Submitted:
02 September 2024
Posted:
04 September 2024
You are already at the latest version
Abstract
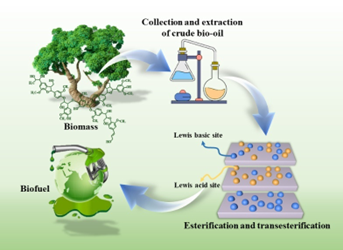
Keywords:
1. Introduction
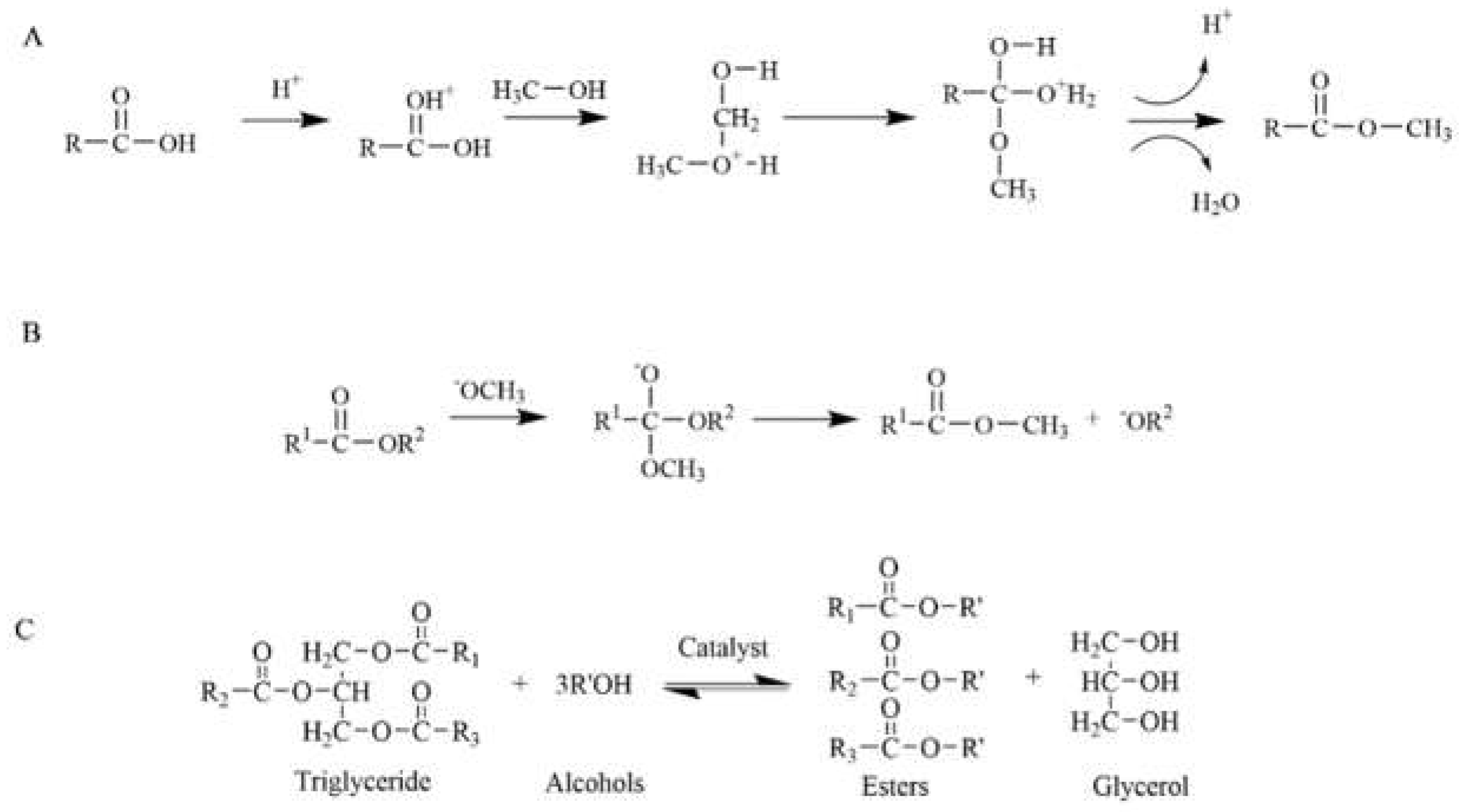
2. Transesterification of Biodiesel
2.1. Fatty Acid Composition and Properties of Biodiesel
2.2. Research Status of Acid-Base Catalyzed Transesterification
3. Lewis Acidic Catalyst
3.1. Research Status of Lewis Acidic Catalyst
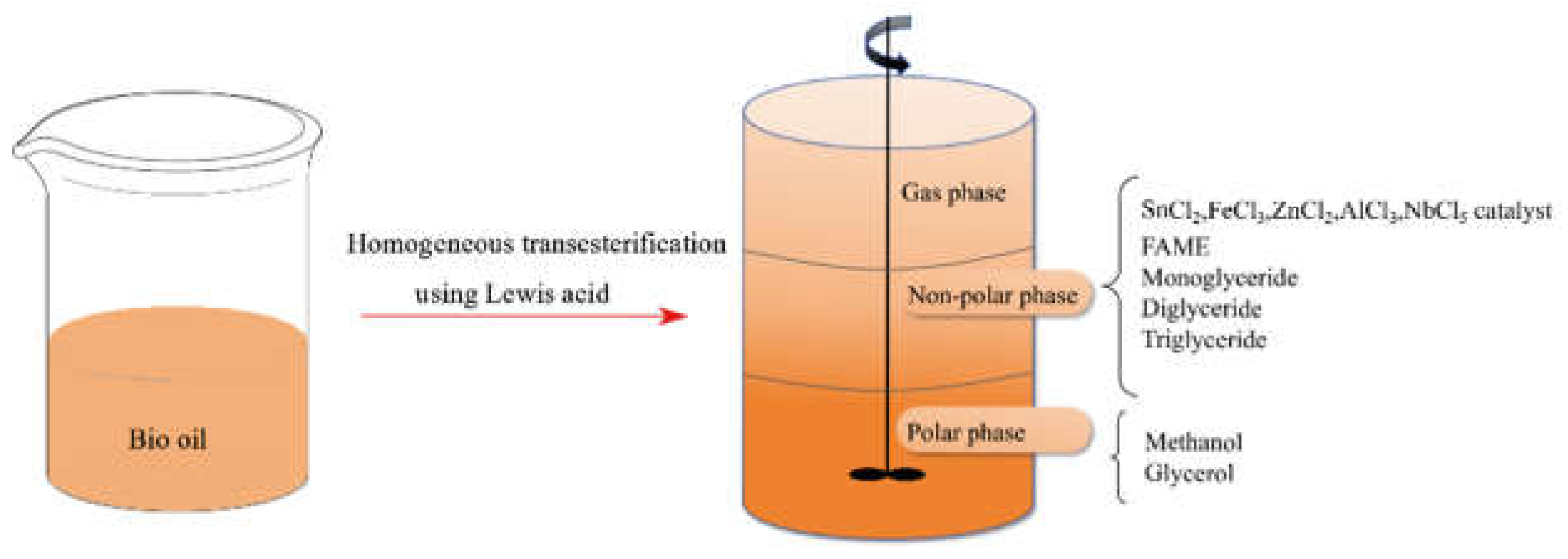
3.2. Heterogeneous Lewis Acidic Catalysis
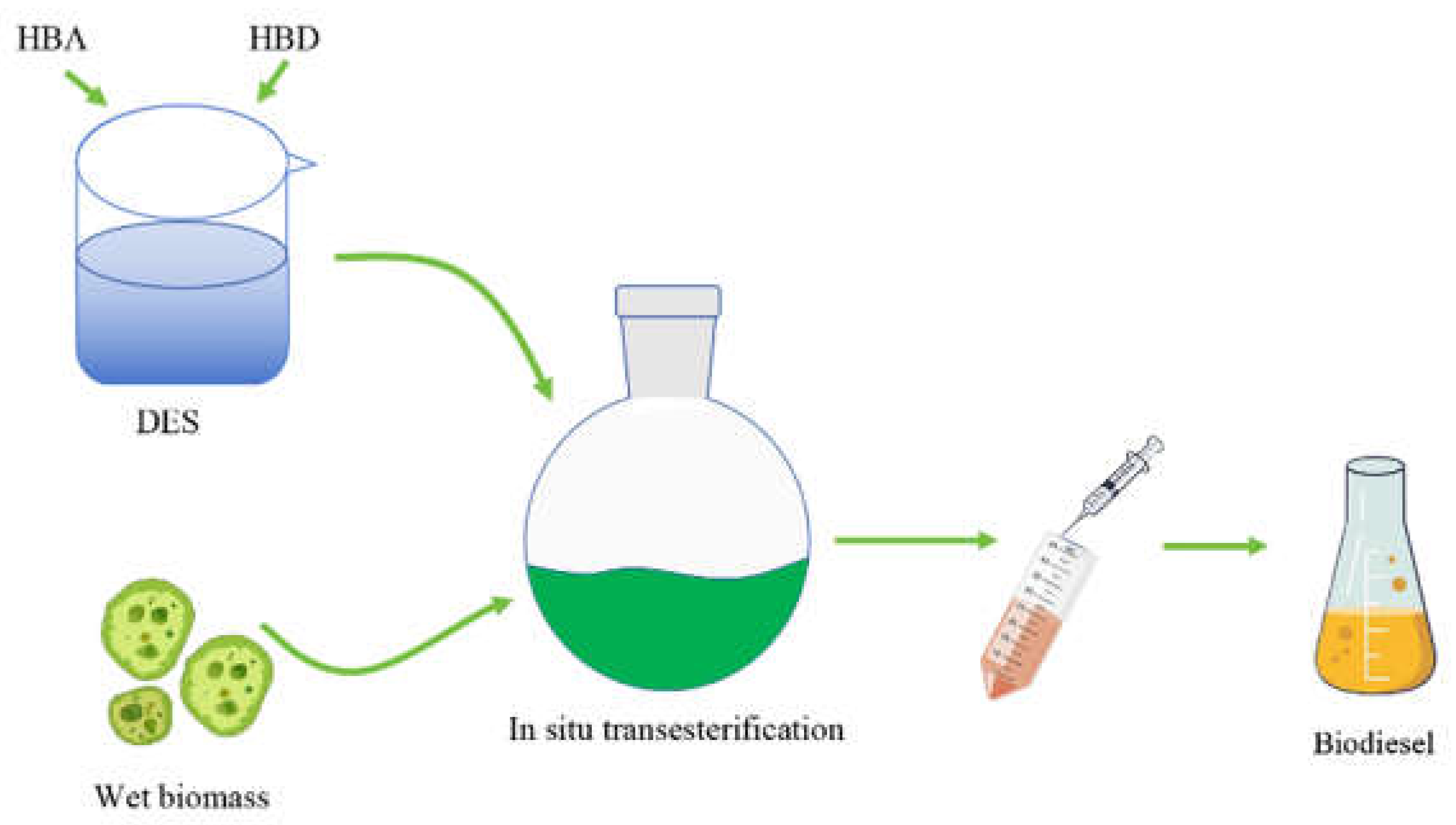
3.3. Lewis Acidic Ionic Liquids and Deep Eutectic Solvents
4. Lewis Basic Catalyst
4.1. Research Status of Lewis Basic Catalysis
4.2. A Solid Alkali Metal Catalyst
4.3. Alkaline Earth Metal Oxide Catalyst
4.4. Lewis Alkaline Sites Loaded on Hydrotalcite
4.5. Alkaline Metal Based Nanomaterials
5. Lewis Acid-Base Bifunctional Catalyst
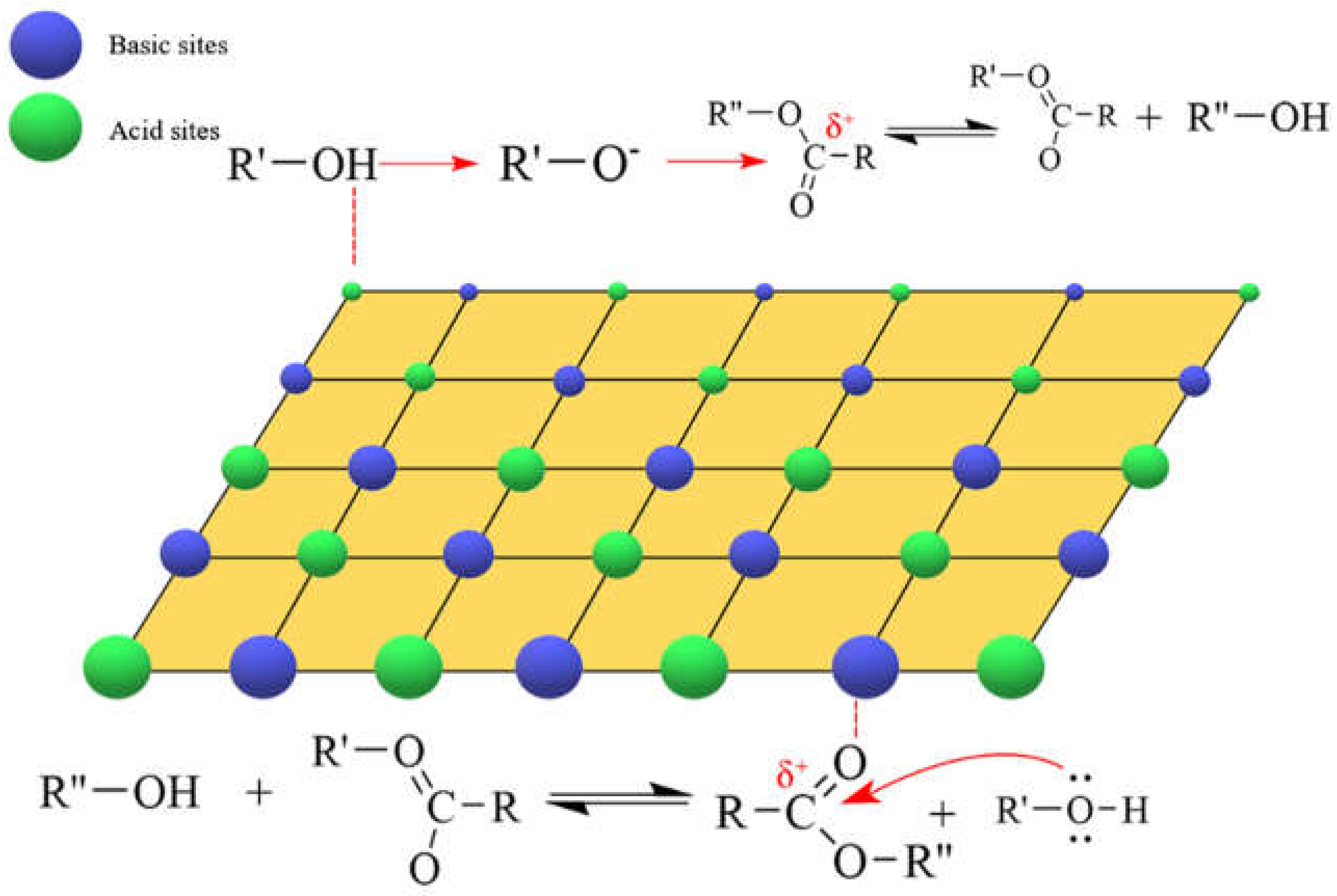
5.1. Metal Oxide Catalysts containing Lewis Acid-Base Sites
5.2. Heteropoly Acid Catalysts with Acid-Base Bifunctional Groups
5.3. Sulfated Solid Acid-Base Amphoteric Catalyst
5.4. Magnetic Functionalized Double Lewis Acid-Base Sites
6. Conclusion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tien Thanh, N.; Mostapha, M.; Lam, M.K.; Ishak, S.; Kanna Dasan, Y.; Lim, J.W.; Tan, I.S.; Lau, S.Y.; Chin, B.L. F.; Hadibarata, T. Fundamental understanding of in-situ transesterification of microalgae biomass to biodiesel: A critical review. Energy Conversion and Management 2022, 270, 116212. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Lee, J.W. In-situ transesterification of wet spent coffee grounds for sustainable biodiesel production. Bioresource Technology 2016, 221, 55–60. [Google Scholar] [CrossRef]
- Kadir, W.N. A.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T. Harvesting and pre-treatment of microalgae cultivated in wastewater for biodiesel production: A review. Energy Conversion and Management 2018, 171, 1416–1429. [Google Scholar] [CrossRef]
