1. Introduction
Immature embryo rescue is one of the most widely used plant in vitro culture techniques for plant breeding, with objectives depending on the plant species.
Prunus breeding programs use these protocols for the development of early ripening cultivars, staring from parents bearing fruits which flesh ripens faster than their embryos. These immature embryos fail to germinate in soil, following the mechanical treatments to remove the endocarp, cold stratification, and germination in pots in greenhouse conditions, which are useful in other
Prunus varieties [
1]. In vitro embryo rescue is used to improve the percentages of viable plant recovery from peach or nectarine[
2,
3,
4,
5,
6,
7,
8,
9,
10,
11], apricot [
12], cherry [
13] and interspecific hybrids [
14,
15,
16], oriented to generate low chilling, early ripening, new varieties for the worldwide commercial fruit production, and particularly in Spain [
17].
First works on peach in vitro embryo rescue were focused on the in-ovule culture [
18,
19,
20,
21] to facilitate embryo development, surrounded by its seed coat and endosperm, prior to embryo excision, and cultured in synthetic media. Since higher embryo rescue efficiencies were obtained with latter fruit maturity stages, and because peach and nectarine breeding programs need to manipulate large number of embryos, direct immature embryo culture was generally adopted in more recent works, even without removing the embryo integuments [
22].
In most works describing in vitro embryo rescue protocols, seeds were dissected out of fruits, and after disinfection and removal of seed coat and endosperm, embryos were cultured in glass tubes with semisolid agar-containing medium. Herein, an alternative medium containing vermiculite, previously used as substrate to enhance rooting of diverse recalcitrant species, such as apple rootstocks and walnuts [
23,
24,
25], chestnuts [
26], hybrid-tea roses [
27], several ornamental
Prunus spp. [
28], and for peaches and nectarines embryo rescue [
5,
29], is massively used herein for embryo rescue and in vitro maintenance of plantlets, without subculture to fresh medium, until external climatic conditions are appropriate for acclimatization and hardening to greenhouse conditions, which is an important step prior to field plantation and selection.
Conventional container sealing films, such as Parafilm [
30], or limited flask ventilation [
31] have been demonstrated to induce stress reactions in some plants. Therefore, an appropriate flask and cap combination for proper ventilation represent an important factor for proper in vitro plant development as shown in rooting of apricot [
32,
33], sugarcane [
34], and sweet chestnut [
35]. Laboratory test tubes, along with caps and appropriate racks to hold them are and important limiting expense when the number of embryos to rescue rise to several thousand a year. A more economic, alternative glass flask, used in the asparagus canning industry, along with an autoclavable oxygen-permeable sealing film to guarantee proper embryo germination and plantlet growth, are described herein.
Reported results so far [
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13] are rarely mentioning the incidence of in vitro embryo contamination due to external or endophytic microorganisms, scaping seed disinfection. Herein, the importance of this value and the need to reduce such incidence to improve the overall embryo rescue efficiency are reported.
Works published so far on Prunus spp. in vitro embryo rescue have been focused on measuring the embryo germination capacities of specific crosses. The present work is reporting the accumulated results of 8 consecutive seasons, providing results for the whole process, from embryo culture to in vitro plantlet development, and later on to acclimatization and hardening to greenhouse conditions, for a broad range of fruit types, including peaches, nectarines, flat peaches, flat nectarines, and apricots.
2. Results
2.1. Contamination and Embryo Size Depending on the Fruit Type
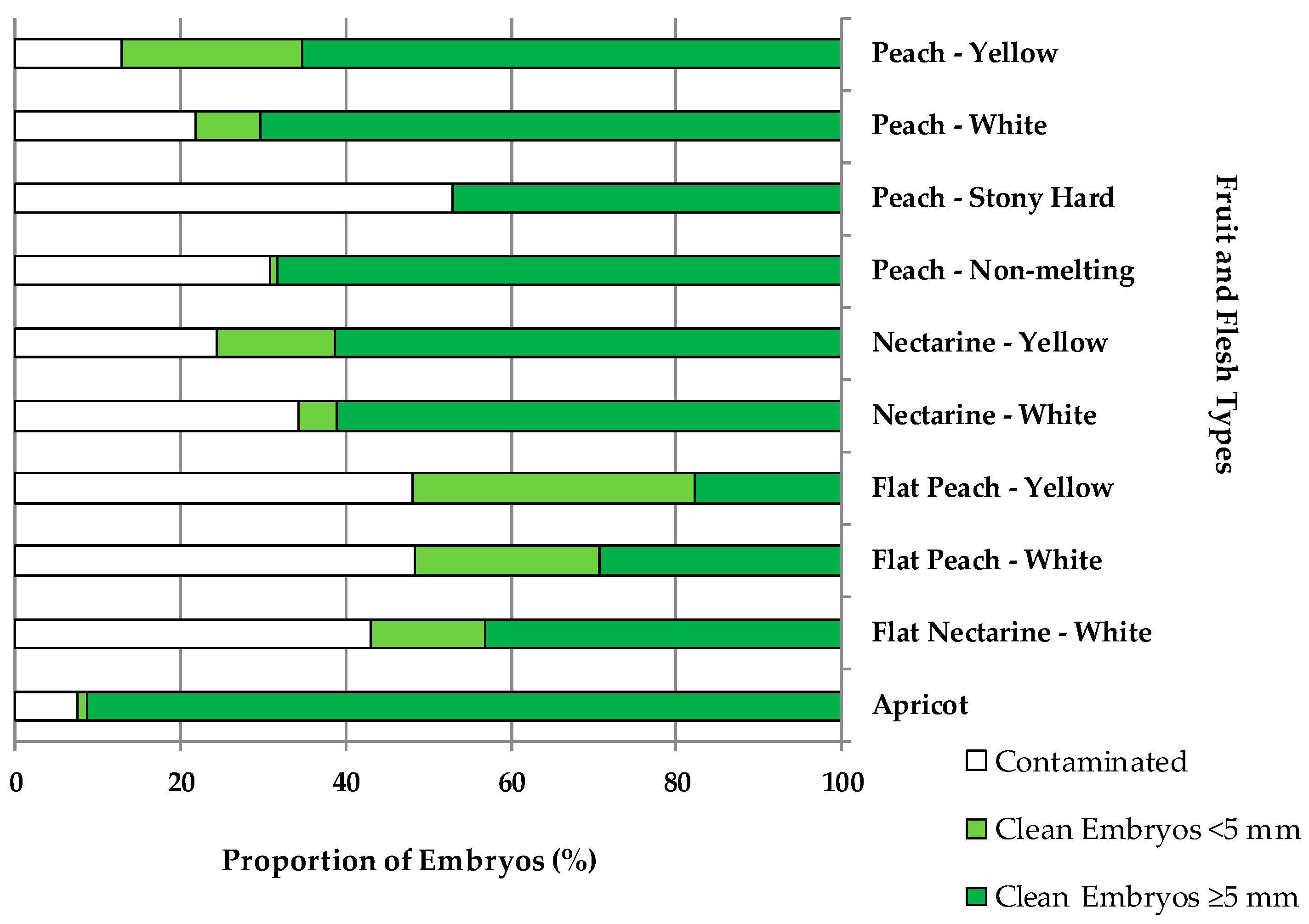
In vitro contamination by exogenous and endogenous microorganisms was found to be dependent on the fruit type (
Figure 1). Apricots (7%), peaches with yellow (13%) and white flesh (22%), nectarines with yellow (24%), peaches with non-melting flesh (31%), and nectarines with white flesh (34%) had the lowest percentages of contamination of the cultured embryos. Instead, the highest percentages of contamination were found on flat nectarines (43%) and flat peaches, with either yellow or white flesh (48%), and on stony hard peaches (53%).
Embryo sizes were also dependent on the fruit type. The highest percentage (34%) of embryos <5 mm was found in flat peaches with yellow flesh. Flat peaches with white flesh and peaches with yellow flesh had both a 22% of embryos <5 mm. Nectarines with yellow flesh and flat nectarines with white flesh had both a 14% of embryos <5 mm. The rest of fruit types had lower percentatges of small embryos, such as 8% in peaches or 5% in nectarines with white flesh, only 1% for apricots and peaches with non-melting flesh, and none for stony hard peaches.
