1. Introduction
Native forests and biodiversity play a pivotal role in ecological, economic, and social sustainability. From an environmental perspective, they act as carbon sinks, helping to reduce the amount of carbon dioxide in the atmosphere. They protect the soil from erosion and maintain the water quality of wetlands and rivers. Also, native forests provide valuable ecosystem services (IPBES, 2019), such as hosting insect pollinators, purifying the air, mitigating the effects of floods, storms and noise pollution (WWF, 2020), dune stabilization in desert areas (Khan et al., 2023), among others. Indeed, links have been demonstrated between biodiversity and improvements in objective measures of health and well-being (Fisher et al., 2023). Even today, many Indigenous Peoples and Local Communities rely on native forests as a source of food and medicine, in addition to their cultural value (de Lange et al., 2023).
The ecological stability of native forests depends on biodiversity; therefore, they are essential for maintaining a healthy and balanced ecosystem, and their conservation is crucial to ensure the availability of resources and services for future generations. In this regard, it is necessary to take measures to protect and conserve them, whether through the creation of protected areas, the implementation of sustainable agricultural and livestock practices, or the promotion of environmental education and awareness among the population. Without immediate action, these areas and their biodiversity are at risk of degradation or even permanent loss, as they harbor endemic organisms that are at risk of extinction. Success or failure will depend on international cooperation efforts such as the Kunming-Montreal Global Biodiversity Framework (2022) or The Forests, Trees and Agroforestry Partnership (2022), as well as the actions of each and every one of us.
Local wild species are essential for informing sustainable management practices and developing innovative, resilient, and sustainable agricultural systems, thus contributing to the well-being of communities and the conservation of our natural heritage. Integrating knowledge of biodiversity into forest management strategies allows for the design of practices that conserve and enhance these ecosystems. For example, identifying and protecting key plants and animals can foster the natural regeneration of the forest and long-term sustainability. Additionally, research on ecological interactions, biological processes, and the detection of key indicators provides crucial information for the restoration of degraded areas, the creation of biological corridors that connect fragmented habitats, and the incorporation of new species into cropping systems (Lindenmayer et al., 2000).
Despite their importance, the area of native forests in Argentina has suffered a loss of 6,631,000 hectares between the years 1990 and 2020. In 2020, an estimated 27,137,000 hectares of native forests were recorded, which represents 10.28% of the country’s total land area (World Bank, 2023). The main causes of deforestation are attributed to the expansion of agriculture, livestock farming, and urbanization (Viglizzo & Jobbágy, 2010; Suwardi & Navia, 2023). In particular, this fact coincides with the global trend of a dramatic acceleration in cropland expansion within protected areas from 2000 to 2019 (Meng et al., 2023). However, despite this situation, there are still areas with low anthropogenic impact, as is the case with the riparian forest along the Uruguay River in the province of Entre Ríos. These areas are crucial for biodiversity due to the vegetation intrusion phenomenon, in which species from the Paranaense Province migrate southward through the Uruguay and Paraná rivers and they adapt to the riparian microclimate (Bertucci et al., 2008; Casas & Albarracín, 2015). In fact, this situation results in an increase in heterogeneity and diversity within and between species and ecosystems, respectively. Mass effect, the occurrence of species outside their core habitats, could also be explained by this phenomenon (Shmida & Wilson, 1985).
A species that stands out in these areas is Hexachlamys edulis (O. Berg) Kausel & D. Legrand (H. edulis), commonly known as “ubajay”. This species is found in areas near watercourses and riparian forests along the Paraná and Uruguay rivers. Furthermore, it is notable for its edible fruit and its potential as a non-timber forest resource of importance for health and nutrition. Without a doubt, H. edulis stands out as a promising species for incorporation into cropping systems to enhance biodiversity and promote sustainable agricultural practices (Vignale & Bisio, 2005; Proença, 2006; Povilonis et al., 2021; Arena et al., 2021, 2023).
Within the framework of studies on the phenotypic variability of H. edulis, which is necessary for subsequent breeding and domestication efforts, it is essential to understand the conservation status of the sampled populations. This understanding can help assess the potential risks faced by this species and determine whether H. edulis populations coexist with different woody and semi-woody plant communities. According to Llorente–Culebras et al. (2023), woody plants were the most frequently studied group in global biodiversity research on protected areas, and these communities support numerous other species while significantly contribute to local biodiversity (Duguma, 2023).
