-16. [CrossRef]
- Abaricia, J.O., et al., Hydrophilic titanium surfaces reduce neutrophil inflammatory response and NETosis. Biomater Sci, 2020. 8(8): p. 2289-2299. [CrossRef]
- Vitkov, L., et al., The initial inflammatory response to bioactive implants is characterized by NETosis. PLoS One, 2015. 10(3): p. e0121359. [CrossRef]
- Campos, V., et al., Characterization of neutrophil adhesion to different titanium surfaces. Bull Mater Sci, 2014. 37(1): p. 9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).