1. Introduction
Platelet functional activity varied in healthy subjects and cardiovascular patients receiving antiplatelet therapy [
1,
2,
3,
4,
5,
6,
7]. Increased platelet activity is a risk factor of thrombotic events, including myocardial infarction and unstable angina (acute coronary syndrome, ACS), being associated with low sensitivity to antiplatelet drugs [
4,
5,
6,
7]. Platelet activity can be affected by such platelet phenotypic features as their size [
8,
9,
10] and the content of “young” reticulated forms [
8,
10,
11].
Early experiments on platelet fractionation and flow cytometry have shown that large platelets have higher functional activity: they better aggregate, express more adhesive molecules (glycoproteins (GP) IIb-IIIa and Ib), contain more intracellular granules and produce greater amounts of thromboxane A2 [
9,
10,
12,
13,
14,
15,
16]. In both experimental and clinical studies platelet size is usually assessed using such indexes as mean platelet volume (MPV), platelet large cell ratio (PLC-R), measured in the impedance-based hematological analyzers, and platelet forward scattering (FSC), measured in flow cytometers [
9,
10,
17].
Reticulated platelets (RP) (another term immature platelets) are “young” platelet forms, recently released into the bloodstream from the bone marrow. They contain residual RNA from megakaryocytes which is destroyed during circulation (platelets do not have nuclei and by themselves do not synthesize RNA
de novo). RP are identified in flow cytometry using RNA specific fluorescent dyes, like thiazole orange (TO) and some others. RP represent a minor platelet subpopulation, on average from 3-5% to about 10% in healthy donors (depending on detection methods) [
10,
11]. Microscopic and flow cytometry studies have shown that RP have a larger size [
10,
11,
14,
15,
18,
19,
20] and higher functional activity than the non-reticulated platelets [
10,
11,
14,
18,
19,
20,
21,
22,
23]. RP have increased ability for aggregation [
18,
22,
23], contain higher number of granules [
20], and expose more activation markers (activated GP IIb-IIIa, P-selectin) after stimulation by different agonists [
14,
19,
20,
21]. RP content correlates with platelet size of the whole platelet population in both healthy subjects [
18,
19] and cardiovascular patients treated with antiplatelet drugs [
21,
24].
In our previous study we have shown that in healthy subjects increased platelet size and RP content are associated with high platelet activity measured by activation-dependent exposure of activated GP IIb-IIIa (fibrinogen receptor) and P-selectin (marker of alpha-granule membranes) using flow cytometry [
19]. Binding of activated GP IIb-IIIa with fibrinogen mediates platelet aggregation and P-selectin is involved in platelet-leukocyte interaction.
In this study we tested the relationships between platelet size indexes and RP percentage (RP, %) and platelet function (exposure of activated GP IIb-IIIa and P-selectin) in cardiovascular patients receiving dual antiplatelet therapy – acetylsalicylic acid (ASA, an inhibitor of cyclooxygenase and thromboxane A2 synthesis) in combination with clopidogrel or ticagrelor (antagonists of P2Y12 ADP receptors) as well as in the control group of patients with risk factors for CHD, but no CHD/ACS and free of antiplatelet drugs.
2. Materials and Methods
Blood Collection and Patients
Blood was collected from CHD patients receiving ASA (75-100 mg/daily) + clopidogrel (75 mg/daily) (n = 55), ACS patients receiving ASA (75-100 mg/daily) + ticagrelor (90 mg x 2/daily) (n = 95) and from patients of the control group (n = 66) with no diagnosed CHD/ACS and free of antiplatelet drugs but with the risk factors for CHD (hypertension, diabetes mellitus, hypercholesterolemia). In ACS patients blood was collected on days 3-5 after the onset of the disease. Main characteristics of the control, CHD, and ACS groups are given in
Appendix A,
Table A1. All groups were comparable in age, while the number of males was greater in CHD and ACS groups. Control group included slightly fewer patients with hypertension and about the same number of patients with diabetes mellitus and hypercholesterolemia in comparison with CHD and ACS groups. There were slightly fewer males and slightly more patients with hypercholesterolemia in CHD in comparison with ACS. All participants were treated or monitored at a Chazov National Medical Research Center of Cardiology and signed informed consent for participation in the study. The study was approved by Ethics Committee of Chazov National Medical Research Center of Cardiology (protocol # 279, 25 April 2022).