The objectives of this study were to (1) record species and families present in the populations where H. edulis grows with special attention to other species with agroforestry potential; (2) explore biodiversity indicators of the plant woody and semi-woody communities in which H. edulis grows; (3) explain the conservation status of each population based on the ratio of native and exotic species and (4) compare the locations based on the similarity of woody and semi-woody plant species accompanying H. edulis trees, by exploring species richness to determine if the species occurs in different plant communities.
2. Materials and Methods
A total of 40 individuals of
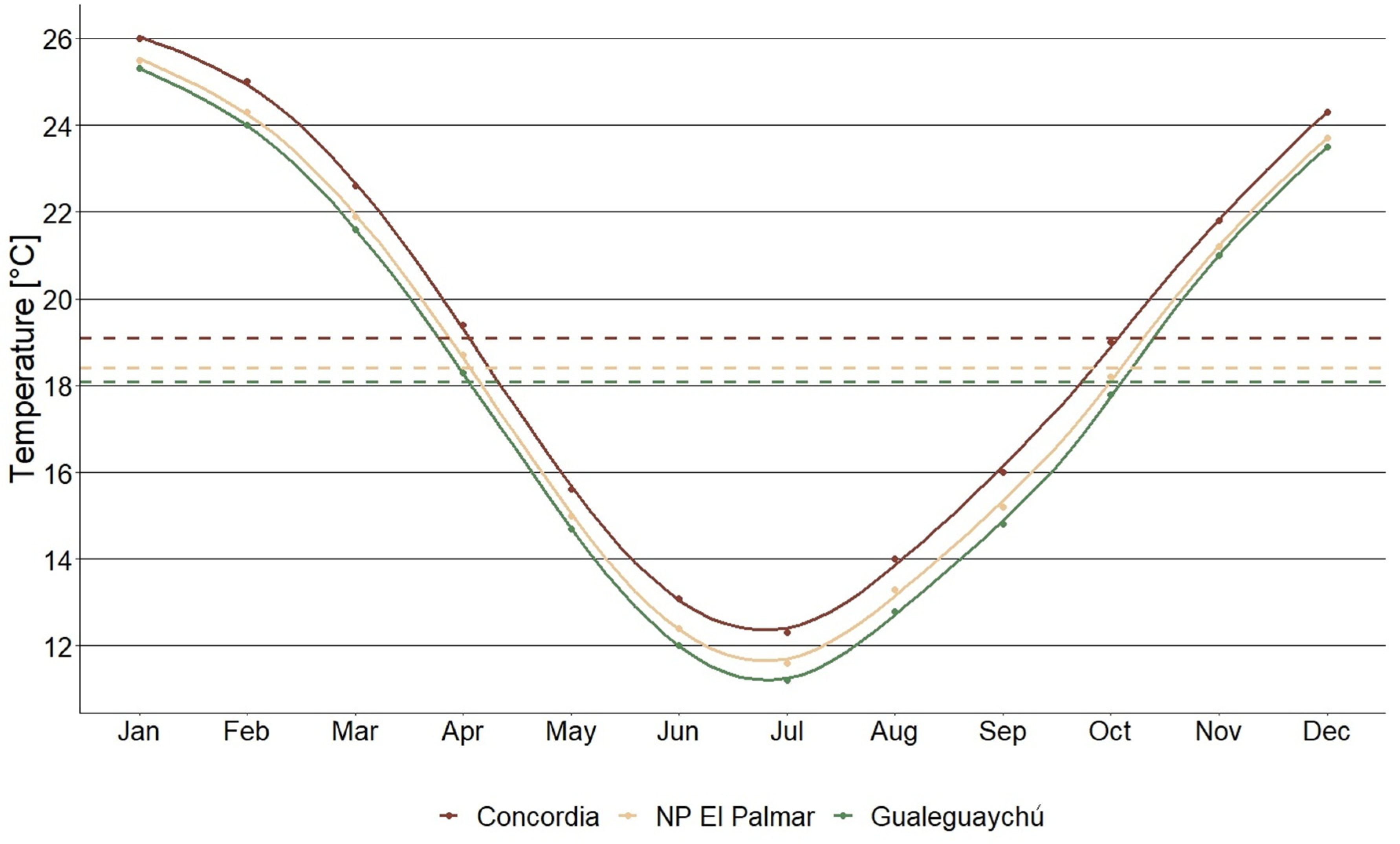
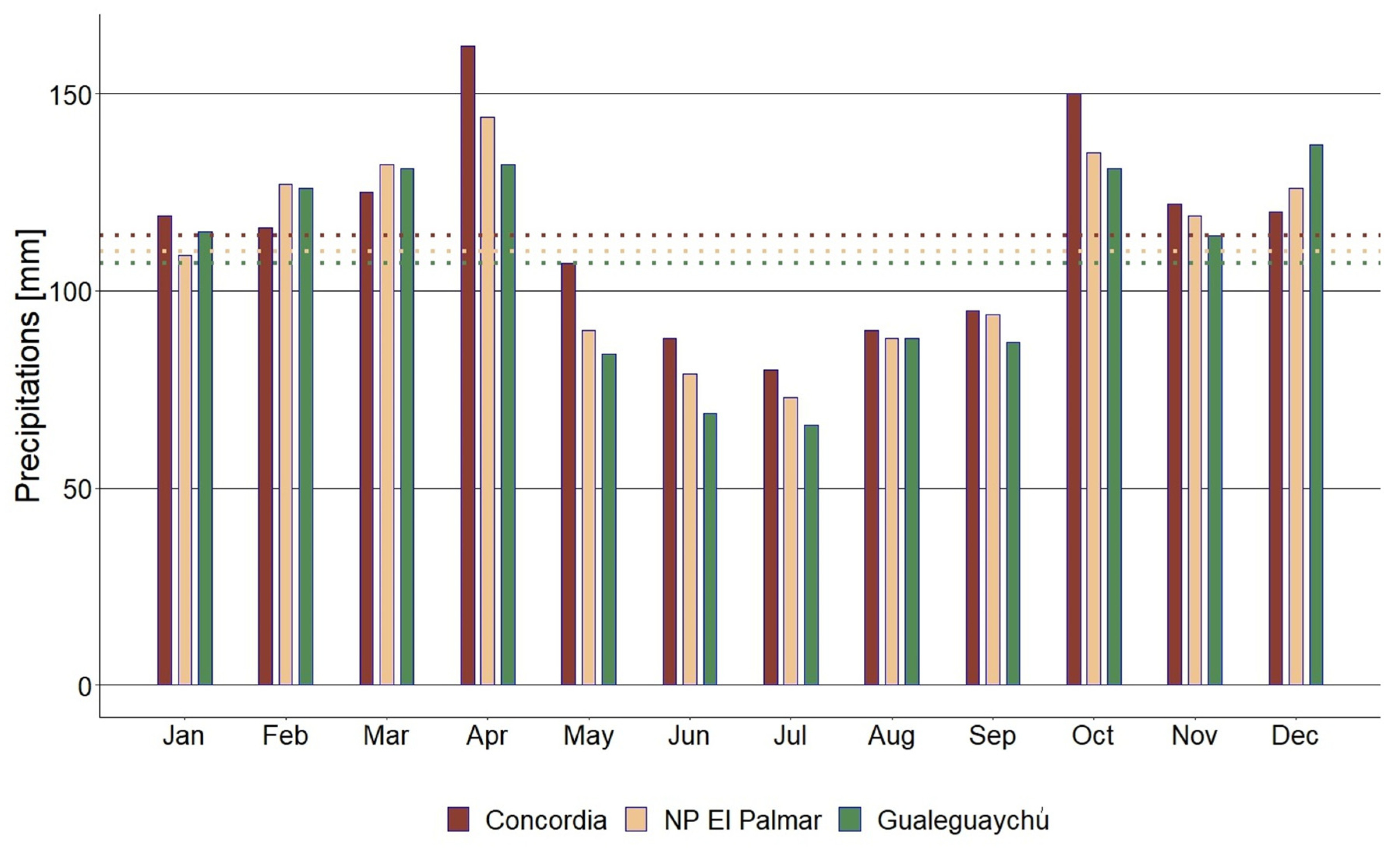
H. edulis grown spontaneously, were selected from three populations in the riparian forest along the Uruguay River within the province of Entre Ríos. The distribution of these selected trees was as follows: 12 individuals in Concordia (-31.28590 S: -57.96305 W) IUCN category not reported, 15 individuals in National Park (NP) El Palmar (-31.86395 S: -58.20998 W) IUCN category II, and 13 individuals in the private reserve El Potrero de San Lorenzo in Gualeguaychú (-33.06490 S: -58.26965 W) IUCN category VI (UNEP –WCMC and IUCN, 2023). The recorded monthly average temperatures and precipitation for each study site between 1991 and 2021 are shown in
Figure 1 and
Figure 2 (SMN, 2023).
Adult trees of
H. edulis with a diameter at breast height greater than 7.5 cm were randomly selected in September 2019, ensuring they were not close to each other (>5 m) and were not dead. The labeled individuals from 154 to 208 belong to Concordia, those labeled from 260 to 288 belong to El Palmar, and among 305 and 364 are from Gualeguaychú. To record and count the accompanying species, a radius of 3 m from the trunk of each selected
H. edulis tree was determined (minimum surveyed area of 28.3 m
2). Taxonomic classification was conducted, and the number of all woody and semi-woody perennial species present was recorded. In addition to species richness as a primary measure of biodiversity (Whittaker, 1977) and to address the complexity present in the structure of a plant community, various indicators were employed, such as evenness, equality, and dominance (
Table 1).
First, vegetal community structure was characterized by calculating species abundance and species richness. Also, Shannon–Wiener index (Shannon & Weaver, 1949) of species diversity (H′), Pielou Equitability (J), Evenness (e
H/S), Dominance (D), Margalef index (M) were calculated at each site according to formulas in
Figure 3 using PAST 3.24 (Hammer
et al., 2001). A distinction was made between native and exotic species, and an anthropic indicator (Ia) was generated (adapted from Napoles, 2016) to indicate the ratio of native to exotic species, where 1 indicates completely native vegetation and 0 indicates completely exotic vegetation. Prior to statistical analyses, normality and homogeneity of variances were assessed for the calculated dependent variables using the Shapiro–Wilks and Levene tests. For each index, an analysis of variance was conducted comparing the three study sites. General linear models were used, either with variance adjustment by site or generalized linear models using a gamma distribution with an identity link function as appropriate. Multiple comparison analyses were performed using the Tukey test (p-value=0.05).
Furthermore, to measure differences in species structure among the studied locations and to compare the heterogeneity of the accompanying vegetation, a presence absence matrix was created. First, species with an occurrence lower than 7.5% were removed. The data was standardized through normalization and then, dissimilarity matrix was calculated based on the Bray–Curtis index. Complementary clustering methods, such as Ward’s merging algorithm for cluster analysis, which delivers mutually exclusive groups, where each group includes members with the highest similarity, providing maximum internal homogeneity (Ward, 1963). Ordination by Principal Coordinates Analysis (PCoA) were used. Discriminant analysis was also performed based on the calculation of Mahalanobis distances, and its significance was evaluated using multi-response permutation procedures (MRPP) with 1000 permutations. All data were analyzed using Rstudio (Posit team, 2023).
3. Results
The total flora of the studied areas was cataloged, revealing 39 families with a richness of 71 species and an abundance of 613 individuals (
Table A1 in Appendix A). Only four species, all of them native, displayed absolute consistency and were found in the three sites:
Acacia caven,
Allophyllus edulis,
Celtis tala, and
Eugenia uruguayensis. The number of native species with the highest fidelity, meaning that they only grow at each site, is 6 in Concordia, 3 in NP El Palmar and 5 in Gualeguaychú (
Table 2).
