Submitted:
02 September 2024
Posted:
03 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
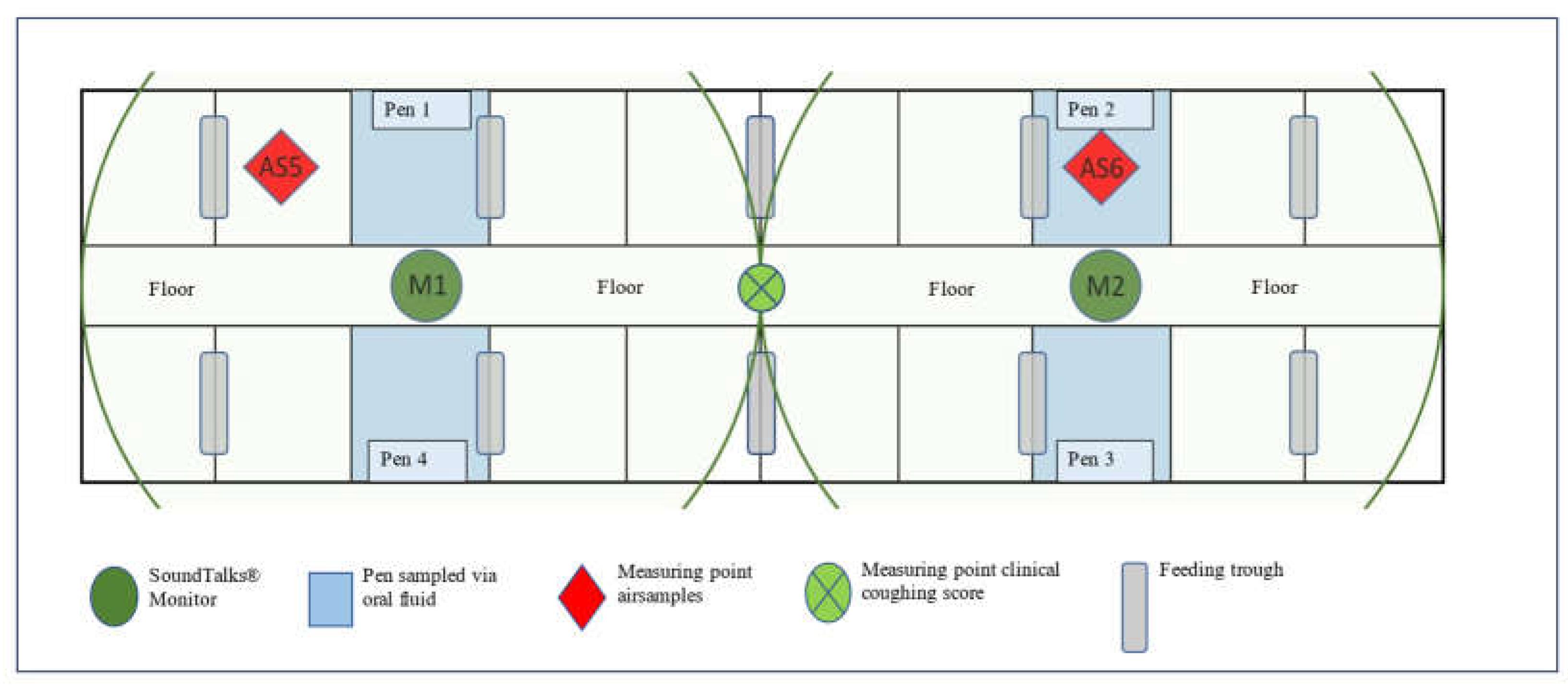
The Farm Description
Study Design and Data Collection
Automated Coughing Monitoring
Clinical Coughing Monitoring
Statistical Analysis
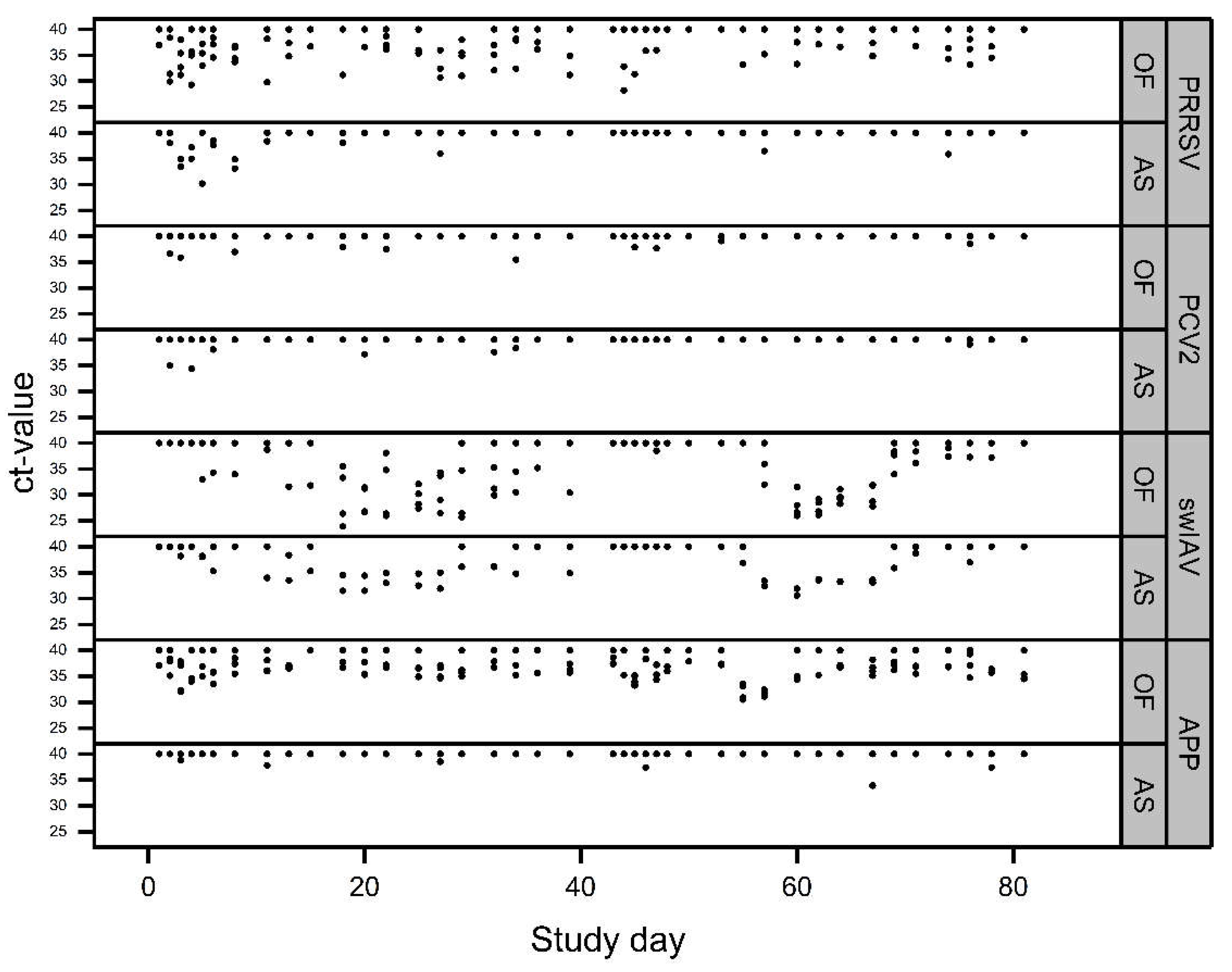
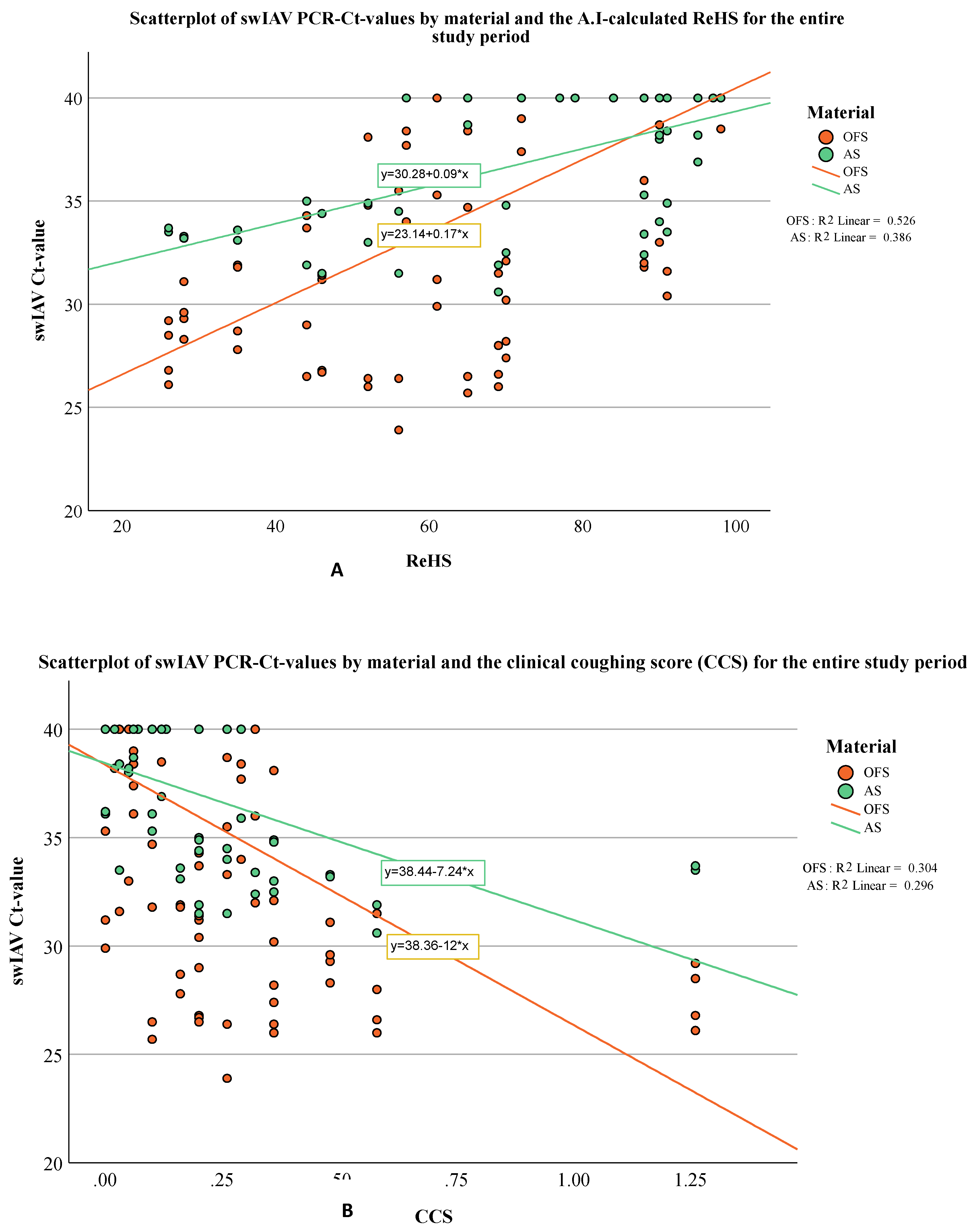
3. Results
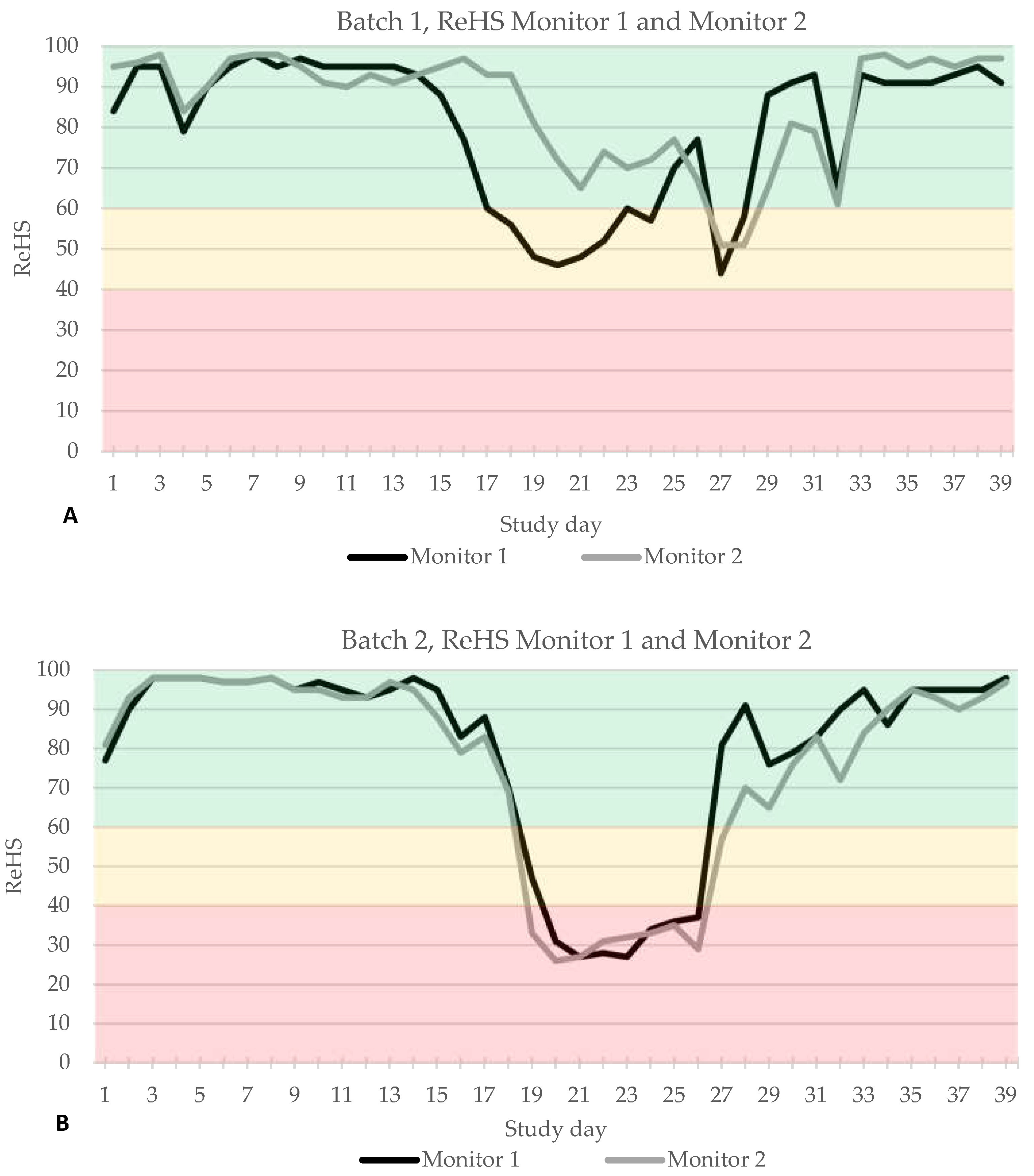
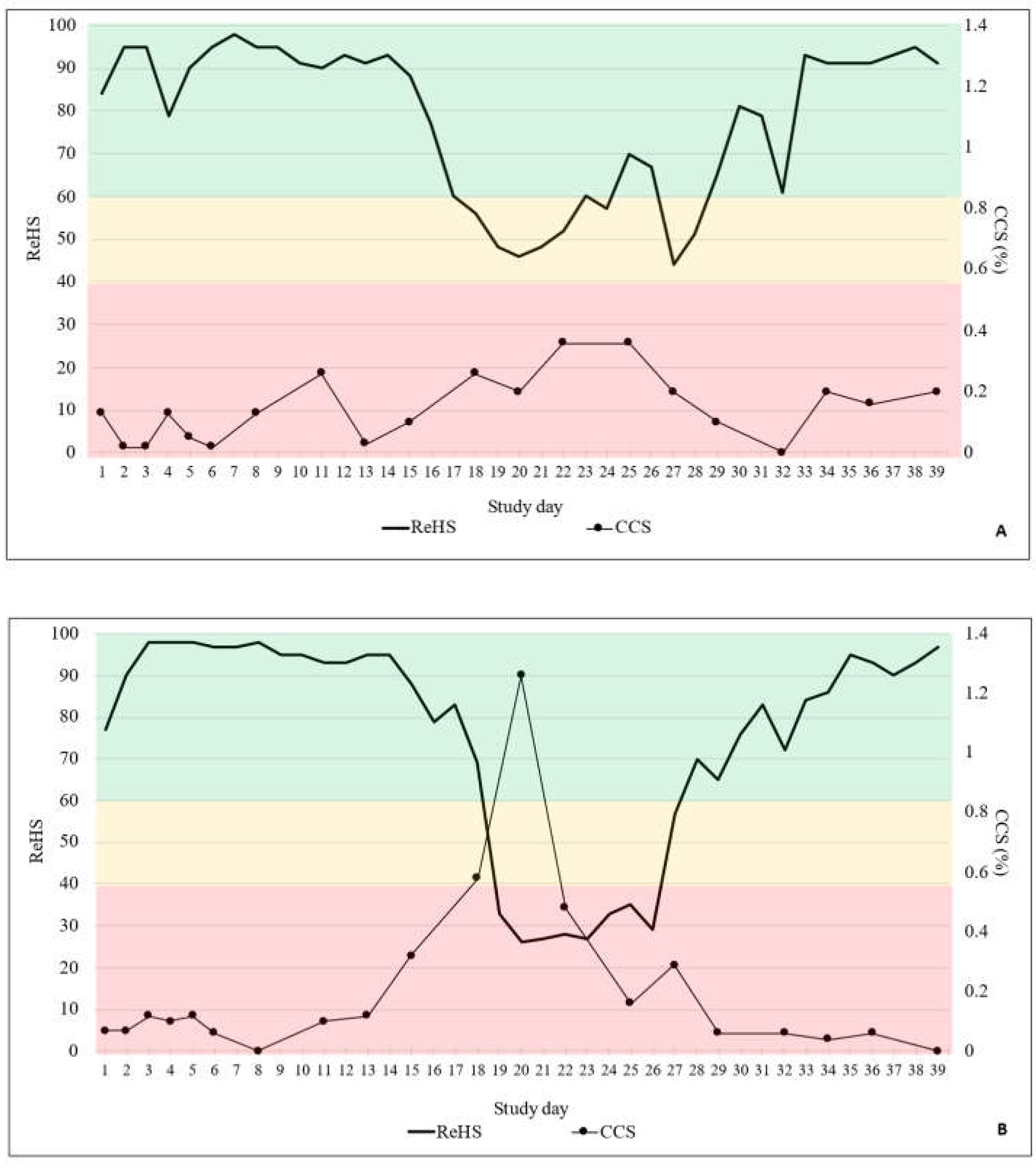
3.1. Coughing Monitoring
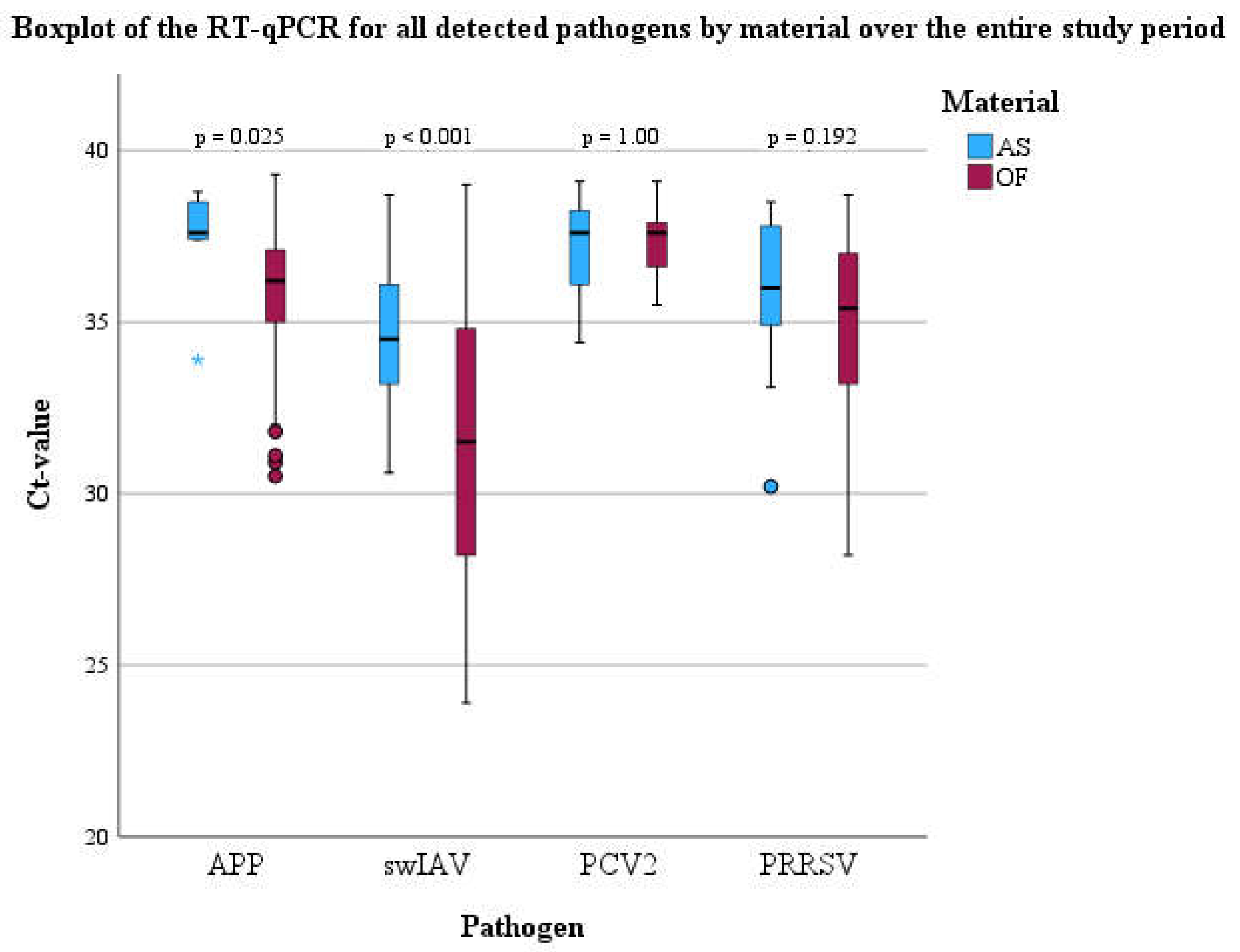
