1. Introduction
Over the past century, neurosurgical oncology has undergone significant evolution, with maximal safe surgical resection and adjuvant therapies now established as cornerstones of treatment (Figure 1). This approach strives to balance the imperative of reducing tumor burden while preserving critical neurovascular functions. Surgical principles for managing Central Nervous System (CNS) tumors have been explored since antiquity. Ancient Chinese findings from as early as 10,000 BCE describe the management of brain tumors whilst trepanned skulls dating back to prehistoric times have been found in France, Mexico and Peru [
1]. The seminal works of William W. Keen, Henry Cushing and Walter Dandy marked a crucial transition to modern neuro-oncology. William Keen's pioneering craniotomy in 1888 for successful brain tumor resection set the stage for subsequent advancements in surgical techniques. Cushing further revolutionized neuro-oncology with innovative and meticulous operative methods significantly improving patient outcomes, as documented through his extensive case series of 2000 intracranial tumors [
2]. Technological breakthroughs like ventriculography and pneumoencephalography, introduced by Walter Dandy, enabled precise localization of brain tumors through the use of contrast agents and air injected into subarachnoid spaces, thereby enhancing diagnostic accuracy and surgical planning [
3,
4]. The advent of surgical microscopes by Gaza Yasargil in 1969 further elevated the precision of brain tumor surgery, laying foundational groundwork for the integration of advanced imaging techniques, molecular diagnostics, and targeted therapies in modern day neurosurgical oncology.
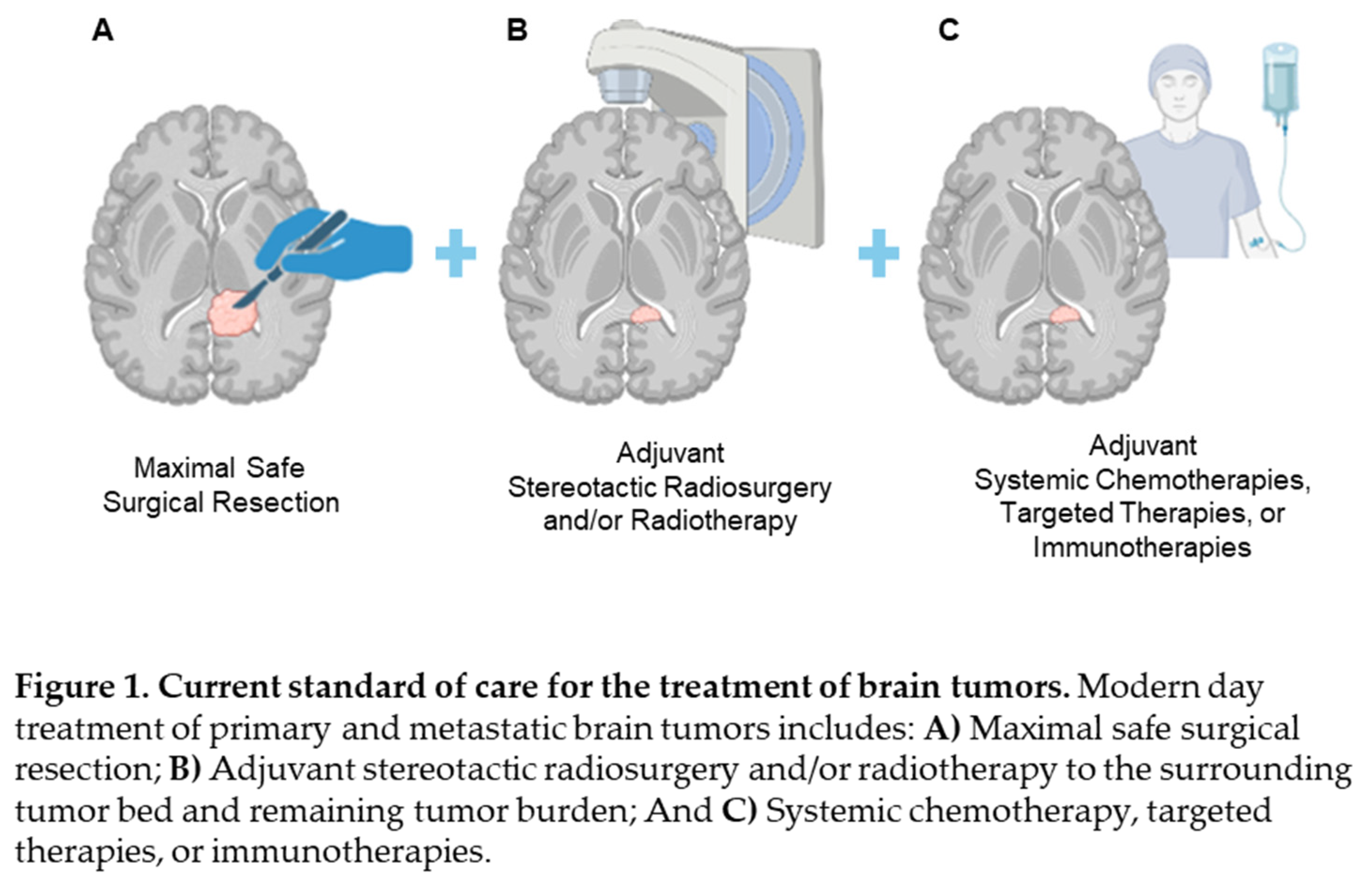
Despite these advancements, significant challenges remain in the standard of care for brain tumor patients. Survival rates for brain tumors remain variable, influenced by tumor-specific factors, patient demographics, and therapeutic variables. For instance, the most aggressive primary adult brain tumor, glioblastoma (GBM), continues to present particularly dismal prognoses, with a median survival of approximately 15 months despite aggressive multimodal therapies involving maximal safe resection, adjuvant radiotherapy, and chemotherapy with agents like temozolomide [
5]. The limited success in improving survival outcomes underscores the persistent challenges and limitations inherent in current therapeutic approaches.
One such challenge is the presence of the blood brain barrier (BBB), which restricts the effective delivery of chemotherapeutics to tumor sites within the CNS [
6]. The BBB consists of tight junctions between endothelial cells, astrocyte foot processes, and pericytes. While small (<400 Da) lipophilic drugs may be able to penetrate the BBB, its structure still poses a considerable obstacle to effective drug delivery including for temozolomide (TMZ), the current standard of care systemic chemotherapy agent for GBM patients, where peak cerebrospinal fluid (CSF) TMZ concentration typically averages only 20% of the circulating plasma concentrations [
