Submitted:
03 September 2024
Posted:
05 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Participants
Study Design
Determination of LTL
Polymerase Chain Reaction (PCR) to Measure LTL
Instruments
Analysis of Data
3. Results
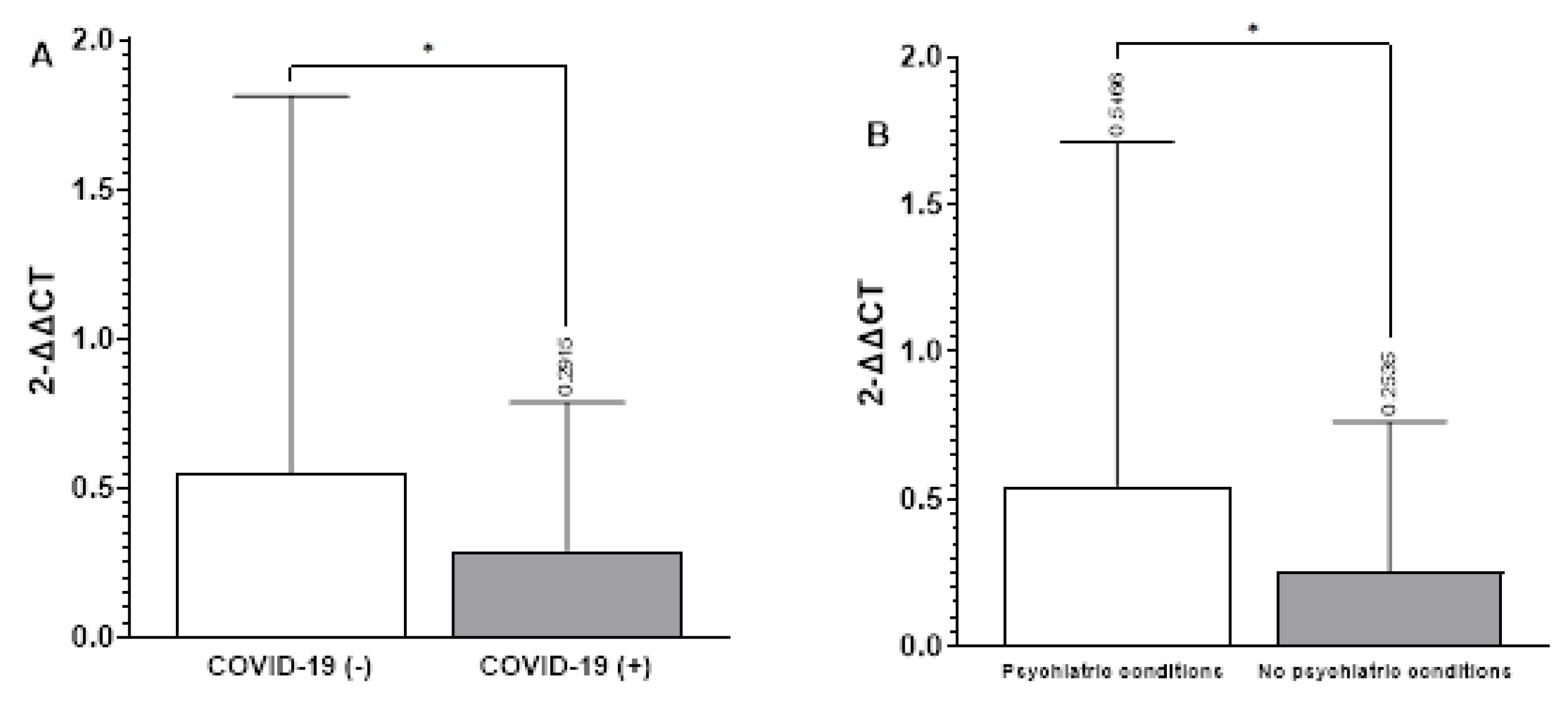
3.1. Relationship between LTL and Groups
3.2. Relationship between LTL and Cognitive Changes in Subjects with Post-COVID-19 Condition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurology 2020, 77, 683–690. [Google Scholar] [CrossRef]
- Mazza, M.G.; Palladini, M.; Poletti, S.; Benedetti, F. Post-COVID-19 Depressive Symptoms: Epidemiology, Pathophysiology, and Pharmacological Treatment. CNS Drugs 2022, 36, 681–702. [Google Scholar] [CrossRef]
- Nalbandian, A.; Desai, A.D.; Wan, E.Y. Post-COVID-19 Condition. Annual Review of Medicine 2023, 74, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. The Lancet Infectious Diseases 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Azcue, N.; Gómez-Esteban, J.C.; Acera, M.; Tijero, B.; Fernandez, T.; Ayo-Mentxakatorre, N.; Pérez-Concha, T.; Murueta-Goyena, A.; Lafuente, J.V.; Prada, Á.; et al. Brain fog of post-COVID-19 condition and Chronic Fatigue Syndrome, same medical disorder? Journal of Translational Medicine 2022, 20, 569. [Google Scholar] [CrossRef] [PubMed]
- Tsilingiris, D.; Tentolouris, A.; Eleftheriadou, I.; Tentolouris, N. Telomere length, epidemiology and pathogenesis of severe COVID-19. European Journal of Clinical Investigation 2020, 50, e13376. [Google Scholar] [CrossRef] [PubMed]
- Mongelli, A.; Barbi, V.; Gottardi Zamperla, M.; Atlante, S.; Forleo, L.; Nesta, M.; Massetti, M.; Pontecorvi, A.; Nanni, S.; Farsetti, A.; et al. Evidence for Biological Age Acceleration and Telomere Shortening in COVID-19 Survivors. International Journal of Molecular Sciences 2021, 22. [Google Scholar] [CrossRef]
- Sanchez-Vazquez, R.; Guío-Carrión, A.; Zapatero-Gaviria, A.; Martínez, P.; Blasco, M.A. Shorter telomere lengths in patients with severe COVID-19 disease. Aging 2021, 13, 1–15. [Google Scholar] [CrossRef]
- dos Santos, G.A.; Pimenta, R.; Viana, N.I.; Guimarães, V.R.; Romão, P.; Candido, P.; de Camargo, J.A.; Hatanaka, D.M.; Queiroz, P.G.S.; Teruya, A.; et al. Shorter leukocyte telomere length is associated with severity of COVID-19 infection. Biochemistry and Biophysics Reports 2021, 27, 101056. [Google Scholar] [CrossRef]
- Wang, Q.; Codd, V.; Raisi-Estabragh, Z.; Musicha, C.; Bountziouka, V.; Kaptoge, S.; Allara, E.; Di Angelantonio, E.; Butterworth, A.S.; Wood, A.M.; et al. Shorter leukocyte telomere length is associated with adverse COVID-19 outcomes: A cohort study in UK Biobank. eBioMedicine 2021, 70. [Google Scholar] [CrossRef]
- Brutto, O.H.D. Cognitive sequelae of COVID-19, a post-pandemic threat. Should we be worried about the brain fog? Arquivos de Neuro-Psiquiatria 2022, 80. [Google Scholar] [CrossRef] [PubMed]
