1. Introduction
The availability of water resources and the resolution of complex water supply issues affecting key economic sectors remain a difficult problem for countries in the Global South, particularly in Africathe the most water-scarce region. According to the World Health Organisation, one in three people is affected by water scarcity. Experts predict that, if the situation does not change, 75% of continent's population will live in regions with water scarcity by 2050 [
1]. To obstacles standing in the way of implementation of specific measures to eliminate water scarcity include the logistical backwardness of the states concerned, the lack of clear environmental plans and a shortage of qualified personnel. To solve these problems, the governments of African states rely on the help of the international community, individual countries and organisations which have finances, highly qualified personnel and the necessary technical equipment.
The problem of public access to clean water is particularly acute in the countries of the Global South, especially in Africa. The hot climate and the lack of necessary disinfectants lead to a rapid proliferation of pathogenic bacteria in the water. Under these conditions, the countries of the Global South are extremely interested in new highly efficient and environmentally friendly water treatment technologies; therefore, the development of appropriate modern equipment can serve as one of the key launching grounds for the development of comprehensive cooperation with friendly countries in the context of the formation of a new world order and the shift of the economic center of development from the West to the East and South.
Water treatment is a complex process that includes several stages of preparation. One of the key stages of traditional treatment methods is the addition of chemicals to suppress the microorganisms remaining after multi-stage aeration and separation of solid suspension by settling [
2,
3,
4,
5]. However, reagent methods have a number of significant disadvantages. For example, the most common chlorine-based chemicals in the water treatment process [
6,
7,
8,
9] lead to the formation of organochlorine compounds and hard-to-remove compounds with metal ions (iron, zinc, manganese, cobalt, lead, copper, cadmium) [
10]. If these substances enter the soil or return to the consumer, they pose a major threat to human health and the environment [
11,
12,
13,
14,
15]. In addition, pathogenic microorganisms that remain or re-enter the treated water can develop resistance due to constant interaction with chlorine and form strains more resistant to antiseptics [
16,
17].
A promising alternative in the field of water treatment methods, which will help to minimise the risks associated with the use of hazardous reagents, reagent-free treatment methods. As the name suggests, these methods do not add any chemical reagents directly to the wastewater. Active substances are produced directly in the treated volume due to physical and physical-chemical processes with virtually zero formation of dangerous reaction by-products [
18,
19]. A special place among these techniques is occupied by advanced oxidation processes (AOPs), which are based on the generation of large amounts of hydroxide radical and other reactive oxygen species, which, due to the high reaction rate, manage both to react with the pollutant and recombine into environmentally friendly substances [
20,
21,
22,
23,
24].
The development of plasma treatment harbours great potential in this area. . However, despite having positive laboratory results, most developments cannot be applied on an industrial scale [
25,
26,
27,
28,
29,
30,
31,
32]. Previously, we carried out work in which we were able to propose a treatment mechanism and develop a design for a flow-through plasma discharge water treatment system, which was called sonoplasma [
33,
34,
35,
36,
37]. This study has demonstrated that plasma treatment in a flow of cavitating liquid can be an effective method of purification from both microbiological contaminants and complex organic pollutants.
Herein we present the results of a study on possible mechanisms of pollutant decomposition carried out to optimise the plasma combustion process and improve the efficiency of the equipment. After optimising the sonoplasma setup industrial tests were carried out with wastewater under real conditions on the site of the biological wastewater treatment plant of a health resort (Republic of Tatarstan, Almetyevsk, Russian Federation).
2. Materials and Methods
The A detailed description of the sonoplasma reactor and the methodology of generation of such a discharge were published in our early work [
33]. The experimental setup with a capacity of 1 m
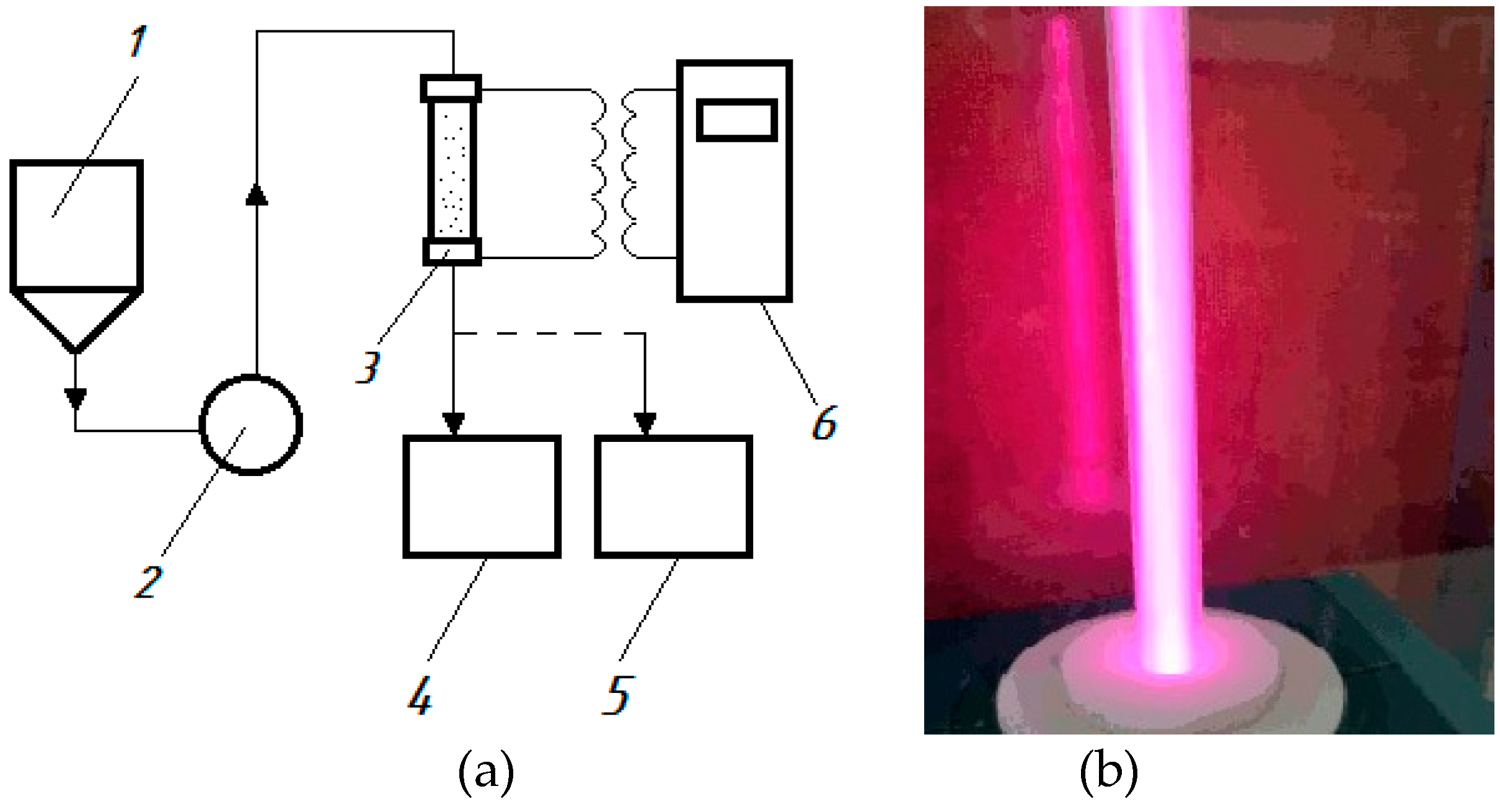
3/h is a flow reactor connected to a generator of high-frequency current pulses, equipped with a hydroacoustic emitter and electrodes. The diagram of the setup is shown in
Figure 1.
From the feed tank (1), wastewater is pumped (2) into the discharge chamber of the reactor (3), where a discharge is formed in the liquid flow using a generator of high-frequency current pulses (6). As a result plasma is generated in the cavitation field, the water is processed and drains into the receiving tank (5). During the processing, samples are taken into the storage tank (4).
Figure 2.
Photograph of the device during the industrial field test. On the left are the feed tank, the reactor and the waste tank. On the right is the generator of high-frequency current pulses.
Figure 2.
Photograph of the device during the industrial field test. On the left are the feed tank, the reactor and the waste tank. On the right is the generator of high-frequency current pulses.
The analysis of the effects of sonoplasma on the pollutants was carried out by treating model solutions of titanium oxysulphate (TiOSO4) and potassium bichromate (K2Cr2O7) in distilled water. Concentrations of 1 g/l and 0.01 g/l were used in the experiment. The obtained solutions were tested using a SF-2000 spectrophotometer (Russia) in the wavelength range from 200 nm to 1000 nm; as a result, light absorption spectra with characteristic spikes were obtained.
The analysis of the efficiency of removal of microbiological contaminants was carried out on real wastewater. The wastewater was taken from the tertiary decantation tank with a capacity of up to 10 m3/h (depending on the time of day, season, weather conditions and discharge peaks), which is located on the premises of the biological water treatment facilities of a health resort in the Republic of Tatarstan, Almetyevsk, Russian Federation. Before the wastewater entered the tertiary decantation tank, it passed through several filtration stages and was then decanted and aerated in two successive stages. The final disinfection stage - treatment with sodium hypochlorite - was replaced by the Sonoplasma process. The wastewater treatment was carried out at room temperature (18-21 °C).
Figure 3.