7]. The complexity of drug delivery to tumor cells is compounded by the blood tumor barrier (BTB). This refers to abnormal neovascularization downstream to hypoxia and angiogenic factor release, which can impede chemotherapy entry in tumor areas [
8]. Drug efflux transport proteins within the BBB also present a major obstacle to chemotherapeutics, alongside the CSF drug washout effect, wherein continuous CSF circulation results in rapid drug clearance, reducing effective concentration and duration of action within the CNS [
9]. Novel technologies which could enhance delivery of therapies across the BBB and BTB could improve treatment outcomes for brain tumor patients.
Our success as tumor surgeons in offering our patients the best outcomes is contingent on our ability to achieve maximal safe resection. However, tumors located in critical or deep brain regions pose significant challenges due to the risk of neurological deficits associated with aggressive resection. Furthermore, the accurate delineation of tumor margins, particularly for diffuse infiltrative tumors such as gliomas, remains a persistent challenge during surgery, often resulting in microscopic residual disease at the tumor periphery [
10]. Fluorescence-guided surgery (FGS) has emerged as a promising adjunct to conventional neurosurgical techniques. Fluorescent agents like 5-aminolevulinic acid (5-ALA), which metabolizes into Protoporphyrin IX (PPIX) within tumor cells and fluoresces under blue light, enhances the intraoperative visualization and delineation of tumor tissue and has demonstrated efficacy in increasing extent of resection in high grade gliomas [
11]. Indocyanine green (ICG), another fluorescent dye in the near infrared (NIR) spectrum, exhibits enhanced specificity to tumor cells due to their accelerated endocytosis and disruption of tight junctions, enabling its preferential accumulation within neoplastic tissue [
12]. ICG enhances tissue penetration, predicts gadolinium enhancement on post-operative magnetic resonance imaging (MRI), and demonstrates higher sensitivity and negative predictive value compared to 5-ALA in detecting neoplastic tissue [
13,
14]. The ability to integrate a tumor-targeting diagnostic moiety with a therapeutic agent (a
theranostic) holds promise in overcoming the conventions of surgical resection followed by adjuvant chemoradiation [
15]. This review explores the potential of theranostics to go “beyond the knife” to transform the field of neurosurgical oncology and significantly improve patient outcomes.
2. Materials and Methods
We performed a review of the literature from 1999 to 2024 on the topics of nanotechnology and theranostics as applied in the fields of neurosurgical oncology and neuro-oncology.