2.2. Embryo Germination Percentatges Depending on the Fruit Type and Embryo Size
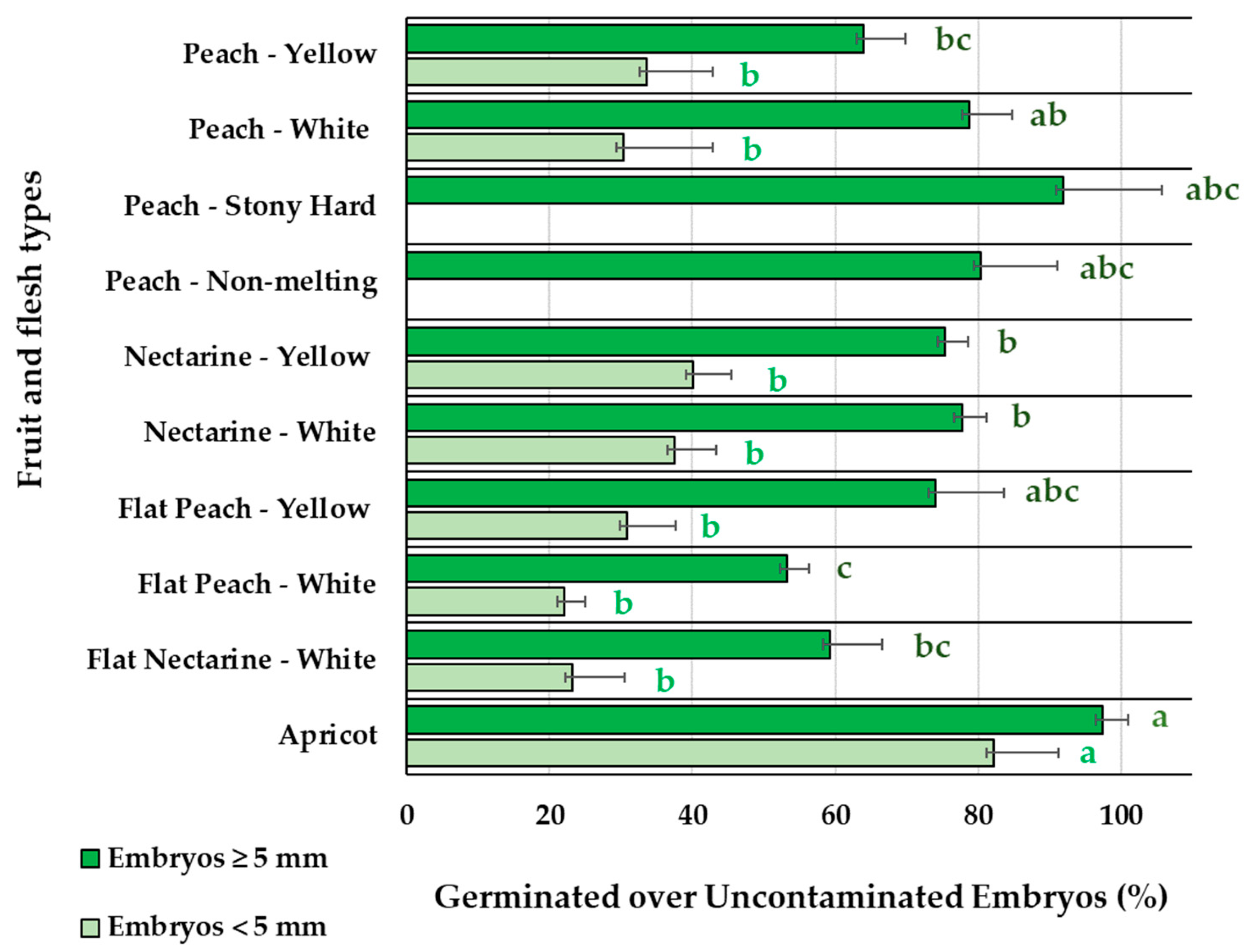
After stratification at 4C, embryo germination for different fruit types, accounted as germinated embryos over uncontaminated embryos (
Figure 2), was significantly lower (p<0.001) for small embryos (< 5 mm) than for big embryos (≥ 5 mm), in all fruit types except for apricot. For small embryos (< 5 mm), no statistically significant differences were observed among peaches and nectarines, with values ranging from 22.1%, for flat peaches with white flesh, to 40.1%, for nectarines with yellow flesh, while apricots had significantly higher embryo germination percentages (82.2%). Instead for big embryos (≥ 5 mm) significant differences were observed among fruit types. Higher embryo germination efficiencies were observed for apricots (97,4%), stony hard peaches (91.9%), non-melting peaches (80.4%), peaches with white flesh (78.7%), and flat peaches with yellow flesh (74.0%). An intermediate level of germination was observed for nectarines with white (77.7%) or yellow flesh (75.3%). The lower germination percentages were registered for peaches with yellow flesh (64.0%), flat nectarines with white flesh (59.3%) and flat peaches with white flesh (53.2%).
2.3. Embryo Germination Percentatges Depending on The use of Vermiculite
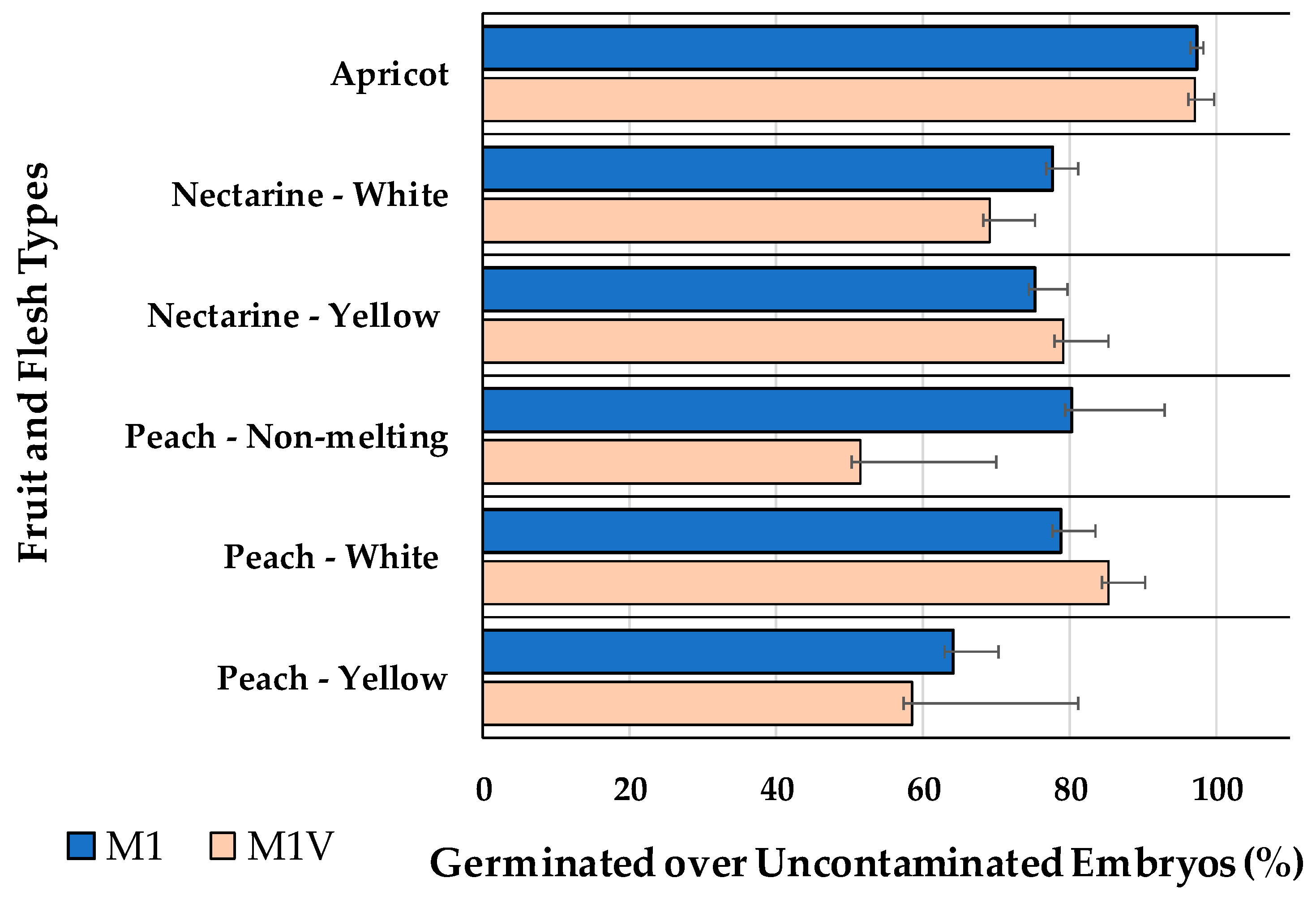
Embryo germination on M1, with no vermiculite, compared to M1V, with vermiculite, assayed for embryos bigger than 5-mm-long is presented in
Figure 3. Flat fruits were not included in this comparison, since though some of their embryos were bigger than 5mm, they had a friable structure, with three cotyledons, easly broken and separated from the embryo axis. The addition of vermiculite to the medium had no statistically significant (P>0.05) effect on the germination percentages of apricots, nectarines or peaches with either white or yellow flesh, and non-melting peaches (
Figure 3).