- Tuntiwiwattanapun, N.; Tongcumpou, C. Sequential extraction and reactive extraction processing of spent coffee grounds: An alternative approach for pretreatment of biodiesel feedstocks and biodiesel production. Industrial Crops and Products 2018, 117, 359–365. [Google Scholar] [CrossRef]
- Mat Husin, M.A.; Mohd Yasin, N.H.; Takriff, M.S.; Jamar, N.H. A review on pretreatment methods for lipid extraction from microalgae biomass. Preparative Biochemistry & Biotechnology 2024, 54, 159–174. [Google Scholar]
- Bauer, G.; Lima, S.; Chenevard, J.; Sugnaux, M.; Fischer, F. Biodiesel via in Situ Wet Microalgae Biotransformation: Zwitter-Type Ionic Liquid Supported Extraction and Transesterification. ACS Sustainable Chemistry & Engineering 2017, 5, 1931–1937. [Google Scholar]
- Chiang, C.-L.; Lin, K.-S.; Shu, C.-W.; Wu, J.C.-S.; Wu, K.C.-W.; Huang, Y.-T. Enhancement of biodiesel production via sequential esterification/tran sesterification over solid superacidic and superbasic catalysts. Catalysis Today 348, 257-269.
- Jin, B.; Duan, P.; Xu, Y.; Wang, B.; Wang, F.; Zhang, L. Lewis acid-catalyzed in situ transesterification/esterification of microalgae in supercritical ethanol. Bioresource Technology 2014, 162, 341–349. [Google Scholar] [CrossRef]
- Encinar, J.M.; Pardal, A.; Sánchez, N.; Nogales, S. Biodiesel by Transesterification of Rapeseed Oil Using Ultrasound: A Kinetic Study of Base-Catalysed Reactions Energies. 2018. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Al-Tikrity, E.T. B.; Khalaf, A.M. Transesterification of non-edible oils over potassium acetate impregnated CaO solid base catalyst. Fuel 2018, 234, 81–93. [Google Scholar] [CrossRef]
- Yang, X.-X.; Wang, Y.-T.; Yang, Y.-T.; Feng, E.-Z.; Luo, J.; Zhang, F.; Yang, W.-J.; Bao, G.-R. Catalytic transesterification to biodiesel at room temperature over several solid bases. Energy Conversion and Management 2018, 164, 112–121. [Google Scholar] [CrossRef]
- Denmark, S.E.; Beutner, G.L. Lewis base catalysis in organic synthesis. Angew Chem Int Ed Engl 2008, 47, 1560–638. [Google Scholar] [CrossRef] [PubMed]