3. Results
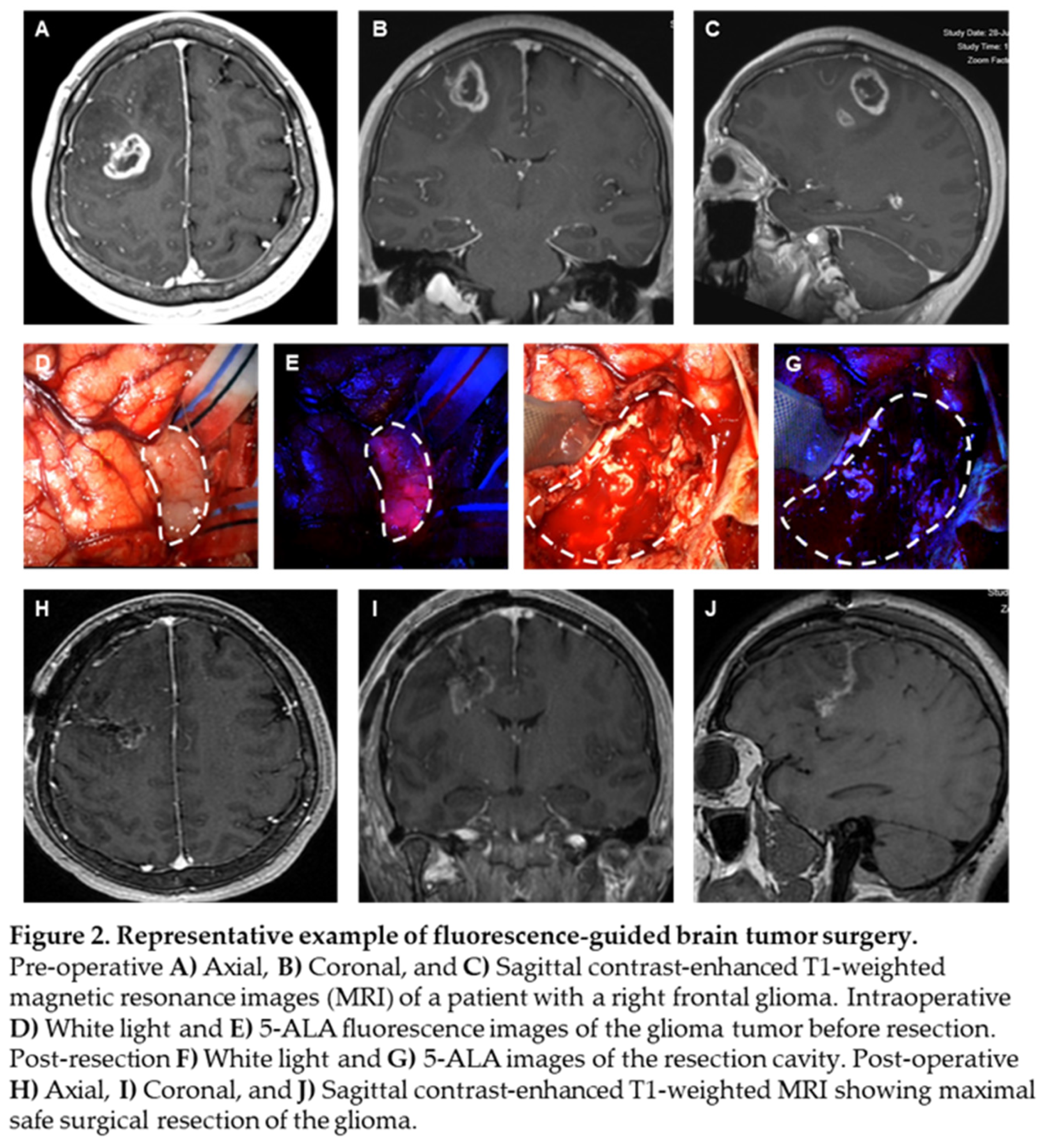
5-ALA, given intravenously at 20 mg/kg 3 hours prior to induction of anesthesia, can effectively identify high grade glioma tissue during surgery. We present a case of a patient with a right frontal, ring-enhancing intra-axial brain lesion, as visualized on axial, coronal, and sagittal MRI scans, respectively (Figure 2A-C). Intraoperative white light microscopy shows flesh colored tumor tissue (Figure 2D, white dashed border), which emits 5-ALA fluorescence (Figure 2E, white dashed border), allowing for the neurosurgeon to achieve maximal extent of resection of tumor tissue without sacrificing neighboring brain tissue. Blood from surrounding tumor vasculature at the time of surgery can obscure the surgeon’s ability to differentiate infiltrative tumor (Figure 2F, white dashed border). Persistence of 5-ALA signal allows for the surgeon to continue resection within a margin of safety (Figure 2G, white dashed border). Post-operative MRI scans demonstrate maximal extent of resection, with effective removal of the bulk of the ring-enhancing tumor (Figure 2H-J) from the patient’s brain and the absence of neurological deficits.