2.4. Effect of Vermiculite on In Vitro Plantlet Growth
A differential plantlet growth in the in vitro culture chamber was observed in M1 than M1V, with vermiculite, as shown in
Figure 4. Both media generated homogeneous plantlets, which could be acclimated to soil. However, the plantlets grown in M1V (
Figure 4B) compared to M1 had longer shoots and roots, with much better developed leaves. M1V-derived plants had more flexible root system, with thinner roots, and abundant secondary roots, compared to plants derived from M1 medium, whose roots were thicker and friable (
Figure 4A) . Root structure of plantlets grown in M1V were much easily removed from tubes, cleaned, manipulated, and transplanted to soil, without breakage, which facilitated acclimatization and hardening in the greenhouse. Aerial part of M1V-derived plantlets was larger in volume, with broader expanded leaves, which were hardened in vitro, facilitating the acclimatization process to soil. In contrast, some of the plantlets grown in M1 medium presented vitrification, and consequently their acclimatization was not viable.
2.5. Embryo Rescue and Viable Plant Production Efficiencies for Different Fruit Types
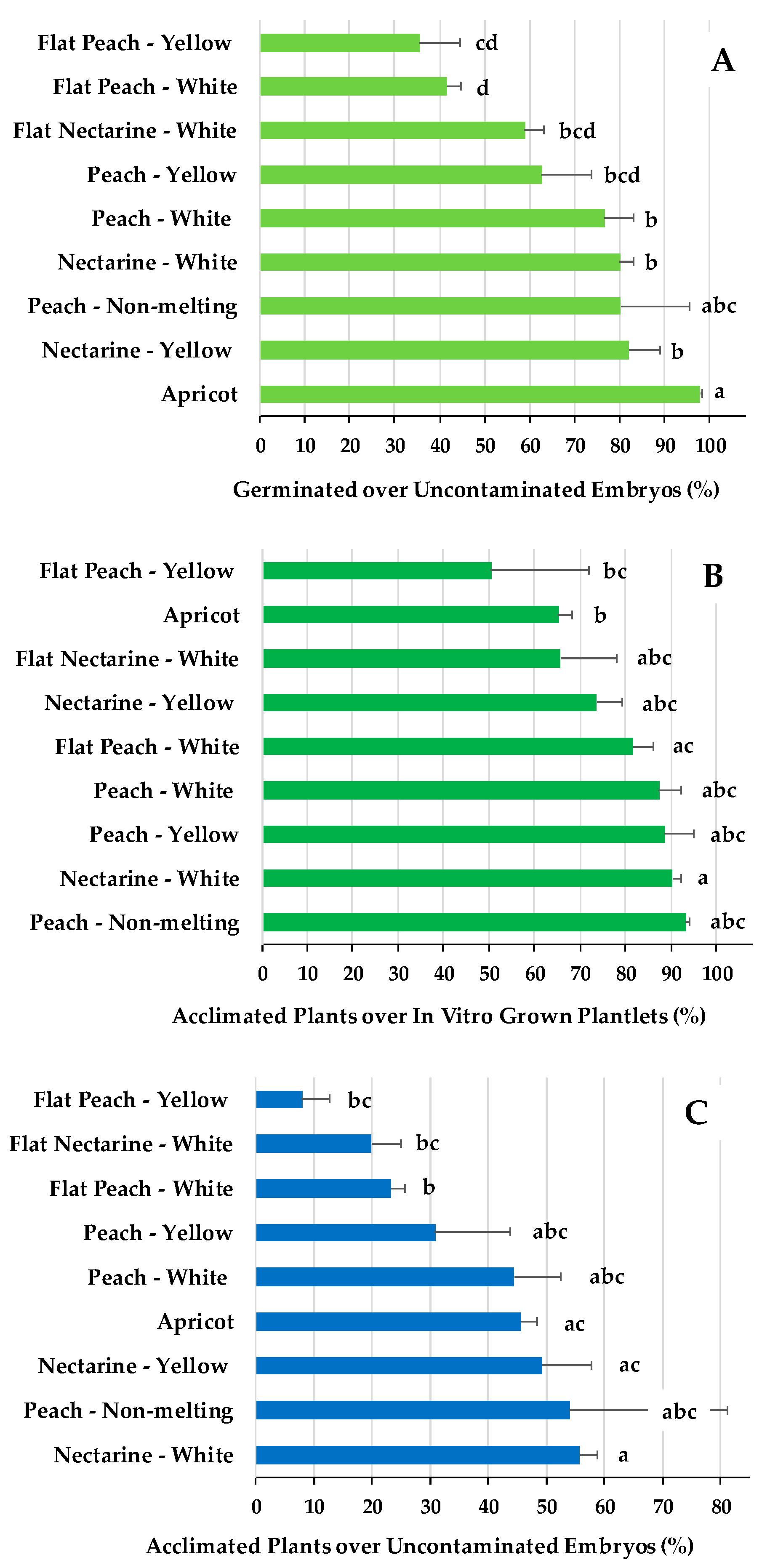
The percentage of in vitro embryo germination over uncontaminated embryos (
Figure 5A) and the percentage of acclimated plants over the in vitro grown plantlets (
Figure 5B) were the two efficiency indexes for which significant differential responses were found among fruit types. An overall efficiency of the whole process, expressed as percentage of acclimated plants over uncontaminated embryos is shown
Figure 5C, for the different fruit types. This index shows the production of viable Prunus spp plants over uncontaminated embryos, transferred to the breeding programs.
The highest efficiency in embryo germination (
Figure 5A) observed for apricot and nectarines with yellow flesh, was associated with a lower efficiency in acclimatization to soil (
Figure 5B), which consequently implied an intermediate overall embryo rescue efficiency (
Figure 5C). The overall lower efficiencies in producing embryo rescued plantlets (
Figure 5C) for flat peaches and flat nectarines, with white or yellow flesh, is associated with both, the lowest embryo germination rates (
Figure 5A) and the lowest or intermediate acclimatization ratios (
Figure 5B). Nectarines with white flesh and peach non-melting are the fruit types with the highest viable plant production ratio (
Figure 5C), which correspond with the highest acclimatization rations (
Figure 5B) and high-intermediate embryo germination efficiencies (
Figure 5A). Peaches, with either white or yellow flesh, had intermediate plant production efficiencies (
Figure 5C), associated with high-intermediate acclimatization ratios (
Figure 5B) and intermediate-low embryo germination efficiencies (
Figure 5A).
3. Discussion
3.1. Contamination and embryo size depending on the fruit type. Fruit and flesh types, their maturity stage at harvest time, and conservation conditions till seed extraction determin
ed their pericarp integrity and softness. Flat peaches, flat nectarines, nectarines with white flesh, and non-melting peaches have abundant split endocarps, and some cracked mesocarps at embryo rescue time. Consequently, seeds were easily contaminated by microorganisms, and their surface sterilization couldn’t avoid the in vitro contamination (
Figure 1). Endocarp integrity, as for stony hard peaches, was not a guaranty of low in vitro contamination, when the exocarp and endocarp are over ripened. When fruits were harvested one or two weeks prior to their commercial harvest the pericarp integrity facilitat
ed the reduction of in vitro embryo contamination levels, as for apricots (7%), peaches with yellow (13%) or white (22%) flesh, and nectarines with yellow flesh (24%). Although such information was not usually presented in previous works [
6,
13,
22,
36], there is a need to reduce the levels of in vitro contamination caused by exo- and endophytes, improving the fruit harvest and storage conditions, seed disinfection, in vitro culture conditions and biological control with beneficial microorganisms [
37,
38,
39].
The high percentage of in vitro embryo contamination in flat fruits was associated with a frequent mold seed contamination while inside the endocarp, which was difficult to eliminate by disinfection, without damaging the embryo. Such contamination is probably related to the split pit formation, a morphological disorder related to the poor adaptation of flat peaches and nectarines to climatic conditions different to the climate of their center of origin in Asia [
17].
Embryo size at fruit harvest time was determined by the fruit type, with a higher proportion of small embryos (<5mm) for flat peaches with yellow flesh (34%), flat peaches with white flesh (22%), peaches with yellow flesh (22%), flat nectarines with white flesh (14%), and nectarines with yellow flesh (14%). The rest of fruit types have proportions of small embryos ranging from 8 to 0%. These proportions of small embryos explained the overall embryo rescue efficiencies for the studied fruit types (
Figure 5C).
3.2. Embryo Germination Percentatges Depending on the Fruit Type and Embryo Size
In the present study, in which 271 crosses from 10 different Prunus fruit types have been tested (
Table 1), lower in vitro germination percentages were observed for smaller embryos (< 5 mm) (
Figure 2), as it was stated previously in peach cultivars [
40]. Some works proposed that embryos should be rescued before abortion and at the same time postponed until embryos were big enough, particularly in interspecific hybrids involving Prunus persica, P. armeniaca, P. salicina, P. cerasifera [
14] and P. salicina, P. armeniaca [
16]. In accordance with it, some works [
9] using the number of weeks after pollination as an indicator for peach embryo development, without specifying the embryo size, found maximum germination percentages at maximum days after pollination (75 and 85 days). In accordance with it, in the present work fruits were harvested at maximum number of days after pollination, right before commercial ripening, saving pericarp integrity.