- Scarabino, D.; Veneziano, L.; Mantuano, E.; Arisi, I.; Fiore, A.; Frontali, M.; Corbo, R.M. Leukocyte Telomere Length as Potential Biomarker of HD Progression: A Follow-Up Study. International Journal of Molecular Sciences 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Clements, M.S.; Roberts, R.O.; Vassilaki, M.; Druliner, B.R.; Boardman, L.A.; Petersen, R.C.; Reynolds, C.A.; Pedersen, N.L.; Hägg, S. Association of telomere length with general cognitive trajectories: a meta-analysis of four prospective cohort studies. Neurobiology of Aging 2018, 69, 111–116. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association,: Arlington, VA, 2013. [Google Scholar]
- Cuevas Diaz, P.; Nicolini, H.; Nolasco-Rosales, G.A.; Juarez Rojop, I.; Tovilla-Zarate, C.A.; Rodriguez Sanchez, E.; Genis-Mendoza, A.D. Telomere Shortening in Three Diabetes Mellitus Types in a Mexican Sample. Biomedicines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Sanabria, M.; Martínez-Magaña, J.; Nicolini-Sánchez, H.; Guzmán-Sánchez, R.; Genis-Mendoza, A.D. Asociación entre la longitud de los telómeros y deterioro cognitivo en adultos mayores. Revista Española de Geriatría y Gerontología 2022, 57, 320–324. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Beaman, S.R.d.; Beaman, P.E.; Garcia-Peña, C.; Villa, M.A.; Heres, J.; Córdova, A.; Jagger, C. Validation of a Modified Version of the Mini-Mental State Examination (MMSE) in Spanish. Aging, Neuropsychology, and Cognition 2004, 11, 1–11. [Google Scholar] [CrossRef]
- Avila-Avila, A.; Sosa-Tinoco, E.; Pacheco-Pacheco, J.; Escobedo-Acosta, M.G.; Bautista-Eugenio, V.; González-García, V.; Blano-Campero, E.J.; Negrete-Redondo, M.I.; Deyta-Pantoja, A.L.; Gutiérrez-Robledo, L.M.F. Guía de instrumentos de evaluación geriátrica integral. 2020. [Google Scholar]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. Journal of the American Geriatrics Society 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Aguilar-Navarro, S.G.; Mimenza-Alvarado, A.J.; Palacios-García, A.A.; Samudio-Cruz, A.; Gutiérrez-Gutiérrez, L.A.; Ávila-Funes, J.A. Validity and reliability of the Spanish Version of the Montreal Cognitive Assessment (MoCA) for the detection of cognitive impairment in Mexico. Revista Colombiana de Psiquiatría (English Edition) 2018, 47, 237–243. [Google Scholar] [CrossRef]
- Patel, R.; Kooner, J.S.; Zhang, W. Comorbidities associated with the severity of COVID-19, and differences across ethnic groups: a UK Biobank cohort study. BMC Public Health 2023, 23, 1566. [Google Scholar] [CrossRef]