Molecular Biological Examinations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McOrist, S. “Defining the Full Costs of Endemic Porcine Proliferative Enteropathy.” Veterinary Journal 170, no. 1 (2005): 8-9. [CrossRef]
- McOrist, S., S. H. Smith, and L. E. Green. “Estimate of Direct Financial Losses Due to Porcine Proliferative Enteropathy.” The Veterinary record 140, no. 22 (1997): 579-81. [CrossRef]
- Alarcon, P., J. Rushton, and B. Wieland. “Cost of Post-Weaning Multi-Systemic Wasting Syndrome and Porcine Circovirus Type-2 Subclinical Infection in England - an Economic Disease Model.” Preventive veterinary medicine 110, no. 2 (2013): 88-102. [CrossRef]
- Renken, C., C. Nathues, H. Swam, K. Fiebig, C. Weiss, M. Eddicks, M. Ritzmann, and H. Nathues. “Application of an Economic Calculator to Determine the Cost of Porcine Reproductive and Respiratory Syndrome at Farm-Level in 21 Pig Herds in Germany.” Porcine health management 7, no. 1 (2021): 3. [CrossRef]
- Boeters, M., B. Garcia-Morante, G. van Schaik, J. Segales, J. Rushton, and W. Steeneveld. “The Economic Impact of Endemic Respiratory Disease in Pigs and Related Interventions - a Systematic Review.” Porcine health management 9, no. 1 (2023): 45. [CrossRef]