In vitro germination rates of small embryos (< 5 mm) were found to be 82.2% in apricot, 40.1% in nectarine, 30% in peach, and 22.1% in flat fruits, slightly better than the 30% reported for hybrids involving
P. cerasifera and
P. armeniaca [
41]. Such germination improvement for small embryos, found in the present work, could be explained by use of M2 medium, which was supplemented with GA
3, known to break the dormancy and promote embryo germination, and BAP, to promote cell division [
2,
42]. The hormonal combination 1μM GA
3 + 1μM BAP present in the M2 medium, was determined in previous works in our laboratory (data not shown) with small embryos (< 5 mm) to be significantly better in promoting germination of nectarines and flat peaches (data not shown) compared to the WPM without hormones, or other hormonal combinations ((I) 1μM GA
3; (II) 1μM BAP; (III) 1μM BAP + 1 μM Kinetin; or (IV) 1μM GA
3 + 1μM BAP + 1 μM Kinetin).
Low embryo germination rates in flat fruits and nectarines (
Figure 2), might be associated to high abortion ratios, due to an abnormal endosperm development [
43] and carbohydrate metabolism [
44]. On the contrary, apricot embryo rescue efficiencies were much higher than other fruit types, reaching highest efficiencies in disinfection and germination. High in vitro germination ratios were in accordance with other studies [
12,
21], for which removing the seed coat improved the germination [
1]. Apricot encountered more difficulties than peach, nectarine, and flat fruits during acclimatization to soil, associated with shoot tip necrosis as a response to lack of calcium in the medium [
45], and the high sensitivity of apricot plants to root asphyxia and soil waterlogging [
46,
47].
Abscisic acid (ABA) accumulation in the seed during embryo maturation, a germination inhibitor [
22], was proposed as the explanation for the poorer germination of big apricots [
12] and
Prunus avium [
13] embryos compared to the small embryos. The present study shows, for any fruit type tested, better germination percentages with the big (> 5 mm) than small (< 5 mm) embryos (
Figure 2), presumably caused by the seed coat and endosperm removal before in vitro embryo culture, the stratification length at 4C, and the culture at 14C after stratification.
The introduction in the present study of a 14C in vitro culture period for 4 weeks, right after stratification at 4C, instead of direct culture to 24C, improved the embryo rescue efficiency with respect to previous studies in our laboratory (data not shown) and is in accordance with previous works recommending lower temperatures of culture for better plantlet development [
19,
36]. At 14C, even though it is not the optimal for in vitro
Prunus spp micropropagation, the axis started to elongate, formed roots and shoot, and leaves grew slower than at 24C, avoiding the apical shoot-tip necrosis observed when germinated embryos were moved directly from 4C to 24C. Apical necrosis has been related to multiple factors, as low boron and calcium content in the culture medium and physical conditions, such as humidity and temperature, that guarantee the translocation of nutrients from the base to the apex [
45,
48,
49]. Periodic reduction of the relative humidity in the in vitro culture flask induced leaf transpiration, root calcium uptake and translocation to the shoot, while constant high relative humidity reduced calcium movement [
50]. Culturing embryos at 14C allowed for a slower growth, less calcium demand and alleviated the incidence of shoot-tip necrosis.
3.3. Embryo Germination Percentatges Depending on the Use of Vermiculite
In this work, vermiculite has been evaluated as an embryo support for the in vitro culture of Prunus spp., in an only-one-medium used from the initial immature embryo culture to the acclimatization of the resulting plantlets. Vermiculite-containing medium had previously been reported as a suitable substrate for rooting during greenhouse acclimatization of chestnut [
26], tangerine [
51], peach [
52] and aloe [
53], and for the in vitro rooting improvement of walnut [
25], grape [
54], hybrid tea rose [
27] and peach [
28].
Earlier, the in vitro embryo rescue of 350 [
5] and 600 [
29] nectarines and peaches, replacing agar with vermiculite, demonstrated a higher percentage of germination with vermiculite than other potting substrates (coconut fiber or charcoal rice husk). After stratification for 9 weeks and culture for 2 more weeks, germination percentage (80-100%) in vermiculite was better compared to the use of medium with agar. In the same sense, in the present study, no statistically significant differences were observed on in vitro embryo germination of apricots, nectarines or peaches (
Figure 3) when cultured in M1 or M1V media. Flat peaches or nectarines were not cultured in M1V, and only in agar-containing media (M1 or M2), due to their size and friable morphology of their embryos. These results suggest that the use of vermiculite in the medium, for embryos bigger than 5-mm-long, would not influence in the germination efficiency of the studied
Prunus spp. fruit types, but help laboratory logistics and process cost reduction.
3.4. Effect of Vermiculite on In Vitro Plantlet Growth
The use of M1V culture medium presented advantages during the in vitro plantlet development and transfer to soil conditions. When plantlets grew in medium with agar as gelling agent (M1), roots twisted on themselves following the tube’s shape and sometimes the shoot became chlorotic or vitrified when kept in the same recipient for a long time. In contrast, plantlets derived from vermiculite-containing medium (M1V) had significantly better root and shoot development. In M1V medium roots ramified and produced secondary roots, presumably caused by the physical obstacle that vermiculite represents and the abundance of aerated spaces with higher oxygen and water levels [
23,
55]. Consequently, plantlets in M1V developed taller shoots with bigger broader leaves (
Figure 4). A better rhizosphere and aerial part favored a successful acclimatization compared to the plantlets grown in agar-containing medium [
23,
25]. Vermiculite-containing medium facilitated the application of rhizosphere microorganisms during embryo rescue, and although rescue efficiencies were not significantly increased, plantlet development and acclimatization to soil were improved [
56].
Plantlets cultured in vermiculite-containing medium could stay longer in the in vitro culture room, without subculture to fresh medium, which represents and advantage over the agar-containing medium, when the external climatological conditions recommend delaying the acclimatization to soil. By adding from 10 to 20 mL of liquid PRU0 medium to each plantlet in M1V, this could remain in vitro for 4 to 8 additional weeks. This avoided plantlets subculture to fresh medium, which supposed a lower labor cost and materials. Prolonging the plants life on in the culture room, the acclimatization was time flexible, resolving the existence of adverse climatic conditions or difficulties in hand labor logistics in the laboratory.
The advantages of M1V over M1 medium go along with the use of the vessels and closures described herein, alternative to the more costly laboratory glass tubes, caps, and racks. They provide more space to thousands of plantlets grown under in vitro conditions, at a minimal cost. Flasks and their closure system, presented herein, are autoclavable, light and air permeable, facilitating the aeration of plantlets in culture. In agreement to our results, improving aeration of the in vitro culture recipients has also been shown to increase the number of usable potatoes microtubes [
57], the regeneration and growth of pepper plantlets [
58] and sugarcane [
34], and increased the number and leaf size during acclimatization in chestnut plantlets [
35]. A good vessel ventilation reduced the stress reactions of
Arabidopsis probably caused by high ethylene and carbon dioxide concentrations in the Petri dishes closed with air-tight tapes [
30,
31].
Prunus GF677 had best multiplication and elongation in round vessels with a filter breathing strip [
59]. Different jar closures were compared for the rooting and acclimatization of apricot plantlets [
32,
33], and the high ethylene concentration in the vessel with hermetic closure affected growth of plant cultures and was associated with lower root number and plant survival in the acclimatization phase. Previous assays with
Pyrus and
Prunus plantlets in coculture with microorganisms demonstrated a CO2 accumulation over 2% in the culture vessels [
60], which resulted to be toxic for plantlet development. Consequently, the used vessels and caps described herein, widely used in the agri-food industry, substantially reduced its cost compared to the laboratory test tubes and resulted to be optimal for embryo rescue.
3.5. Embryo Rescue and Viable Plant Production Efficiencies for Different Fruit Types
As commonly found in any plant in vitro culture methodology, the genotype, herein indicated as fruit type, has a significant differential effect on in vitro embryo rescue, from the germination to acclimatization (
Figure 2 and
Figure 5). Major part of the studies on embryo rescue have been focused on the different culture conditions to improve germination, in peaches and nectarines, showing differential responses of the varieties [
4,
8,
9]. Herein, an optimized protocol, over several seasons in our conditions, has been applied to different fruit types. Germination ratios presented in this work in peach and nectarine are like those published previously [
7,
8,
22].
Independently of the embryo size, apricots had the highest embryo germination ratios. When embryos were smaller than 5mm no differences were found among different types of peaches and nectarines, and when embryos were bigger than 5mm best germination percentages were recorded for nectarines and peaches rather than flat fruit types. Differential in vitro contamination by exophytes and endophytes (
Figure 1), capacities for in vitro embryo germination (
Figure 5A), and differential acclimatization capacities to soil (
Figure 5B) explained the overall ratios for plant production of each fruit type (
Figure 5C). These values have been presented for the first time as a measure of the feasibility of the protocol used for in vitro embryo rescue applied to
Prunus plant breeding programs, for which a great number of embryos must be handled.