- Zapelini, I.W.; Silva, L.L.; Cardoso, D. The role of alcohols on the accessibility of basic ≡SiO− sites in hybrid silicas for catalytic transesterification. Braz. J. Chem. Eng 2023, 41, 409–416. [Google Scholar] [CrossRef]
- She, Q.; Qiu, M.; Li, K.; Liu, J.; Zhou, C. Acidic and basic sites on the surface of sodium montmorillonite active for catalytic transesterification of glycerol to glycerol carbonate. Applied Clay Science 2023, 238, 106916. [Google Scholar] [CrossRef]
- Eid, J.G.; de Paula, G.M.; Cardoso, D. Heterogeneous transesterification catalyzed by silicas containing basic sites. Molecular Catalysis 2022, 531, 112631. [Google Scholar] [CrossRef]
- Abdelgaid, M.; Mpourmpakis, G. Structure–Activity Relationships in Lewis Acid–Base Heterogeneous Catalysis. ACS Catalysis 2022, 12, 4268–4289. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, L.; Liang, R.; Liu, X.; Yan, G.; Zhu, S.; Wang, X. Bifunctional Lewis acid-base nanocatalysts with dual active sites for strengthened coupling of alcohol conversion and H2 evolution. International Journal of Hydrogen Energy 2024, 51, 1598–1607. [Google Scholar] [CrossRef]
- Munyentwali, A.; Li, H.; Yang, Q. Review of advances in bifunctional solid acid/base catalysts for sustainable biodiesel production. Applied Catalysis A: General 2022, 633, 118525. [Google Scholar] [CrossRef]
- Vicente, G.; Carrero, A.; Rodríguez, R.; del Peso, G.L. Heterogeneous-catalysed direct transformation of microalga biomass into Biodiesel-Grade FAMEs. Fuel 2017, 200, 590–598. [Google Scholar] [CrossRef]
- Bouaid, A.; Vázquez, R.; Martinez, M.; Aracil, J. Effect of free fatty acids contents on biodiesel quality. Pilot plant studies. Fuel 2016, 174, 54–62. [Google Scholar] [CrossRef]
- Supeno, M.; Sihotang, J.P.; Panjaitan, Y.V.; Damanik, D.S. Y.; Tarigan, J.B.; Sitepu, E.K. Room temperature esterification of high-free fatty acid feedstock into biodiesel. RSC Advances 2023, 13, 33107–33113. [Google Scholar] [CrossRef]
- Manikandan, G.; Kanna, P.R.; Taler, D.; Sobota, T. Review of Waste Cooking Oil (WCO) as a Feedstock for Biofuel—Indian Perspective Energies, 2023.