The second stage of aeration (above) and the site where water samples are taken from the tertiary decanting tank (below).
Figure 3.
The second stage of aeration (above) and the site where water samples are taken from the tertiary decanting tank (below).
Before starting the experiments, the feed tank, the storage tank, the pump and all connecting tubes were pre-filled with three percent hydrogen peroxide solution and stored for at least 2 hours. After exposure, all the elements were washed with clean distilled water to prevent distortion of the results.
Sampling was carried out through the drain pipe of the experimental unit. Water for processing was poured into the receiving tank through the neck. Clean plastic canisters with a volume of 10 liters were used as containers for the processed samples. The sample volume was also 10 liters.
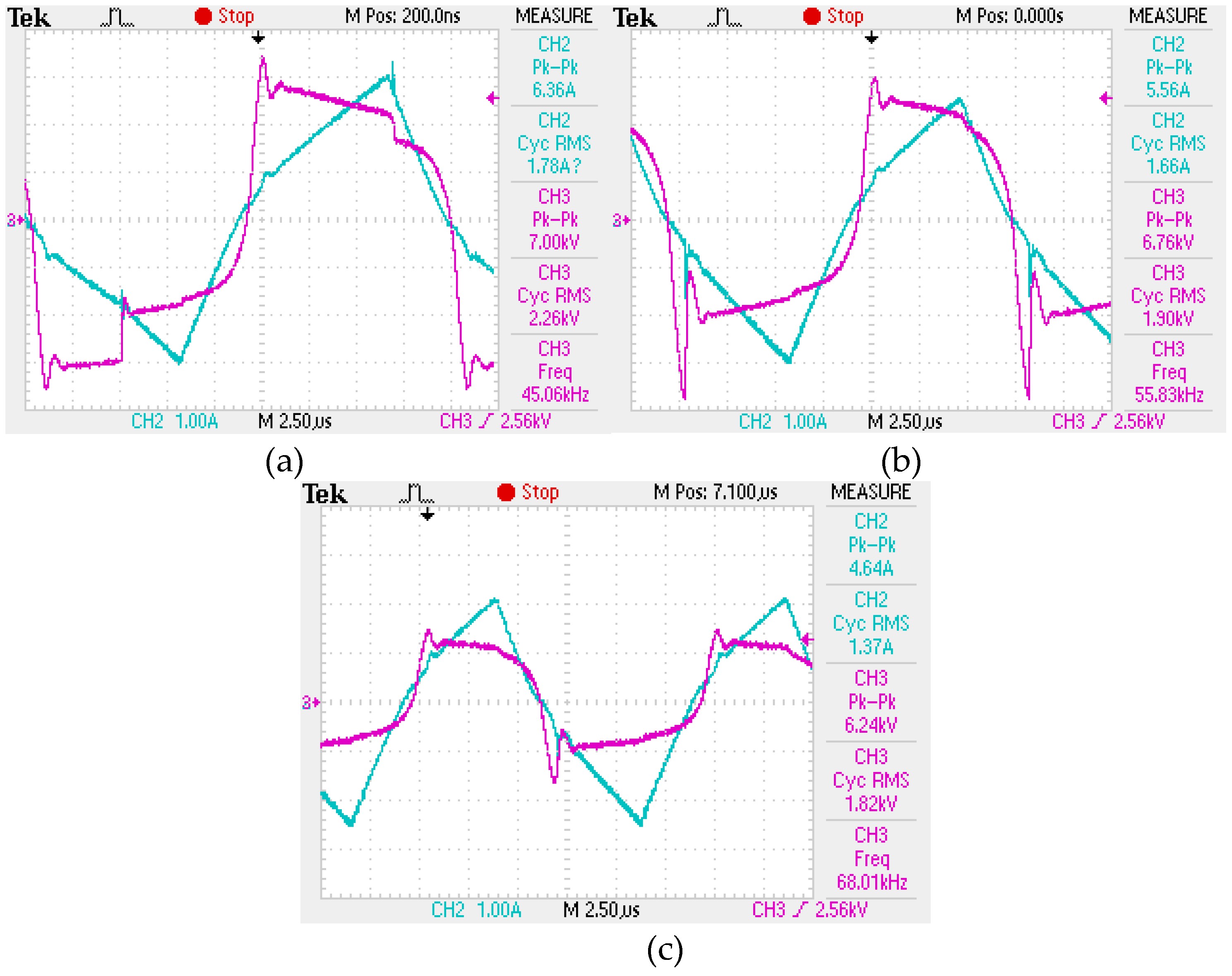
During the experiment, 7 samples were taken, including a control sample of the wastewater. The treatment process was carried out at three frequencies of electrical pulses (40 kHz, 55 kHz and 68 kHz), each for two complete processing cycles. A list of the obtained samples is presented in
Table 1. The characteristics of the generated electrical pulses during treatment are shown in
Figure 4.
Microbiological analysis was carried out by culturing bacteria in Petri dishes, followed by counting the bacteria forming colonies by microscopic analysis.