Few studies have reported results on the differential acclimatization of the plantlets resultant from the embryo rescue [
56]. In the present work, results come from larger numbers of embryos (
Table 1), derived from different fruit types, varieties, and seasons than in previous works [
52], in which a high percentage of acclimatization was obtained in peach seeds but after using a rooting medium before acclimatization, which is difficult to apply in programs involving many embryos.
4. Materials and Methods
4.1. Plant Material
A total of 271 controlled crosses between early ripening peach, nectarine, flat peach or flat nectarine accessions, all belonging to Prunus persica, and apricot, Prunus armeniaca, were used in this study, resulting in the harvest of 63549 fruits during eight consecutive seasons, from 2014 to 2021. Most of the Prunus persica fruits were derived from IRTA’s breeding program developed at the experimental orchard located at Gimenells, northeastern Spain. Some other Prunus persica fruits were provided by two Spanish private breeding programs. The Prunus armeniaca fruits were provided by a French breeding company, located at southeast France. Fruits form each cross were harvested 10 days before commercial ripening, transported and stored between 1 and 3 C, in conventional cold rooms at IRTA Fruitcentre, till embryo dissection was performed. The in vitro germination efficiencies were recorded for all the 271 crosses, and for a subset of 142 crosses, efficiencies were evaluated for in vitro embryo germination, in vitro plantlet development, and acclimatization to greenhouse conditions.
Table 1.
Number of directed crosses, fruits and rescued embryos used in the present work. .
Table 1.
Number of directed crosses, fruits and rescued embryos used in the present work. .
| Species |
Fruit Type |
Flesh Type |
Crosses |
Fruits |
Embryos |
|
Prunus persica (L.) Batsch |
Peach |
Yellow |
18 |
2778 |
2838 |
|
Prunus persica (L.) Batsch |
Peach |
White |
16 |
3984 |
3469 |
|
Prunus persica (L.) Batsch |
Peach |
Non-melting (Pavia) |
8 |
434 |
391 |
|
Prunus persica (L.) Batsch |
Peach |
Stony Hard (Albino) |
3 |
717 |
660 |
|
Prunus persica (L.) Batsch |
Flat Peach |
Yellow |
7 |
1350 |
1341 |
|
Prunus persica (L.) Batsch |
Flat Peach |
White |
65 |
10792 |
10553 |
|
Prunus persica (L.) Batsch |
Nectarine |
Yellow |
53 |
20121 |
18329 |
|
Prunus persica (L.) Batsch |
Nectarine |
White |
47 |
14684 |
12555 |
|
Prunus persica (L.) Batsch |
Flat Nectarine |
White |
14 |
4842 |
2009 |
| Prunus armeniaca |
Apricot |
- |
40 |
3846 |
3811 |
| Total |
|
|
271 |
63548 |
55956 |
4.2. Embryo Dissection and Culture
Peach and nectarine fruits were opened with the help of pruning scissors. Apricot fruit flesh was removed with a knife and the kernel was open with a nutcracker. Seeds were extracted from the kernel, if required with the help of forceps, trying not to damage neither the seed coat nor the embryos. To reduce microbial contaminations, seeds with teguments, in groups of 10 seeds, were disinfected with 1% NaClO for 10 minutes, followed by three rinses in sterile distilled water. Seeds were dissected at naked eye or with the help of dissecting scope, under aseptic conditions, removing the seed coat and endosperm, trying to keep the embryo (cotyledons, plumule and radicle) as integer as possible. After that, embryos were cultured into different types of culture vessels and media formulations, depending on the embryo size.
4.3. Culture Media
All embryos were cultured in a basal medium composed of Woody Plant Medium (WPM) [
61] basal salts and vitamins (Duchefa Biochemie B.V., 2003 RV Haarlem, The Netherlands), amended with 30 g/L sucrose, and 9 g/L agar (Quimivita, Barcelona, Spain), adjusted to pH 5.7 before autoclaving. For embryos bigger than 5–mm-long, two different media were used, named M1 and M1V. Both were based on the basal medium, and M1V was amended with 125% Vermiculite (50/40, v/v, vermiculite/semisolid medium). Vermiculite (Termita-3, Castillo Arnedo, Calahorra, Spain) was dispensed into the culture vessels before the semisolid basal medium was added. Embryos smaller than 5-mm-long of maximum diameter, were cultured in M2 medium. This medium was the basal medium supplemented 1µM gibberellic acid (GA3), and 1µM 6-benzylaminopurine (BAP). BAP was added to the medium before autoclaving, while GA3 was dissolved in dimethyl sulfoxide (DMSO) and added to the medium after its sterilization and cooling till 50 C. The final DMSO concentration was 0.05% (v/v, DMSO/medium). M1 and M1V once in the following culture vessels were sterilized for 20 minutes at 121 C. While M2 was sterilized in bottles and dispensed to Petri dishes after adding the GA3.
4.4. Containers Used for Embryo Rescue
Embryos bigger than 5-mm-long were cultured in either (1) glass tubes of 38-mm-wide and 160-mm-high with aerated polypropylene cap (Ref L10568, LineaLAB, Badalona, Spain) (
Figure 6C and 7C), or (2) glass jars of 42-mm-wide and 170-mm-high (Ref 7cyl TO48, Apiglass, Barcelona, Spain) closed with a plastic layer P30PXNP (Ref PET 30 Melinex, ILPRA Systems S.L., Mataró, Spain) (
Figure 6D and 7D). This film is autoclavable, transparent, and with an Oxigen Gas Transmission Rate <110 cm3/m2 day. A square of 10 × 10cm was cut and fixed at the mouth of the jar with a PVC ring (47 mm internal diameter and 20 mm height). Each glass tube or jar contained 90 mL of M1V medium. Alternatively, embryos with a size bigger than 5-mm-long were cultured in glass tubes of 24-mm-wide and 150-mm-high with aerated polypropylene caps (Ref N-51A KIMAX, Scharlab, S.L., Barcelona, Spain) (
Figure 6B and 7B), each containing 20 mL of M1 medium. Petri dishes, 60-mm-wide and 15-mm-height (Ref 82.1194.500, Sarstedt, La Roca del Vallès, Spain) (
Figure 6A and 7A), each containing 10 mL of M2 medium, were used to culture embryos smaller than 5-mm-long, one in each dish.
4.5. Cold stratification and Induction of Embryo Germination
Petri dishes, racks of tubes or jars with embryos were wrapped with plastic film, placed inside of dark plastic bags, and cultured in a dark cold chamber set at 4C, for the stratification during 3 months. After this time, a first germination control was done, recording (1) contaminated cultures, which were eliminated, (2) number of clean embryos (C), and (3) number of germinated embryos (G). Embryos started their germination when their cotyledons were unfolded, and the embryo axis started to elongate, as shown in
Figure 6. Not germinated embryos were brought back to the cold culture room at 4C for 2 additional months. At this time, a second germination control was done; recording the same parameters (C and G). These values were added to the values recorded in the first germination control. The ratio G/C, expressed in a percentage was calculated for each cross.
4.6. Plantlet Growth till Acclimatization
Germinated embryos after the first control and all embryos after the second control, were moved to an in vitro culture chamber at 14C and 150-200 µmol m-2 s-1 of light intensity with a 12/12h photoperiod, for a 1-month-long culture period. In the case of smaller embryos, germinated in a Petri dishes (
Figure 7A), were transplanted to tubes with M1 medium, to increase the available space for their development under this culture conditions. One month later and depending on the plantlet development, tubes and flasks with plantlets (
Figure 7B, C and D) were moved to a culture chamber at 24C, and 150-200 µmol m-2 s-1 of light intensity with a 16/8h photoperiod, to accelerate the plantlet growth. Embryos rescued in M1V medium (
Figure 7C and D), when necessary were supplemented with 20 mL of liquid PRU0 medium in each vessel. This medium was half WPM basal salts and vitamins, amended with 50 µM FeEDTA, 6 µM CuSO4·5H2O, 30 g/L sucrose, adjusted to pH 5.7 before autoclaving. From 5 to 7-month post embryo dissection and culture, the number of developed plantlets (P), such as those in
Figure 7B, C and D, was recorded for each cross.