- Aviv, A. Short telomeres and severe COVID-19: The connection conundrum. eBioMedicine 2021, 70. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, F.; Shi, Y.; Chen, Y.; Shi, B.; Yu, G. Causal association of epigenetic aging and COVID-19 severity and susceptibility: A bidirectional Mendelian randomization study. Frontiers in Medicine 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Haridoss, M.; Ayyasamy, L.; Bagepally, B.S. Is COVID-19 severity associated with telomere length? A systematic review and meta-analysis. Virus Genes 2023, 59, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, A.; Sanaie, S.; Eskandari, M.; Behrouzi, N.; Taghizadeh, M.; Roudbari, F.; Emamalizadeh, B.; Sohrabifar, N.; Kazeminasab, S. Association between leukocyte telomere length and COVID-19 severity. Egyptian Journal of Medical Human Genetics 2023, 24, 37. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.J.; Susser, E.; Arbeev, K.G.; Yashin, A.I.; Levy, D.; Verhulst, S.; Aviv, A. Telomere-length dependent T-cell clonal expansion: A model linking ageing to COVID-19 T-cell lymphopenia and mortality. eBioMedicine 2022, 78. [Google Scholar] [CrossRef]
- Grand, R.J. SARS-CoV-2 and the DNA damage response. Journal of General Virology 2023, 104. [Google Scholar] [CrossRef]
- Retuerto, M.; Lledó, A.; Fernandez-Varas, B.; Guerrero-López, R.; Usategui, A.; Lalueza, A.; García-García, R.; Mancebo, E.; Paz-Artal, E.; Sastre, L.; et al. Shorter telomere length is associated with COVID-19 hospitalization and with persistence of radiographic lung abnormalities. Immunity & Ageing 2022, 19, 38. [Google Scholar] [CrossRef]
- Reeves, J.; Kooner, J.S.; Zhang, W. Accelerated ageing is associated with increased COVID-19 severity and differences across ethnic groups may exist. Frontiers in Public Health 2022, 10. [Google Scholar] [CrossRef]
- Levstek, T.; Kozjek, E.; Dolžan, V.; Trebušak Podkrajšek, K. Telomere Attrition in Neurodegenerative Disorders. Frontiers in Cellular Neuroscience 2020, 14. [Google Scholar] [CrossRef]
- Fu, J.; Ji, X.; Liu, J.; Chen, X.; Shang, H. Meta-analysis of the Connection Between Alzheimer Disease and Telomeres. Alzheimer Disease & Associated Disorders 2022, 36. [Google Scholar]
- Gampawar, P.; Schmidt, R.; Schmidt, H. Telomere length and brain aging: A systematic review and meta-analysis. Ageing Research Reviews 2022, 80, 101679. [Google Scholar] [CrossRef]
- Koh, Z.Y.; Law, F.; Chew, J.; Ali, N.; Lim, W.S. Impact of Coronavirus Disease on Persons with Dementia and Their Caregivers: An Audit Study. Ann Geriatr Med Res 2020, 24, 316–320. [Google Scholar] [CrossRef]
- Bersani, F.S.; Lindqvist, D.; Mellon, S.H.; Penninx, B.W.J.H.; Verhoeven, J.E.; Révész, D.; Reus, V.I.; Wolkowitz, O.M. Telomerase activation as a possible mechanism of action for psychopharmacological interventions. Drug Discovery Today 2015, 20, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Epel, E.S.; Prather, A.A. Stress, Telomeres, and Psychopathology: Toward a Deeper Understanding of a Triad of Early Aging. Annual Review of Clinical Psychology 2018, 14, 371–397. [Google Scholar] [CrossRef] [PubMed]