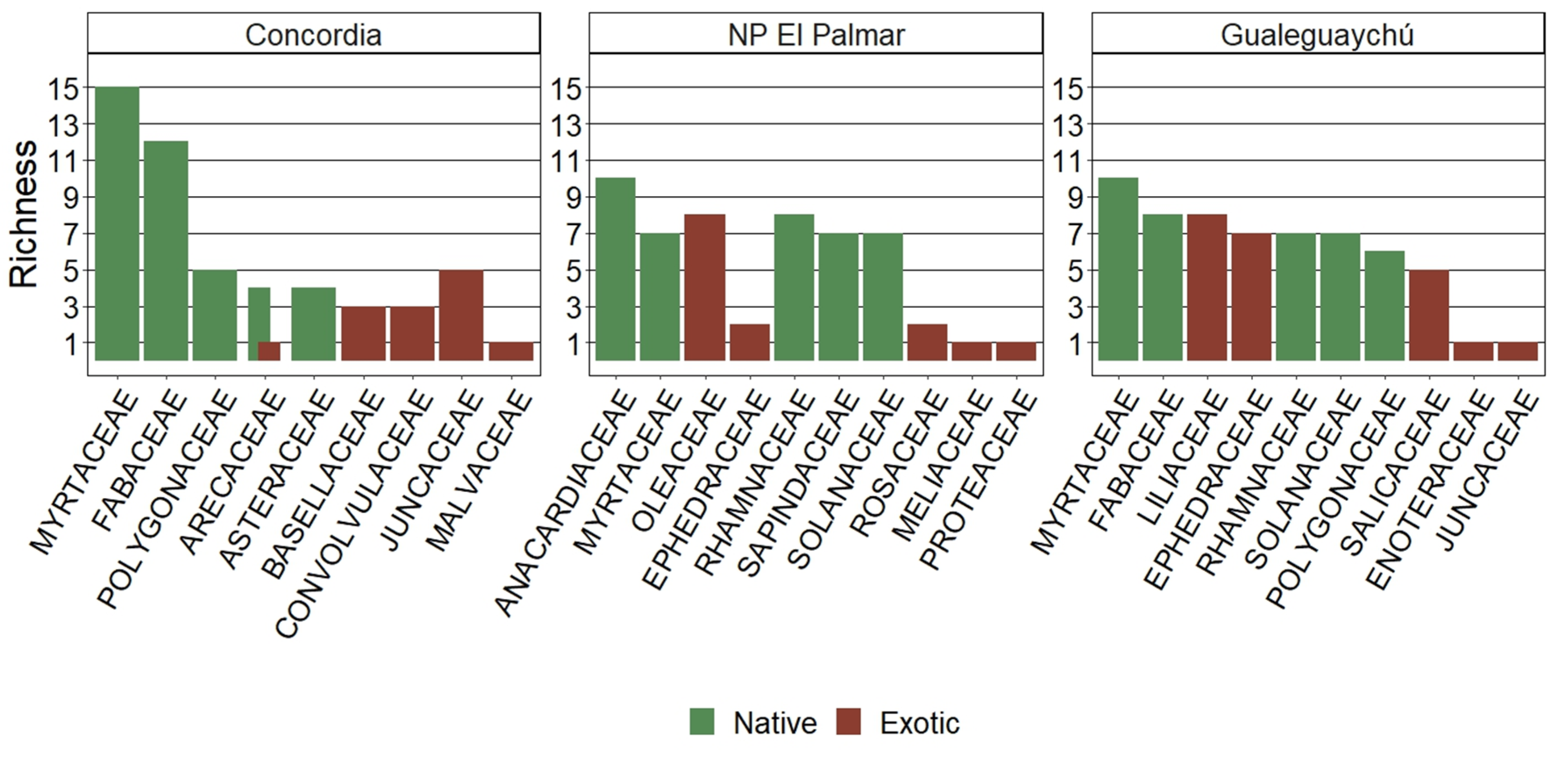
The individuals of the Myrtaceae family had the highest relevance, as it was the family with the greatest representation of native species in Concordia and Gualeguaychú, and the second highest in NP El Palmar, following the Anacardiaceae family (
Figure 3).
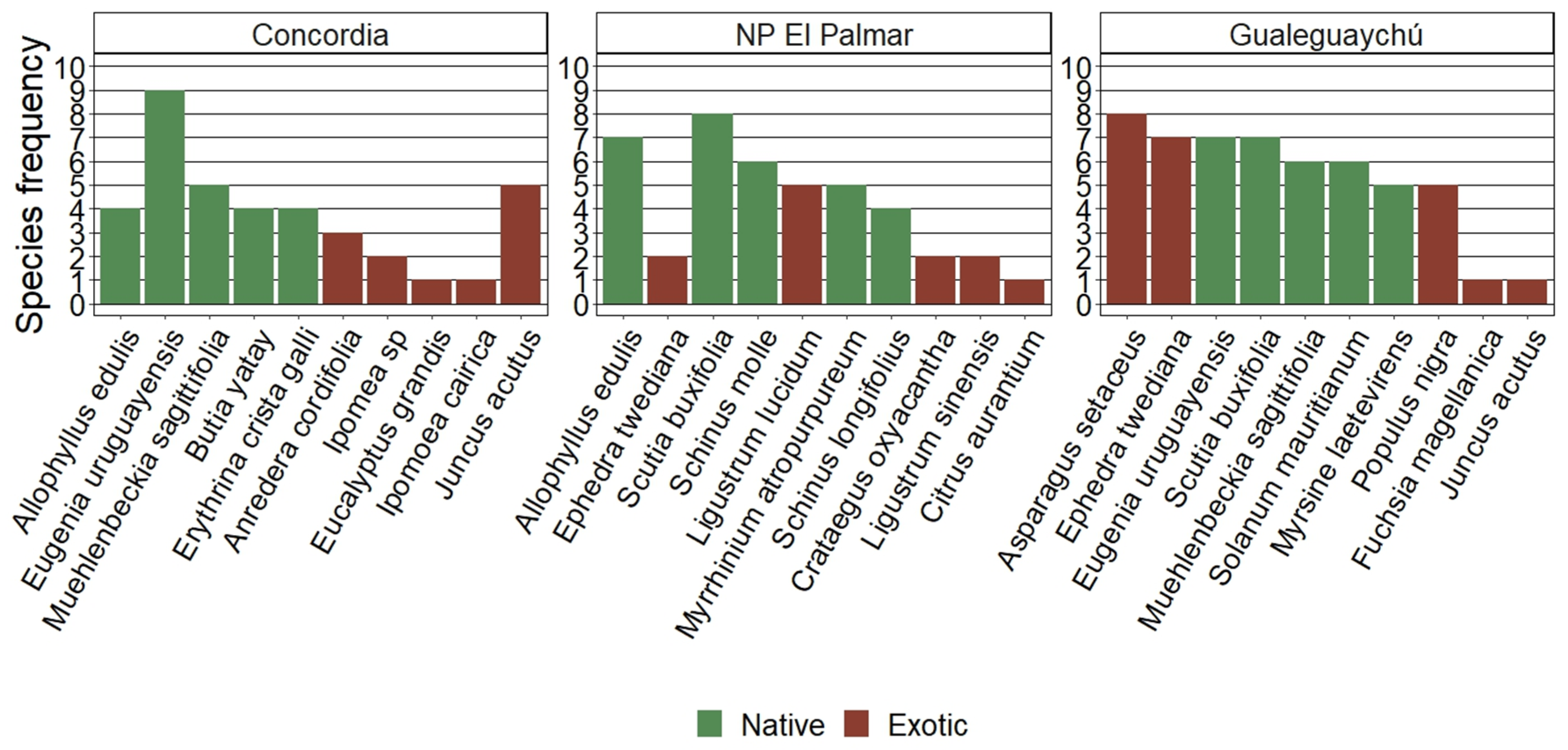
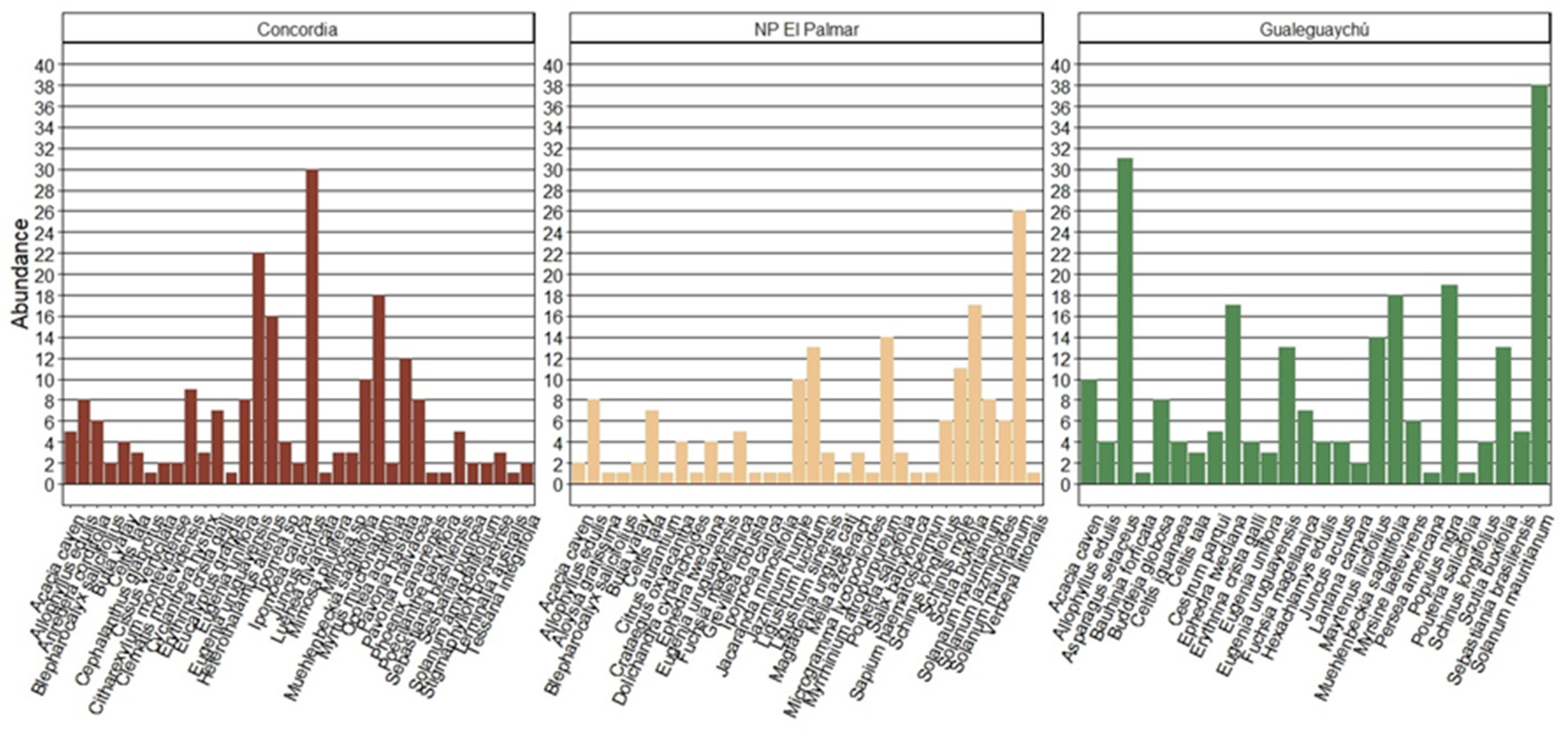
The most frequent native species at the sites were
Eugenia uruguayensis (17),
Scutia buxifolia (15), and
Allophyllus edulis (13), while the most frequent exotic species were
Ephedra twediana (9),
Asparagus setaceus (8), and
Juncus acutus (6) (
Figure 4).