Blood was taken from the antecubital vein using 18 g needles into 5% EDTA and 3.8% sodium citrate at a blood/anticoagulant ratio 9/1.
Platelet Count, Mean Platelet Volume (MPV) and Platelet Large Cell Ratio (PLC-R)
Platelet count, MPV and PLC-R were measured in EDTA-anticoagulated blood in an Abacus Junior B hematological analyzer (Diatron Ltd., Austria). Patients with thrombocytopenia (platelet count < 100 x 109/l) were excluded from the study.
Reticulated Platelets (RP)
RP were identified in EDTA-anticoagulated blood by thiazole orange (TO) staining as described earlier [
19]. In samples without TO, 5 µl whole blood was mixed with 990 µl BD FACS Flow reagent (BD Bioscience, San Jose, CA) and 5 µl CD42b-APC (BD Biosciences, San Jose, CA). In samples with TO, 5 µl whole blood was mixed with 940 µl BD FACS Flow reagent, 5 µl CD42b-APC and 50 µl 10 µg/ml TO solution. The samples were incubated for 30 min at room temperature, centrifuged at 2500 g for 3 min, and the pellet was resuspended in 350 µl BD FACS Flow reagent. Analysis was performed in a BD FACSCantoTM II flow cytometer using BD FACS DivaTM Software (BD Biosciences, San Jose, CA). Platelets were gated according to their size and staining with CD42b-APC; 10000 events in the platelet gate were analyzed. Platelets were considered as TO positive (RP) when their fluorescence exceeded that of > 99% platelets in sample without TO and their percentage (RP, %) was calculated
Platelet forward Scattering (FSC)
Platelet FSC mean values were determined by flow cytometry in the same sample as RP (see above) and expressed in arbitrary units (a.u.).
Platelet Function
Platelet function was assessed in citrated blood using flow cytometry by measuring activated GP IIb-IIIa and P-selectin exposure (binding of PAC-1 and CD62P antibodies respectively) as described earlier [
19]. Blood was diluted to 1:6 ratio with Tyrode/HEPES solution without CaCl2 (137 mМ NaCl, 2.7 mМ KCl, 0.36 mМ NaH2PO4, 0.1% dextrose, 5 mМ HEPES, pH 7.35, 1 mM MgCl2), containing 0.35% BSA. 60 µl diluted samples was supplemented with 3 µl CD42b-APC and either with 10 µl PAC-1-FITC (BD Biosciences, San Jose, CA) or 5 µl CD62P-FITC (IMTEK, Moscow) or 5 µl mouse IgG-FITC (IMTEK, Moscow). In the control group platelets were not activated or activated with thrombin receptor activating peptide (TRAP, sequence SFLLRN, a generous gift of Mikhail V. Ovchinnikov, Chazov National Medical Research Center of Cardiology) 10 µM or ADP (AppliChem GmbH, Darmstadt, Germany) 20, 5 or 2.5 µM and in CHD/ACS groups with TRAP 10 µM and ADP 20 and 5 µM in the absence or presence epinephrine (AppliChem GmbH, Darmstadt, Germany) 20 µM. Samples with antibodies were incubated for 15 min at 37
oC in the dark, fixed with an equal volume 2% paraformaldehyde, diluted by 250 µl BD FACS Flow reagent and analyzed in a BD FACSCantoTM II flow cytometer using BD FACS DivaTM Software. Platelets were gated according to their size and CD42b-APC staining; 10000 events in the platelet gate were analyzed. Mean fluorescence intensity (MFI, a.u.) for PAC-1-FITC and CD62P-FITC binding and the percentages of PAC-1 and CD62P positive platelets (PAC-1+ and CD62P+) were evaluated. Platelets were considered as PAC-1+ and CD62P+ when their fluorescence exceeded that of 95% platelets in control samples (non-activated platelets for PAC-1 and non-activated platelets with mouse IgG-FITC for CD62P).
Interleukin 6 (IL6)
IL6 was measured in plasma prepared from EDTA-anticoagulated blood (1500 g, 15 min x 2) using DuoSet Human IL6 kit (R&D Systems, Minneapolis, MN) according to the manufacturer’s instruction.
Statistics
Statistical analysis was performed using Statistica 12 software (Stat. Soft., USA). Parametric statistics was used since most variables fitted normal distribution according to Shapiro-Wilks test. Data are presented as means ± standard deviations (SD). Intergroup differences were estimated using Student’s t-test for means for comparison of quantitative parameters and Chi-square test for comparison of qualitative parameters. Correlations were assessed using Pearson test.