The analysis of the content of hydrogen sulphide, chlorides and iron was carried out by photometric methods using a photocolorimeter KFK-2 (Russia) in the wavelength range of 670±25 nm, 450±25 nm and 500±25 nm. The hydrogen index was measured using the AMTAST AMT03 water quality meter (USA) with an accuracy of ± 0.01. The density was determined using a set of aerometers AON-1 (Russia) with an accuracy of 1 g/cm3. The mass concentration of ions was measured using the titrimetric method.
All reagents used in the experiments had a degree of purity not lower than “PURE”.
3. Results
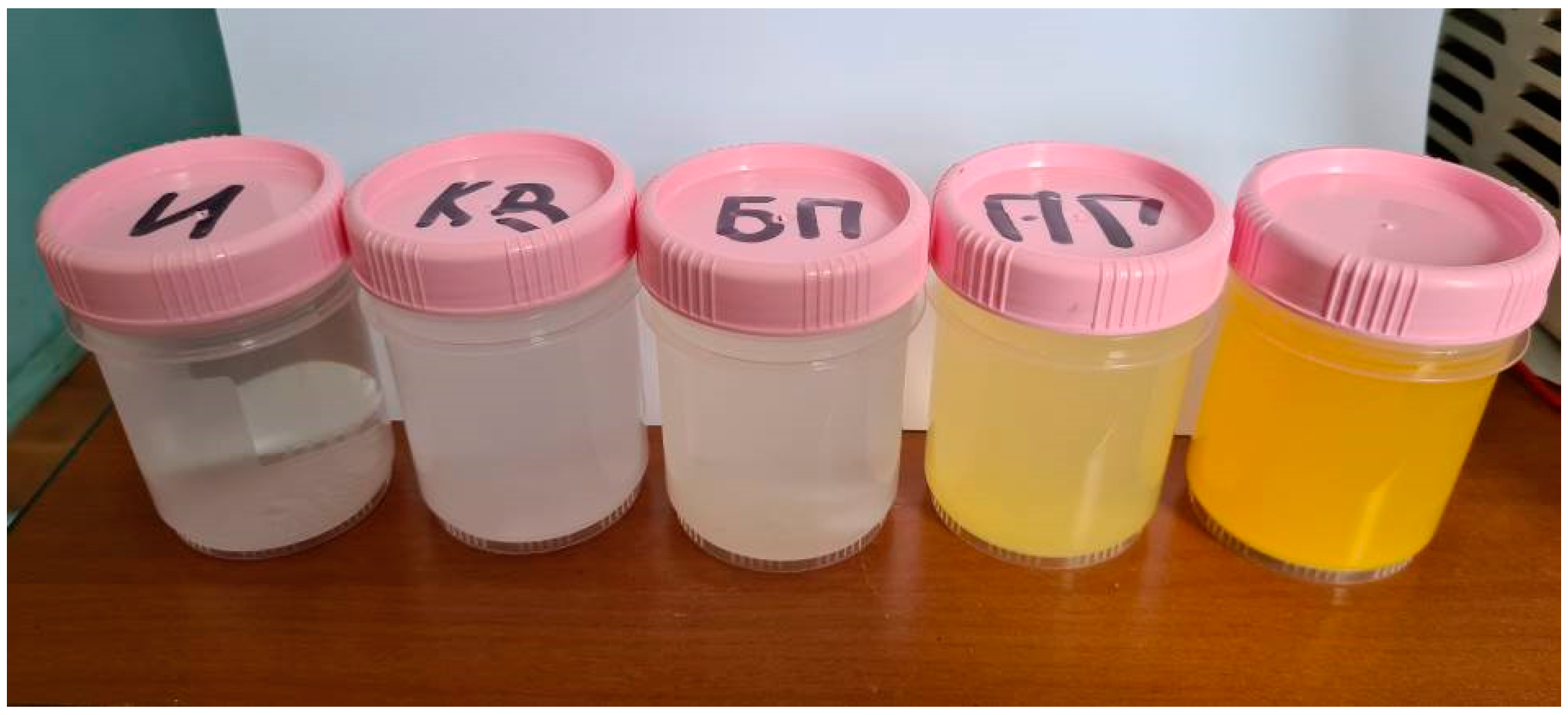
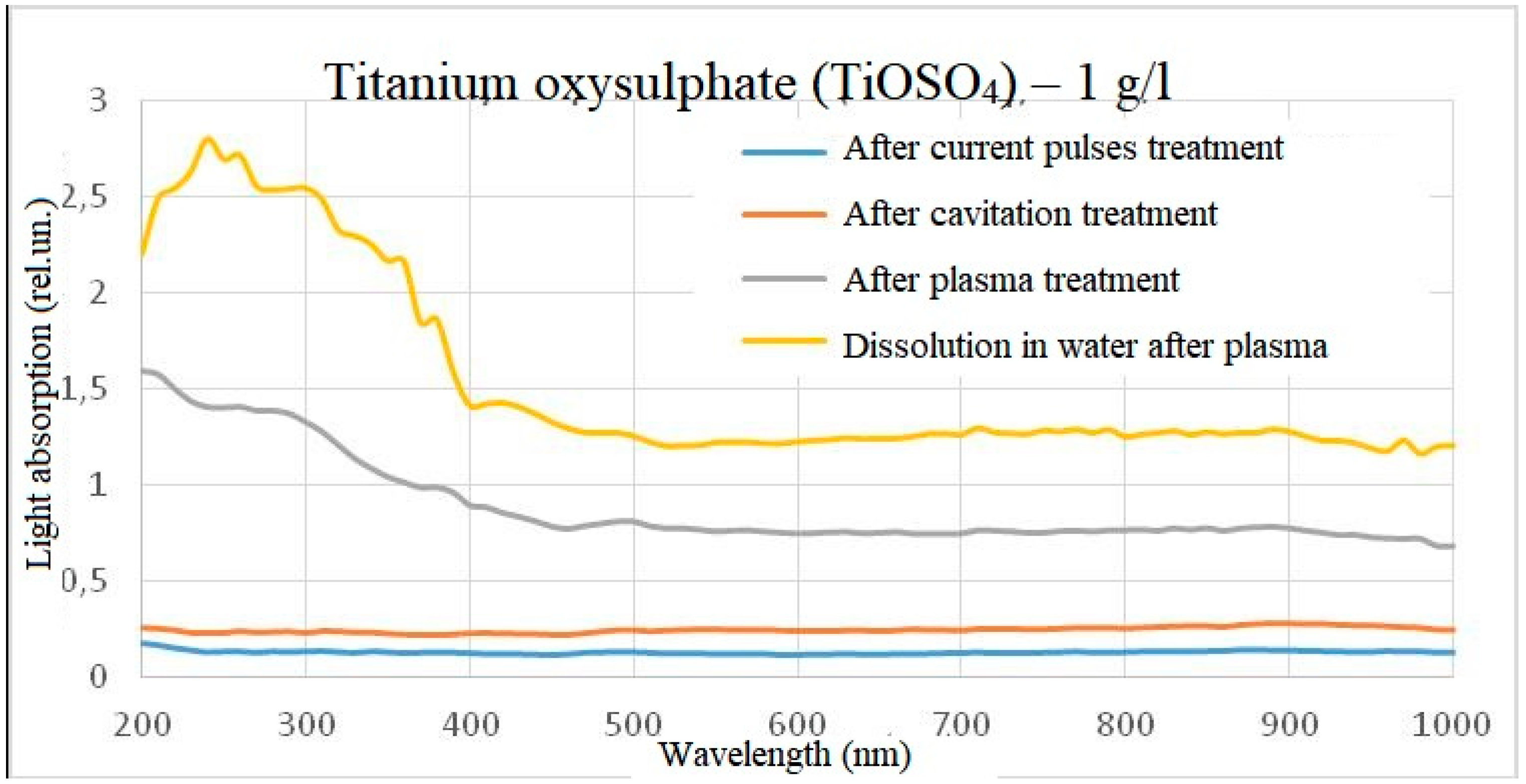
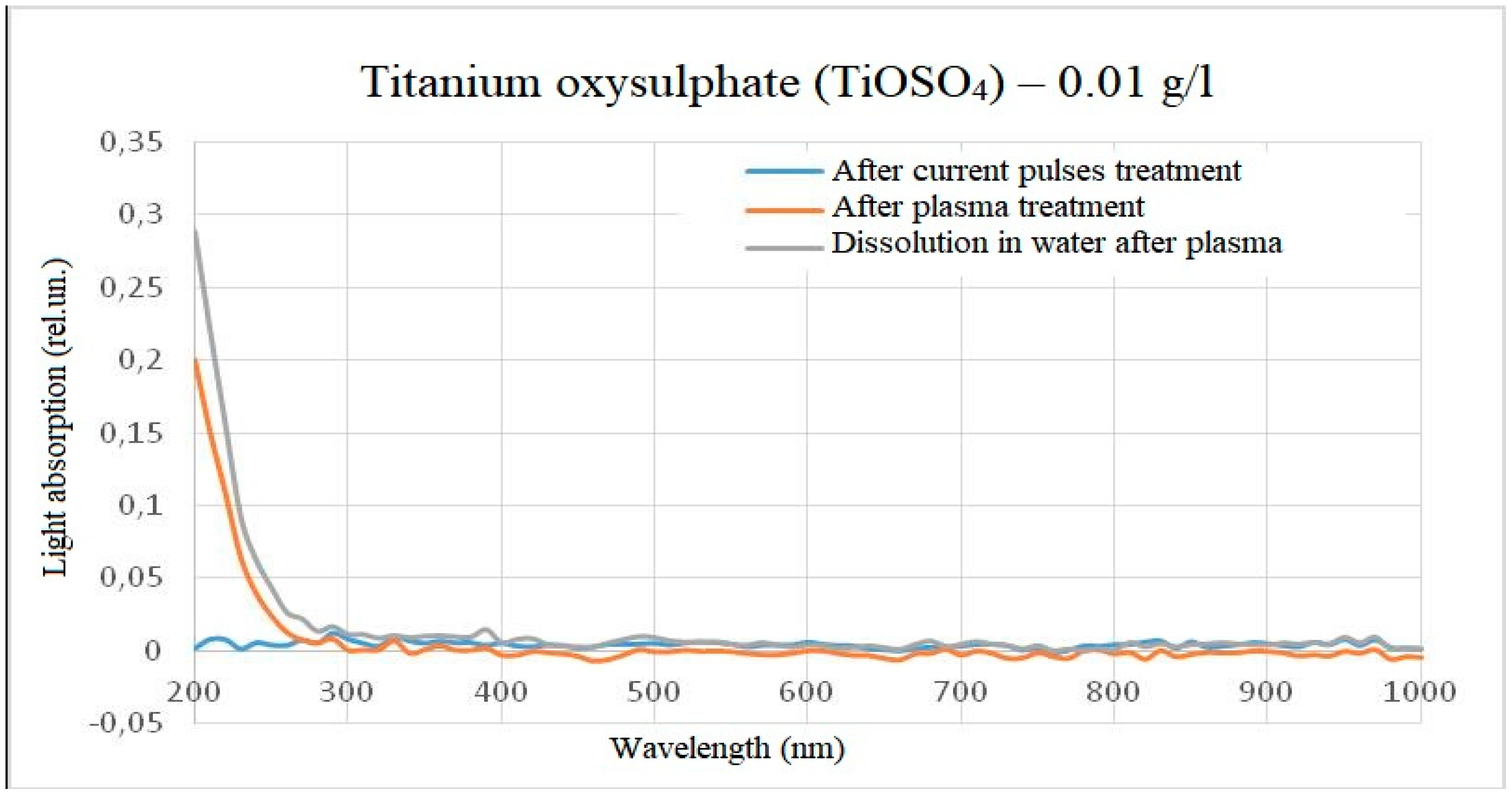
When water containing 1 g/l of titanium oxysulphate in plasma was treated, an increase in absorbance was observed over the entire visible wavelength range, with the solution acquiring a slight yellow tinge. When dissolving the same amount of titanium oxysulphate in water pre-treated with plasma, the result was similar, but the optical dissipation was higher and the yellow colouration more pronounced. A clear increase in the wavelength range from 200 to 400 nm can also be observed in the dilution sample.
No significant changes were observed in the solution either when the solution was treated with high-frequency electrical pulses without plasma discharge or when the solution was treated only with hydroacoustic cavitation. The visual observations are confirmed by photospectrometric data. The photograph of the samples and the spectrum of light absorption intensity in the wavelength range from 200 nm to 1000 nm are shown in
Figure 5 and
Figure 6. The spectra were obtained with a preliminary subtraction of the initial light absorption and reflect the changesin the samples after processing.