Some registered native species worth highlighting for possible incorporation into farming systems to improve biodiversity are Allophyllus edulis, Butia yatay, Eugenia uniflora, Eugenia uruguayensis and Muehlenbeckia sagittifolia among others. Allophyllus edulis, commonly known as the “chal-chal” is a small tree or shrub. This species holds significant potential for incorporation into farming systems due to its edible fruits, which are rich in antioxidants, potassium, and exhibit high levels of nitrogen-free extract (Schmeda-Hirschmann et al., 2005). Moreover, the plant has medicinal properties; traditionally, it has been used to treat various ailments, including gastrointestinal disorders and inflammation, as well as an indicator of contaminated areas for bioremediation (Aguilar et al., 2023).
Butia yatay, known as the “yatay palm” produces edible dates. These fruits are not only nutritious but also have the potential to be developed into value-added products such as sweets, beverages, and preserves. It is a significant nectar source for honey production (Brelis et al., 2023) and the leaves can be utilized in handicrafts, providing a sustainable source of income for local communities (Barbieri et al., 2014). Additionally, the yatay palm plays a crucial ecological role by providing habitat and food for wildlife, making it a valuable species for enhancing biodiversity in farming systems.
Eugenia uniflora also known as “Surinam cherry” or “pitanga” is a small tree or shrub that offers a wealth of benefits. It produces delicious sweet-tart fruits rich in nutrients and boasts various culinary uses. Additionally, this species has valuable medicinal properties. Traditionally, it has been used to treat digestive issues and respiratory problems (Schapoval et al., 1994; Kuhn et al., 2015). Recent research highlights Eugenia uniflora’s exciting potential. Studies have shown genetic variability within the species, suggesting possibilities for breeding programs. The essential oils possess bioactive properties, including antioxidant and anti-Leishmania activities (Cipriano et al., 2021; Rodrigues et al., 2023). Furthermore, the plant’s ornamental value and its role in attracting pollinators make it a perfect candidate for agroforestry systems. Overall, Eugenia uniflora’s potential extends to both agricultural and industrial applications.
Eugenia uruguayensis has been found to possess a range of beneficial properties. It’s leaf oil contains compounds such as limonene, 1,8-cineole, α-pinene, and caryophyllene oxide, which have potential antimicrobial and anti-inflammatory effects (Dellacassa et al., 1997). The use of Eugenia species, including E. uruguayensis and E. uniflora in agroforestry systems has been highlighted as profitable alternatives for agricultural production (Lamarca et al., 2013).
Muehlenbeckia sagittifolia, also known as “wire vine” is a hardy climbing plant that produces small, edible fruits. These fruits can be harvested and utilized in local cuisines, while the plant itself is often used for its ornamental value in landscaping and medicinal use (Di Gristina & Raimondo, 2022). Its robust growth habit makes it suitable for erosion control and as a living fence in farming systems. By incorporating Muehlenbeckia sagittifolia into agroforestry practices, farmers can benefit from its multipurpose use while promoting soil conservation and biodiversity.
Incorporating these native species and certainly others into farming systems can significantly contribute to biodiversity, provide new economic opportunities, and support sustainable agricultural practices. Future studies should explore their full potential and develop best practices for their cultivation and use.
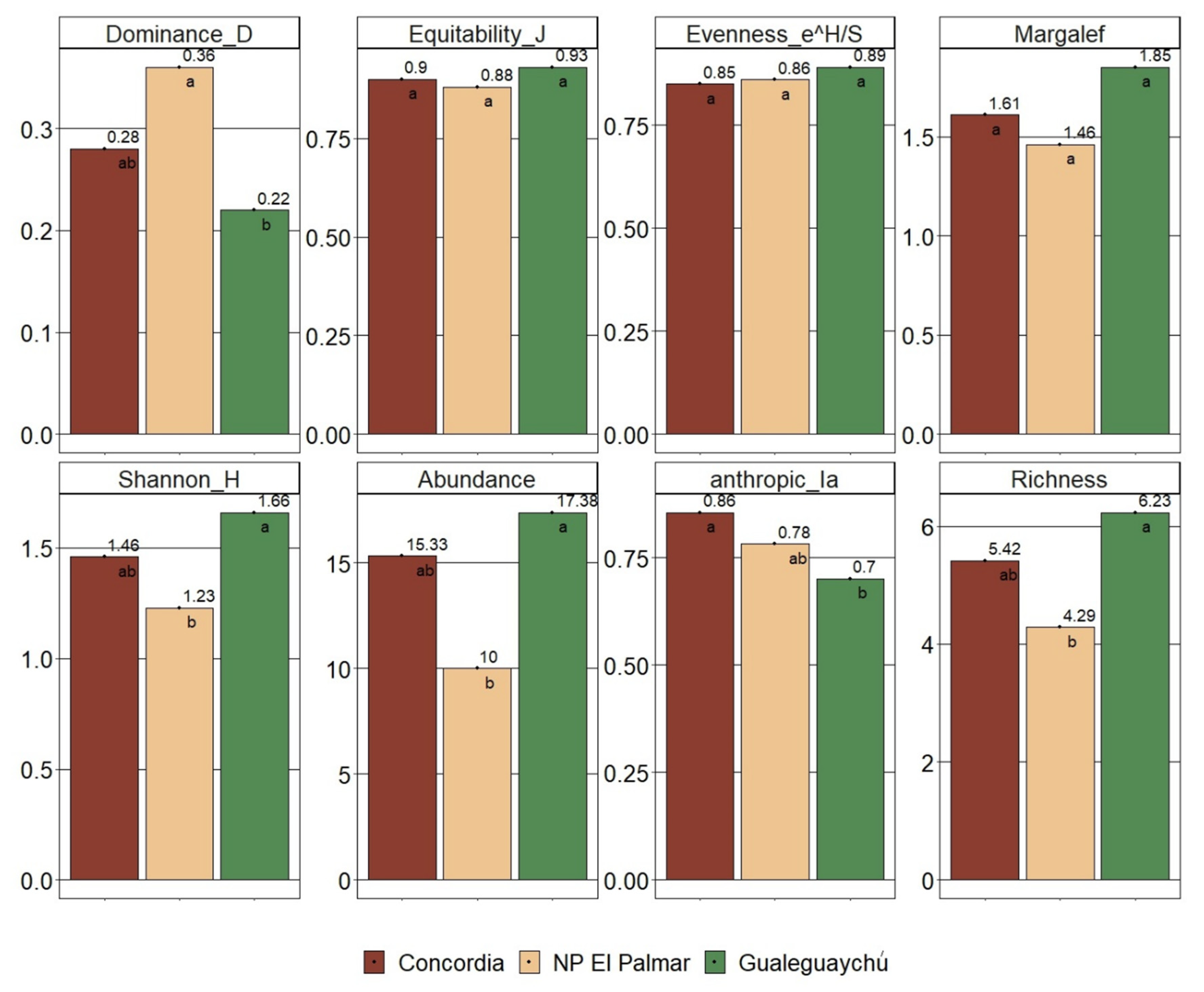
The average number of individuals and species was significantly different between Gualeguaychú (17.38 and 6.23) and NP El Palmar (10.00 and 4.29), while Concordia did not show any differentiation (15.33 and 5.42 respectively). According to Margalef index, there are not statistical differences between populations (
Figure 5). Equitability was similar for the three populations and the uniformity explained by evenness (e
H/S) for each site was also very similar. Dominance was significantly higher in NP El Palmar (0.36) than in Gualeguaychú (0.22), although without differences with Concordia (0.28). Also, the ecological diversity calculated using the Shannon–Weaver index shows significant differences between NP El Palmar (1.23) and Gualeguaychú (1.66). Lastly, the anthropic indicator value was 0.85 for Concordia, 0.78 for NP El Palmar and 0.70 for Gualeguaychú.