3. Results
Platelet size indexes (MPV, PLC-R and FSC), RP, % and exposure of activated GP IIb-IIIa and P-selectin after platelet activation with different agonists were measured in the control (without CHD/ACS receiving no antiplatelet drugs), CHD (ASA + clopidogrel) and ASC (ASA + ticagrelor) groups of patients (for patient characteristics – see
Appendix A,
Table A1). Intergroup differences for platelet size indexes and RP, % were insignificant, reaching statistical significance only for MPV between CHD and ACS groups (
Table 1). In all groups correlations between different platelet size indexes were strong and significant (r > 0.5, p < 0.001) although MPV and P-LCR were measured in a hematological analyzer and FSC in a flow cytometer (
Table 1). Mostly strong and highly significant correlations were also detected between RP, % and size indexes (r > 0.5, p < 0.001). Weaker but still significant correlations were found only between RP, % and MPV in CHD and ACS groups (r = 0.348 and r = 0.278 respectively, p < 0.01) (
Table 1).
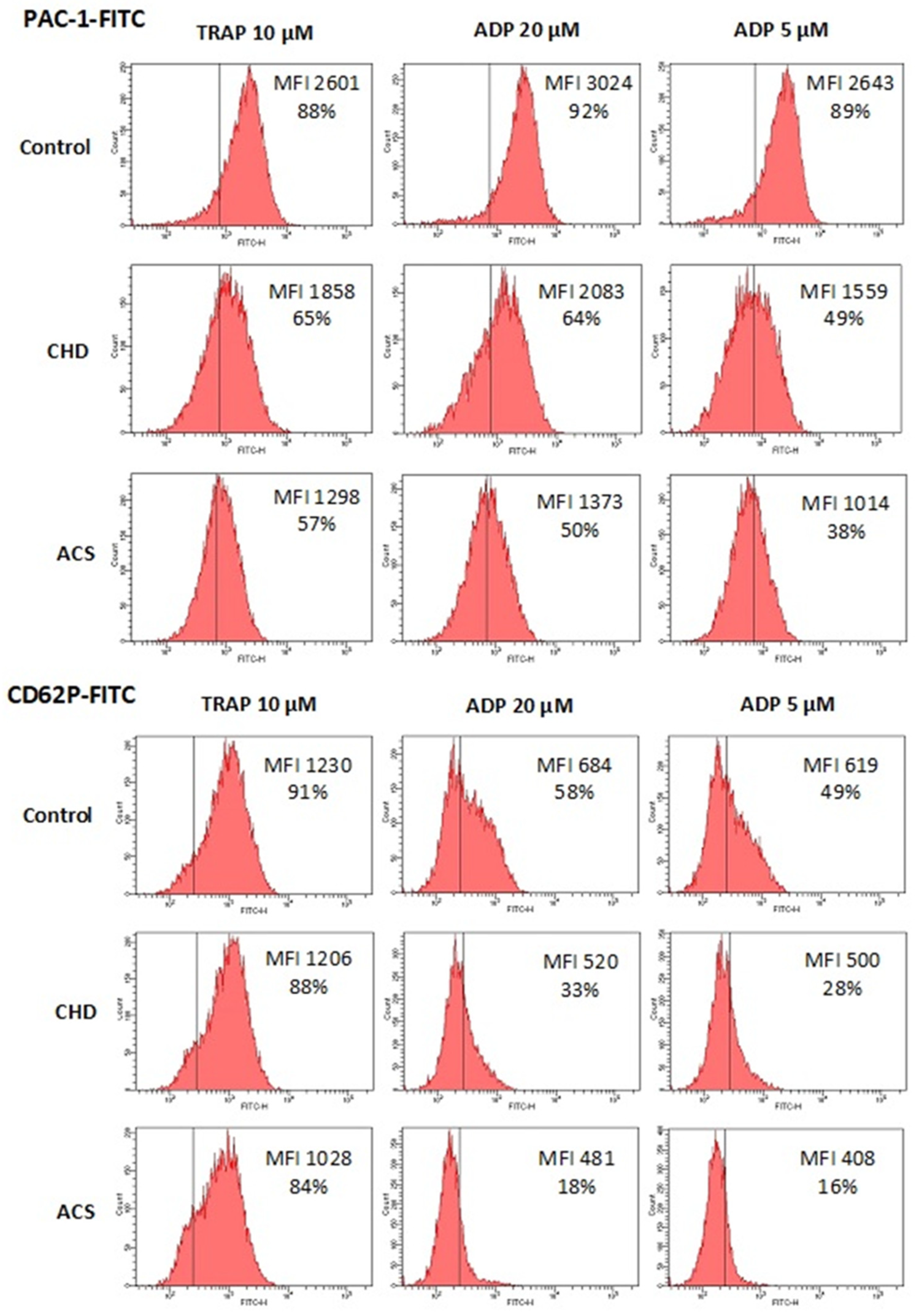
Platelet function was assessed by exposure of two activation markers, activated GP IIb-IIIa (PAC-1 antibody binding) and P-selectin (CD62P antibody binding). In control group (no antiplatelet drugs) platelets were activated by TRAP 10 µM, ADP 20, 5 and 2.5 µM. In CHD and ACS groups receiving strong dual antiplatelet therapy we omitted the lowest ADP dose (2.5 µM) and all other agonists were added not only alone but also in combination with 20 µM epinephrine, which potentiates their effects on platelet activation (see