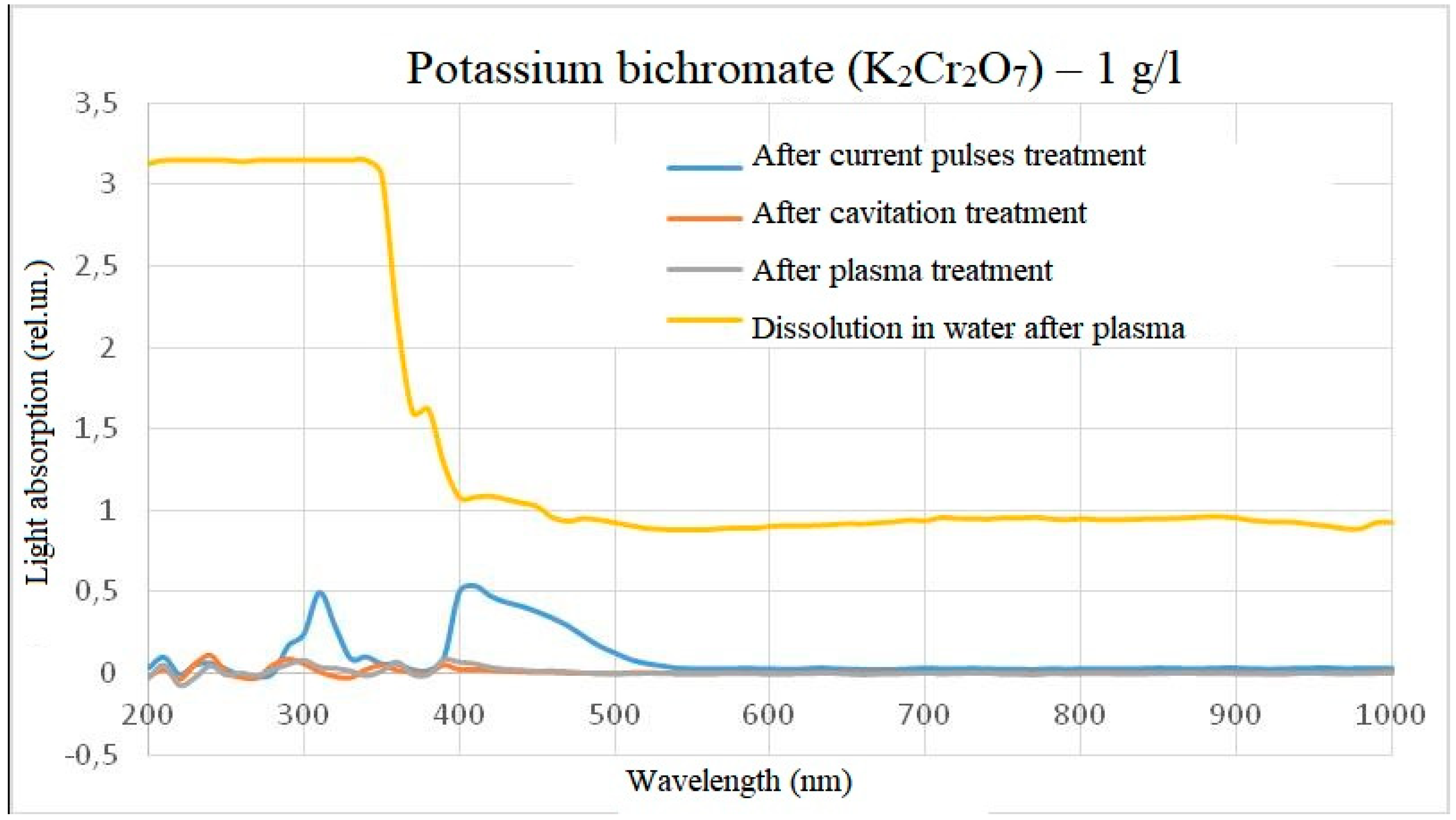
The treatment of potassium bichromate with a concentration of 1 g/l showed the following results: despite the fact that the samples visually virtually did not differ, when potassium bichromate was dissolved in water after plasma treatment to a concentration of 1 g/l, the optical density in the range from 200 to 350 nm increased significantly relative to the control sample. At the same time, treatment with electric pulses without plasma formation increased the intensity of light absorption at the same wavelengths as the initial substance. When treating a solution of potassium bichromate in plasma or after treatment with hydroacoustic cavitation, the intensity of light absorption virtually did not change. The photo of the samples and the spectra are shown in
Figure 7 and
Figure 8.
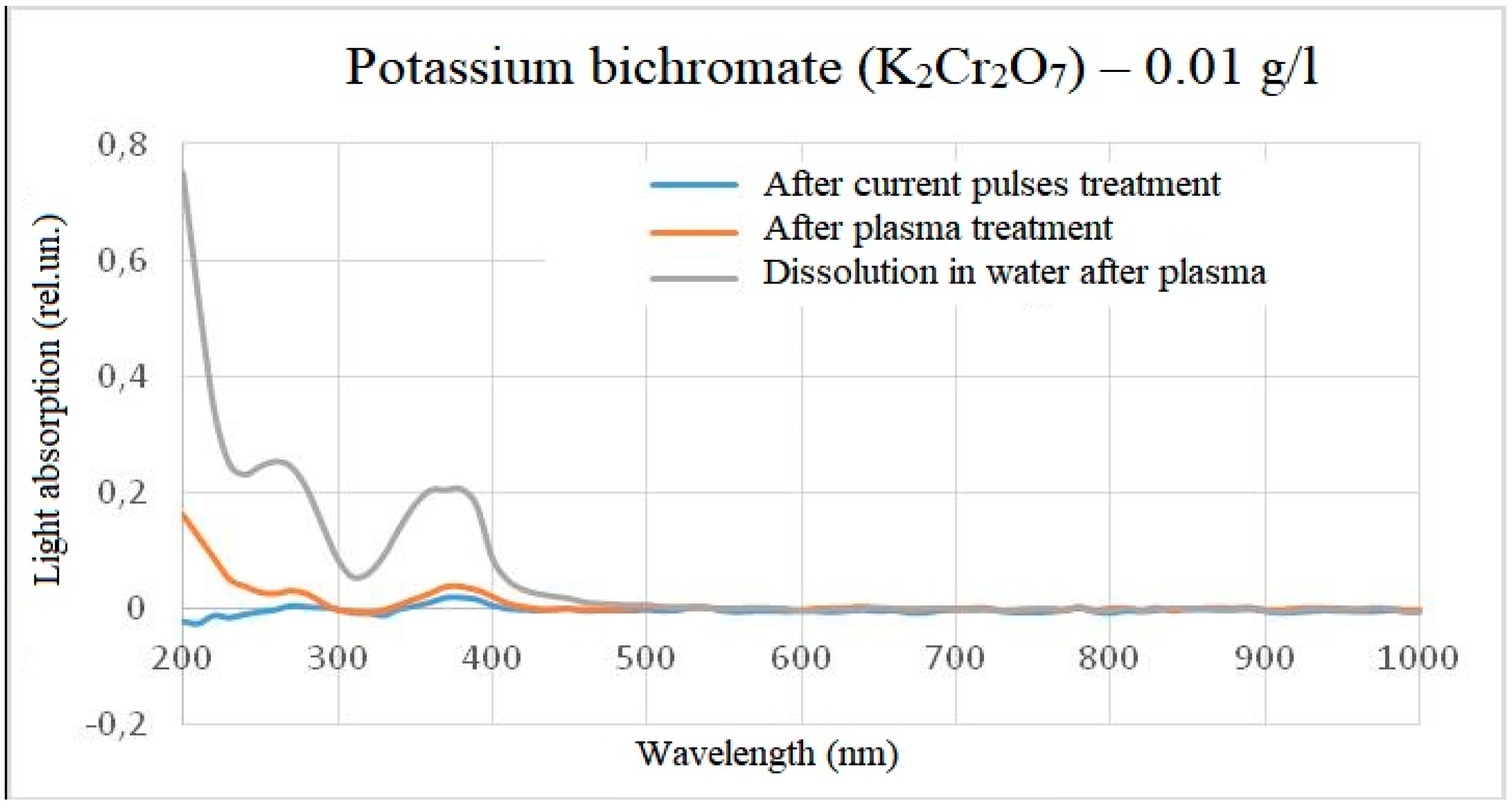
For reducing the effect of light scattering, a similar experiment was conducted, but with a reduced concentration of starting substances by 100 times (up to 0.01 g/l). At this concentration, the solutions became much more transparent, and, consequently, the light absorption over the entire visible range became lower. However, when processing samples of both titanium oxysulphate and potassium bichromate, the result did not change as a whole and repeated the experimental results at concentrations of 1 g/l. The results are shown in
Figure 9,
Figure 10,
Figure 11 and
Figure 12.
The microbiological analysis conducted showed that the concentration of coliform bacteria after plasma discharge treatment of wastewater decreased by 68.75% compared to the control sample, which is 10 times less than the maximum permissible values for wastewater discharge in the territory of the Russian Federation. A summary table with the results of the microbiological study is shown in
Table 2.
This result was observed in any treatment mode. In this regard, subsequent experiments were carried out at a frequency of 68 kHz, as the least energy-intensive mode of operation of the equipment.
The second treatment cycle resulted in a 100% lethality of microorganisms, which allows concluding that sequential treatment of the liquid flow with two plasma reactors can significantly increase the efficiency of microbiological purification.
When analysing the concentration of coliphages and Escherichia coli in all treated samples, the above-mentioned microorganisms were not detected, which allows concluding the 100% efficiency after 1 treatment cycle.
Viable eggs of helminths (ascaris, whipworm, threadworm, toxocara, fasciola, taeniidae, dwarf tapeworm) as well as viable cysts of pathogenic intestinal protozoa were not found in either the control sample or the treated samples.
Chemical analysis of treated wastewater (which was taken from an industrial site nearby) showed a significant decrease in the concentration of harmful inorganic substances. The results of the chemical analysis of the treated water are presented in
Table 3.
During the first treatment cycle, the amount of hydrogen sulphide was reduced by 61.53%, and the chlorides were completely removed. The second treatment cycle allowed the residual concentration of hydrogen sulphide to be completely removed. Thus, the plasma discharge water treatment method can be used not only for microbiological contamination, but also as a method of cleaning from chemical contaminants.