4.7. Acclimatization to Greenhouse Conditions
Plantlets with a good shoot and root development (
Figure 7B, C and D) were removed from the containers, cleaned up with tap water and planted in trays containing a peat:vermiculite mix substrate (2:1, v/v). Vermiculite was the same used in M1V medium. Peat substrate was the Exclusive (Gebr.Brill Substrate GmbH & Co. KG, Georgsdorf, Germany). Plants were acclimated in plastic tunnels (
Figure 8A), within a conventional greenhouse, designed to decrease the atmospheric relative humidity gradually and automatically, from 100 to 60% during a 3 to 4-week-log period. Acclimatization tunnels had a soil temperature above 22/18 ºC (day/night) and a photoperiod of 16 h light, supplemented with LED lights (SUP12100DC, AlternativaLED, Spain) to extend the day light hours, at 230 μmol m-2 s-1 PAR at leaf level. After this period, trays were removed from the tunnels and cultured in a greenhouse for their hardening, watered by a mist system until they were hard enough to be transferred into an evaluation plot. At this point (
Figure 8B), the number of acclimated plants (A) was determined for each cross.
5. Conclusions
In vitro immature embryo rescue efficiencies measured as viable plants transferred to field plots, from early ripening peach, nectarine, and apricot varieties, primarily depend on the fruit type, embryo size, fruit quality at harvest and conservation prior the seed dissection out of the pericarp. Endo- and exophytic embryo contamination must be reduced or controlled to improve the rescue of viable plants.
Overall efficiencies of the protocol described herein run from 8 to 23% acclimated plants over uncontaminated embryos for flat peaches and flat nectarines, 31 to 54% for peaches, 49 to 56% for nectarines, and 46% for apricots. Embryo germination after culture at 4C, and acclimatization of plantlets to soil are the variables determining the differential embryo rescue efficiencies for the different fruit types.
The use of vermiculite in the culture medium, compared to agar-containing medium, had no detrimental effects on the in vitro embryo germination and was beneficial for the development of the rhizosphere, stem, and foliar area, which were favorable for the plantlet acclimatization to soil.
Adaptation of affordable glass containers, used in the food industry, instead of more expensive laboratory glass tubes, and the use of gas permeable films to close the flask, make of the protocol described herein a feasible and reliable methodology for Prunus spp breeding programs, in which manipulating thousands of embryos is necessary.
Author Contributions
Conceptualization, M.C., E.C., and R.D.-S.; methodology, M.C., E.C., and R.D.-S.; software, M.C. and R.D.-S.; validation, M.C. and R.D.-S.; formal analysis, M.C. and R.D.-S.; investigation, M.C., E.C., and R.D.-S.; resources, M.C. and R.D.-S.; data curation, M.C. and R.D.-S.; writing—original draft preparation, M.C. and R.D.-S.; writing—review and editing, M.C. and R.D.-S.; visualization, M.C. and R.D.-S.; supervision, M.C. and R.D.-S.; project administration, R.D.-S.; funding acquisition, R.D.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The CERCA Programme/Generalitat de Catalunya, and IRTA.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. Access to plant material names or identifications is restricted due to private breeding programs interests.
Acknowledgments
We acknowledge Christian Bois and Arthur Bois (Newcot, France) for apricot fruits; José Martín Charlez (Vif International, Spain) for peach and nectarine fruits, as well as Cristian Fontich, Celia Cantin, Carolina Font, Gemma Reig, and Luis Asin (IRTA, Fruticulture Program) for most fruits used for the present work. Also thank Sandra Franquesa (IRTA, Plant in Vitro Culture Laboratory), for unvaluable technical support for the present work. Aswell, we acknowledge the work done as undergraduate or graduate students, in practices at the Plant in Vitro Culture Laboratory, between 2014 and 2024, to Albert Solé, Amanda Prieto, Anna Calvet, Bernat Capdevila, Bernat Nadal, Blanca Fernández, Carlos Rolando, Daniel Cantabella, Ester Mora, Eva Alsina, Gemma Puigarnau, Jaume Bagué, Joel Begué, Júlia Amorós, Khedidja Gasmi, Laia López, Laia Ribalta, Maria Fiñana, Maria Juan, Maria Martin, Marta Bosch, Nadia Cambra, Natàlia Garcia, Neus Mas, Nicolau Llebòt, Núria Solsona, Oliver Sorolla, Pilar Fernández, and Silvia Colonques (all from UdL, Lleida); Maria Almira (UAB, Barcelona); Alexandre Vicens (UVic-UCC, Vic-Manresa); Oriol Piqué (UB, Barcelona); Jan Callejón (UPV, Valencia); Sotirios Pilafidis, Fragkiskos Papakonstantinou, and Fevronia Vanioti (all from Agricultural University of Athens, Greece); Neslihan Kalkin (Uludag University, Turkey); Alberto Iannuzzi (Università Tuscia, Italy); and Lucia Monti (Università Bologna, Italy).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Szymajda, M.; Żurawicz, E.; Maciorowski, R.; Pruski, K. Stratification Period Combined with Mechanical Treatments Increase Prunus Persica and Prunus Armeniaca Seed Germination. Dendrobiology 2019, 81, 47–57. [Google Scholar] [CrossRef]
- Rizzo, M.; Bassi, D.; Byrne, D.; Porter, K. Growth on Immature Peach (Prunus Persica L. Batsch.) Embryos on Different Media. Acta Hort. 1998, 465, 141–144. [Google Scholar] [CrossRef]
- Sinclair, J.W.; Byrne, D.H. In Vitro Growth of Immature Peach Embryos as Related to Carbohydrate Source and PH. Acta Hort. 2002, 592, 157–159. [Google Scholar] [CrossRef]
- Hamill, S.D.; Beppu, K.; Topp, B.L.; Russell, D.M.; Defaveri, J. Effects of Media and Fruit Ripeness on Germination and Transplanting of In Vitro Cultured Embryos from Low-Chill Peach and Nectarine. Acta Hort. 2005, 694, 145–148. [Google Scholar] [CrossRef]
- Promchot, S.; Boonprakob, U. Replacing Agar with Vermiculite, Coconut Fiber and Charcoal Rice Husk in Culture Media for Embryo Rescue of Immature Nectarines Seeds. Thai J Agric Sci 2007, 40, 167–173. [Google Scholar]
- Mansvelt, E.L.; Pieterse, W.-M.; Shange, S.B.D.; Mabiya, T.C.; Cronjé, C.; Balla, I.; Ham, H.; Rubio-Cabetas, M.-J. Embryo Rescue of Prunus Persica: Medium Composition Has Little Influence on Germination. Acta Hort. 2015, 1084, 207–210. [Google Scholar] [CrossRef]
- Singh, H.; Thakur, A.; Jawandha, S.K. Summer Stratification and Germination: A Viable Option for Recovery of Hybrid Seedlings in Low Chill Peach and Nectarines. Indian Journal of Horticulture 2017, 74, 151–155. [Google Scholar] [CrossRef]
- Devi, I.; Singh, H.; Thakur, A. Effect of Developmental Stage and Medium on Embryo Culture of Low Chill Peach Hybrids. Curr Sci 2017, 113, 1771–1775. [Google Scholar] [CrossRef]
- Sundouri, A.S.; Singh, H.; Gill, M.I.S.; Thakur, A.; Sangwan, A.K. In-Vitro Germination of Hybrid Embryo Rescued from Low Chill Peaches as affected by Stratification Period and Embryo Age. Indian J. Hort. 2014, 71, 151–155. [Google Scholar]
- Mancuso, M.L.; Caruso, T.; Germanà, M.A. Peach Breeding Programme for Early Ripening, Low Chilling Requirement Cultivars: Embryo Rescue and Somatic Embryogenesis. Acta Hort. 2002, 592, 125–129. [Google Scholar] [CrossRef]
- Sinclair, J.W.; Byrne, D.H. Improvement of Peach Embryo Culture Through Manipulation of Carbohydrate Source and PH. HORTSCIENCE 2003, 38, 582–585. [Google Scholar] [CrossRef]
- Burgos, L.; Ledbetter, C.A. Improved Efficiency in Apricot Breeding: Effects of Embryo Development and Nutrient Media on in Vitro Germination and Seedling Establishment. Plant Cell Tissue Organ Cult 1993, 35, 217–222. [Google Scholar] [CrossRef]
- Dulić, J.; Ognjanov, V.; Ercisli, S.; Miodragović, M.; Barać, G.; Ljubojević, M.; Dorić, D. In Vitro Germination of Early Ripening Sweet Cherry Varieties(Prunus Avium L.) at Different Fruit Ripening Stages. Erwerbs-Obstbau 2016, 58, 113–118. [Google Scholar] [CrossRef]