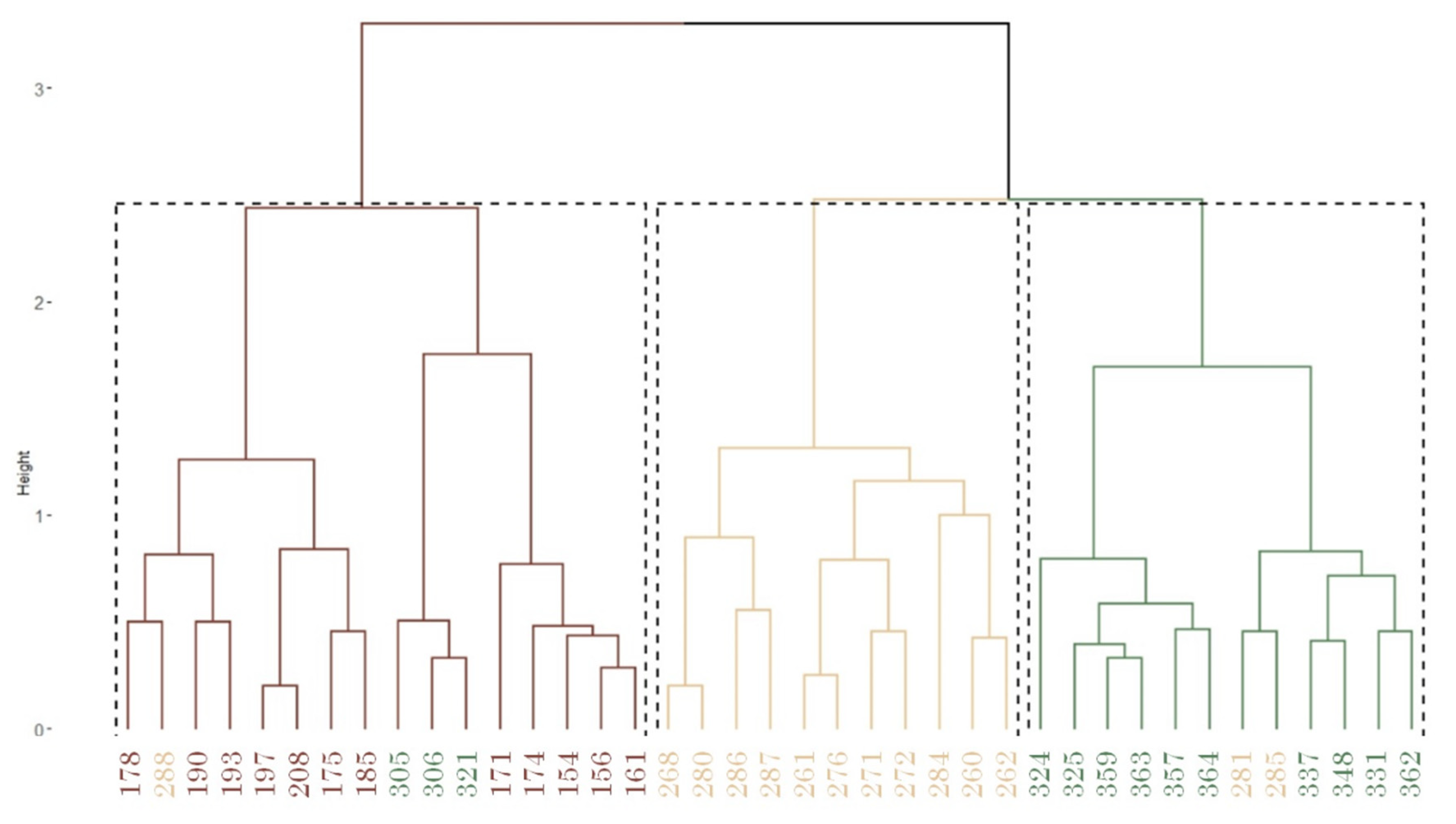
Cluster analysis (
Figure 6) shows a grouping of the censuses into 3 clusters. Each cluster is represented by the censuses from each population, except for 288, 305, 306, 321, 281, and 285. This means that 85% of the censuses are grouped within the censuses from the same population. NP El Palmar and Gualeguaychú show a lower distance explained by vegetation composition.
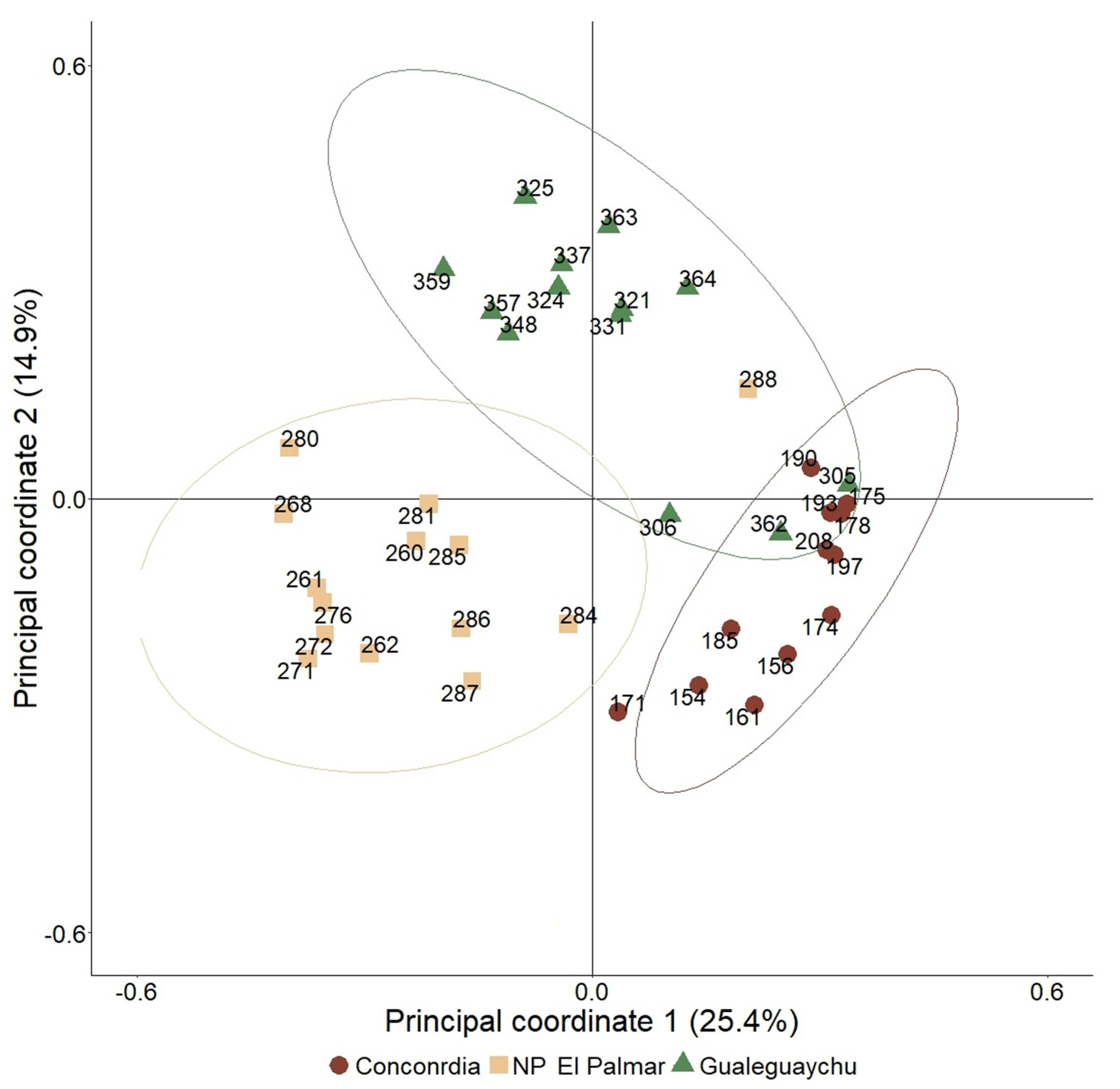
Consistent with the cluster analysis, the representation of the two principal coordinates in the PCoA (
Figure 7) shows a clustering of censuses from the same locality, except for 288. In other words, the clustering into three main groups predominantly includes censuses from the same populations. Principal coordinates explain the 22.5 and 14.2% of the variation. Also, in the populations of NP El Palmar and Gualeguaychú, a greater dispersion is observed in the multidimensional scaling compared to Concordia, which appears to have a more homogeneous vegetation composition. The MRPP analysis of species composition changes showed significant differences among the sampled populations (delta = 0.001).