4. Discussion
On the basis of the spectra obtained, it can be assumed that the chemical reactions at the time of plasma treatment in the reactor and the chemical reactions at the outlet of the reactor are different, but on the other hand are similar in nature and linked to each other. For example, in the light absorption spectrum of titanium oxysulphate, an increase in the intensity of light absorption relative to the initial sample and a slight deviation in wavelength was detected, and when diluting titanium oxysulphate in plasma-treated water, a similar increase in intensity occurs relative to the initial sample, but the intensity of light absorption is noticeably higher, and the peak becomes more pronounced and more shifted towards short wavelengths. At the same time, the effect on the spectrum of electric pulses without initialisation of an electric discharge and the effect on the spectrum of hydroacoustic cavitation was excluded, since under such processing modes the spectra did not differ from the control one. This result was observed both when processing a solution with a concentration of 1 g/l of titanium oxysulphate and when processing a solution with a concentration of 0.01 g/l.
In the case of potassium bichromate, the main change in the light absorption spectrum occurs when it is diluted in plasma-treated water, while treatment directly in plasma did not give an obvious change in the light absorption spectrum relative to the control sample. This means that either there is no reaction in the reactor, or the reaction products do not affect the overall light absorption spectrum, or the peak of light absorption is beyond the wavelength measurement range, for example, when processing a solution with a concentration of potassium bichromate 0.01 g/l after plasma treatment, a deviation in the intensity of light absorption at very short wavelengths was detected. This spectra behavior also indicates the difference between chemical reactions occurring in the reactor and outside the reactor. A slight intensity episode during the electric pulses treatment of solutions can be explained by a measurement error or impurities, since no such episode was observed at a concentration of 0.01 g/l. The difference between the spikes at dilution of 1 g/l and 0.01 g/l in water after plasma can be explained by high light scattering, which does not allow distinguishing individual peaks spikes.
Thus, both experimental results indicate that during plasma treatment, one active substance is formed in the cavitation field, which, as it moves in the reactor, recombines into a more stable active form and continues to interact or remains in dissolved form. Such active forms can be hydroxide ions and the hydrogen peroxide formed from them.
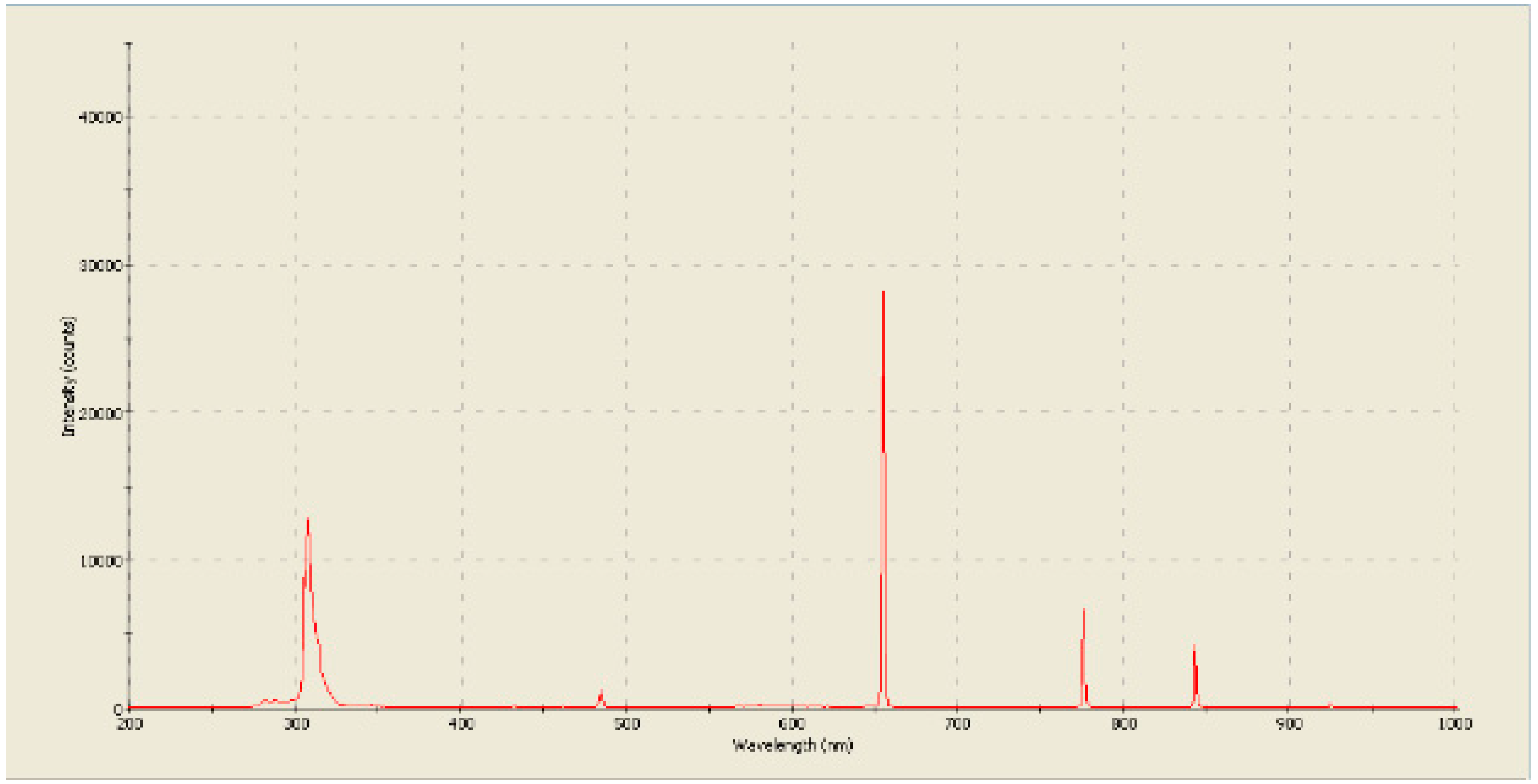
In our previous researches, the analysis of the radiation spectrum (
Figure 14) indicated the formation of hydrogen and a large number of hydroxide ions [
32,
34]. At the same time, in our other studies on the effect of plasma on antibiotics, it was found that the residual active forms of oxygen are most likely hydrogen peroxide [
33].
Thus, it can be assumed that at the moment of formation of a cavitation bubble and at the moment of electrical breakdown, bubbles filled with a vapor-gas mixture consisting mainly of water vapor, hydroxide ions and atomic hydrogen are formed.
Firstly, water vapor is filled and further converted into atomic hydrogen and hydroxide radical through the dissociation process.
Then the conversion of hydroxide radicals occurs. Since the reaction time of the hydroxyl group is very short, even compared to the lifetime of the bubble [
38], it can be assumed that this process occurs even before the process of diffusing reaction products through the phase boundary begins, that is, part of the hydroxyl group forms hydroxyl radicals, which combine into hydrogen peroxide [
39], while atomic hydrogen passes into a diatomic gas only after the collapse of the cavitation bubble.
This process can proceed even faster when exposed to ultraviolet rays on the surface of metal oxides [
40,
41]. For example, titanium oxysulphate does not enter the reactor in its pure form, but as a suspension of nanoscale particles
On this basis, it can be assumed that the main chemical reagent at the time of plasma burning is the hydroxyl group, while the residual active oxygen forms are predominantly hydrogen peroxide.
Provided that cavitation treatment and chemical interaction due to the dissociation process and the products formed during it do not significantly affect the light absorption spectra, it can be assumed that the hydrogen formed participates in the reduction of sulphuric acid or is released as a gas, while the hydroxide group completely reacts with the substance or is converted into hydrogen peroxide. Concomitant substances such as ozone or radicals formed as a result of recombination of hydrogen peroxide in simple reactions make an insignificant contribution and are formed in very small concentrations, but they can still form a synergistic effect during the decomposition of complex organic compounds, initiating the beginning of chemical interaction.
Based on the assumption above, the effect of plasma on inorganic compounds, in particular on chlorides and hydrogen sulphide, is possible due to the powerful oxidative effect of OH radicals produced in large quantities. Given the large amounts of sulphate ions (SO
42-) found in the samples of treated water, it can be assumed that the hydrogen sulphide molecule also dissociates into atomic hydrogen and a sulphur atom under the influence of an electric current, after which sulphur continues to oxidise in a large excess of the hydroxyl radical. At the same time, only a small part of the sulphate ions forms sulphuric acid with free hydrogen, while the bulk of the hydrogen is released as a gas.
After release, the sulphate ion with great probability reacts with free trivalent iron to form iron sulphate.
This assumption is supported by a drop in the concentration of iron (III) in the sample after treatment.
However, other possible reactions involving iron, for example, the formation of iron (III) oxide and hydroxide, are not excluded.
The decomposition of chlorides also occurs in several stages, where free chlorine is partially released as a gas, and part of it reacts with the iron present, forming a salt.
The decrease in the concentration of carbonates can be explained by the formation of carbon dioxide with the formation of hydrocarbonate, as a product of intermediate reactions.
The increase in some indicators after the second cycle of plasma discharge treatment can be explained by accumulated reactive oxygen species (in particular hydrogen peroxide), as well as the formation of small, difficult-to-remove hydrogen bubbles [
42,
43,
44,
45], which, during the repeated dissociation process, react with intermediate or final products of the chemical interaction of plasma with wastewater.