- Liu, W.; Chen, X.; Liu, G.; Liang, Q.; He, T.; Feng, J. Interspecific Hybridization of Prunus Persica with P. Armeniaca and P. Salicina Using Embryo Rescue. Plant Cell Tissue Organ Cult 2007, 88, 289–299. [Google Scholar] [CrossRef]
- Schulze, J.A.; Lattier, J.D.; Contreras, R.N. In Vitro Germination of Immature Prunus Lusitanica Seed. HortScience 2017, 52, 1122–1124. [Google Scholar] [CrossRef]
- Sallom, A.; Fatahi, R.; Zamani, Z.; Ebadi, A. Optimization in Vitro Conditions for Plum × Apricot Embryo Rescue and Modeling Some Critical Factors by Using Artificial Neural Networks Technology. Sci Hortic 2021, 289. [Google Scholar] [CrossRef]
- Romeu, J.F.; Sánchez, M.C.; García-Brunton, J. Potential Productivity Evolution of Flat Peach Cultivars (Prunus Persica Var. Platycarpa) Grown in Different Climatic Conditions of Southeast of Spain. Sci Hortic 2015, 197, 687–696. [Google Scholar] [CrossRef]
- Pinto, A.C.Q.; Dethier Rogers, S.M.; Byrne, D.H. Growth of Immature Peach Embryos in Response to Media, Ovule Support Method, and Ovule Perforation. HORTSCIENCE 1994, 29, 1081–1083. [Google Scholar] [CrossRef]
- Anderson, N.; Byrne, D.H.; Ramming, D.W. In Ovule Culture Success as Affected by Sugar Source and Fruit Storage Duration in Nectarine. Acta Hortic. 2006, 713, 89–92. [Google Scholar] [CrossRef]
- Ramming, D.W.; Emershad, R.L.; Foster, C. In Vitro Factors During Ovule Culture Affect Development and Conversion of Immature Peach and Nectarine Embryos. HORTSCIENCE 2003, 38, 424–428. [Google Scholar] [CrossRef]
- Yildirim, H.; Tilkat, E.; Onay, A.; Çetin Ozen, H. In Vitro Embryo Culture of Apricot, Prunus Armeniaca L. Cv. Hacıhaliloğlu. International Journal of Science & Technology 2007, 2, 99–104. [Google Scholar]
- Perez-Jimenez, M.; Guevara-Gazquez, A.; Carrillo-Navarro, A.; Cos-Terrer, J. How Carbon Source and Seedcoat Influence the in Vitro Culture of Peach (Prunus Persica l. Batsch) Immature Seeds. HortScience 2021, 56, 136–137. [Google Scholar] [CrossRef]
- Navatel, J.; Bourrain, L. Influence of the Physical Structure of the Medium on in Vitro Rooting. Adv. Hort. Sci 1994, 8, 57–59. [Google Scholar]
- Tuan, P.N.; Meier-Dinkel, A.; Höltken, A.M.; Wenzlitschke, I.; Winkelmann, T. Factors Affecting Shoot Multiplication and Rooting of Walnut (Juglans Regia L.) in Vitro. Acta Hortic 2017, 1155, 525–530. [Google Scholar] [CrossRef]
- Dolcet-Sanjuan, R.; Claveria, E.; Gruselle, R.; Meier-Dinkel, A.; Jay-Allemand, C.; Gaspar, T. Practical Factors Controlling in Vitro Adventitious Root Formation from Walnut Shoot Microcuttings. Journal of the American Society for Horticultural Science 2004, 129. [Google Scholar] [CrossRef]
- Oakes, A.D.; Desmarais, T.; Powell, W.A.; Maynard, C.A. Improving Rooting and Shoot Tip Survival of Micropropagated Transgenic American Chestnut Shoots. HortScience 2016, 51, 171–176. [Google Scholar] [CrossRef]
- Kumari, S.; Singh, K.P.; Singh, S.K.; Kumar, S.; Sarkhel, S. Establishment of in Vitro Propagation Protocol for Hybrid Tea Rose Cv. Raktagandha. Indian Journal of Horticulture 2017, 74, 245–250. [Google Scholar] [CrossRef]
- Kalinina, A.; Brown, D.C.W. Micropropagation of Ornamental Prunus Spp. and GF305 Peach, a Prunus Viral Indicator. Plant Cell Rep 2007, 26, 927–935. [Google Scholar] [CrossRef]
- Hamill, S.; Promchot, S.; Bignell, G.; Giles, J.; Topp, B. Vermiculite Improves Early Development and Survival of Low Chill Stone-Fruit Embryos Rescued In Vitro. Acta Hort. 2009, 829, 79–84. [Google Scholar] [CrossRef]
- Xu, L.; Li, S.; Shabala, S.; Jian, T.; Zhang, W. Plants Grown in Parafilm-Wrapped Petri Dishes Are Stressed and Possess Altered Gene Expression Profile. Front Plant Sci 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Matuszkiewicz, M.; Koter, M.D.; Filipecki, M. Limited Ventilation Causes Stress and Changes in Arabidopsis Morphological, Physiological and Molecular Phenotype during in Vitro Growth. Plant Physiology and Biochemistry 2019, 135, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Noferini, M. Effect of the Type of Closure for Culture Bottles on Micropropagation Efficiency of Apricot. Sci Hortic 2013, 161, 306–313. [Google Scholar] [CrossRef]
- Cati, M.; Gennari, F.; Marino, G. Effect of Culture Jar Seal on in Vitro Rooting and Subsequent Acclimatization of Three Italian Apricot Varieties. Sci Hortic 2014, 168, 120–123. [Google Scholar] [CrossRef]
- Puc, I.; Alegria, J.; Daniels, D.; Guerra, D.; Williams, S. Effects of Four Different Capping Systems in the Micropropagation of Sugarcane (Saccharum) Variety B79-474. International Journal of Scientific Engineering and Research 2018, 6, 13–16. [Google Scholar]
- Sáez, P.L.; Bravo, L.A.; Latsague, M.I.; Toneatti, M.J.; Coopman, R.E.; Álvarez, C.E.; Sánchez-Olate, M.; Ríos, D.G. Influence of in Vitro Growth Conditions on the Photosynthesis and Survival of Castanea Sativa Plantlets during Ex Vitro Transfer. Plant Growth Regul 2015, 75, 625–639. [Google Scholar] [CrossRef]
- Anderson, N.; Byrne, D.H.; Sinclair, J.; Millie Burrell, A. Cooler Temperature During Germination Improves the Survival of Embryo Cultured Peach Seed. RESOURCES HORTSCIENCE 2002, 37, 402–403. [Google Scholar] [CrossRef]
- Cantabella, D.; Teixidó, N.; Solsona, C.; Casanovas, M.; Torres, R.; Dolcet-Sanjuan, R. Acidification of the Culture Medium as a Strategy to Control Endophytic Contaminations in Prunus Spp. Rootstocks Cultured in GreenTray TIS Bioreactor. Sci Hortic 2021, 290. [Google Scholar] [CrossRef]
- Cantabella, D.; Mendoza, C.R.; Teixidó, N.; Vilaró, F.; Torres, R.; Dolcet-Sanjuan, R. GreenTray® TIS Bioreactor as an Effective in Vitro Culture System for the Micropropagation of Prunus Spp. Rootstocks and Analysis of the Plant-PGPMs Interactions. Sci Hortic 2022, 291. [Google Scholar] [CrossRef]
- Cantabella, D.; Dolcet-Sanjuan, R.; Teixidó, N. Using Plant Growth-Promoting Microorganisms (PGPMs) to Improve Plant Development under in Vitro Culture Conditions. Planta 2022, 255. [Google Scholar] [CrossRef]
- Hesse, C.O.; Kester, D.E. Germination of Embryos of Prunus Related to Degree of Embryo Development of Method of Handling. Proceedings of the American Society for Horticultural Science 1955, 251–264. [Google Scholar]
- Arbeloa, A.; Elena Daorden, M.; García, E.; Andreu, P.; Marín, J.A. In Vitro Culture of “Myrobalan” (Prunus Cerasifera Ehrh.) Embryos. Hortscience 2009, 44, 1672–1674. [Google Scholar] [CrossRef]
- Mehanna, H.T.; Martin, G.C.; Nishijima, C. Effects of Temperature, Chemical Treatments and Endogenous Hormone Content on Peach Seed Germination and Subsequent Seedling Growth. Sci Hortic 1985, 27, 63–73. [Google Scholar] [CrossRef]
- JiHong, D.; DanDan, W.; ZhengWen, Y.; MingShen, S.; HuiJuan, Z.; XiongWei, L.; XiaNan, Z. Study on the Development of Fruit and Embryo of Flat Peach. Acta Agriculturae Shanghai 2018, 34, 100–105. [Google Scholar]
- Guo, J.; Cao, K.; Yao, J.-L.; Deng, C.; Li, Y.; Zhu, G.; Fang, W.; Chen, C.; Wang, X.; Wu, J.; et al. Reduced Expression of a Subunit Gene of Sucrose Non-Fermenting 1 Related Kinase, PpSnRK1βγ, Confers Flat Fruit Abortion in Peach by Regulating Sugar and Starch Metabolism. BMC Plant Biol 2021, 21, 88. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tornero, O.; Burgos, L. Different Media Requirements for Micropropagation of Apricot Cultivars. Plant Cell Tissue Organ Cult 2000, 63, 133–141. [Google Scholar] [CrossRef]
- Nicolás, E.; Torrecillas, A.; Dell’Amico, J.; Alarcón, J.J. The Effect of Short-Term Flooding on the Sap Flow, Gas Exchange and Hydraulic Conductivity of Young Apricot Trees. Trees - Structure and Function 2005, 19, 51–57. [Google Scholar] [CrossRef]