- Yu, H.; Niu, S.; Lu, C.; Li, J.; Yang, Y. Preparation and esterification performance of sulfonated coal-based heterogeneous acid catalyst for methyl oleate production. Energy Conversion and Management 2016, 126, 488–496. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Miguel, V.U.; Errazu, A.F. Heterogeneous esterification of oil with high amount of free fatty acids. Fuel 2007, 86, 906–910. [Google Scholar] [CrossRef]
- Ganesan, S.; Nadarajah, S.; Chee, X.Y.; Khairuddean, M.; Teh, G.B. Esterification of free fatty acids using ammonium ferric sulphate-calcium silicate as a heterogeneous catalyst. Renewable Energy 2020, 153, 1406–1417. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Zulkurnain, N.; Rosid, S.J. M.; Azid, A.; Endut, A.; Toemen, S.; Ismail, S.; Abdullah, W.N. W.; Aziz, S.M.; Yusoff, N.M.; Rosid, S.M.; Nasir, N.A. Catalytic Transesterification of Coconut Oil in Biodiesel Production: A Review. Catalysis Surveys from Asia 2022, 26, 129–143. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Martínez, G.; Nogales-Delgado, S. Transesterification of Soybean Oil through Different Homogeneous Catalysts: Kinetic Study Catalysts [Online], 2022.
- Johnson, M.B.; Wen, Z. Production of Biodiesel Fuel from the Microalga Schizochytrium limacinum by Direct Transesterification of Algal Biomass. Energy & Fuels 2009, 23, 5179–5183. [Google Scholar]
- Tiwari, P.; Garg, S. Study of reversible kinetic models for alkali-catalyzed Jatropha curcas transesterification. Biomass Conversion and Biorefinery 2016, 6, 61–70. [Google Scholar] [CrossRef]
- Liu, Y.; Lotero, E.; Goodwin, J.G.; Lu, C. Transesterification of triacetin using solid Brønsted bases. Journal of Catalysis 2007, 246, 428–433. [Google Scholar] [CrossRef]
- Georgogianni, K.G.; Katsoulidis, A.K.; Pomonis, P.J.; Manos, G.; Kontominas, M.G. Transesterification of rapeseed oil for the production of biodiesel using homogeneous and heterogeneous catalysis. Fuel Processing Technology 2009, 90, 1016–1022. [Google Scholar] [CrossRef]
- Stojković, I.J.; Stamenković, O.S.; Povrenović, D.S.; Veljković, V.B. Purification technologies for crude biodiesel obtained by alkali-catalyzed transesterification. Renewable and Sustainable Energy Reviews 2014, 32, 1–15. [Google Scholar] [CrossRef]
- Liu, X.; Piao, X.; Wang, Y.; Zhu, S.; He, H. Calcium methoxide as a solid base catalyst for the transesterification of soybean oil to biodiesel with methanol. Fuel 2008, 87, 1076–1082. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, W.; Jia, L.; Guo, X. Sodium phosphate solid base catalysts for production of novel biodiesel by transesterification reaction. RSC Advances 2023, 13, 26700–26708. [Google Scholar] [CrossRef]
- Kouider Elouahed, S.; Asikin-Mijan, N.; Alsultan G, A.; Kaddour, O.; Yusop, M.R.; Mimoun, H.; Samidin, S.; Mansir, N.; Taufiq-Yap, Y.H. Optimization of the activity of Mo7-Zn3/CaO catalyst in the transesterification of waste cooking oil into sustainable biodiesel via response surface methodology. Energy Conversion and Management 2024, 303, 118185. [Google Scholar] [CrossRef]
- Xie, W.; Zhao, L. Production of biodiesel by transesterification of soybean oil using calcium supported tin oxides as heterogeneous catalysts. Energy Conversion and Management 2013, 76, 55–62. [Google Scholar] [CrossRef]
- Ma, X.; Liu, F.; Helian, Y.; Li, C.; Wu, Z.; Li, H.; Chu, H.; Wang, Y.; Wang, Y.; Lu, W.; Guo, M.; Yu, M.; Zhou, S. Current application of MOFs based heterogeneous catalysts in catalyzing transesterification/esterification for biodiesel production: A review. Energy Conversion and Management 2021, 229, 113760. [Google Scholar] [CrossRef]
- Shahidul Islam, M.; Robin Hart, C.; Casadonte, D. Ultrasound-assisted solid Lewis acid-catalyzed transesterification of Lesquerella fendleri oil for biodiesel synthesis. Ultrasonics Sonochemistry 2022, 88, 106082. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; Ramos, M.J.; Rodríguez, J.F.; Pérez, Á. Tin compounds as Lewis acid catalysts for esterification and transesterification of acid vegetable oils. Fuel Processing Technology 2013, 106, 321–325. [Google Scholar] [CrossRef]
- Guan, Q.; Shang, H.; Liu, J.; Gu, J.; Li, B.; Miao, R.; Chen, Q.; Ning, P. Biodiesel from transesterification at low temperature by AlCl3 catalysis in ethanol and carbon dioxide as cosolvent: Process, mechanism and application. Applied Energy 2016, 164, 380–386. [Google Scholar] [CrossRef]
- Soriano, N.U.; Venditti, R.; Argyropoulos, D.S. Biodiesel synthesis via homogeneous Lewis acid-catalyzed transesterification. Fuel 2009, 88, 560–565. [Google Scholar] [CrossRef]
- Chin, S.Y.; Ahmad, A.L.; Mohamed, A.R.; Bhatia, S. Characterization and activity of zinc acetate complex supported over functionalized silica as a catalyst for the production of isopropyl palmitate. Applied Catalysis A: General 2006, 297, 8–17. [Google Scholar] [CrossRef]
- Di Serio, M.; Tesser, R.; Dimiccoli, M.; Cammarota, F.; Nastasi, M.; Santacesaria, E. Synthesis of biodiesel via homogeneous Lewis acid catalyst. Journal of Molecular Catalysis A: Chemical 2005, 239, 111–115. [Google Scholar] [CrossRef]
- Reinoso, D.M.; Damiani, D.E.; Tonetto, G.M. Zinc carboxylic salts used as catalyst in the biodiesel synthesis by esterification and transesterification: Study of the stability in the reaction medium. Applied Catalysis A: General 2012, 449, 88–95. [Google Scholar] [CrossRef]
- Kolet, M.; Atrash, M.; Molina, K.; Zerbib, D.; Albo, Y.; Nakonechny, F.; Nisnevitch, M. Sol–Gel Entrapped Lewis Acids as Catalysts for Biodiesel Production. Molecules 2020, 25, 5936. [Google Scholar] [CrossRef] [PubMed]