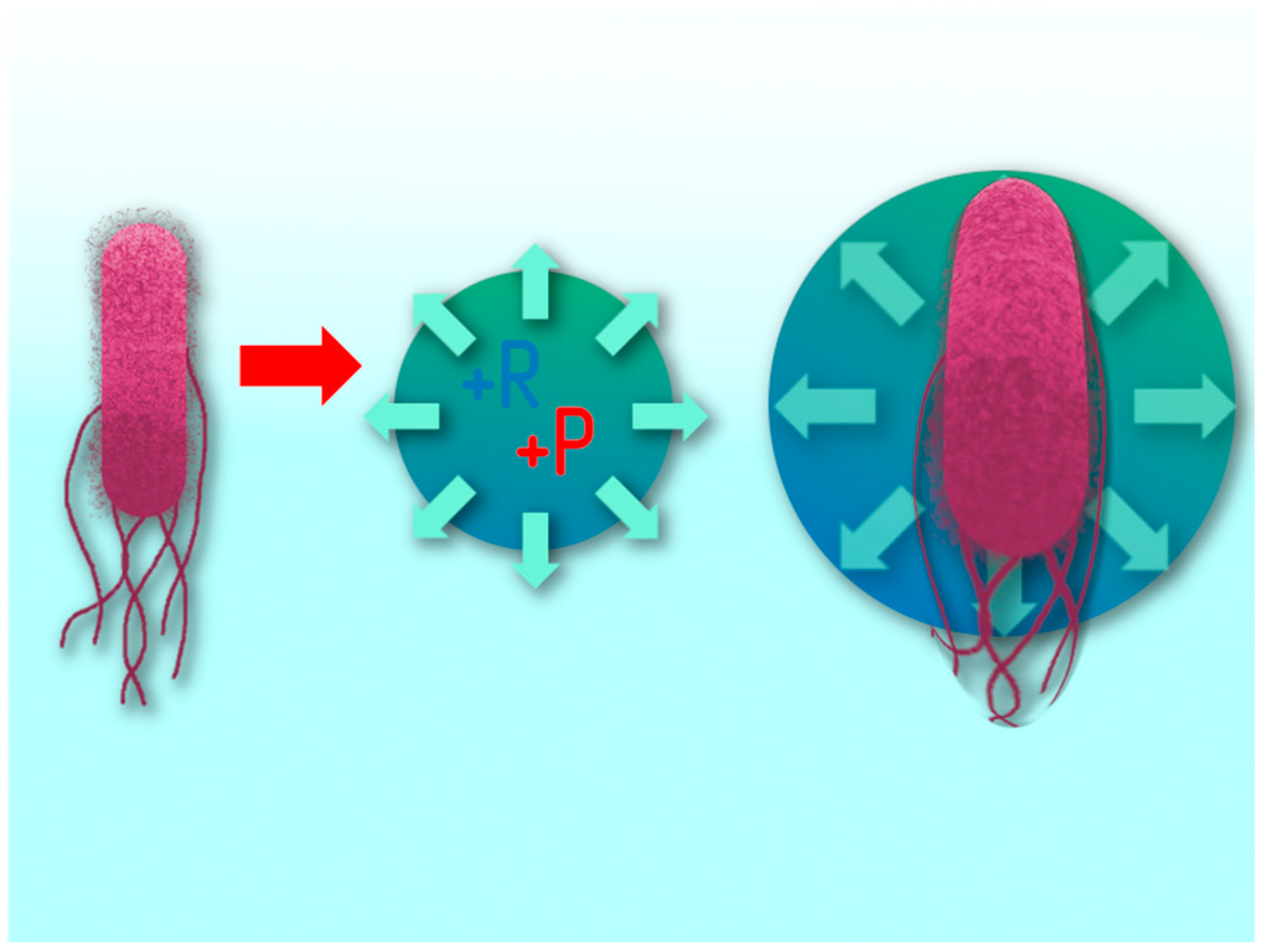
The process of exposure of a plasma discharge in a cavitation field to microbiological contamination takes place in several stages. The first stage is the mechanical action on cells by hydroacoustic cavitation. At the moment the microorganism enters the cavitation bubble, it stretches due to the pressure difference (
Figure 15), which leads to a violation of its protective layer [
46]. Some of the cells can be completely destroyed at the moment of collapse of the bubble. However, this exposure factor is not always fatal for the cell. Even after breaking the outer layer, the cell can remain viable.
The main stage of exposure to cells is the physico-chemical and chemical processes occurring at the time of discharge formation in cavitation bubbles.
In part, the lethality of microorganisms is due to the high temperature and pressure that are formed when an electric discharge penetrates into the bubbles [
47]. However, due to the fact that the nature of process is local, thermal exposure is more an auxiliary cleaning mechanism than the main factor.
One of the factors of the physical-chemical effect on microorganisms is the interaction of current with cells, which leads to chemical transformations of the elements of which it consists (chemical reactions with carbon, hydrogen, oxygen and nitrogen) [
48,
49]. Since the main chemical substance of the cell is water, first of all, the process of dissociation of the water molecule occurs with the release of a powerful oxidiser, the hydroxide ion, which continues to destroy the internal organs of the cell.
If the cell has not been exposed to current, then the main chemical effect is a chemical reaction with the active substances formed in the treated medium. Since the main chemical in the treated effluents is also water, due to a similar dissociation process, water molecules formed in large quantities of OH radicals and hydrogen peroxide tend to come from the outside into the cell, also causing a powerful antibacterial effect. This process is facilitated by the previously described mechanism of pressure increase in the bubble, which allows active substances to penetrate into the cell through the damaged membrane.
Other chemical agents, such as released oxygen and the resulting ozone, can also be involved in the process of chemical exposure to cells.
The last stage of the exposure process is the formation of stable reactive oxygen species, which continue to react with microorganisms for a certain time. Our previous research showed that the hydrogen peroxide formed with varying degrees of contamination can persist for up to several days. [
34]
5. Conclusions
Currently, there are already many conventional technologies for water purification and treatment that are offered to countries in the Global South. It is obvious that in the conditions of intense competition for emerging markets, it is necessary to offer something innovative that has undeniable advantages over traditional methods and is developed taking into account the specifics of the problems characteristic of the countries of the Global South (difficulties in the supply of chemicals, lack of access to uninterrupted power supply and necessary financing, etc.).
During the study, it was found that the main active chemicals formed from the treated water during Sonoplasma treatment are hydroxide radicals and hydrogen peroxide. Hydroxide is formed only in bubbles and is partially converted to peroxide even before diffusing through the phase boundary. The effect of prolonged exposure is mainly achieved by the chemical action of hydrogen peroxide. Both substances have a high reaction rate and pose no chemical hazard when released into water.
The observed destruction of hydrogen sulphide and chlorides showed that plasma in the cavitation field can be used as a method for treating chemical contaminants.
The installation for the treatment of wastewater with flow plasma made it possible to reduce the values of indicators of microbiological contamination, which in general allows us to conclude that the developed method has been successfully applied in real conditions of wastewater treatment. In view of the results obtained, the possibility of replacing the existing stage of reagent treatment with sonoplasma treatment should be investigated.
Author Contributions
Conceptualization, A.K. and V.B.; methodology, M.S.; software, R.N.; validation, A.K., V.B.; formal analysis, I.F.; investigation, I.F., M.S. and R.N.; resources, R.N.; data curation, I.F.; writing—original draft preparation, I.F.; writing—review and editing, A.K. and G.C.; visualization, I.F.; supervision, A.K. and G.C.; project administration, A.K.; funding acquisition, I.A. All authors have read and agreed to the published version of the manuscript.
Funding
The article was prepared within the project and supported by the grant from Ministry of Science and Higher Education of the Russian Federation program for research projects in priority areas of scientific and technological development (Agreement № 075-15-2024-546).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest
References
- The United Nations world water development report 2018: nature-based solutions for water. UNESCO, 2018. Р. 15. https://unesdoc.unesco.org/ark:/48223/ pf0000261424 (date of access 11.06.2024).
- Petrov, M. P., & Shagidullin, R. R. (2011). Anthropogenic load on water bodies and problems of functioning of biological treatment facilities. Georesources, (2 (38)), 14-20.
- Azina A.R., Barieva E.R., & Serazeeva E.V. (2016). Improvement of wastewater treatment technology. Bulletin of the Magistracy, (12-2 (63)), 14-16.
- Gubeidullin, H. H., Shigapov, I. I., & Kadyrova, A.M. (2012). Wastewater aeration in livestock farms. Bulletin of the Ulyanovsk State Agricultural Academy, (4 (20)), 114-117.
- Korovushkin, A. A., & Pominchuk, Yu. A. (2011). Optimisation of the use of activated sludge aeration in wastewater treatment plants. Agrarian Bulletin of the Urals, (2), 65.
- Smirnov A.D., Gerasimov M.M., Tkachev A.A., and Sverdlikov A.A. Wastewater disinfection: traditional and new technologies. Innovations and Investments, No. 9, 2018, pp. 173-175.
- A. I. Azhgirevich. Production, properties and features of chlorine application in water treatment products. Ecology of urbanised territories, No. 4, 2019, pp. 96-104.
- Reshnyak, V. I., & Posashkova, S. E. (2012). Disinfection of wastewater. Bulletin of the Admiral S. O. Makarov State University of Marine and River Fleet, (2 (14)), 177-182.