Table 2). Exposure of activated GP IIb-IIIa and P selectin in response to the same agonists (TRAP 10 µM, ADP 20, 5 µM) was expectedly lower in CHD and ACS in comparison with the control group due to applied antiplatelet therapy (all differences were significant except for TRAP 10 µM, CD62P+, % in CHD group) (
Figure 1 and
Table 2). A decrease in platelet activity was greater in ACS in comparison to CHD since ticagrelor is a more potent P2Y12 antagonist than clopidogrel, although differences were not significant for some activation indexes (predominantly for the most powerful agonist – TRAP 10 µM + Epinephrine, 20 µM) (
Figure 1 and
Table 2)
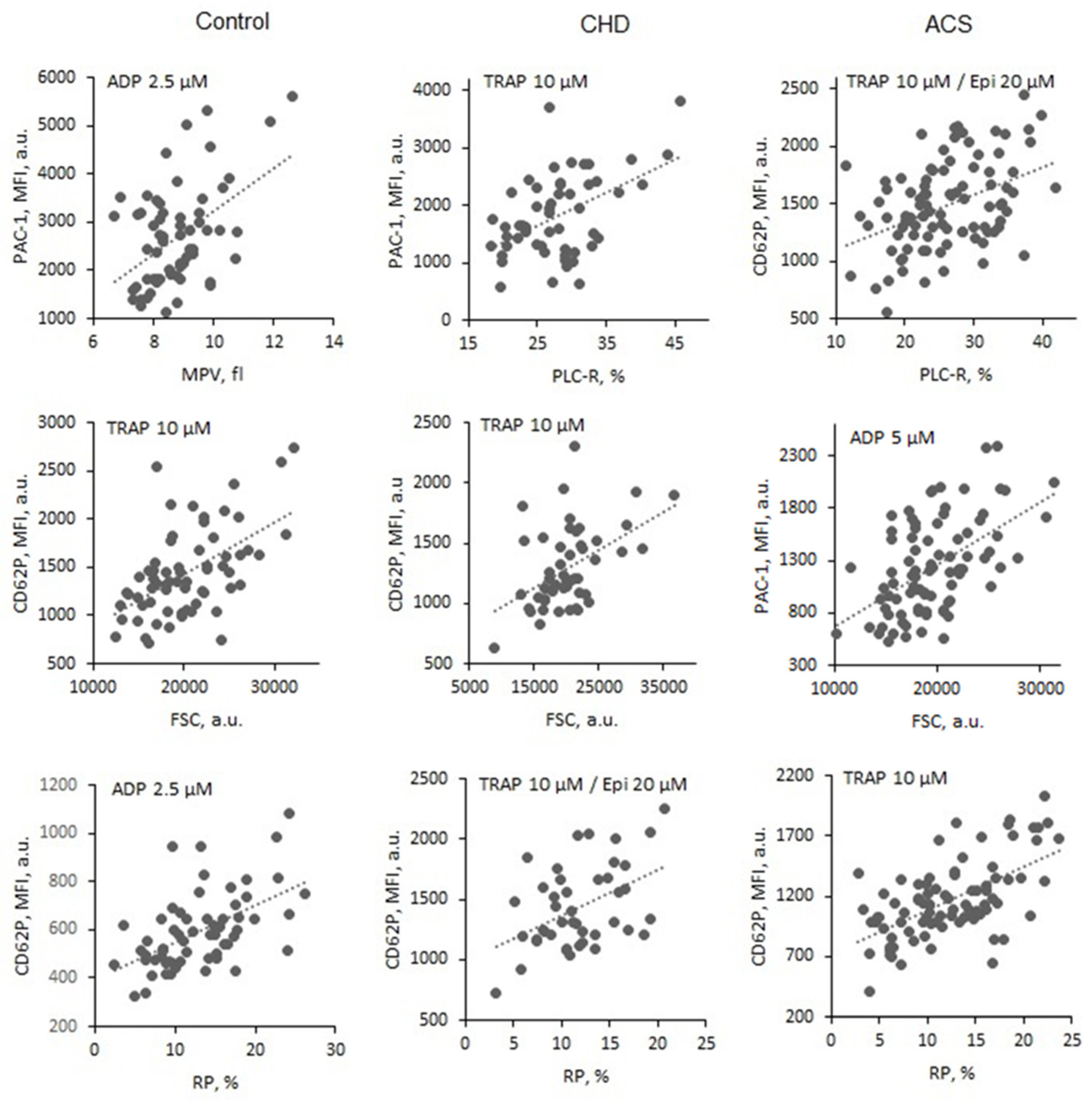
In the control group (no antiplatelet drugs) increased platelet size (assessed by all indexes) and increased RP, % were associated with increased exposure of both markers, activated GP IIb-IIIa and P-selectin (PAC-1-FITC and CD62-FITC MFI levels) after platelet activation with all applied agonists (TRAP 10 µM, ADP 20, 5 and 2.5 µM) (
Figure 2 and
Table 3). Moderate to high correlations were detected (r from 0.300 to 0.551) with the high significance (p mainly < 0.001 or < 0.01) (
Table 3).