- Mello, V.M.; Pousa, G.P. A. G.; Pereira, M.S. C.; Dias, I.M.; Suarez, P.A. Z. Metal oxides as heterogeneous catalysts for esterification of fatty acids obtained from soybean oil. Fuel Processing Technology 2011, 92, 53–57. [Google Scholar] [CrossRef]
- Raia, R.Z.; da Silva, L.S.; Marcucci, S.M. P.; Arroyo, P.A. Biodiesel production from Jatropha curcas L. oil by simultaneous esterification and transesterification using sulphated zirconia. Catalysis Today 2017, 289, 105–114. [Google Scholar] [CrossRef]
- Du, Y.; Liu, S.; Ji, Y.; Zhang, Y.; Wei, S.; Liu, F.; Xiao, F.-S. Synthesis of Sulfated Silica-Doped Tin Oxides and Their High Activities in Transesterification. Catalysis Letters 2008, 124, 133–138. [Google Scholar] [CrossRef]
- Li, D.; Feng, W.; Chen, C.; Chen, S.; Fan, G.; Liao, S.; Wu, G.; Wang, Z. Transesterification of Litsea cubeba kernel oil to biodiesel over zinc supported on zirconia heterogeneous catalysts. Renewable Energy 2021, 177, 13–22. [Google Scholar] [CrossRef]
- Hechelski, M.; Ghinet, A.; Louvel, B.; Dufrénoy, P.; Rigo, B.; Daïch, A.; Waterlot, C. From Conventional Lewis Acids to Heterogeneous Montmorillonite K10: Eco-Friendly Plant-Based Catalysts Used as Green Lewis Acids. ChemSusChem 2018, 11, 1249–1277. [Google Scholar] [CrossRef]
- Shestakova, P.; Popova, M.; Szegedi, Á.; Lazarova, H.; Nga Luong, T.K.; Trendafilova, I.; Mihály, J.; Parac-Vogt, T.N. Hybrid catalyst with combined Lewis and Brønsted acidity based on ZrIV substituted polyoxometalate grafted on mesoporous MCM-41 silica for esterification of renewable levulinic acid. Microporous and Mesoporous Materials 2021, 323, 111203. [Google Scholar] [CrossRef]
- Aziz, M.A. A.; Puad, K.; Triwahyono, S.; Jalil, A.A.; Khayoon, M.S.; Atabani, A.E.; Ramli, Z.; Majid, Z.A.; Prasetyoko, D.; Hartanto, D. Transesterification of croton megalocarpus oil to biodiesel over WO3 supported on silica mesoporous-macroparticles catalyst. Chemical Engineering Journal 2017, 316, 882–892. [Google Scholar] [CrossRef]
- Duan, Y.; Ding, R.; Shi, Y.; Fang, X.; Hu, H.; Yang, M.; Wu, Y. Synthesis of Renewable Diesel Range Alkanes by Hydrodeoxygenation of Palmitic Acid over 5% Ni/CNTs under Mild Conditions. Catalysts 2017, 7, 81. [Google Scholar] [CrossRef]
- Shu, Q.; Zou, W.; He, J.; Lesmana, H.; Zhang, C.; Zou, L.; Wang, Y. Preparation of the F--SO42-/MWCNTs catalyst and kinetic studies of the biodiesel production via esterification reaction of oleic acid and methanol. Renewable Energy 2019, 135, 836–845. [Google Scholar] [CrossRef]
- Shu, Q.; Tang, G.; Liu, F.; Zou, W.; He, J.; Zhang, C.; Zou, L. Study on the preparation, characterization of a novel solid Lewis acid Al3+-SO42−/MWCNTs catalyst and its catalytic performance for the synthesis of biodiesel via esterification reaction of oleic acid and methanol. Fuel 2017, 209, 290–298. [Google Scholar] [CrossRef]
- Ibrahim, M.L.; Nik Abdul Khalil, N.N. A.; Islam, A.; Rashid, U.; Ibrahim, S.F.; Sinar Mashuri, S.I.; Taufiq-Yap, Y.H. Preparation of Na2O supported CNTs nanocatalyst for efficient biodiesel production from waste-oil. Energy Conversion and Management 2020, 205, 112445. [Google Scholar] [CrossRef]
- Hasan, Z.; Jun, J.W.; Jhung, S.H. Sulfonic acid-functionalized MIL-101(Cr): An efficient catalyst for esterification of oleic acid and vapor-phase dehydration of butanol. Chemical Engineering Journal 2015, 278, 265–271. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, Y.; Li, Y.; Wen, Z.; Shen, X.; Zhao, Y. Application of functional metal anionic Lewis acid ionic liquids in the alkylation of chlorobenzene/SOCl2. RSC Advances 2023, 13, 11635–11641. [Google Scholar] [CrossRef]
- Ullah, Z.; Khan, A.S.; Muhammad, N.; Ullah, R.; Alqahtani, A.S.; Shah, S.N.; Ghanem, O.B.; Bustam, M.A.; Man, Z. A review on ionic liquids as perspective catalysts in transesterification of different feedstock oil into biodiesel. Journal of Molecular Liquids 2018, 266, 673–686. [Google Scholar] [CrossRef]
- Panchal, B.; Chang, T.; Qin, S.; Sun, Y.; Wang, J.; Bian, K. Optimization of soybean oil transesterification using an ionic liquid and methanol for biodiesel synthesis. Energy Reports 2020, 6, 20–27. [Google Scholar] [CrossRef]
- Han, X.; Yan, W.; Hung, C.-T.; He, Y.; Wu, P.-H.; Liu, L.-L.; Huang, S.-J.; Liu, S.-B. Transesterification of soybean oil to biodiesel by tin-based Brønsted-Lewis acidic ionic liquid catalysts. Korean Journal of Chemical Engineering 2016, 33, 2063–2072. [Google Scholar] [CrossRef]
- Xie, W.; Wan, F. Immobilization of polyoxometalate-based sulfonated ionic liquids on UiO-66-2COOH metal-organic frameworks for biodiesel production via one-pot transesterification-esterification of acidic vegetable oils. Chemical Engineering Journal 2019, 365, 40–50. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, F.; Li, L.; Yuan, B.; Xie, C.; Yu, S. Lewis acidic deep eutectic solvents as catalysts for rosin polymerization. New Journal of Chemistry 2023, 47, 20144–20150. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, X.-F.; Wang, Z.; Zheng, T.; Yao, J. Deep eutectic solvent with bifunctional Brønsted-Lewis acids for highly efficient lignocellulose fractionation. Bioresource Technology 2022, 347, 126723. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-K.; Hong, S.; Wen, J.-l.; Ma, C.-Y.; Tang, L.; Jiang, H.; Chen, J.-J.; Li, S.; Shen, X.-J.; Yuan, T.-Q. Lewis Acid-Facilitated Deep Eutectic Solvent (DES) Pretreatment for Producing High-Purity and Antioxidative Lignin. ACS Sustainable Chemistry & Engineering 2020, 8, 1050–1057. [Google Scholar]
- Chen, H.; Sun, C.; Hu, Y.; Xia, C.; Sun, F.; Zhang, Z. Reaction characteristics of metal-salt coordinated deep eutectic solvents during lignocellulosic pretreatment. Journal of Environmental Chemical Engineering 2023, 11, 109531. [Google Scholar] [CrossRef]