- Drovovozova T.I., Panenko N.N., and Kulakova E.S.. Improving the sanitary and environmental safety of wastewater. International Scientific Research Journal, No. 4-4 (58), 2017, pp. 39-42.
- Kashchenko O.V., Kiselyov K.A. Influence of chlorine and its derivatives on the environment. Modern scientific research and innovation. 2022. № 3.
- Vishnevetsky V.Yu., Ledyaeva V.S.. On the issue of hygienic assessment of the content of organochlorine compounds in drinking waterю Engineering Bulletin of the Don, vol. 39, No. 4-2, 2015, pp. 23.
- Krasnova Tamara Andreevna, Skolubovich Yuri Leonidovich, Gogina Elena Sergeevna, and Volkov Dmitry Dmitrievich. Investigation of the effect of the type of chlorine-containing disinfectant on the quality of drinking water and the effectiveness of water treatment technologyю Construction: Science and Education, No. 3, 2019, pp. 9.
- Makotrina L.V., Zverkova A.S. The effect of disinfection of drinking water with chlorine on human health. News of Higher Educational Institutions. Investment. Construction. Real Estate, No. 1 (1), 2011, pp. 87-94.
- Karataev O.R. Comparative assessment of chlorinating preparations in the treatment of swimming pool waterю Proceedings of the Kazan State University of Architecture and Civil Engineering, No. 1 (11), 2009, pp. 221-225.
- Lutsevich I.N. Hygienic assessment of the transformation of complex organic substances formed as a result of disinfection of drinking water with chlorine. Kazan Medical Journal, vol. 84, No. 2, 2003, pp. 142-145.
- Alyoshnya V.V., Zhuravlev P.V., Panasovets O.P., Sedova D.A. Experimental study of the effect of active chlorine on pathogenic and potentially pathogenic microorganisms. ZNiSO. 2018. №10 (307).
- Grenkova T. A., Selkova E. P., Gusarova M. P., Ershova O. N., Alexandrova I. A., Sazykina S. Yu., Kurdyumova N. V. Control over the resistance of microorganisms to antibiotics, antiseptics and disinfectants. Epidemiology and vaccine prevention. 2014. №1 (74).
- Dubrovskaya O. G. Kulagin V. A. Reagent-free treatment of industrial wastewater containing heavy metals based on hydrothermodynamic cavitation technology. SFU journal. Engineering and technology. 2019. No.4.
- Khan V. A., Lerner M. I., Myshkin V. F., Tshe A. A. Development of a reagent-free water treatment complex. KubGAU Scientific Journal. 2013. No.86.
- Zhelovitskaya A.V., Dresvyannikov A.F., Chudakova O.G. Application of promising oxidative processes for wastewater treatment containing pharmaceuticals (review) Bulletin of Kazan Technological University, vol. 18, No. 20, 2015, pp. 73-79.
- Kyrii S., Krimets G., Kosogina I., Astrelin I., Fedorenko O. Applying AOPs for antibiotics excstraction from wastewater. Norwegian Journal of Development of the International Science, no. 26-2, 2019, pp. 26-31.
- Vyalkova E. I., Glushchenko E. S., Velizhanina T. S., Osipova E. Yu. Analysis of physical-chemical methods of domestic wastewater treatment in northern settlements. Bulletin of the Tomsk State University of Architecture and Civil Engineering, vol. 22, No. 1, 2020, pp. 152-163.
- Zhelovitskaya A.V., Ermolaeva E. A., Dresvyannikov A. F. Oxidation of organic compounds using a hydroxide radical generated in solutions by chemical and electrochemical methods. Bulletin of Kazan Technological University, No. 6, 2008, pp. 211-229.
- Kabanov M. A., Ivantsova N. A., Koval K. A., Balakina E. S. Photo-oxidative methods in the purification of organic compounds. review. Advances in Chemistry and Chemical Technology, vol. 34, No. 12 (235), 2020, pp. 24-27.
- A.V. Kravchenko, V. S. Kublanovsky. Application of low-temperature plasma electrolysis for wastewater treatment. Electronic Materials Processing, No. 3, 2003, pp. 70-75.
- Odaryuk V. A., Tronin S. Ya. Plasma chemical technologies for industrial wastewater treatment, gas emissions, oil refining, hard domestic waste (HDW) and industrial waste. Technologies of civil safety, vol. 11, No. 3 (41), 2014, pp. 46-51.
- E.X. Viitmaa , B.X. Brodskaya , U.E. Kirso , L.K. Lesment. The complex effect of surface electrical discharges on polluted watersю Hygiene and Sanitation, No. 5, 1984, pp. 10-11.
- Shutov D.A., Bogdanov P.V., Ivanov A.N. Method of destruction of organic pollutants in aqueous solutions under the action of a low-temperature plasma jet. Proceedings of higher educational institutions. Chemistry and Chemical Technology, vol. 58, No. 2, 2015, pp. 73-77.
- Bulychev N.A., Gridneva E.S., Kisterev E.V., Zolezzi A. The use of plasma discharge for the synthesis of nanomaterials and the creation of water sterilisation technology. Bulletin of the Voronezh State Technical University, vol. 8, No. 5, 2012, pp. 115-122.
- Abdullin I. Sh., Zheltukhin V. S. HF plasma surface cleaning at low pressure. Bulletin of the Kazan Technological University, no. 1, 2003, pp. 149-154.
- Yakushin R. V., Kolesnikov V. A., Brodsky V. A., Chistolinov A.V., Perfilieva A.V., Solovyova I. N. Oxidative degradation of organic substances in a barrier discharge and prospects for using the method in wastewater treatment of printed circuit board production. Advances in chemistry and chemical technology, vol. 31, No. 6 (187), 2017, pp. 40-42.
- Nazarov V. D., Garaev I. F., Nazarov M. V., Rusakovich A. A., and Solovyov V. B. Physical-chemical methods of purification and disinfection of wastewater from tuberculosis and infectious diseases hospitals. Bashkir Chemical Journal, vol. 14, No. 4, 2007, pp. 134-138.
- Abramov, V.O., Abramova, A.V., Cravotto, G., Nikonov, R.V., Fedulov, I.S., & Ivanov, V.K.. Flow-mode water treatment under simultaneous hydrodynamic cavitation and plasma. Ultrasonics Sonochemistry, 2020, 70. [CrossRef]
- Abramov V.O., Abramova (Kamler) A.V., Bayazitov V.M., Kameneva S.V., Veselova V.O., Kozlov D.A., Sozarukova M., Baranchikov A.E., Fedulov I.S., Nikonov R.B., Fast Degradation of Tetracycline and Ciprofloxacin in Municipal Water under Hydrodynamic Cavitation/Plasma with CeO2 Nanocatalyst. Processes 2022, 10(10), 2063.
- A.V. Abramova (Kamler), V.O. Abramov, V.M. Bayazitov , R.V. Nikonov, I.S. Fedulov, A. A. Golubov, T. Karabassov, A.S. Vasenko, G. Cravotto. A new method for water treatment: an electric discharge in cavitating liquid. ESS-JSS-AOSS 1st JOINT SONOCHEMISTRY CONFERENCE, Japan, November 2021.
- T. Karabassov, A S Vasenko, V M Bayazitov, A A Golubov, I S Fedulov, A V Abramova. Electrical Discharge in a Cavitating Liquid under an Ultrasound Field. Journal of Physical Chemistry Letters, 14 (49), 10880-10885. [CrossRef]
- Abramova (Kamler) A.V., Bayazitov V.M., Fedulov I.S., Nikonov R.V., Sister V.G., Cravotto G. Influence of acoustic oscillations on continuous-flow water disinfection. Processes. 2020. Т. 8. № 10. С. 1-7. [CrossRef]
- Yankovsky O. Yu., Eismont Yu. A. Hydroxyl radical as a product and potential effector of the oxygen-dependent antimicrobial leukocyte system. Biological Communications. 2003. No. 2.
- Zhu C, Francisco JS. Production of hydrogen peroxide enabled by microdroplets. Proc Natl Acad Sci U S A. 2019 Sep 24;116(39):19222-19224. [CrossRef]
- Xiangzhong Li, Chuncheng Chen, Jincai Zhao. Mechanism of Photodecomposition of H2O2 on TiO2 Surfaces under Visible Light Irradiation. Langmuir 2001 17 (13), 4118-4122. [CrossRef]
- V.Diesen, M. Jonsson. Formation of H2O2 in TiO2 Photocatalysis of Oxygenated and Deoxygenated Aqueous Systems: A Probe for Photocatalytically Produced Hydroxyl Radicals. The Journal of Physical Chemistry C 2014 118 (19), 10083-10087. [CrossRef]
- Yslamidinov A.Y., Abdaliev U.K., Tashpolotov Y. Formation of cavitation bubbles during passage of a water jet through a laval nozzle // International Journal of Applied and Fundamental Research. – 2016. – No. 7-5. – pp. 776-778;
- Mikhailov V.A., Salamatov Yu.P. Chemical effects for invention algorithms. M.: Solon Press, 2021.
- V.S. Borovkov, I. E. Karaichev. Hydrophysical factors affecting the formation of bubbles during water aeration. Ecology of urbanised Territories, 4, 2017.