We previously published on two different theranostic tools with significant translational potential for use in neuro-oncology. One technology involves the formulation of liposomal nanoparticles which are capable of packaging water soluble small molecules such as TMZ in its aqueous center and hydrophobic small molecules such as the bromodomain inhibitor JQ1 in its lipid bilayer, allowing for delivery of dual combination therapies (Figure 3A) [
16]. These liposomal nanoparticles can be further functionalized on their surface with proteins such as transferrin, which have been shown to enable receptor-mediated transcytosis across the BBB, and fluorophores, allowing for fluorescence detection. In an intracranial orthotopic xenograft mouse model of GBM, we demonstrated that these transferrin-functionalized nanoparticles were capable of crossing the BBB and BTB, attaching to the surface of intracranial glioma tumors which inherently overexpress transferrin receptors on their cell membranes (Figure 3C, transferrin receptors) [
16]. The ability to achieve tumor specific delivery of combination therapies across the BBB led to decreased tumor burden, prolonged surgical and relative reduction in systemic drug toxicity profiles in glioma-bearing mice.
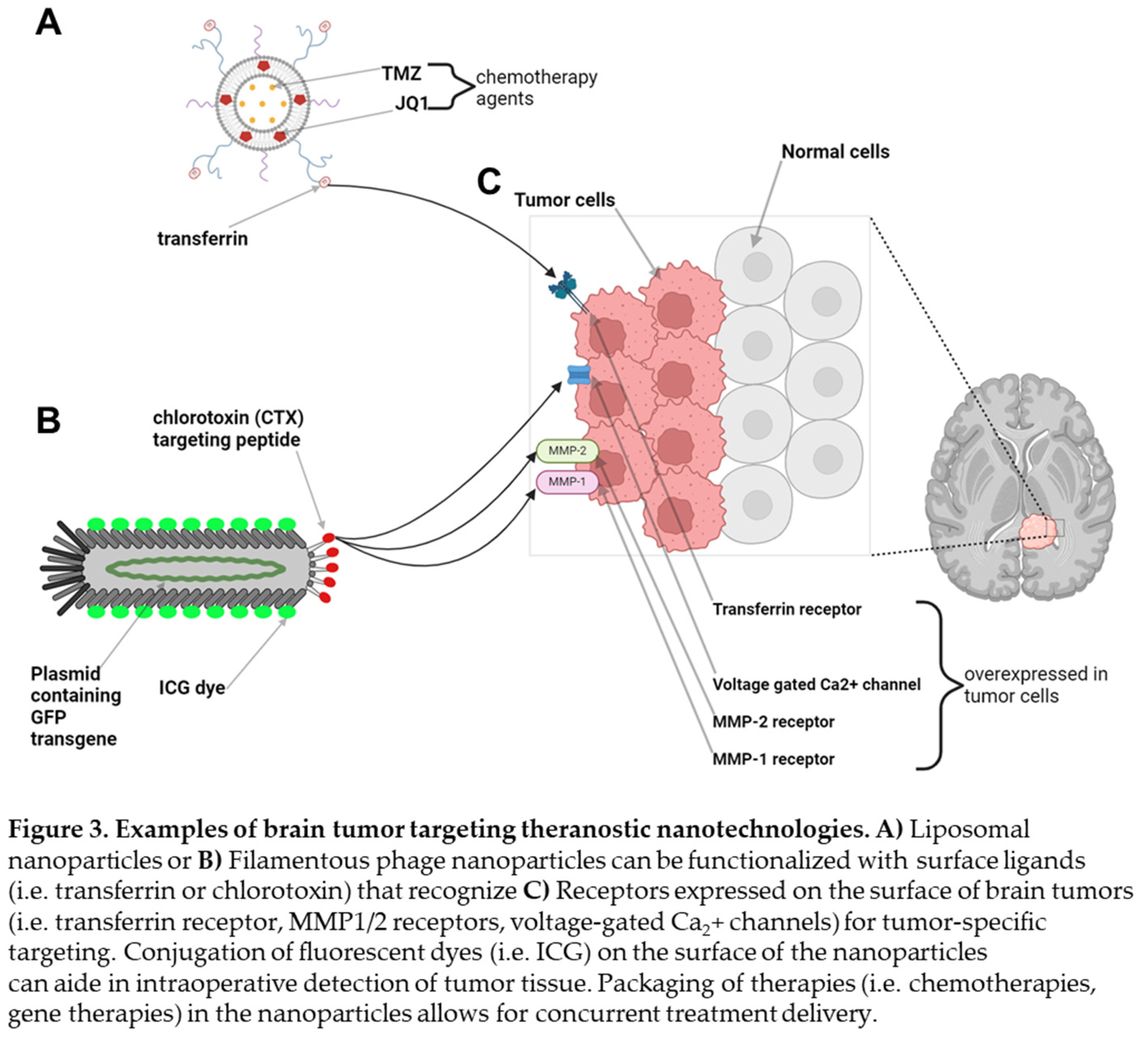
We recently published our ability to leverage filamentous M13 bacteriophage as a theranostic for tumor imaging in the short-wave infrared (SWIR) spectrum using a patient-derived orthotopic xenograft mouse model of GBM (Figure 3B). Filamentous phage particles are narrow in diameter (5 nm), modular in length, and genetically tunable with the ability to express transgene plamids. Similar to liposomal nanoparticles, phage particles have surface peptides that can be conjugated with fluorophores and small molecules. We produced ultrashort (50 nm) M13 “inho” phage particles that expressed the 28 amino acid chlorotoxin (CTX) peptide, known to recognize the MMP1/2 receptors on the surface of glioma cells [
17]. We then conjugated ICG fluorophores on the surface of inho phage particles and delivered them intravenously in a patient-derived xenograft mouse model of GBM, enabling intracranial detection of brain tumors in mice using a SWIR imaging system (Figure 3C) [