- Kitagishi, Y.; Kobayashi, M.; Kikuta, K.; Matsuda, S. Roles of PI3K/AKT/GSK3/mTOR Pathway in Cell Signaling of Mental Illnesses. Depression Research and Treatment 2012, 2012, 752563. [Google Scholar] [CrossRef] [PubMed]
- Mayén-Lobo, Y.G.; Martínez-Magaña, J.J.; Pérez-Aldana, B.E.; Ortega-Vázquez, A.; Genis-Mendoza, A.D.; Dávila-Ortiz de Montellano, D.J.; Soto-Reyes, E.; Nicolini, H.; López-López, M.; Monroy-Jaramillo, N. Integrative Genomic–Epigenomic Analysis of Clozapine-Treated Patients with Refractory Psychosis. Pharmaceuticals 2021, 14. [Google Scholar] [CrossRef]
- Wei, H.; Aucoin, J.; Kuntapay, G.R.; Justice, A.; Jones, A.; Zhang, C.; Santos, H.P., Jr.; Hall, L.A. The prevalence of nurse burnout and its association with telomere length pre and during the COVID-19 pandemic. PLOS ONE 2022, 17, e0263603. [Google Scholar] [CrossRef]
- Campisi, M.; Cannella, L.; Celik, D.; Gabelli, C.; Gollin, D.; Simoni, M.; Ruaro, C.; Fantinato, E.; Pavanello, S. Mitigating cellular aging and enhancing cognitive functionality: visual arts-mediated Cognitive Activation Therapy in neurocognitive disorders. Frontiers in Aging Neuroscience 2024, 16. [Google Scholar] [CrossRef]
- Jylhävä, J.; Pedersen, N.L.; Hägg, S. Biological Age Predictors. eBioMedicine 2017, 21, 29–36. [Google Scholar] [CrossRef]
- Gardner, M.; Bann, D.; Wiley, L.; Cooper, R.; Hardy, R.; Nitsch, D.; Martin-Ruiz, C.; Shiels, P.; Sayer, A.A.; Barbieri, M.; et al. Gender and telomere length: Systematic review and meta-analysis. Experimental Gerontology 2014, 51, 15–27. [Google Scholar] [CrossRef]
- Hägg, S.; Jylhävä, J. Sex differences in biological aging with a focus on human studies. eLife 2021, 10, e63425. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Crimmins, E.M. Mortality and morbidity in ageing men: Biology, Lifestyle and Environment. Reviews in Endocrine and Metabolic Disorders 2022, 23, 1285–1304. [Google Scholar] [CrossRef]
- Ahmed, W.; Lingner, J. Impact of oxidative stress on telomere biology. Differentiation 2018, 99, 21–27. [Google Scholar] [CrossRef]
- Shalev, I.; Entringer, S.; Wadhwa, P.D.; Wolkowitz, O.M.; Puterman, E.; Lin, J.; Epel, E.S. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology 2013, 38, 1835–1842. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Nolasco-Rosales, G.A.; Alonso-García, C.Y.; Hernández-Martínez, D.G.; Villar-Soto, M.; Martínez-Magaña, J.J.; Genis-Mendoza, A.D.; González-Castro, T.B.; Tovilla-Zarate, C.A.; Guzmán-Priego, C.G.; Martínez-López, M.C.; et al. Aftereffects in Epigenetic Age Related to Cognitive Decline and Inflammatory Markers in Healthcare Personnel with Post-COVID-19: A Cross-Sectional Study. Int J Gen Med 2023, 16, 4953–4964. [Google Scholar] [CrossRef]