In CHD (ASA + clopidogrel) and ACS (ASA + ticagrelor) groups platelets were activated by TRAP 10 µM, ADP 20, 5 µM ± epinephrine 20 µM. In many cases significant correlations between platelet size indexes, RP, % and activated GP IIb-IIIa and P-selectin (PAC-1-FITC and CD62-FITC MFI levels) were detected regardless the effect of strong antiplatelet drugs (some r values > 0.5) (
Figure 2 and
Table 4). More frequently significant correlations were established for activated GP IIb-III than for P-selectin particularly after platelet activation with ADP or ADP + epinephrine. Most rarely significant correlations were detected between MPV and both activation markers (
Table 4).
Plasma IL6 content was measured in the majority of patients in the control (n = 45), CHD (n = 44) and ACS groups (n = 82). All mean values varied in the range of 40-50 pg/ml and did not differ significantly between groups. In the control group platelet size (all indexes) and RP, % correlated with IL6 content: r - 0,641 (p < 0.001), 0.398 (p < 0.01), 0.520 (p < 0.001) and 0.430 (p < 0.01) for MPV, PLC-R, FSC and RP, % respectively. These correlations were determined by the presence of several patients with extremely high IL6 levels (> 100 pg/ml) since their exclusion made correlations weak and not significant. Patients with high IL6 content were identified in CHD and ACS groups as well, but no correlations were detected between platelet size indexes, RP, % and IL6 content in those groups (r < 0.1 everywhere).