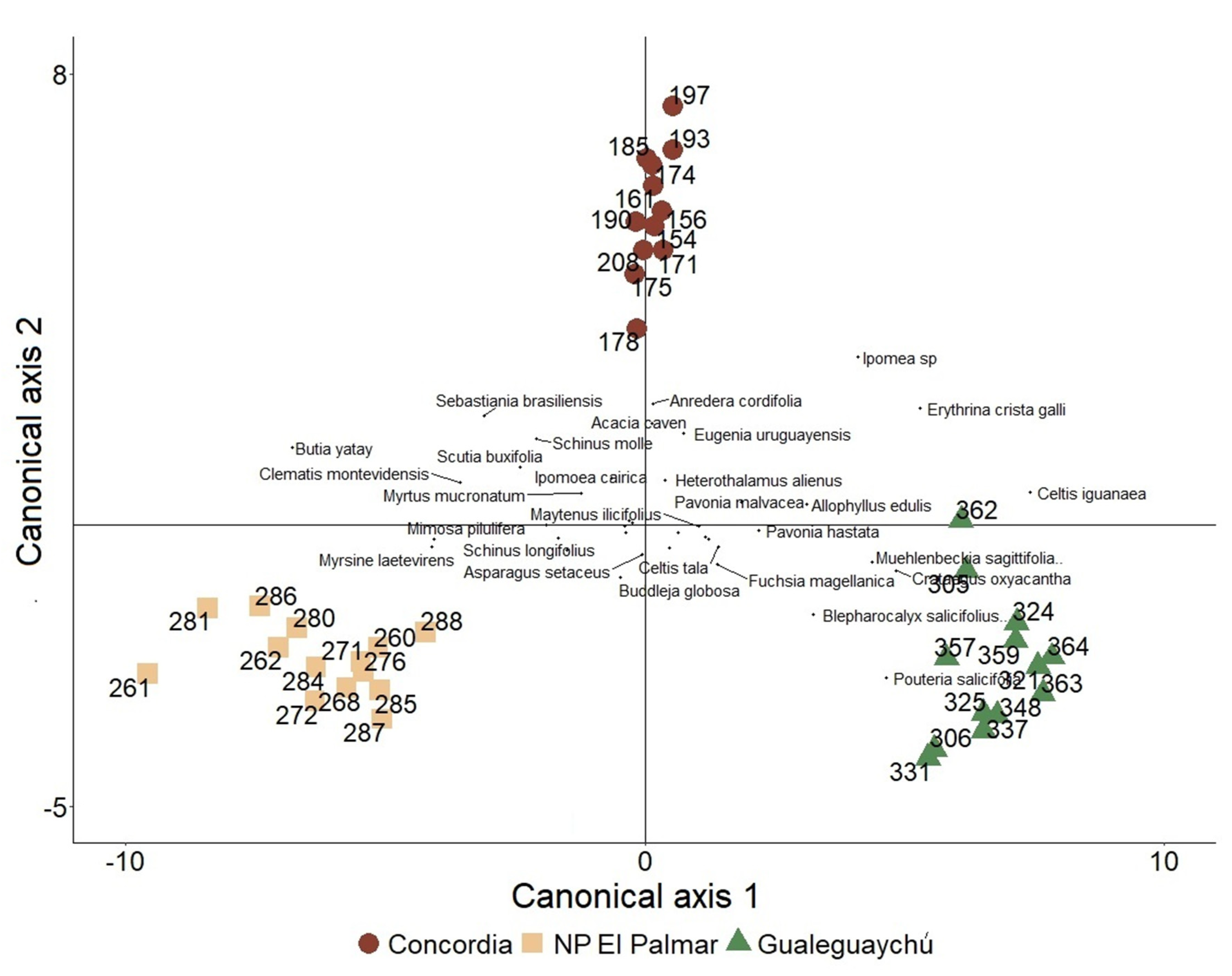
In the discriminant analysis, the maximum separation between groups and the relative location of the species represented in the discriminant canonical axes can be observed (
Figure 8). The separation between groups is complete and the apparent error rate from cross-validation was 0%. This validation implies that if one data is obtained from one of these populations without knowing which one, and we represent its position in this multidimensional space, we would correctly identify the population with a success probability of 100%.
4. Discussion
The consistency in species diversity among the studied locations, as observed in this study and supported by Oliveira–Filho’s research (2017), underscores the reliability of the obtained results. Concordia exhibits a high species richness with 75 registered species, albeit with a moderate coincidence rate of 31.4%. On the other hand, NP El Palmar displays a richness of 71 species, with a relatively higher coincidence rate of 34.3%. Gualeguaychú, while having a lower species richness with 60 species, still maintains a noteworthy coincidence rate of 33.3%. Hence, a considerable number of species and families associated with H. edulis has been successfully recorded in the studied populations. While in terms of alpha diversity, the obtained results and the compared literature seem quite disparate, it should be noted that the bibliographic source consists of a multi-year project that draws from many other sources, which could explain the lower number of species in this study. However, this information allows us to assert that the degree of species representativeness among sites is, at the very least, similar.
In general, regarding richness, abundance, Shannon index and Dominance, less favorable conditions are observed in NP El Palmar compared to Gualeguaychú. The lowest values of richness and abundance recorded in NP El Palmar may be attributed to the majority of H. edulis trees being isolated and located outside areas of dense vegetation. As stated by Micou (2003) in the riparian areas with dense and closed vegetation and a significant presence of Ligustrum lucidum, which is considered an invasive species and a threat to the conservation of regional biodiversity, H. edulis trees were not found. These reasons might lead to the assumption that H. edulis is, in turn, adapted to specific stands within the landscape’s heterogeneity.
Overall, it is suggested that for the three sites, there is a group of species that are relatively dominant in each community, while others have a less pronounced presence. This indicates a non-uniform distribution of species abundance, but the value is not extremely high, which could be moderately considered positive in terms of biodiversity. The higher dominance in NP El Palmar could be explained due to many species were surveyed only once, while a few species were recorded more frequently, such as Solanum mauritianum (26), Scutia buxifolia (17), and Myrrhinium atropurpureum (14). Solanum mauritianum (38) and Asparagus setaceus (31) were two species being markedly dominant in Gualeguaychú, while only three species were recorded once. Same trend was observed with Pielou equitability although no differences were found.
While the Shannon index has significant differences between NP El Palmar and Gualeguaychú, we cannot strictly conclude differences in site biodiversity based on this indicator, as it measures entropy or the state of the complexity rather than biodiversity (Lou & González–Oreja, 2012). In other words, we could conclude that there is greater biological complexity but not necessarily greater diversity per se in Gualeguaychú compared to NP El Palmar. Furthermore, the indicators that measure biodiversity more strictly, such as Evenness and Margalef, showed that there are no conclusive differences between the sites where H. edulis grows.
Broadly speaking, the values of biodiversity indicators might seem not very encouraging, but it should be kept in mind that the surveys were only restricted to woody or semi-woody species, and the precipitation and average temperature regime corresponds to a subtropical to temperate transition zone, and it is known that these climatic variables are correlated with the quantity of species and individuals (Gentry, 1982). It should also be considered that comparative differences with other studies could be due to the fact that the surveys were based on species associated with a single species.
The relationships between native and exotic species and individuals are a way for showing the impact of human activity. In this sense, the anthropic indicator highlighted that Gualeguaychú shows the lower ratio of native to exotic species. It is important to note that while biodiversity indicators may seem favorable, they could represent exotic species and individuals that interact with the habitat and alter the original balance. Therefore, intended introductions of trees and shrubs have led to widespread and problematic invasions that have had negative impacts on ecosystems around the world (Richardson & Rejmánek. 2011).
Also, it is demonstrated that individuals of H. edulis are found in different vegetation communities of the riparian forest of the Uruguay River and are adapted to different conditions of biodiversity, competition, and environment in terms of accompanying woody and semi-woody species. Other studies have successfully set a precedent for combining PCoA with Hierarchical Cluster Analysis (Perelman & Puhl, 2023), as they perform a hierarchical clustering of the observations and then represent them in an ordination obtained with PCoA using the same distance measure used in the clustering, for example as done by Ulloa et al. (2011). Even, other studies have confirmed differences in the composition of tree vegetation in the gallery forests of the Uruguay River according to climatic, physiographic and edaphic environment (Arturi & Juárez, 1997; Batista et al., 2014). In the Cluster Analysis, NP El Palmar and Gualeguaychú show a lower distance explained by vegetation composition. The similarities could be explained by the species: Solanum mauritianum, Scutia buxifolia, Schinus longifolius, and Fuchsia magellanica present in all three sites.
The three statistical analysis tools, i.e., cluster analysis, PCoA, and discriminant analysis, complement each other and consistently demonstrate that the plant communities where H. edulis spontaneously grows are different, and the composition of vegetation among populations is heterogeneous. Additionally, as shown by Cluster Analysis, a closer similarity is observed between El Palmar and Gualeguaychú, that could be explained by a greater difference in annual average temperature and precipitation compared to Concordia.
5. Conclusions
This study successfully recorded a considerable number of species and families associated with H. edulis in the studied populations indicating a diverse plant community within the habitat of H. edulis.
Among the species grown along with the ubajay this study successfully identified five promising native plant species for agroforestry applications: Allophyllus edulis, Butia yatay, Eugenia uniflora, Eugenia uruguayensis and Muehlenbeckia sagittifolia. These species offer a wealth of benefits including edible fruits, medicinal properties, ecological advantages for biodiversity and bioremediation, economic opportunities through fruit production and handicrafts and support for sustainable agricultural practices.
Biodiversity indicators, including richness, Shannon index and dominance revealed variations among the studied sites. These indicators provide insights into the ecological complexity and community structure within each population.