- Thacker, E. L. “Immunology of the Porcine Respiratory Disease Complex.” Vet Clin North Am Food Anim Pract 17, no. 3 (2001): 551-65. [CrossRef]
- Eddicks, Matthias, Lina Eddicks, Julia Stadler, Walter Hermanns, and Mathias Ritzmann. “Der Porcine Respiratory Disease Complex (Prdc)–Eine Klinische Übersicht.” Tierärztliche Praxis Ausgabe G: Großtiere/Nutztiere 49, no. 02 (2021): 120-32.
- Harms, P. A., P. G. Halbur, and S. D. Sorden. “Three Cases of Porcine Respiratory Disease Complex Associated with Porcine Circovirus Type 2 Infection.” Journal of Swine Health and Production 10, no. 1 (2002): 27-30. [CrossRef]
- Kim, J., H. K. Chung, and C. Chae. “Association of Porcine Circovirus 2 with Porcine Respiratory Disease Complex.” Veterinary Journal 166, no. 3 (2003): 251-56. [CrossRef]
- Opriessnig, T., L. G. Gimenez-Lirola, and P. G. Halbur. “Polymicrobial Respiratory Disease in Pigs.” Animal health research reviews / Conference of Research Workers in Animal Diseases 12, no. 2 (2011): 133-48.
- Deblanc, C., and G. Simon. “Involvement of Swine Influenza a Viruses in the Porcine Respiratory Disease Complex.” Virologie (Montrouge) 21, no. 5 (2017): 225-38.