- Liu, W.; Wang, F. p-Toluenesulfonic Acid-based Deep Eutectic Solvent as Transesterification Catalyst for Biodiesel Production. Journal of Oleo Science 2018, 67, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Potchamyou Ngatcha, A.D.; Zhao, A.; Zhang, S.; Xiong, W.; Sarker, M.; Xu, J.; Alam, M.A. Determination of active sites on the synthesis of novel Lewis acidic deep eutectic solvent catalysts and kinetic studies in microalgal biodiesel production. RSC Advances 2023, 13, 10110–10122. [Google Scholar] [CrossRef]
- Alam, M.A.; Deng, L.; Ngatcha, A.D. P.; Fouegue, A.D. T.; Wu, J.; Zhang, S.; Zhao, A.; Xiong, W.; Xu, J. Biodiesel production from microalgal biomass by Lewis acidic deep eutectic solvent catalysed direct transesterification. Industrial Crops and Products 2023, 206, 117725. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Peng, S.-Y.; Wang, H.; Zhang, Z.-Q.; Xu, Y.-P.; Sun, J.; Xu, Z.-N.; Guo, G.-C. Enhanced catalytic activity for CO esterification to dimethyl oxalate via increasing Lewis basic sites in Pd/MgAl-LDO catalyst. Catalysis Communications 2023, 184, 106781. [Google Scholar] [CrossRef]
- Zhang, S.; He, H.; Sun, F.; Zhao, N.; Du, J.; Pan, Q.; Zhu, G. A novel adenine-based zinc(II) metal-organic framework featuring the Lewis basic sites for heterogeneous catalysis. Inorganic Chemistry Communications 2017, 79, 55–59. [Google Scholar] [CrossRef]
- Liu, X.; Fan, W.; Zhang, M.; Li, G.; Liu, H.; Sun, D.; Zhao, L.; Zhu, H.; Guo, W. Enhancing light hydrocarbon storage and separation through introducing Lewis basic nitrogen sites within a carboxylate-decorated copper–organic framework. Materials Chemistry Frontiers 2018, 2, 1146–1154. [Google Scholar] [CrossRef]
- Naganawa, Y.; Abe, H.; Nishiyama, H. Design of bifunctional chiral phenanthroline ligand with Lewis basic site for palladium-catalyzed asymmetric allylic substitution. Chemical Communications 2018, 54, 2674–2677. [Google Scholar] [CrossRef]
- Hassan, H.M. A.; Alhumaimess, M.S.; Kamel, M.M.; Alsohaimi, I.H.; Aljaddua, H.I.; Aldosari, O.F.; Algamdi, M.S.; Mohamed, R.M. K.; El-Aassar, M.R. Electrospinning NH2-MIL-101/PAN nanofiber mats: A promising catalyst with Lewis acidic and basic bifunctional sites for organic transformation reactions. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2022, 642, 128659. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, Y.; Pan, J.; Liu, C.; Hu, Y.; Gao, Z.; Zhuang, X. Flexible nanofibrous membranes of dual metallic metal–organic framework with enhanced Lewis basic sites and high loading mass for efficient CO2 capture. Journal of Colloid and Interface Science 2023, 651, 200–210. [Google Scholar] [CrossRef]
- Machorro, J.J.; Lazaro, A.L.; Espejel-Ayala, F.; Coutiño-Gonzalez, E.; Chavarria-Hernandez, J.C.; Godínez, L.A.; Rodríguez-Valadez, F.J. The Roles of the Structure and Basic Sites of Sodium Titanates on Transesterification Reactions to Obtain Biodiesel Catalysts [Online], 2019.
- Cannilla, C.; Bonura, G.; Arena, F.; Rombi, E.; Frusteri, F. How surface and textural properties affect the behaviour of Mn-based catalysts during transesterification reaction to produce biodiesel. Catalysis Today 2012, 195, 32–43. [Google Scholar] [CrossRef]
- Lingfeng, C.; Guomin, X.; Bo, X.; Guangyuan, T. Transesterification of Cottonseed Oil to Biodiesel by Using Heterogeneous Solid Basic Catalysts. Energy & Fuels 2007, 21, 3740–3743. [Google Scholar]
- Pampararo, G.; Debecker, D.P. Sodium Aluminate-Catalyzed Biodiesel Synthesis. ACS Sustainable Chemistry & Engineering 2023, 11, 10413–10421. [Google Scholar]
- Singh, H.; Ali, A. Esterification as well as transesterification of waste oil using potassium imbued tungstophosphoric acid supported graphene oxide as heterogeneous catalyst: Optimization and kinetic modeling. Renewable Energy 2023, 207, 422–435. [Google Scholar] [CrossRef]
- Tavizón-Pozos, J.A.; Chavez-Esquivel, G.; Suárez-Toriello, V.A.; Santolalla-Vargas, C.E.; Luévano-Rivas, O.A.; Valdés-Martínez, O.U.; Talavera-López, A.; Rodriguez, J.A. State of Art of Alkaline Earth Metal Oxides Catalysts Used in the Transesterification of Oils for Biodiesel Production. Energies 2021, 14, 1031. [Google Scholar] [CrossRef]
- Cabrera-Munguia, D.A.; González, H.; Barreto-Gutiérrez, M.; Gutiérrez-Alejandre, A.; Rico, J.L.; Solís-Casados, D.A. Tuning the Basic Properties of ZnAl Hydrotalcites Modified with Ce Applied to Transesterification of Soybean Oil. Catalysis Letters 2020, 150, 1957–1969. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, H.; Liu, Z.; Miao, R.; He, L.; Guan, Q. Walnut-shaped calcium oxide-cancrinite spheres for transesterification of waste frying oil. Renewable Energy 2023, 208, 229–239. [Google Scholar] [CrossRef]
- Singh, V.; Hameed, B.H.; Sharma, Y.C. Economically viable production of biodiesel from a rural feedstock from eastern India, P. pinnata oil using a recyclable laboratory synthesized heterogeneous catalyst. Energy Conversion and Management 2016, 122, 52–62. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Luo, W.; Yang, G.; Miao, C.; Fu, J.; Xing, S.; Fan, P.; Lv, P.; Wang, Z. Sustainable biodiesel production via transesterification by using recyclable Ca2MgSi2O7 catalyst. Fuel 2017, 196, 306–313. [Google Scholar] [CrossRef]
- Alkimim, I.P.; Silva, L.L.; Cardoso, D. Synthesis of hybrid spherical silicas and application in catalytic transesterification reaction. Microporous and Mesoporous Materials 2017, 254, 37–44. [Google Scholar] [CrossRef]
- Kubota, Y.; Nishizaki, Y.; Ikeya, H.; Saeki, M.; Hida, T.; Kawazu, S.; Yoshida, M.; Fujii, H.; Sugi, Y. Organic–silicate hybrid catalysts based on various defined structures for Knoevenagel condensation. Microporous and Mesoporous Materials 2004, 70, 135–149. [Google Scholar] [CrossRef]