4. Discussion
We examined the effects of two individual phenotypic factors, namely platelet size assessed by different indexes (MPV, PLC-R and FSC) and RP content (% in the entire platelet population) on platelet functional activity in cardiovascular patients. Previously, we revealed a direct association between platelet size indexes and RP, % and exposure of activated GP IIb-IIIa and P-selectin in healthy volunteers free of drugs affecting platelet function [
19]. In the present study we confirmed these results in a slightly different group also free of antiplatelet drugs. This group (the control one) consisted of patients with risk factors but without diagnosed CHD. Two other groups of patients with CHD and ACS received strong dual antiplatelet therapy, ASA + clopidogrel and ASA + ticagrelor, respectively. Antiplatelet drugs inhibited platelet function, inhibitory effect being potentially influenced by individual variations of pharmacodynamics and/or pharmacokinetics (particularly for clopidogrel when platelets are affected by active clopidogrel metabolite produced in the liver). However, even under these conditions we detected the influence of platelet size and RP, % on platelet functional activity. Significant correlations were detected less frequently than in the control group but some correlations were still strong (r > 0.5) and highly significant. Guthikonda et al. [
14] analyzed GP IIb-IIIa and P-selectin exposure induced by 10 µM ADP in CHD patients receiving ASA + clopidogrel who were stratified into tertiles according to RP, %. They reported differences for activated GP IIb-IIIa but not P-selectin exposure between tertiles with high and low RP content. Here we failed to obtain correlations of ADP-induced exposure of both markers with RP, % in comparable CHD group (ASA + clopidogrel) and the cause of the discrepancy in results on activated GP IIb-IIIa is unclear. However, we revealed significant correlations of activated GP IIb-IIIa and RP, % in ACS group (ASA + ticagrelor) confirming the influence of RP content on this reaction in patients receiving strong antiplatelet therapy. Most authors studying the effects of platelet size and RP content on routine platelet aggregation response reported positive correlations in patients receiving ASA + clopidogrel but not ASA + ticagrelor [
8,
10,
11]. Very low ADP-induced aggregation rates in patients treated with ticagrelor (in some cases close to zero level) which is more potent blocker of P2Y12 ADP receptor than clopidogrel may account for the lack of observed effects. We have demonstrated correlations of platelet size indexes and RP, % with ADP- (as well as TRAP-) induced activated GP IIb-IIIa exposure (a reaction preceding fibrinogen binding and aggregation) in ACS patients treated with ASA + ticagrelor. Presumably, this is due to relatively high levels of PAC-1 antibody binding to activated GP IIb-IIIa even under the action of ASA + ticagrelor (at least far from zero level) particularly in the presence of epinephrine. For CD62P exposure significant interactions of size indexes and RP, % in both groups were obtained mainly in TRAP-activated platelets. Thus, the data obtained in our study indicated that increased platelet size and RP content can diminish effects on platelet function even of such strong dual therapy as ASA + ticagrelor.
High platelet functional activity in patients with increased platelet size and RP content could explain why these laboratory parameters serve as a moderate risk factor of thrombotic events in cardiovascular patients, including those receiving dual antiplatelet therapy. The validity of these factors was estimated in separate clinical studies and meta-analysis [
9,
10,
11,
25,
26].
Both platelet size and RP content reflects the intensity of platelet production by megakaryocytes in the bone marrow. Presumably in some patients with accelerated thrombocytosis the release of “young” (reticulated) and large platelets is enchanced. Thrombopoietin and IL6 are two major regulators of megakaryocyte maturation and functioning [
27,
28]. In two studies including our own one modest although significant association were reported between increased platelet size (assessed by MPV index) and amount of TPO in blood plasma in cardiovascular patients [
16,
29]. In the present investigation we attempted to access interactions of size indexes and RP, % with IL6 levels. We established significant correlations only in the control but not CHD and ACS. Thus, our data do not allow to suggest a significant role of increased IL6 in regulation of platelet production and determination of platelet size and RP content at least in CHD and ACS patients.
Author Contributions
Design and concept, D.V.P., A.V.M.; methodology, V.V.B, O.N.S., N.V.G., patients’ selection and characteristics, A.K.A, V.V.V.; experiments and data collection, V.V.B, O.N.S., N.V.G.; data analysis, V.V.B., O.N.S., A.V.M., writing and editing, V.V.B., O.N.S., D.V.P., A.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Russian Science Foundation, grant # 22-15-00005.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Chazov National Medical Research Center of Cardiology (protocol # 279, 25 April 2022).
Informed Consent Statement
Written informed consent was received from all participants.
Acknowledgments
The authors like to thank Dr. Mikhail V. Ovchinnikov (Chazov National Medical Research Center of Cardiology) for supplying with TRAP reagent.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Table 1.
A. Main characteristics of the control (no antiplatelet drugs), CHD (ASA + clopidogrel) and ACS (ASA + ticagrelor) groups of patients.
Table 1.