- Schmidt, C., S. P. Cibulski, C. P. Andrade, T. F. Teixeira, A. P. Varela, C. M. Scheffer, A. C. Franco, L. L. de Almeida, and P. M. Roehe. “Swine Influenza Virus and Association with the Porcine Respiratory Disease Complex in Pig Farms in Southern Brazil.” Zoonoses and public health 63, no. 3 (2016): 234-40. [CrossRef]
- Thacker, E. L., P. G. Halbur, R. F. Ross, R. Thanawongnuwech, and B. J. Thacker. “Mycoplasma Hyopneumoniae Potentiation of Porcine Reproductive and Respiratory Syndrome Virus-Induced Pneumonia.” Journal of clinical microbiology 37, no. 3 (1999): 620-7. [CrossRef]
- Chung, Y., S. Oh, J. Lee, D. Park, H. H. Chang, and S. Kim. “Automatic Detection and Recognition of Pig Wasting Diseases Using Sound Data in Audio Surveillance Systems.” Sensors (Basel) 13, no. 10 (2013): 12929-42. [CrossRef]
- Pessoa, J., M. Rodrigues da Costa, E. García Manzanilla, T. Norton, C. McAloon, and L. Boyle. “Managing Respiratory Disease in Finisher Pigs: Combining Quantitative Assessments of Clinical Signs and the Prevalence of Lung Lesions at Slaughter.” Prev Vet Med 186 (2021): 105208. [CrossRef]
- Silva, Apsp, G. Y. Storino, F. S. M. Ferreyra, M. Zhang, E. Fano, D. Polson, C. Wang, R. J. Derscheid, J. J. Zimmerman, M. J. Clavijo, and B. L. Arruda. “Cough Associated with the Detection of Mycoplasma Hyopneumoniae DNA in Clinical and Environmental Specimens under Controlled Conditions.” Porcine Health Manag 8, no. 1 (2022): 6. [CrossRef]
- Wang, S., H. Jiang, Y. Qiao, S. Jiang, H. Lin, and Q. Sun. “The Research Progress of Vision-Based Artificial Intelligence in Smart Pig Farming.” Sensors (Basel) 22, no. 17 (2022). [CrossRef]
- Jorquera-Chavez, M., S. Fuentes, F. R. Dunshea, R. D. Warner, T. Poblete, R. S. Morrison, and E. C. Jongman. “Remotely Sensed Imagery for Early Detection of Respiratory Disease in Pigs: A Pilot Study.” Animals (Basel) 10, no. 3 (2020). [CrossRef]
- Neethirajan, S. “Affective State Recognition in Livestock-Artificial Intelligence Approaches.” Animals (Basel) 12, no. 6 (2022). [CrossRef]
- Neethirajan, Suresh. “Recent Advances in Wearable Sensors for Animal Health Management.” Sensing and Bio-Sensing Research 12 (2017): 15-29. [CrossRef]
- Arulmozhi, E., A. Bhujel, B. E. Moon, and H. T. Kim. “The Application of Cameras in Precision Pig Farming: An Overview for Swine-Keeping Professionals.” Animals (Basel) 11, no. 8 (2021). [CrossRef]
- Esener, N., M. J. Green, R. D. Emes, B. Jowett, P. L. Davies, A. J. Bradley, and T. Dottorini. “Discrimination of Contagious and Environmental Strains of Streptococcus Uberis in Dairy Herds by Means of Mass Spectrometry and Machine-Learning.” Scientific reports 8, no. 1 (2018): 17517. [CrossRef]
- Ezanno, P., S. Picault, G. Beaunee, X. Bailly, F. Munoz, R. Duboz, H. Monod, and J. F. Guegan. “Research Perspectives on Animal Health in the Era of Artificial Intelligence.” Veterinary research 52, no. 1 (2021): 40. [CrossRef]
- Comas, Maria, Christopher J. Gordon, Brian G. Oliver, Nicholas W. Stow, Gregory King, Pawan Sharma, Alaina J. Ammit, Ronald R. Grunstein, and Craig L. Phillips. “A Circadian Based Inflammatory Response – Implications for Respiratory Disease and Treatment.” Sleep Science and Practice 1, no. 1 (2017): 18. [CrossRef]
- Hernandez-Garcia, J., N. Robben, D. Magnee, T. Eley, I. Dennis, S. M. Kayes, J. R. Thomson, and A. W. Tucker. “The Use of Oral Fluids to Monitor Key Pathogens in Porcine Respiratory Disease Complex.” Porcine health management 3 (2017): 7. [CrossRef]
- Ramirez, A., C. Wang, J. R. Prickett, R. Pogranichniy, K. J. Yoon, R. Main, J. K. Johnson, C. Rademacher, M. Hoogland, P. Hoffmann, A. Kurtz, E. Kurtz, and J. Zimmerman. “Efficient Surveillance of Pig Populations Using Oral Fluids.” Preventive veterinary medicine 104, no. 3-4 (2012): 292-300. [CrossRef]
- Kleinmans, M., K. Fiebig, R. Tabeling, H. Swam, A. Duivelshof-Crienen, M. Ritzmann, and M. Eddicks. “Explorative Field Study on the Use of Oral Fluids for the Surveillance of Actinobacillus Pleuropneumoniae Infections in Fattening Farms by an Apx-Real-Time Pcr.” Vet Sci 9, no. 10 (2022). [CrossRef]
- Schott, F., K. Hoffmann, E. Sarno, P. D. Bangerter, R. Stephan, G. Overesch, M. Haessig, X. Sidler, and R. Graage. “Evaluation of Oral Fluids for Surveillance of Foodborne and Zoonotic Pathogens in Pig Farms.” J Vet Diagn Invest 33, no. 4 (2021): 655-63.
- Deffner, P., R. Maurer, V. Cvjetkovic, W. Sipos, R. Krejci, M. Ritzmann, and M. Eddicks. “Cross-Sectional Study on the in-Herd Prevalence of Mycoplasma Hyopneumoniae at Different Stages of Pig Production.” The Veterinary record (2022): e1317.