- Zhao, S.; Yi, H.; Tang, X.; Kang, D.; Yu, Q.; Gao, F.; Wang, J.; Huang, Y.; Yang, Z. Mechanism of activity enhancement of the Ni based hydrotalcite-derived materials in carbonyl sulfide removal. Materials Chemistry and Physics 2018, 205, 35–43. [Google Scholar] [CrossRef]
- Álvarez, M.G.; Plíšková, M.; Segarra, A.M.; Medina, F.; Figueras, F. Synthesis of glycerol carbonates by transesterification of glycerol in a continuous system using supported hydrotalcites as catalysts. Applied Catalysis B: Environmental 2012, 113-114, 212–220. [Google Scholar] [CrossRef]
- Coumans, F.J. A. G.; Mitchell, S.; Schütz, J.; Medlock, J.; Pérez-Ramírez, J. Hydrotalcite-Derived Mixed Oxides for the Synthesis of a Key Vitamin A Intermediate Reducing Waste. ACS Omega 2018, 3, 15293–15301. [Google Scholar] [CrossRef]
- Dahdah, E.; Estephane, J.; Taleb, Y.; El Khoury, B.; El Nakat, J.; Aouad, S. The role of rehydration in enhancing the basic properties of Mg–Al hydrotalcites for biodiesel production. Sustainable Chemistry and Pharmacy 2021, 22, 100487. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, B.; Wang, C.; Tian, Z.; Qu, W.; Ma, H.; Xu, R. Basicities and transesterification activities of Zn–Al hydrotalcites-derived solid bases. Green Chemistry 2014, 16, 2604–2613. [Google Scholar] [CrossRef]
- Sánchez Faba, E.M.; Ferrero, G.O.; Dias, J.M.; Eimer, G.A. Thermo-chemically tuning of active basic sites on nanoarchitectured silica for biodiesel production. Molecular Catalysis 2020, 481, 110171. [Google Scholar] [CrossRef]
- Wang, H.; Yan, S.; Salley, S.O.; Simon Ng, K.Y. Support effects on hydrotreating of soybean oil over NiMo carbide catalyst. Fuel 2013, 111, 81–87. [Google Scholar] [CrossRef]
- Ryoo, R. Birth of a class of nanomaterial. Nature 2019, 575, 40–41. [Google Scholar] [CrossRef]
- Davoodbasha, M.; Pugazhendhi, A.; Kim, J.-W.; Lee, S.-Y.; Nooruddin, T. Biodiesel production through transesterification of Chlorella vulgaris: Synthesis and characterization of CaO nanocatalyst. Fuel 2021, 300, 121018. [Google Scholar] [CrossRef]
- Kumari, N.; Aulakh, M.K.; Sareen, S.; Sharma, A.; Sohal, H.S.; Verma, M.; Mehta, S.K.; Mutreja, V. Greener Synthesis of Zirconium-Based Nanocatalyst for Transesterification. Topics in Catalysis 2022, 65, 1811–1820. [Google Scholar] [CrossRef]
- Al-Abbasi, A.; Almahdi, F.; Almaky, M.; Izriq, R.; Milad, A.; Salim, S.; Najar, A. BaO as a heterogeneous nanoparticle catalyst in oil transesterification for the production of FAME fuel. Inorganic Chemistry Communications 2023, 158, 111620. [Google Scholar] [CrossRef]
- Silva, I.F.; Rios, R.D. F.; Savateev, O.; Teixeira, I.F. Carbon Nitride-Based Nanomaterials as a Sustainable Catalyst for Biodiesel Production. ACS Applied Nano Materials 2023, 6, 9718–9727. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Yang, C.-M.; Nguyen Hoang, T.T.; Tsai, D.-H. Porous magnesia-alumina composite nanoparticle for biodiesel production. Fuel 2021, 285, 119203. [Google Scholar] [CrossRef]
- Widiarti, N.; Bahruji, H.; Holilah, H.; Ni’mah, Y.L.; Ediati, R.; Santoso, E.; Jalil, A.A.; Hamid, A.; Prasetyoko, D. Upgrading catalytic activity of NiO/CaO/MgO from natural limestone as catalysts for transesterification of coconut oil to biodiesel. Biomass Conversion and Biorefinery 2023, 13, 3001–3015. [Google Scholar] [CrossRef]
- Ghasemi, I.; Haghighi, M.; Bekhradinassab, E.; Ebrahimi, A. Ultrasound-assisted dispersion of bifunctional CaO-ZrO2 nanocatalyst over acidified kaolin for production of biodiesel from waste cooking oil. Renewable Energy 2024, 225, 120287. [Google Scholar] [CrossRef]
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Lee, H.V.; Taufiq-Yap, Y.H. A new route for the synthesis of La-Ca oxide supported on nano activated carbon via vacuum impregnation method for one pot esterification-transesterification reaction. Chemical Engineering Journal 2016, 304, 61–71. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, S.; Niu, S.; Lyu, Z.; Han, K.; Hu, X. Halloysite nanotube functionalized with La-Ca bimetallic oxides as novel transesterification catalyst for biodiesel production with molecular simulation. Energy Conversion and Management 2020, 220, 113138. [Google Scholar] [CrossRef]
- Lee, H.V.; Juan, J.C.; Taufiq-Yap, Y.H. Preparation and application of binary acid–base CaO–La2O3 catalyst for biodiesel production. Renewable Energy 2015, 74, 124–132. [Google Scholar] [CrossRef]
- Nuguid, R.J. G.; Ortino-Ghini, L.; Suskevich, V.L.; Yang, J.; Lietti, L.; Kröcher, O.; Ferri, D. Interconversion between Lewis and Brønsted–Lowry acid sites on vanadia-based catalysts. Physical Chemistry Chemical Physics 2022, 24, 4555–4561. [Google Scholar] [CrossRef] [PubMed]
- Marberger, A.; Ferri, D.; Elsener, M.; Kröcher, O. The Significance of Lewis Acid Sites for the Selective Catalytic Reduction of Nitric Oxide on Vanadium-Based Catalysts. Angewandte Chemie International Edition 2016, 55, 11989–11994. [Google Scholar] [CrossRef]
- Mulyatun, M.; Prameswari, J.; Istadi, I.; Widayat, W. Synthesis Method Effect on the Catalytic Performance of Acid–Base Bifunctional Catalysts for Converting Low-Quality Waste Cooking Oil to Biodiesel. Catalysis Letters 2024, 154, 4837–4855. [Google Scholar] [CrossRef]
- Kesica, Z.; Lukic, I.; Zdujic, M.; Liu, H.; Skala, D. Mechanochemically Synthesized CaO ZnO Catalyst For Biodiesel Production. Procedia Engineering 2012, 42, 1169–1178. [Google Scholar] [CrossRef]
- Mansir, N.; Hwa Teo, S.; Lokman Ibrahim, M.; Yun Hin, T.-Y. Synthesis and application of waste egg shell derived CaO supported W-Mo mixed oxide catalysts for FAME production from waste cooking oil: Effect of stoichiometry. Energy Conversion and Management 2017, 151, 216–226. [Google Scholar] [CrossRef]