18]. Taken together, our ability to combine existing advanced intraoperative neurosurgical techniques with translational theranostic technologies may allow us to address the large unmet need in offering our brain tumor patients significant survival benefits.
4. Discussion
Stanford University is internationally acclaimed for its pioneering work in neurosurgery, neuro-oncology, and technological innovation. Dr. John R. Adler Jr. at Stanford Neurosurgery pioneered Cyberknife robotic stereotactic radiosurgery (SRS) for the treatment of brain and spine neurosurgical conditions [
19]. Under the current co-director leadership of Dr. Steven D. Chang in the Department of Neurosurgery and Dr. Scott G. Soltys in the Department of Radiation Oncology, we previous published on the treatment of our first 7,000 patients coming through the Cyberknife radiosurgery program between the years of 1999 to 2018 [
20]. To date, the Cyberknife program has treated over
11,000 patients. This review presents our vision of converging translational theranostic tumor-targeting platforms to further augment the current clinical platforms of image-guided surgery, FGS, SRS, and delivery of precision cancer therapies to our patients (Figure 4A).
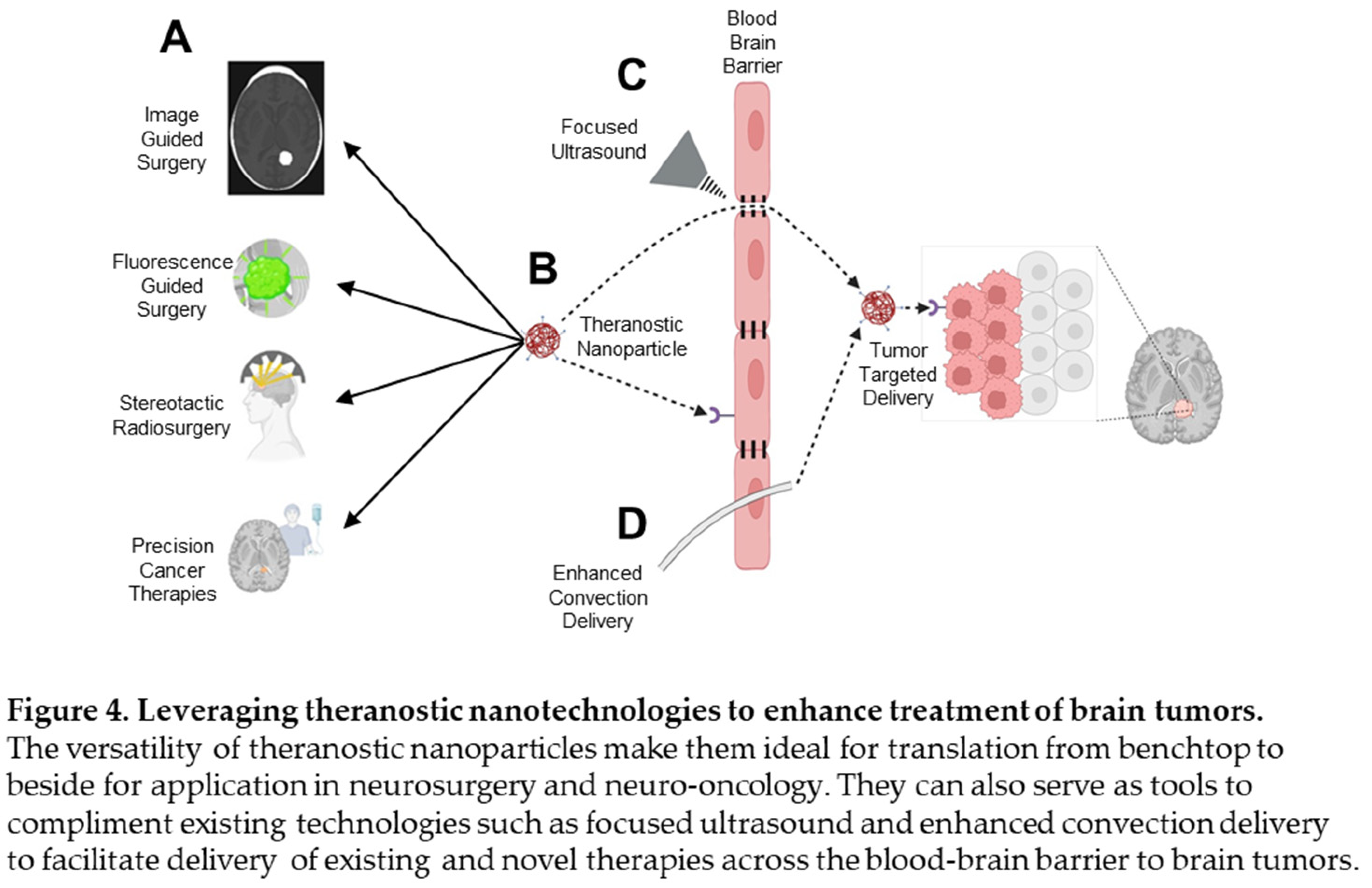
Whilst gadolinium-based contrast imaging is the gold standard in image-guided neurosurgery, its non-specific accumulation in areas of BBB disruption can blur the distinction between tumor tissue and inflamed margins [
21]. Theranostic agents targeting tumor-specific receptors could potentially improve tumor resolution, reduce non-specific contrast accumulation, and integrate diagnostic and therapeutic functions into a single platform, offering real-time feedback on therapeutic efficacy. Current fluorophores for use in FSG also face notable limitations. 5-ALA has limited penetration depth and lack of benefit in lower grade gliomas [
22], and fluorescein sodium accumulates in edematous and surgically inflamed non-tumor tissues due to reliance on BBB leakage [