- Tedeschini, E., S. Pasqualini, C. Emiliani, E. Marini, A. Valecchi, C. Laoreti, S. Ministrini, B. Camilloni, R. Castronari, L. Patoia, F. Merante, S. Baglioni, E. De Robertis, M. Pirro, A. Mencacci, and L. Pasqualini. “Monitoring of Indoor Bioaerosol for the Detection of Sars-Cov-2 in Different Hospital Settings.” Front Public Health 11 (2023): 1169073.
- Song, L., C. Wang, G. Jiang, J. Ma, Y. Li, H. Chen, and J. Guo. “Bioaerosol Is an Important Transmission Route of Antibiotic Resistance Genes in Pig Farms.” Environ Int 154 (2021): 106559. [CrossRef]
- Nguyen, N. T. T., Y. T. H. Luu, T. D. Hoang, H. X. Nguyen, T. D. Dao, V. N. Bui, and G. C. Gray. “An Epidemiological Study of Streptococcus Suis Prevalence among Swine at Industrial Swine Farms in Northern Vietnam.” One Health 13 (2021): 100254. [CrossRef]
- Anderson, B. D., J. A. Lednicky, M. Torremorell, and G. C. Gray. “The Use of Bioaerosol Sampling for Airborne Virus Surveillance in Swine Production Facilities: A Mini Review.” Front Vet Sci 4 (2017): 121. [CrossRef]
- Anderson, B. D., M. Yondon, E. S. Bailey, E. K. Duman, R. A. Simmons, A. G. Greer, and G. C. Gray. “Environmental Bioaerosol Surveillance as an Early Warning System for Pathogen Detection in North Carolina Swine Farms: A Pilot Study.” Transboundary and emerging diseases 68, no. 2 (2021): 361-67. [CrossRef]
- Prost, K., H. Kloeze, S. Mukhi, K. Bozek, Z. Poljak, and S. Mubareka. “Bioaerosol and Surface Sampling for the Surveillance of Influenza a Virus in Swine.” Transboundary and emerging diseases 66, no. 3 (2019): 1210-17. [CrossRef]
- Dee, S., S. Otake, S. Oliveira, and J. Deen. “Evidence of Long Distance Airborne Transport of Porcine Reproductive and Respiratory Syndrome Virus and Mycoplasma Hyopneumoniae.” Veterinary research 40, no. 4 (2009): 39. [CrossRef]
- Torremorell, M., C. Pijoan, K. Janni, R. Walker, and H. S. Joo. “Airborne Transmission of Actinobacillus Pleuropneumoniae and Porcine Reproductive and Respiratory Syndrome Virus in Nursery Pigs.” American journal of veterinary research 58, no. 8 (1997): 828-32. [CrossRef]
- Nathues, H., J. Spergser, R. Rosengarten, L. Kreienbrock, and E. Grosse Beilage. “Value of the Clinical Examination in Diagnosing Enzootic Pneumonia in Fattening Pigs.” Veterinary Journal 193, no. 2 (2012): 443-7. [CrossRef]
- Prickett, J., R. Simer, J. Christopher-Hennings, K. J. Yoon, R. B. Evans, and J. J. Zimmerman. “Detection of Porcine Reproductive and Respiratory Syndrome Virus Infection in Porcine Oral Fluid Samples: A Longitudinal Study under Experimental Conditions.” J Vet Diagn Invest 20, no. 2 (2008): 156-63. [CrossRef]
- Landis, J. Richard, and Gary G. Koch. “The Measurement of Observer Agreement for Categorical Data.” Biometrics 33, no. 1 (1977): 159-74. [CrossRef]
- Duerlinger, S., C. Knecht, S. Sawyer, G. Balka, M. Zaruba, T. Ruemenapf, C. Kraft, P. H. Rathkjen, and A. Ladinig. “Efficacy of a Modified Live Porcine Reproductive and Respiratory Syndrome Virus 1 (Prrsv-1) Vaccine against Experimental Infection with Prrsv Aut15-33 in Weaned Piglets.” Vaccines (Basel) 10, no. 6 (2022). [CrossRef]
- Ingram, D. L., and M. J. Dauncey. “Circadian Rhythms in the Pig.” Comparative Biochemistry and Physiology Part A: Physiology 82, no. 1 (1985): 1-5.
- Polson D., Playter S., Berckmans D., Cui Z., Quinn B. “Determining the Optimal Placement and Configuration of an Audio-Based Sensor Platform to Enable Improved Detection and Characterisation of Clinical Respiratory Episodes in Large Growing Pig Airspaces and Sites.” In Proceedings of the European Conference on Precision Livestock Farming, pp. 490-95. Cork, Ireland, 26–29 August 2019.
- Neira, V., M. Allerson, C. Corzo, M. Culhane, A. Rendahl, and M. Torremorell. “Detection of Influenza a Virus in Aerosols of Vaccinated and Non-Vaccinated Pigs in a Warm Environment.” PLoS One 13, no. 5 (2018): e0197600. [CrossRef]
- Madapong, A., K. Saeng-Chuto, A. Tantituvanont, and D. Nilubol. “Safety of Prrsv-2 Mlv Vaccines Administrated Via the Intramuscular or Intradermal Route and Evaluation of Prrsv Transmission Upon Needle-Free and Needle Delivery.” Scientific reports 11, no. 1 (2021): 23107. [CrossRef]
- Biernacka, K., P. Karbowiak, P. Wrobel, T. Chareza, M. Czopowicz, G. Balka, C. Goodell, R. Rauh, and T. Stadejek. “Detection of Porcine Reproductive and Respiratory Syndrome Virus (Prrsv) and Influenza a Virus (Iav) in Oral Fluid of Pigs.” Research in veterinary science 109 (2016): 74-80. [CrossRef]
- Kittawornrat, A., J. Prickett, W. Chittick, C. Wang, M. Engle, J. Johnson, D. Patnayak, T. Schwartz, D. Whitney, C. Olsen, K. Schwartz, and J. Zimmerman. “Porcine Reproductive and Respiratory Syndrome Virus (Prrsv) in Serum and Oral Fluid Samples from Individual Boars: Will Oral Fluid Replace Serum for Prrsv Surveillance?” Virus research 154, no. 1-2 (2010): 170-6.