A. Main characteristics of the control (no antiplatelet drugs), CHD (ASA + clopidogrel) and ACS (ASA + ticagrelor) groups of patients.
| |
Control |
CHD |
ACS |
| n |
66 |
55 |
95a
|
| Age (mean ± SD), years |
62 ± 13 |
65 ± 11 |
62 ± 10
p(CHD) = 0.052 |
| Male/female, n/n |
30/36 |
37/18* |
78/17***
p(CHD) = 0.038 |
| Hypertension, n (%) |
47 (71%) |
51 (93%)** |
84 (88%)**
p(CHD) = 0.398 |
| Diabetes mellitus, n (%) |
10 (15%) |
15 (27%) |
22 (23%)
p(CHD) = 0.573 |
| Hypercholesterolemia, n (%) |
22 (33%) |
27 (49%) |
30 (32%)
p(CHD) = 0.033 |
References
- Yee DL, Sun CW, Bergeron AL, Dong JG, Bray PF. Aggregometry detects platelet hyperreactivity in healthy individuals. Blood 2005; 106: 2723–2729. [CrossRef]
- Yakushkin VV, Zyuryaev IT, Khaspekova SG, Sirotkina OV, Ruda MYa, Mazurov AV. Glycoprotein IIb-IIIa content and platelet aggregation in healthy volunteers and patients with acute coronary syndrome. Platelets 2011; 22: 243-251. [CrossRef]
- De Gaetano G, Santimone I, Gianfagna F, Iacoviello L, Cerletti C. Variability of platelet indices and function: acquired and genetic factors. In: Gresele P, Born GVR, Patrono C, Page CP, editors. Handbook of experimental pharmacology 2010. Antiplatelet agents. Springer-Verlag, Berlin: Heidelberg; 2012. p. 395-434.
- Wisman PP, Roest M, Asselbergs FW, de Groot PG, Moll FL, van der Graaf Y, de Borst GJ. Platelet-reactivity tests identify patients at risk of secondary cardiovascular events: a systematic review and meta-analysis. Thromb Haemost 2014; 12: 736-747. [CrossRef]
- Aradi D, Kirtane A, Bonello L, Gurbel PA, Tantry US, Huber K, Freynhofer MK, ten Berg J6, Janssen P, Angiolillo DJ, Siller-Matula JM, Marcucci R, Patti G, Mangiacapra F, Valgimigli M, Morel O, Palmerini T, Price MJ, Cuisset T, Kastrati A, Stone GW, Sibbing D. Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J 2015; 36: 1762-1771. [CrossRef]
- Reny, JL, Fontana P, Hochholzer W, Neumann FJ., ten Berg J, Janssen PW, Geisler, T., Gawaz M, Marcucci R, Gori, A. M., Cuisset T, Alessi MC, Berdagué P, Gurbel PA., Yong G, Angiolillo DJ, Aradi D, Beigel R, Campo, G., Combescure C. Vascular risk levels affect the predictive value of platelet reactivity for the occurrence of MACE in patients on clopidogrel. Thromb Haemos. 2016; 115: 823-825. [CrossRef]
- Larsen PD, Holley AS, Sasse A, Al-Sinan A, Fairley S, Harding SA. Comparison of multiplate and verifyNow platelet function tests in predicting clinical outcome in patients with acute coronary syndromes. Thromb Res 2017; 152: 14-19. [CrossRef]
- Freynhofer MK, Gruber SC, Grove EL, Weiss TW, Wojta J, Huber K. Antiplatelet drugs in patients with enhanced platelet turnover: biomarkers versus platelet function testing. Thromb Haemost 2015; 114: 459–468. [CrossRef]
- Handtke S, Thiele T. Large and small platelets – (When) do they differ? J Thromb Haemost 2020; 18: 1256-1267. [CrossRef]
- Bodrova VV, Shustova ON, Khaspekova SG, Mazurov AV. Laboratory markers of platelet production and turnover. Biochemistry (Moscow) 2023; 88 (Suppl. 1): S39-S51. [CrossRef]
- Hannawi B, Hannawi Y, Kleiman NS. Reticulated platelets: changing focus from basics to outcomes. J Thromb Haemost 2018; 118: 1517-1527. [CrossRef]
- Thompson C.B., Jakubowsky J.A. The pathophysiology and clinical relevance of platelet heterogeneity. Blood 1988; 72: 1-8.