The anthropic indicator highlighted the impact of human activity, with differences in the ratio of native to exotic species among the populations, suggesting varying degrees of human influence and potential conservation challenges.
Statistical analyses, such as cluster analysis, PCoA, and discriminant analysis, consistently demonstrated differences in plant communities among the populations. These variations reflect the heterogeneity of the landscape and the adaptability of H. edulis to different ecological conditions and associated species.
Results obtained can serve as baseline information for future research on the dynamics of vegetation in these areas and on H. edulis studied species. Finally, these findings contribute to understanding how wild species like H. edulis adapt to different plant communities, which might be valuable for developing new agroecological approaches or identifying potential companion planting species in future agricultural systems.
Funding
This work was supported by the University of Morón [PICTO-UM-2019-00003] and CONICET [PIP 11220200102292CO].
Acknowledgments
We would like to especially thank Susana Luisa Stoffella for her contributions. We appreciate the collaboration of INTA Concordia, Establecimiento Pampa Azul, the National Park El Palmar and El Potrero de San Lorenzo reserve.
Appendix A
Table A1.
Presence (P) and absence (A) of native and exotic species and abundance in Concordia, NP El Palmar and Gualeguaychú.
Table A1.
Presence (P) and absence (A) of native and exotic species and abundance in Concordia, NP El Palmar and Gualeguaychú.
| Family |
Species |
Concordia |
NP El Palmar |
Gualeguaychú |
|
| Native |
|
| FABACEAE |
Acacia caven |
P |
P |
P |
|
| SAPINDACEAE |
Allophyllus edulis |
P |
P |
P |
|
| LILIACEAE |
Asparagus setaceus |
A |
A |
P |
|
| FABACEAE |
Bauhinia forficata |
A |
A |
P |
|
| MYRTACEAE |
Blepharocalyx salicifolius |
P |
P |
A |
|
| LOGANIACEAE |
Buddleja globosa |
A |
A |
P |
|
| ARECACEAE |
Butia yatay |
P |
P |
A |
|
| ULMACEAE |
Celtis iguanaea |
A |
A |
P |
|
| ULMACEAE |
Celtis tala |
P |
P |
P |
|
| RUBIACEAE |
Cephalanthus glabrotus |
P |
A |
A |
|
| SOLANACEAE |
Cestrum parqui |
A |
A |
P |
|
| VITACEAE |
Cissus verticilata |
P |
A |
A |
|
| RUTACEAE |
Citrus aurantium |
A |
P |
A |
|
| RANUNCULACEAE |
Clematis montevidensis |
P |
A |
A |
|
| CUCURBITACEAE |
Cyclanthera hystrix |
P |
A |
A |
|
| BIGNONIACEAE |
Dolichandra cynanchoides |
A |
P |
A |
|
| FABACEAE |
Erythrina crista galli |
P |
A |
P |
|
| MYRTACEAE |
Eugenia uniflora |
P |
A |
P |
|
| MYRTACEAE |
Eugenia uruguayensis |
P |
P |
P |
|
| ENOTERACEAE |
Fuchsia magellanica |
A |
P |
P |
|
| ASTERACEAE |
Heterothalamus alienus |
P |
A |
A |
|
| MYRTACEAE |
Hexachlamys edulis |
A |
A |
P |
|
| BIGNONIACEAE |
Jacaranda mimosifolia |
A |
P |
A |
|
| TILIACEAE |
Luehea divaricata |
P |
A |
A |
|
| BIGNONIACEAE |
Magfadenia unguis cati |
A |
P |
A |
|
| CELASTRACEAE |
Maytenus ilicifolius |
A |
A |
P |
|
| POLYPODIACEAE |
Microgramma lycopodioides |
A |
P |
A |
|
| FABACEAE |
Mimosa pilulifera |
P |
A |
A |
|
| FABACEAE |
Mimosa sp |
P |
A |
A |
|
| POLYGONACEAE |
Muehlenbeckia sagittifolia |
P |
A |
P |
|
| MYRTACEAE |
Myrrhinium atropurpureum |
A |
P |
A |
|
| MYRSINACEAE |
Myrsine laetevirens |
A |
A |
P |
|
| MYRTACEAE |
Myrtus mucronatum |
P |
A |
A |
|
| LAURACEAE |
Ocotea acutifolia |
P |
A |
A |
|
| MALVACEAE |
Pavonia malvacea |
P |
A |
A |
|
| FABACEAE |
Poecilanthe parviflora |
P |
A |
A |
|
| SAPOTACEAE |
Pouteria salicifolia |
A |
P |
P |
|
| EUPHORBIACEAE |
Sapium haematospermun |
A |
P |
A |
|
| ANACARDIACEAE |
Schinus longifolius |
A |
P |
P |
|
| ANACARDIACEAE |
Schinus molle |
A |
P |
A |
|
| RHAMNACEAE |
Scutia buxifolia |
A |
P |
P |
|
| EUPHORBIACEAE |
Sebastiania brasiliensis |
P |
A |
P |
|
| FABACEAE |
Sesbania punicea |
P |
A |
A |
|
| SOLANACEAE |
Solanum amygdalifolium |
P |
A |
A |
|
| SOLANACEAE |
Solanum jazminoides |
A |
P |
A |
|
| SOLANACEAE |
Solanum mauritianum |
A |
P |
P |
|
| MALPIGHIACEAE |
Stigmaphyllon bonarense |
P |
A |
A |
|
| COMBRETACEAS |
Terminalia australis |
P |
A |
A |
|
| ASTERACEAE |
Tessaria integrifolia |
P |
A |
A |
|
| VERBENACEAE |
Verbena littoralis |
A |
P |
A |
|
| Exotic |
|
| VERBENACEAE |
Aloysia gratissima |
A |
P |
A |
|
| BASELLACEAE |
Anredera cordifolia |
P |
A |
A |
|
| VERBENACEAE |
Citharexylum montevidense |
P |
A |
A |
|
| ROSACEAE |
Crataegus oxyacantha |
A |
P |
A |
|
| EPHEDRACEAE |
Ephedra twediana |
A |
P |
P |
|
| MYRTACEAE |
Eucalyptus grandis |
P |
A |
A |
|
| PROTEACEAE |
Grevillea robusta |
A |
P |
A |
|
| CONVOLVULACEAE |
Ipomea sp |
P |
A |
A |
|
| CONVOLVULACEAE |
Ipomoea cairica |
P |
P |
A |
|
| OLEACEAE |
Jazminum humile |
A |
P |
A |
|
| JUNCACEAE |
Juncus acutus |
P |
A |
P |
|
| VERBENACEAE |
Lantana camara |
A |
A |
P |
|
| OLEACEAE |
Ligustrum lucidum |
A |
P |
A |
|
| OLEACEAE |
Ligustrum sinensis |
A |
P |
A |
|
| MELIACEAE |
Melia azederach |
A |
P |
A |
|
| MALVACEAE |
Pavonia hastata |
P |
A |
A |
|
| LAURACEAE |
Persea americana |
A |
A |
P |
|
| ARECACEAE |
Phoenix canariensis |
P |
A |
A |
|
| SALICACEAE |
Populus nigra |
A |
A |
P |
|
| SALICACEAE |
Salix babylonica |
A |
P |
A |
|
| Total |
35 |
32 |
26 |
|
References
- Aguilar, M.V.M., Kuinchtner, C.C., Wertonge, G.S. et al. Tolerance and sensitivity of Inga marginata and Allophylus edulis to copper excess. Trees 37, 781–796 (2023). [CrossRef]
- Arena, M. E., Povilonis, I., Borroni, V., Constenla, D., Radice, S., 2021. Changes in physicochemical properties at different development stages of Hexachlamys edulis fruit, an underutilized South American species. Heliyon (7) 11. [CrossRef]
- Arena, M. E., Povilonis, I. S., Borroni, V., Pérez, E., Pellegrino, N., Cacciatore, C., Radice, S., 2023. Changes in Carbohydrates, Organic Acids, and Minerals at Different Development Stages of Hexachlamys edulis Fruit, a Wild South American Species with Horticultural Potential. Horticulturae. 9(3):314. [CrossRef]
- Arturi, M.F. & Juarez, M. C., 1997. Composición de las comunidades arbóreas de la Isla Martín García en relación a un gradiente ambiental. Ecología Austral: 7:65–72.
- Barbieri, R. L., Costa Gomes, J. C., Alercia, A., & Padulosi, S. (2014). Agricultural Biodiversity in Southern Brazil: Integrating Efforts for Conservation and Use of Neglected and Underutilized Species. Sustainability, 6(2): 741-757. [CrossRef]
- Batista, W. B., Rolhauser, A. G., Biganzoli, F., Burkart, S. E., Goveto, L., Maranta, A., Pignataro, A., Genoveva Morandeira, N. S., & Rabadán, M., 2014. Las comunidades vegetales de La Sabana del Parque Nacional El Palmar (Argentina). Darwiniana, nueva serie, 2(1), 5–38. URL: www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S0011–67932014000100001&lng=es&tlng=en.