- Kittawornrat, A., Y. Panyasing, C. Goodell, C. Wang, P. Gauger, K. Harmon, R. Rauh, L. Desfresne, I. Levis, and J. Zimmerman. “Porcine Reproductive and Respiratory Syndrome Virus (Prrsv) Surveillance Using Pre-Weaning Oral Fluid Samples Detects Circulation of Wild-Type Prrsv.” Veterinary Microbiology 168, no. 2-4 (2014): 331-9. [CrossRef]
- Kristensen, C. S., O. Angen, M. Andreasen, H. Takai, J. P. Nielsen, and S. E. Jorsal. “Demonstration of Airborne Transmission of Actinobacillus Pleuropneumoniae Serotype 2 between Simulated Pig Units Located at Close Range.” Veterinary Microbiology 98, no. 3-4 (2004): 243-9. [CrossRef]
- Tobias, T. J., A. Bouma, J. van den Broek, A. van Nes, A. J. Daemen, J. A. Wagenaar, J. A. Stegeman, and D. Klinkenberg. “Transmission of Actinobacillus Pleuropneumoniae among Weaned Piglets on Endemically Infected Farms.” Preventive veterinary medicine 117, no. 1 (2014): 207-14. [CrossRef]
- Nielsen, G. B., J. P. Nielsen, J. Haugegaard, S. C. Leth, L. E. Larsen, C. S. Kristensen, K. S. Pedersen, H. Stege, C. K. Hjulsager, and H. Houe. “Comparison of Serum Pools and Oral Fluid Samples for Detection of Porcine Circovirus Type 2 by Quantitative Real-Time Pcr in Finisher Pigs.” Porcine health management 4 (2018): 2. [CrossRef]
- Fachinger, V., R. Bischoff, S. B. Jedidia, A. Saalmueller, and K. Elbers. “The Effect of Vaccination against Porcine Circovirus Type 2 in Pigs Suffering from Porcine Respiratory Disease Complex.” Vaccine 26, no. 11 (2008): 1488-99. [CrossRef]
- Kixmoeller, M., M. Ritzmann, M. Eddicks, A. Saalmueller, K. Elbers, and V. Fachinger. “Reduction of Pmws-Associated Clinical Signs and Co-Infections by Vaccination against Pcv2.” Vaccine 26, no. 27-28 (2008): 3443-51. [CrossRef]
| Pathogen | Name of the used assay | Manufacturer |
|---|---|---|
| PRRSV | Virotype PRRSV NA/EU | INDICAL BIOSCIENCE GmbH; Leipzig, Germany |
| PCV 2 | Virotype PCV 2/PCV 3 | INDICAL BIOSCIENCE GmbH; Leipzig, Germany |
| swIAV | Virotype Influenza A RT-PCR | INDICAL BIOSCIENCE GmbH; Leipzig, Germany |
| M. hyopneumoniae | EXOone Mycoplasma hyopneumoniae | exopol; San Mateo de Gállego, Zaragoza, Spain |
| A. pleuropneumoniae | EXOone Actinobacillus pleuropneumoniae | exopol; San Mateo de Gállego, Zaragoza, Spain |
| B1 | B2 | Entire study period | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OFs (n = 64) |
AS (n = 32) |
p-value | OFs (n = 60) |
AS (n = 30) |
p-value | OFs (n = 124) |
AS (n = 62) |
p-value | ||
| PRRSV | 65.0 % (52/80) |
32.5 % (13/40) |
< 0.001 | 27.5 % (22/80) |
5.0 % (2/40) |
0.003 | 46.3 % (74/160) |
18.8 % (15/80) |
< 0.001 | |
| PCV2 | 7.5 % (6/80) |
15.0 % (6/40) |
0.211 | 5.0 % (4/80) |
2.5 % (1/40) |
0.664 | 6.3 % (10/160) |
8.8 % (7/80) |
0.594 | |
| swIAV | 45.0 % (36/80) |
57.5 % (23/40) |
0.246 | 36.3 % (29/80) |
35.0 % (14/40) |
1.00 | 40.6 % (65/160) |
46.3 % (37/80) |
0.410 | |
| APP1 | 63.7 % (51/80) |
7.5 % (3/40) |
<0.001 | 70.0 % (56/80) |
7.5 % (3/40) |
< 0.001 | 66.9 % (107/160) |
7.5 % (6/80) |
< 0.001 | |
| M. hyo2 | 0.0 % (0/64) |
0.0 % (0/32) |
- | 0.0 % (0/60) |
0.0 % (0/30) |
- | 0.0 % (0/124) |
0.0 % (0/62) |
n.d.* | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