- Yan, S.; Tong, T.; Li, Y.; Khan, S.U.; Zhao, J.; Wang, S.; Wang, X. Production of Biodiesel Through Esterification Reaction Using Choline Exchanging Polytungstoboronic Acids as Temperature-Responsive Catalysts. Catalysis Surveys from Asia 2017, 21, 151–159. [Google Scholar] [CrossRef]
- Lapis, A.A. M.; de Oliveira, L.F.; Neto, B.A. D.; Dupont, J. Ionic Liquid Supported Acid/Base-Catalyzed Production of Biodiesel. ChemSusChem 2008, 1, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, H.; Zhang, H.; Pan, H.; Yang, S. Efficient Catalytic Production of Biodiesel with Acid-Base Bifunctional Rod-Like Ca-B Oxides by the Sol-Gel Approach. Materials, 2019, 12, 83. [Google Scholar] [CrossRef]
- Cheng, J.; Guo, H.; Yang, X.; Mao, Y.; Qian, L.; Zhu, Y.; Yang, W. Phosphotungstic acid-modified zeolite imidazolate framework (ZIF-67) as an acid-base bifunctional heterogeneous catalyst for biodiesel production from microalgal lipids. Energy Conversion and Management 2021, 232, 113872. [Google Scholar] [CrossRef]
- Lee, G.; Lee, C.; Kim, H.; Jeon, Y.; Shul, Y.-G.; Park, J. Bifunctional 1,2,4-Triazole/12-Tungstophosphoric Acid Composite Nanoparticles for Biodiesel Production. Nanomaterials 2022, 12, 4022. [Google Scholar] [CrossRef]
- Mansir, N.; Taufiq-Yap, Y.H.; Rashid, U.; Lokman, I.M. Investigation of heterogeneous solid acid catalyst performance on low grade feedstocks for biodiesel production: A review. Energy Conversion and Management 2017 141, 171–182. [CrossRef]
- Rechnia-Gorący, P.; Malaika, A.; Kozłowski, M. Acidic activated carbons as catalysts of biodiesel formation. Diamond and Related Materials 2018, 87, 124–133. [Google Scholar] [CrossRef]
- Rocha, P.D.; Oliveira, L.S.; Franca, A.S. Sulfonated activated carbon from corn cobs as heterogeneous catalysts for biodiesel production using microwave-assisted transesterification. Renewable Energy 2019, 143, 1710–1716. [Google Scholar] [CrossRef]
- Luo, Y.; Mei, Z.; Liu, N.; Wang, H.; Han, C.; He, S. Synthesis of mesoporous sulfated zirconia nanoparticles with high surface area and their applies for biodiesel production as effective catalysts. Catalysis Today 2017, 298, 99–108. [Google Scholar] [CrossRef]
- Bora, A.P.; Konda, L.D. N. V. V.; Pasupuleti, S.; Durbha, K.S. Synthesis of MgO/MgSO4 nanocatalyst by thiourea–nitrate solution combustion for biodiesel production from waste cooking oil. Renewable Energy 2022, 190, 474–486. [Google Scholar] [CrossRef]
- Shobhana, G.; Asikin-Mijan, N.; AbdulKareem-Alsultan, G.; Sivasangar, S.; Izham, S.M.; Taufiq-Yap, Y.H. Biodiesel production via simultaneous esterification and transesterification of chicken fat oil by mesoporous sulfated Ce supported activated carbon. Biomass and Bioenergy 2020, 141, 105714. [Google Scholar]
- Li, H.; Liu, F.; Ma, X.; Cui, P.; Guo, M.; Li, Y.; Gao, Y.; Zhou, S.; Yu, M. An efficient basic heterogeneous catalyst synthesis of magnetic mesoporous Fe@C support SrO for transesterification. Renewable Energy 2020, 149, 816–827. [Google Scholar] [CrossRef]
- Lani, N.S.; Ngadi, N.; Mohammed Inuwa, I.; Anako Opotu, L.; Zakaria, Z.Y.; Haron, S. A cleaner approach with magnetically assisted reactor setup over CaO-zeolite/Fe3O4 catalyst in biodiesel production: Evaluation of catalytic performance, reusability and life cycle assessment studies. Journal of Cleaner Production 2023, 419, 138329. [Google Scholar] [CrossRef]
- Banerjee, S.; Rout, S.; Banerjee, S.; Atta, A.; Das, D. Fe2O3 nanocatalyst aided transesterification for biodiesel production from lipid-intact wet microalgal biomass: A biorefinery approach. Energy Conversion and Management 2019, 195, 844–853. [Google Scholar] [CrossRef]
- Xie, W.; Zang, X. Covalent immobilization of lipase onto aminopropyl-functionalized hydroxyapatite-encapsulated-γ-Fe2O3 nanoparticles: A magnetic biocatalyst for interesterification of soybean oil. Food Chemistry 2017, 227, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Mansoorsamaei, Z.; Mowla, D.; Esmaeilzadeh, F.; Dashtian, K. Sustainable biodiesel production from waste cooking oil using banana peel biochar-Fe2O3/Fe2K6O5 magnetic catalyst. Fuel 2024, 357, 129821. [Google Scholar] [CrossRef]
- Kouzu, M.; Hidaka, J.-s. Purification to remove leached CaO catalyst from biodiesel with the help of cation-exchange resin. Fuel 2013, 105, 318–324. [Google Scholar] [CrossRef]
- Xia, S.; Li, J.; Chen, G.; Tao, J.; Li, W.; Zhu, G. Magnetic reusable acid-base bifunctional Co doped Fe2O3–CaO nanocatalysts for biodiesel production from soybean oil and waste frying oil. Renewable Energy 2022, 189, 421–434. [Google Scholar] [CrossRef]
- Kannapiran, N.; Muthusamy, A.; Renganathan, B.; Ganesan, A.R.; Savithiri, S.; Meena, S.S. Magnetic, Dielectric and Ethanol Gas Sensing Properties of Poly(o-phenylenediamine)/(MnNi)Fe2O4 Nanocomposites and Quantum Chemical Calculations of (MnNi)Fe2O4. Journal of Inorganic and Organometallic Polymers and Materials 2022, 32, 2173–2191. [Google Scholar] [CrossRef]
- Rahmanivahid, B.; Ajamein, H.; Zakizadeh, T.; Nayebzadeh, H. Fabrication of super basic BaxMg(1-x)Fe2O4 magnetic spinel nanocatalyst toward biodiesel production. Materials Research Bulletin 2023, 165, 112321. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Fang, Z.; Yang, X.-X.; Yang, Y.-T.; Luo, J.; Xu, K.; Bao, G.-R. One-step production of biodiesel from Jatropha oils with high acid value at low temperature by magnetic acid-base amphoteric nanoparticles. Chemical Engineering Journal 2018, 348, 929–939. [Google Scholar] [CrossRef]
- Guo, H.; Cheng, J.; Mao, Y.; Qian, L.; Shao, Y.; Yang, W. Fabricating different coordination states of cobalt as magnetic acid-base bifunctional catalyst for biodiesel production from microalgal lipid. Fuel 2022, 322, 124172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