- Bertucci, A., Haretche, F., Olivaro, C., & Vázquez, A., 2008. Prospección química del bosque en galería de río Uruguay. Revista Brasileira de Farmacognosia, 18(1), 21–25.
- Brelis, L., Busch, V., & Sanguinetti, A. (2023). Palynological and physicochemical characterization of honey from Butia yatay palm savannas in Argentina. Intech Open. [CrossRef]
- Casas, R. R., & Albarracin, G. F., 2015. El deterioro del suelo y del ambiente en la Argentina (Tomo I). Editorial Prosa.
- Cipriano, R., Maia, B., & Deschamps, C. (2021). Chemical variability of essential oils of Eugenia uniflora L. genotypes and their antioxidant activity. Anais da Academia Brasileira de Ciencias, 93 1, e20181299. [CrossRef]
- de Lange, E., Sze, J.S., Allan, J. et al., 2023. A global conservation basic income to safeguard biodiversity. Nat Sustain. [CrossRef]
- Dellacassa, E., Lorenzo, D., Mondello, L., & Cotroneo, A. 1997. Uruguayan Essential Oils. Part VII. Composition of Leaf Oil of Eugenia uruguayensis Camb. var. uruguayensis (Myrtaceae). Journal of Essential Oil Research, 9(3), 295–297. [CrossRef]
- Duguma, D. W., Law, E., Shumi, G. et al., 2023. Spatial predictions for the distribution of woody plant species under different land–use scenarios in southwestern Ethiopia. Landsc Ecol 38, 1249–1263. [CrossRef]
- Fisher, J. C., Dallimer, M., Irvine, K.N. et al., 2023. Human well–being responses to species’ traits. Nat Sustain. [CrossRef]
- Gentry, A. H., 1982. Patterns of neotropical plant species diversity. Evolutionary Biology 15, 1–84.
- Hammer O., Harper D. A. T., Ryan P. D., 2001. PAST: Paleontological Statistics software package for education and data analysis. Palaeontologia Electronica 4(1):1–9.
- Khan, A. U., Abbas, A., Sharif, F., Mansoor, A., Siddiq, Z., 2023. Conserving the threatened woody vegetation on dune slopes: Monitoring the decline and designing adaptive strategies for restoration. Nature Conservation 53: 165–182. [CrossRef]
- IPBES. 2019. Global assessment report of the Intergovernmental Science–Policy Platform on Biodiversity and Ecosystem Services, Brondízio, E. S., Settele, J., Díaz, S., Ngo, H. T. (eds). IPBES secretariat, Bonn, Germany. 1144 pages. ISBN: 978–3–947851–20–1.
- Kuhn, A., Tedesco, M., Laughinghouse, H., Flores, F., Silva, C., Canto-Dorow, T., & Tedesco, S. (2015). Mutagenic and antimutagenic effects of Eugenia uniflora L. by the Allium cepa L. test. Caryologia, 68, 25 - 30. [CrossRef]
- Kunming – Montreal Global Biodiversity Framework CBD. 2022. URL: www.cbd.int/doc/c/e6d3/cd1d/daf663719a03902a9b116c34/cop–15–l–25–en.pdf.
- Lamarca, E. V., Baptista, W., Rodrigues, D. S., & Oliveira-Júnior, C. J. F. 2013. Contribuições do conhecimento local sobre o uso de Eugenia spp. em sistemas de policultivos e agroflorestas. Revista Brasileira de Agroecologia, 8(2), 119-130.
- Lindenmayer, D., Margules, C., & Botkin, D. 2000. Biodiversity Indicators for Ecologically Sustainable Forestry. Conservation Biology, 14(4), 942-949.
- Llorente–Culebras, S., Ladle R. J., Santos A. M. C., 2023. Publication trends in global biodiversity research on protected areas, Biological Conservation, Volume 281, 2023, 109988, ISSN 0006–3207.
- Lou J., González–Oreja, J. A., 2012. Midiendo la diversidad biológica: más allá del índice de Shannon Acta zoológica lilloana 56 (1–2): 3–14.
- Meng, Z., Dong, J., Ellis, E. C. et al., 2023. Post–2020 biodiversity framework challenged by cropland expansion in protected areas. Nat Sustain. [CrossRef]
- Micou, A. P., 2003. Riesgo ambiental por invasiones biológicas en una zona con alto valor de conservación Las cuencas de El Palmar, Entre Ríos [Tesis de grado]. Universidad de Buenos Aires, Buenos Aires, Argentina.
- Nápoles, R., 2016. Ecological Indexes for assessment anthropization – conservation of unit of vegetations, ecosystems, landscape and territory. Acta Botánica Cubana. 215(3), 328–335.
- Oliveira–Filho, A. T., 2017. NeoTropTree, Flora arbórea da Região Neotropical: Um banco de dados envolvendo biogeografia, diversidade e conservação. Universidade Federal de Minas Gerais.
- Perelman, S. B., & Puhl, L. E., 2023. Abordaje multivariado en estudios botánicos y ecológicos. Darwiniana, Nueva Serie, 11(1), 272–294. [CrossRef]
- Posit team. 2023. RStudio: Integrated Development Environment for R. Posit Software, PBC, Boston, MA. Recuperado de http://www.posit.co/.
- Povilonis, I., Arena, M. E., & Radice, S., 2021. Hexachlamys edulis (Berg) Kausel & Legrand, “ubajay”, a native fruit species from South America. Advances in Horticultural Science, 35(4), 389–397. [CrossRef]
- Proença, C. E. B., 2006. Proposal to Conserve the Name Myrcianthes edulis against Psidium amygdalinum (Myrtaceae). Taxon, 55(2), 536–537.
- Richardson, D. M. and Rejmánek, M., 2011, Trees and shrubs as invasive alien species – a global review. Diversity and Distributions, 17, 788–809. [CrossRef]
- Rodrigues, K., Amorim, L., Oliveira, J., Dias, C., Moraes, D., Andrade, E., Maia, J., Carneiro, S., & Carvalho, F. (2013). Eugenia uniflora L. Essential Oil as a Potential Anti-Leishmania Agent: Effects on Leishmania amazonensis and Possible Mechanisms of Action. Evidence-based. Complementary and Alternative Medicine: eCAM. [CrossRef]
- Schapoval, E., Silveira, S., Miranda, M., Alice, C., & Henriques, A. (1994). Evaluation of some pharmacological activities of Eugenia uniflora L. Journal of ethnopharmacology 44 3, 137-42. [CrossRef]
- Shannon, C. & Weaver, W., 1949. The mathematical theory of communication. University of Illinois Press, Urbana–USA.
- Schmeda-Hirschmann, G., Feresin, G., Tapia, A., Hilgert, N., & Theoduloz, C. (2005). Proximate composition and free radical scavenging activity of edible fruits from the Argentinian Yungas. Journal of the Science of Food and Agriculture, 85, 1357–1364.
- Shmida, A., & Wilson, M. V., 1985. Biological Determinants of Species Diversity. Journal of Biogeography, 12(1), 1–20. [CrossRef]
- SMN. 2023. Servicio Meteorológico Nacional. Argentina.
- Suwardi, A. B. & Navia, Z. I., 2023. Sustainable Use and Management of Wild Edible Fruit Plants: A Case Study in the Ulu Masen Protected Forest, West Aceh, Indonesia, Journal of Sustainable Forestry, 42:8, 811–830. [CrossRef]
- The Forests, Trees and Agroforestry Partnership. 2022. URL: www.foreststreesagroforestry.org/wp–content/uploads/2022/10/The–FTA–Partnership–Charter.pdf.
- UNEP–WCMC and IUCN. 2023. Protected Planet: The World Database on Protected Areas (WDPA) and World Database on Other Effective Area–based Conservation Measures (WD–OECM) [Online], July 2023, Cambridge, UK: UNEP–WCMC and IUCN. Available at: www.protectedplanet.net.
- Ulloa, W., C. M. Baeza, V. L. Finot, A. Marticorena & E. Ruiz., 2011. Micromorfología de la lemma de los géneros Polypogon, Agropogon y Agrostis (Poaceae) en Chile. Journal of the Botanical Research Institute of Texas 5(1):237–253.
- Viglizzo, E. F., & Jobbágy, E., 2010. Expansión de la Frontera Agropecuaria en Argentina y su Impacto Ecológico–Ambiental. Ediciones INTA, 9–16.
- Vignale, B., & Bisio, L., 2005. Selección de frutales nativos en Uruguay. Agrociencia, 9(1–2), 41–51.
- Ward, J. H., 1963. Hierarchical grouping to optimize an objective function. J. Amer. Stat. Assoc. 58, 236–244.
- Whittaker, R. H., 1977. Evolution of species diversity in land communities, Evol. Bioi. 10, 1–87.
- World Bank. 2023. Recuperado de www.worldbank.org/.
- WWF. 2020. Living planet report – bending the curve of biodiversity loss. Gland, Switzerland. Recuperado de www.wwf.ch/de/.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).