23]. Nanoparticle-based delivery systems can potentially address these issues by enhancing targeting accuracy, improving tumor margin delineation and broadening applicability across a variety of CNS tumors (Figure 4B). An early phase clinical trial using pegylated nanoliposomal irinotecan combined with metronomic TMZ was tested in recurrent glioblastoma patients, though without specific tumor-targeting mechanisms, a further indication of the gradual coming of age of theranostic technology in the field of neuro-oncology [
24].
The Stanford Cyberknife radiosurgery program continues to push the frontiers in the use of SRS for the treatment of brain and spine tumors. Our vision to apply theranostic technologies to deliver agents that can further enhance the radiobiological effect of radiation on tumors, for example, can be a game changer. Such agents can include radiosensitizers which enhance tumor cell susceptibility to radiation, reduce repopulation during SRS, and achieve greater tumor control with reduced radiation dose, minimizing damage to surrounding tissue [
25]. The modular nature of theranostics also allows us to deliver multimodal therapies that can be both additive and/or synergistic in their tumoricidal effects [
26,
27]. We can further leverage existing tools for BBB disruption such as focused ultrasound technology (Figure 4C) and convection enhanced delivery (Figure 4D) to optimize therapeutic delivery.
Whilst theranostic technologies hold considerable promise, several limitations must be acknowledged. Immunogenicity remains a significant concern, particularly with the use of viral vectors and bacteriophage-based systems [
28,29]. Nanoparticle-based delivery systems can also elicit varying effects on the innate immune response, with the potential to induce both immune overactivation and immunosuppression [30]. Furthermore, the ultrasmall size and large surface area of nanoparticles, whilst facilitating receptor interactions at the tumor site, also promotes organ accumulation and mediates toxicity. Nanoparticles have been associated with the accumulation of reactive oxygen species (ROS), mitochondrial damage, inflammation, cellular apoptosis and DNA damage across a variety of organ systems including the respiratory, nervous, endocrine and reproductive systems [31]. Continued research is necessary to fully understand the pharmacological properties and long-term effects of nanoparticle therapies in humans.