- Rowe, R.N.; Catlin, P.B. Differential Sensitivity to Waterlogging and Cyanogenesis by Peach, Apricot, and Plum Roots. J. Amer. Soc. Hort. Sci. 1971, 96, 305–308. [Google Scholar] [CrossRef]
- Cid, M.; Pina, D.; Capote, I.; Escalona, M.; Daquinta Gradaile, M. Estrategias Para Disminuir La Necrosis Apical En La Multiplicación y El Enraizamiento in Vitro Del Pistacho. Rev Colomb Biotecnol 2016, 18, 97–105. [Google Scholar] [CrossRef]
- Kataeva, N. V.; Alexandrova, I.G.; Butenko, R.G.; Dragavtceva, E. V. Effect of Applied and Internal Hormones on Vitrification and Apical Necrosis of Different Plants Cultured in Vitro. Plant Cell Tissue Organ Cult 1991, 27, 149–154. [Google Scholar] [CrossRef]
- Carrasco-Cuello, F.; Jené, L.; Dolcet-Sanjuan, R.; Quiñones, A.; Rufat, J.; Torres, E. Differential Response to Calcium-Labelled (44Ca) Uptake and Allocation in Two Peach Rootstocks in Relation to Transpiration under in Vitro Conditions. Sci Hortic 2024, 326. [Google Scholar] [CrossRef]
- Kumar, R.; Kaul, M.K.; Saxena, S.N.; Kumar, P.; Singh, A.K. Micro Propagation Technique in Kinnow Mandarin (Citrus Reticulata). Indian Journal of Agricultural Sciences 2016, 86, 297–302. [Google Scholar] [CrossRef]
- Kumar, A.; L. Arora, R. Rapid in Vitro Multiplication of Early Maturing Peaches and Their in Vivo Acclimatization. Indian Journal of Horticulture 2007, 64, 258–262. [Google Scholar]
- Baskaran, P.; Kumari, A.; Naidoo, D.; Staden, J. Van In Vitro Propagation and Biochemical Changes in Aloe Pruinosa. Ind Crops Prod 2015, 77, 51–58. [Google Scholar] [CrossRef]
- Dev, R.; Singh, S.K.; Singh, A.K.; Verma, M.K. Comparative in Vitro Multiplication of Some Grape (Vitis Vinifera) Genotypes. Indian Journal Of Agricultural Sciences 2015, 85, 1477–1483. [Google Scholar] [CrossRef]
- Hoang, N.N.; Kitaya, Y.; Shibuya, T.; Endo, R. Development of an in Vitro Hydroponic Culture System for Wasabi Nursery Plant Production—Effects of Nutrient Concentration and Supporting Material on Plantlet Growth. Sci Hortic 2019, 245, 237–243. [Google Scholar] [CrossRef]
- Cantabella, D.; Dolcet-Sanjuan, R.; Casanovas, M.; Solsona, C.; Torres, R.; Teixidó, N. Inoculation of in Vitro Cultures with Rhizosphere Microorganisms Improve Plant Development and Acclimatization during Immature Embryo Rescue in Nectarine and Pear Breeding Programs. Sci Hortic 2020, 273. [Google Scholar] [CrossRef]
- Alix, M.J.; Savvides, S.; Blake, J.; Herrmann, R.; Hornung, R. Effects of Illumination Source, Culture Ventilation and Sucrose on Potato (Solanum Tuberosum) Microtuber Production under Short Days. Annals of Applied Biology 2001, 139, 175–187. [Google Scholar] [CrossRef]
- Mohamed, M.A.-H.; Alsadon, A.A. Effect of Vessel Type and Growth Regulators on Micropropagation of Capsicum Annuum. Biol Plant 2011, 55, 370–374. [Google Scholar] [CrossRef]
- Kornova, K.M.; Popov, S.K. Effect of the In Vitro Container Type on Growth Characteristics of the Microplants in In Vitro Propagation of GF 677. In Proceedings of the I Balkan Symposium On Fruit Growing ; Zhivondov, A and Gercheva, P and Koumanov, K., Ed.; Int Soc Horticultural Science: PO BOX 500, 3001 Leuven 1, Belgium, 2009; Vol. 825, pp. 277–281.
- Cantabella, D.; Teixidó, N.; Segarra, G.; Torres, R.; Casanovas, M.; Dolcet-Sanjuan, R. Rhizosphere Microorganisms Enhance in Vitro Root and Plantlet Development of Pyrus and Prunus Rootstocks. Planta 2021, 253. [Google Scholar] [CrossRef]
- McCown, B.H.; Lloyd, G. Woody Plant Medium (WPM) - A Mineral Nutrient Formulation for Microculture of Woody Plant-Species. HortScience 1981, 16, 453–453. [Google Scholar]
Figure 1.
Percentage of contaminated embryos, clean embryos <5 mm, and clean embryos ≥5 mm, over the total number of embryos cultured in vitro for different fruit and flesh types.
Figure 1.
Percentage of contaminated embryos, clean embryos <5 mm, and clean embryos ≥5 mm, over the total number of embryos cultured in vitro for different fruit and flesh types.
Figure 2.
Percentage of germinated over uncontaminated embryos, of sizes <5 mm or ≥5 mm, for different fruit and flesh types. Bars represent the standard error. Means with the same letter, within each embryo size, are not significantly different according to Tukey HSD (P=0.05).
Figure 2.
Percentage of germinated over uncontaminated embryos, of sizes <5 mm or ≥5 mm, for different fruit and flesh types. Bars represent the standard error. Means with the same letter, within each embryo size, are not significantly different according to Tukey HSD (P=0.05).
Figure 3.
Percentage of germinated over uncontaminated embryos, for different fruit types, cultured in M1 or M1V media. Bars represent the standard error. Means within each fruit and flesh type are not significantly different.
Figure 3.
Percentage of germinated over uncontaminated embryos, for different fruit types, cultured in M1 or M1V media. Bars represent the standard error. Means within each fruit and flesh type are not significantly different.
Figure 4.
Plantlets from embryos grown in M1 (A) and in M1V medium (B). Solid bar is equivalent to 1 cm length.
Figure 4.
Plantlets from embryos grown in M1 (A) and in M1V medium (B). Solid bar is equivalent to 1 cm length.
Figure 5.
Embryo rescue efficiencies in percentages of (A) in vitro germinated over uncontaminated embryos, (B) acclimated plants in the greenhouse over the in vitro developed plantlets, and (C) acclimated plants over uncontaminated embryos. Means with the same letter, within each efficiency rate, are not significantly different according to Tukey HSD (P=0.05).
Figure 5.
Embryo rescue efficiencies in percentages of (A) in vitro germinated over uncontaminated embryos, (B) acclimated plants in the greenhouse over the in vitro developed plantlets, and (C) acclimated plants over uncontaminated embryos. Means with the same letter, within each efficiency rate, are not significantly different according to Tukey HSD (P=0.05).
Figure 6.
In vitro germinated immature Prunus spp embryos in the control post stratification, at 4C, in (A) 60-mm-wide Petri dishes, (B) 24-mm-wide tubes, (C) 38-mm-wide tubes, and (D) 42-mm-glass jars. White solid bar in each picture is equivalent to 1 cm length.
Figure 6.
In vitro germinated immature Prunus spp embryos in the control post stratification, at 4C, in (A) 60-mm-wide Petri dishes, (B) 24-mm-wide tubes, (C) 38-mm-wide tubes, and (D) 42-mm-glass jars. White solid bar in each picture is equivalent to 1 cm length.
Figure 7.
Prunus spp plantlets from embryos grown at (A) 14C in Petri dishes containing M2, and at 24C in (B) 24-mm-wide tubes containing M1 medium, in (C) 38-mm-wide tubes containing M1V medium, and in (D) 42-mm-glass jars containing M1V medium. White solid bar in each picture is equivalent to 1 cm length.
Figure 7.
Prunus spp plantlets from embryos grown at (A) 14C in Petri dishes containing M2, and at 24C in (B) 24-mm-wide tubes containing M1 medium, in (C) 38-mm-wide tubes containing M1V medium, and in (D) 42-mm-glass jars containing M1V medium. White solid bar in each picture is equivalent to 1 cm length.
Figure 8.
Prunus spp plantlets derived from embryo rescue during (A) the initial phase of acclimatization to soil and (B) after hardening in the greenhouse and ready to be transferred to the field. White solid bars in each picture are equivalent to 10 cm length.
Figure 8.
Prunus spp plantlets derived from embryo rescue during (A) the initial phase of acclimatization to soil and (B) after hardening in the greenhouse and ready to be transferred to the field. White solid bars in each picture are equivalent to 10 cm length.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).