- Leytin V., Shapiro H., Novikov I., Radney J. Flow cytometric analysis of the platelet surface area and surface density of glycoprotein IIb-IIIa of unactivated human platelets of various sizes. Biochem Biophys Res Commun 1996; 226: 94–100. [CrossRef]
- Guthikonda S, Alviar CL, Vaduganathan M, Arikan M, Tellez A, DeLao T, Granada JF, Dong J-F, Kleiman NS, Lev EI. Role of reticulated platelets and platelet size heterogeneity on platelet activity after dual antiplatelet therapy with aspirin and clopidogrel in patients with stable coronary artery disease. J Am Coll Cardiol 2008; 52: 743-749. [CrossRef]
- Mangalpally K, Siqueiros-Garcia A, Vaduganathan M, Dong J-F, Kleiman NS, Guthikonda S. Platelet activation patterns in platelet size sub-populations: differential responses to aspirin in vitro. J Thromb Thrombolysis 2010; 30: 251–26211. [CrossRef]
- Khaspekova SG, Zyuryaev IT, Yakushkin VV, Sirotkina OV, Zaytseva NO, Ruda MYa, Panteleev MA, Mazurov AV. Relationships of glycoproteins IIb-IIIa and Ib content with mean platelet volume and their genetic polymorphisms. Blood Coagul Fibrinolysis 2014; 25: 128-134. [CrossRef]
- Connor D, Rabbolini D, Morel-Kopp M-C, Fixter K, Donikian D, Kondo M, Chan O, Jarvis S, Chen W, Brighton T, Chen V, Ward C, Joseph J. The utility of flow cytometric platelet forward scatter as an alternative to mean platelet volume. Platelets 2022; 22: 1-7. [CrossRef]
- Guthikonda S, Lev EI, Patel R, Delao T, Bergeron AL, Dong J-F, Kleiman NS. Reticulated platelets and uninhibited COX-1 and COX-2 decrease the antiplatelet effects of aspirin. J Thromb Haemost 2007; 5: 490-496. [CrossRef]
- Bodrova VV, Shustova ON, Khaspekova SG, Mazurov AV. Platelet reticulated forms, size indexes and functional activity. Interactions in healthy volunteers. Platelets 2022; 33: 398-403. [CrossRef]
- Hille L, Lenz M, Vlachos A, Grüning B, Hein L, Neumann F-J, Nührenberg TG, Trenk D. Ultrastructural, transcriptional, and functional differences between human reticulated and non-reticulated platelets. J Thromb Haemost 2020; 18: 2034-2046. [CrossRef]
- Lador A, Leshem LD, Spectre G, Abelow A, Kornowski R, Lev EI. Characterization of surface antigens of reticulated immature platelets. J Thromb Thrombolysis 2017; 44: 291-297. [CrossRef]
- McBane RD, Gonzalez C, Hodge DO, Wysokinski WE. Propensity for young reticulated platelet recruitment into arterial thrombi. J Thromb Thrombolysis 2014; 37: 148-154. [CrossRef]
- Armstrong PC, Hoefer T, Knowles RB, Tucker AT, Hayman MA, Ferreira PM, Chan MV, Warner TD. Newly formed reticulated platelets undermine pharmacokinetically short-lived antiplatelet therapies. Arterioscler Thromb Vasc Biol 2017; 37: 949-956. [CrossRef]
- Cesari F, Marcucci R, Caporale R, Paniccia R, Romano E, Gensini G-F, Abbate R, Gori A-M. Relationship between high platelet turnover and platelet function in high-risk patients with coronary artery disease on dual antiplatelet therapy. J Thromb Haemost 2008; 99: 930-935. [CrossRef]
- Sansanayudh N, Numthavaj P, Muntham D, Yamwong S, McEvoy M, Attia J, Sritara P, Thakkinstian A. Prognostic effect of mean platelet volume in patients with coronary artery disease. A systematic review and meta-analysis. Thromb Haemost 2015; 114: 1299–1309. [CrossRef]
- Zhaol Y, Lai R, Zhang Y, Shi D. The prognostic value of reticulated platelets in patients with coronary artery disease: a systematic review and meta-analysis. Front Cardiovasc Med 2020; 7: 57-61. [CrossRef]
- Kaushansky K. Thrombopoiesis. Semin Hematol, 2015; 52: 4-11. [CrossRef]
- Baatout S. Interleukin-6 and megakaryocytopoiesis: an update. Ann Hematol 1996; 73; 157-162. [CrossRef]
- Senaran H, Ileri M, Altinbaş A, Koşar A, Yetkin E, Oztürk M, Karaaslan Y, Kirazli S. Thrombopoietin and mean platelet volume in coronary artery disease. Clin Cardiol 2001; 24: 405–408. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).