Moreover, the transition from benchtop to beside presents significant challenges. Whilst preclinical models have demonstrated success in overcoming the BBB and achieving targeted delivery, translating into clinical practice remains complex. This complexity arises in part from the inability of preclinical models to fully replicate the heterogeneity of human tumors, particularly in terms of the tumor microenvironment and BBB characteristics [32]. Finally, the scalability and reproducibility of nanoparticle manufacturing processes also poses a substantial challenge, exacerbated by regulatory requirements and the high financial barriers associated with the development, testing and production of theranostic technologies [33]. Nevertheless, the growing involvement of clinical scientists in nanotherapeutics research will invariably accelerate the translation of these innovations into clinical settings to improve the treatment and survival outcomes for brain tumor patients.
Author Contributions
Conceptualization, S.G., F.C.L.; methodology, S.G., F.C.L.; resources, S.G., F.C.L., D.J.P., S.D.C.; data curation, S.G., F.C.L., D.J.P.; writing—original draft preparation, S.G., F.C.L.; writing—review and editing, S.G., F.C.L., Y.H., D.A.B., D.J.P., S.D.C.; supervision, D.J.P., S.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the descriptive nature of this review not affecting nor involving the well-being of human subjects.
Informed Consent Statement
Patient consent was waived due to all images being stripped of all patient identifiers.
Data Availability Statement
Not applicable.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
-
Principles of Neuro-Oncology; Springer Nature: Dordrecht, GX, Netherlands, 2021.
- Cairns, H. The Ultimate Results of Operations for Intracranial Tumours: A Study of a Series of Cases after a Nine-Year Interval. . 1936, 8, 421–92. [Google Scholar] [PubMed]
- Dandy, W.E. VENTRICULOGRAPHY FOLLOWING THE INJECTION OF AIR INTO THE CEREBRAL VENTRICLES. Ann. Surg. 1918, 68, 5–11. [Google Scholar] [CrossRef]
- Dandy, W.E. Röntgenography of the brain after the injection of air into the spinal canal. Ann. Surg. 1919, 70, 397–403. [Google Scholar] [CrossRef]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef]
- Sonabend, A.M.; Gould, A.; Amidei, C.; Ward, R.; A Schmidt, K.; Zhang, D.Y.; Gomez, C.; Bebawy, J.F.; Liu, B.P.; Bouchoux, G.; et al. Repeated blood–brain barrier opening with an implantable ultrasound device for delivery of albumin-bound paclitaxel in patients with recurrent glioblastoma: a phase 1 trial. Lancet Oncol. 2023, 24, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.A.; Stupp, R. Plasma and Cerebrospinal Fluid Population Pharmacokinetics of Temozolomide in Malignant Glioma Patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef]
- Ortiz, R.; Perazzoli, G.; Cabeza, L.; Jiménez-Luna, C.; Luque, R.; Prados, J.; Melguizo, C. Temozolomide: An Updated Overview of Resistance Mechanisms, Nanotechnology Advances and Clinical Applications. Curr. Neuropharmacol. 2021, 19, 513–537. [Google Scholar] [CrossRef]
- Deeken, J.F.; Löscher, W. The Blood-Brain Barrier and Cancer: Transporters, Treatment, and Trojan Horses. Clin. Cancer Res. 2007, 13, 1663–1674. [Google Scholar] [CrossRef]
- Sanai, N.; Berger, M.S. GLIOMA EXTENT OF RESECTION AND ITS IMPACT ON PATIENT OUTCOME. Neurosurgery 2008, 62, 753–766. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Onda, N.; Kimura, M.; Yoshida, T.; Shibutani, M. Preferential tumor cellular uptake and retention of indocyanine green forin vivotumor imaging. Int. J. Cancer 2016, 139, 673–682. [Google Scholar] [CrossRef]