- Cao, X.; Li, W.; Wang, T.; Ran, D.; Davalos, V.; Planas-Serra, L.; Pujol, A.; Esteller, M.; Wang, X.; Yu, H. Accelerated biological aging in COVID-19 patients. Nature Communications 2022, 13, 2135. [Google Scholar] [CrossRef]
| Variable | F, Percentage, M ± S.D. | F, P |
|---|---|---|
| Age (years) | 42.06 ± 11.67 | |
| Sex | ||
| Female | 106, 41.4% | |
| Male | 148, 57.8% | |
| Groups | ||
| I | 75, 29.3% | |
| II | 39, 14.8% | |
| III | 62, 24.2% | |
| IV | 80, 31.3% | |
| Diagnosis | ||
| Mood and emotional disorders | 12, 15% | |
| Neurodevelopmental disorders | 17, 21% | |
| Neurodegenerative disorders | 5, 6% | |
| Schizophrenia | 80, 70% | |
| Telomere length (2-ΔΔCT) | 0.46 ± 1.28 | |
| Sex | ||
| Female | 0.58 ± 0.36 | 0.17 |
| Male | 1.58 ± 0.98 | |
| Telomere length vs age (years) | ||
| Very short | 42.34 ± 11.15 | 2.671, 0.048 |
| Short | 45.22 ± 11.92 | |
| Medium | 41.05 ± 11.68 | |
| Large | 39.63 ± 11.31 | |
| LTL (2-ΔΔCT) | GIM ± S.D. | GIIM ± S.D. | GIIIM ± S. D | GIVM ± S. D | P |
|---|---|---|---|---|---|
| Very short | 0.004±0.0015 | 0.005±0.0004 | 00.002±0.0012 | 0.0035±0.0024 | |
| Short | 0.01±0.005 | 0.023±0.023 | 0.040±0.027 | 0.012±0.0047 | |
| Medium | 0.062±0.03 | 0.061±0.030. | 0.059±0.023 | 0.064±0.026 | |
| Large | 1.052±1.74* | 0.6±0.56 | 0.62±0.79 | 0.49±0.65 | 0.022 |
| Assessment | LTL | MOCA | MMSE | F | df | p | ƞp2 |
|---|---|---|---|---|---|---|---|
| Survey I | 1.457 | 6 | 0.198 | 0.185 | |||
| Very short | 23.20 ± 2.68 | 28.00 ± 4.30 | |||||
| Short | 23.00 ± 2.98 | 29.90 ± 4.93 | |||||
| Medium | 23.80 ± 1.92 | 33.40 ± 1.82 | |||||
| Large | 25.75 ± 2.50 | 29.75 ± 1.50 | |||||
| Survey II | 1.490 | 4 | 0.226 | 0.142 | |||
| Very short | 26.67 ± 3.22 | 34.33 ± 1.16 | |||||
| Short | - | - | |||||
| Medium | 24.50 ± 3.92 | 30.30 ± 2.75 | |||||
| Large | 25.78 ± 2.73 | 29.22 ± 4.12 |
| Variable | Survey I | Survey II | p |
|---|---|---|---|
| MMSE vs LTL | |||
| Very short | 34 ±1.41 | 27.66 ± 4.51 | 0.163 |
| Short | - | - | - |
| Medium | 29.37 ± 2.9 | 32.45±2.46 | 0.023 |
| Large | 28.83 ± 036 | 29.66±4.18 | 0.732679 |
| MOCA vs LTL | |||
| Very short | 22.25±3.41 | 21.67± 4.16 | 0.37 |
| Short | 25.50 ±.71 | 25.50 ±.1.21 | 0.87 |
| Medium | 24.33±3.05 | 22.45 ± 3.50 | 0.79 |
| Large | 23± 0.5 | 22.78±4.38 | 0.22 |
| Cognitive changes | No cognitive changes | X2, p | |
|---|---|---|---|
| MMSE | 6, 15 % | 34, 85 % | |
| Very short | 3, 50.0% | 7, 20.6% | 3.92, 0.27 |
| Short | 1, 16.7% | 9, 26.5% | |
| Medium | 0, 0.0% | 10, 29.4% | |
| Large | 2, 33.3% | 8, 23.5% | |
| MoCA | 29, 72.5 % | 11, 27.5 % | |
| Very short | 9, 31.0% | 1, 9.1% | 4.39, 0.22 |
| Short | 5, 17.2% | 5, 45.5% | |
| Medium | 7, 24.1%, | 3, 27.3% | |
| Large | 8, 27.6% | 2, 18.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