- Lee, J.Y.; Thawani, J.P.; Pierce, J.; Zeh, R.; Martinez-Lage, M.; Chanin, M.; Venegas, O.; Nims, S.; Learned, K.; Keating, J.; et al. Intraoperative Near-Infrared Optical Imaging Can Localize Gadolinium-Enhancing Gliomas During Surgery. Neurosurgery 2016, 79, 856–871. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.; Salinas, R.; De Ravin, E.; Teng, C.W.; Li, C.; Abdullah, K.G.; Buch, L.; Hussain, J.; Ahmed, F.; Dorsey, J.; et al. Near-Infrared Imaging with Second-Window Indocyanine Green in Newly Diagnosed High-Grade Gliomas Predicts Gadolinium Enhancement on Postoperative Magnetic Resonance Imaging. Mol. Imaging Biol. 2019, 22, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- D'Angelo, M.; Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Dominguez-Benot, R.; Pitari, G.; Ippoliti, R.; Cimini, A. Theranostic Nanomedicine for Malignant Gliomas. Front. Bioeng. Biotechnol. 2019, 7, 325. [Google Scholar] [CrossRef]
- Barajas, R.F.; E Hamilton, B.; Schwartz, D.; McConnell, H.L.; Pettersson, D.R.; Horvath, A.; Szidonya, L.; Varallyay, C.G.; Firkins, J.; Jaboin, J.J.; et al. Combined iron oxide nanoparticle ferumoxytol and gadolinium contrast enhanced MRI define glioblastoma pseudoprogression. Neuro-Oncology 2019, 21, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.G.; Walker, D.G.; Miller, D.M.; Butte, P.; Morrison, B.; Kittle, D.S.; Hansen, S.J.; Nufer, K.L.; A Byrnes-Blake, K.; Yamada, M.; et al. Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults With Newly Diagnosed or Recurrent Gliomas. Neurosurgery 2019, 85, E641–E648. [Google Scholar] [CrossRef]
- Tsedev, U.; Lin, C.-W.; Hess, G.T.; Sarkaria, J.N.; Lam, F.C.; Belcher, A.M. Phage Particles of Controlled Length and Genome for In Vivo Targeted Glioblastoma Imaging and Therapeutic Delivery. ACS Nano 2022, 16, 11676–11691. [Google Scholar] [CrossRef]
- Adler JR, Jr., Murphy MJ, Chang SD, Hancock SL. Image-guided robotic radiosurgery. Neurosurgery. 1999 Jun;44(6):1299-306; discussion 306-7. PMID10371630.
- Fatima, N.; Meola, A.; Ding, V.Y.; Pollom, E.; Soltys, S.G.; Chuang, C.F.; Shahsavari, N.; Hancock, S.L.; Gibbs, I.C.; Adler, J.R.; et al. The Stanford stereotactic radiosurgery experience on 7000 patients over 2 decades (1999–2018): looking far beyond the scalpel. J. Neurosurg. 2021, 135, 1725–1741. [Google Scholar] [CrossRef]
- Iyad, N.; Ahmad, M.S.; Alkhatib, S.G.; Hjouj, M. Gadolinium contrast agents- challenges and opportunities of a multidisciplinary approach: Literature review. Eur. J. Radiol. Open 2023, 11, 100503. [Google Scholar] [CrossRef]
- Mirza, A.B.; Lavrador, J.P.; Christodoulides, I.; Boardman, T.M.; Vastani, A.; Al Banna, Q.; Ahmed, R.; Norman, I.C.F.; Murphy, C.; Devi, S.; et al. 5-Aminolevulinic Acid-Guided Resection in Grade III Tumors—A Comparative Cohort Study. Neurosurg. 2022, 22, 215–223. [Google Scholar] [CrossRef]
- Stummer, W. Fluorescein in brain metastasis and glioma surgery. Acta Neurochir. 2015, 157, 2199–2200. [Google Scholar] [CrossRef]
- Elinzano, H.; Toms, S.; Robison, J.B.; Mohler, A.B.; Carcieri, A.B.; Cielo, D.B.; Donnelly, J.; Disano, D.; Vatketich, J.; Baekey, J.; et al. Nanoliposomal Irinotecan and Metronomic Temozolomide for Patients with Recurrent Glioblastoma. Am. J. Clin. Oncol. 2020, 44, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Han, Y.; Zhang, X.; Ma, H.; Li, L.; Yu, R.; Liu, H. Application of New Radiosensitizer Based on Nano-Biotechnology in the Treatment of Glioma. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Floyd, S.R.; Pacold, M.E.; Huang, Q.; Clarke, S.M.; Lam, F.C.; Cannell, I.G.; Bryson, B.D.; Rameseder, J.; Lee, M.J.; Blake, E.J.; et al. The bromodomain protein Brd4 insulates chromatin from DNA damage signalling. Nature 2013, 498, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.C.; Kong, Y.W.; Huang, Q.; Han, T.-L.V.; Maffa, A.D.; Kasper, E.M.; Yaffe, M.B. BRD4 prevents the accumulation of R-loops and protects against transcription–replication collision events and DNA damage. Nat. Commun. 2020, 11, 1–20. [Google Scholar] [CrossRef]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).