- C.-L, Kuo & Chen, Chin-Ta & Ho, Chao-Ching. Research on nano H2/O2 bubble generating mechanism and characteristics. Frontiers in Chemistry. 2022, 10. [CrossRef]
- Zevnik J, Dular M. Cavitation bubble interaction with compliant structures on a microscale: A contribution to the understanding of bacterial cell lysis by cavitation treatment. Ultrason Sonochem. 2022 Jun;87:106053. [CrossRef]
- Yushkov, Yu. G. Investigation of the initiation of an electric discharge in water during the development of electrohydraulic technology / Yu. G. Yushkov, A. S. Klimov, E. A. Grichnevsky, A. Yu. Yushkov. — Text: direct // Technical sciences: theory and practice: materials of the I International Scientific Conference (Chita, April 2012). — Chita: Publishing House Molodoy Ucheny, 2012. — pp. 139-141.
- Bilich G. L., Kryzhanovsky V. A. Biology. Full course: In 4 volumes — 5th edition, expanded and revised. - Onyx, 2009. — p. 20. — 864 p. — ISBN 978-5-488-02311-6.
- Green N., Stout W., Taylor D. Biology: in 3 vol. — Mir, 1993. — Vol. 1. — pp. 105-112. — 456 p. — ISBN 5-03-003685-7.
Figure 2.
Experimental setup for generation of an electrical discharge in a cavitating flow: a) diagram: 1 – feed tank; 2 – pump; 3 – reactor; 4 – storage tank; 5 – receiving tank, 6 – generator of high-frequency current pulses (power supply); b) – photograph of the reactor in operation.
Figure 2.
Experimental setup for generation of an electrical discharge in a cavitating flow: a) diagram: 1 – feed tank; 2 – pump; 3 – reactor; 4 – storage tank; 5 – receiving tank, 6 – generator of high-frequency current pulses (power supply); b) – photograph of the reactor in operation.
Figure 4.
The oscillogram of current pulses: a – 40 kHz; b – 55 kHz; c – 68 kHz.
Figure 4.
The oscillogram of current pulses: a – 40 kHz; b – 55 kHz; c – 68 kHz.
Figure 5.
Photo of titanium oxysulphate samples (from left to right): initial solution (1 g/l), after cavitation, after treatment with electric pulses without plasma, after plasma treatment, after dissolution in water after plasma treatment.
Figure 5.
Photo of titanium oxysulphate samples (from left to right): initial solution (1 g/l), after cavitation, after treatment with electric pulses without plasma, after plasma treatment, after dissolution in water after plasma treatment.
Figure 6.
Light absorption spectrum of titanium oxysulphate (1 g/l) after treatment (minus the initial spectrum).
Figure 6.
Light absorption spectrum of titanium oxysulphate (1 g/l) after treatment (minus the initial spectrum).
Figure 7.
Photo of potassium bichromate samples (from left to right): initial solution (1 g/l), after cavitation, after treatment with electric pulses without plasma, after plasma treatment, after dissolution in water after plasma treatment.
Figure 7.
Photo of potassium bichromate samples (from left to right): initial solution (1 g/l), after cavitation, after treatment with electric pulses without plasma, after plasma treatment, after dissolution in water after plasma treatment.
Figure 8.
The light absorption spectrum of potassium bichromate (1 g/l) after treatment (minus the initial spectrum).
Figure 8.
The light absorption spectrum of potassium bichromate (1 g/l) after treatment (minus the initial spectrum).
Figure 9.
Photo of titanium oxysulphate samples (from left to right): the initial solution (0.01 g/l), after treatment with electric pulses without plasma, after plasma treatment, after dissolution in water after plasma treatment.
Figure 9.
Photo of titanium oxysulphate samples (from left to right): the initial solution (0.01 g/l), after treatment with electric pulses without plasma, after plasma treatment, after dissolution in water after plasma treatment.
Figure 10.
Light absorption spectrum of titanium oxysulphate (0.01 g/l) after treatment (minus the initial spectrum).
Figure 10.
Light absorption spectrum of titanium oxysulphate (0.01 g/l) after treatment (minus the initial spectrum).
Figure 11.
Photo of potassium bichromate samples (from left to right): the initial solution (0.01 g/l), after treatment with electric pulses without plasma, after plasma treatment, after dissolution in water after plasma treatment.
Figure 11.
Photo of potassium bichromate samples (from left to right): the initial solution (0.01 g/l), after treatment with electric pulses without plasma, after plasma treatment, after dissolution in water after plasma treatment.
Figure 12.
The light absorption spectrum of potassium bichromate (0.01 g/l) after treatment (minus the initial spectrum).
Figure 12.
The light absorption spectrum of potassium bichromate (0.01 g/l) after treatment (minus the initial spectrum).
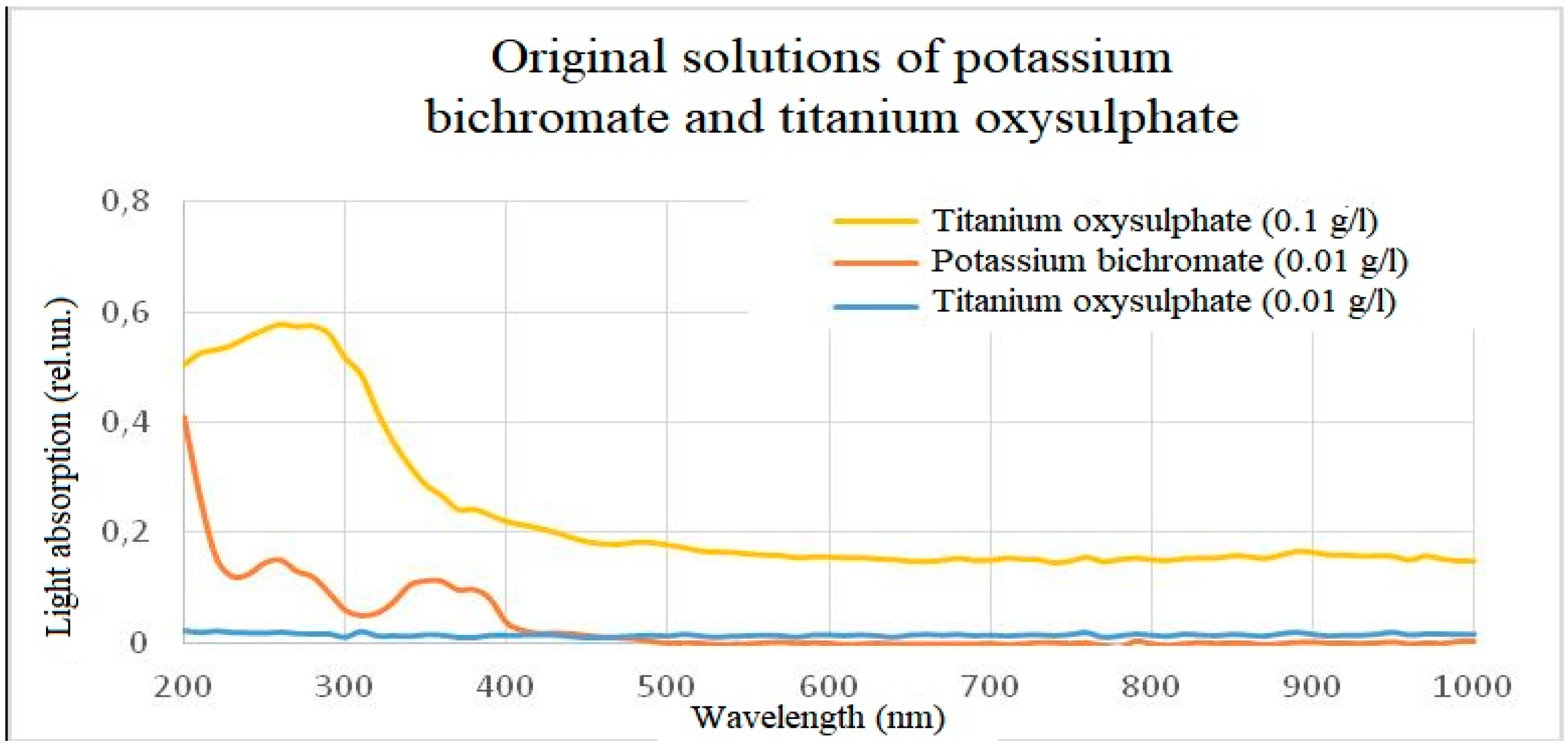
Figure 13.
Light absorption spectra of initial solutions of titanium oxisulphate and potassium bichromate.
Figure 13.
Light absorption spectra of initial solutions of titanium oxisulphate and potassium bichromate.
Figure 14.
The spectrum of the glow of plasma burning in the flow of an incoming liquid. [
34].
Figure 14.
The spectrum of the glow of plasma burning in the flow of an incoming liquid. [
34].
Figure 15.
The Escherichia coli cell trapped by the cavitation bubble increases in size due to an increase in internal pressure in the bubble)
Figure 15.
The Escherichia coli cell trapped by the cavitation bubble increases in size due to an increase in internal pressure in the bubble)
Table 1.
Table of samples obtained.
Table 1.
Table of samples obtained.
| Entries |
Frequency, kHz |
Treatment cycle |
| 1А |
40 |
1 |
| 2А |
40 |
2 |
| 1B |
55 |
1 |
| 2B |
55 |
2 |
| 1C |
68 |
1 |
| 2C |
68 |
2 |
| K |
Control |
Control |
Table 2.
Results of microbiological tests of water samples.
Table 2.
Results of microbiological tests of water samples.
| Indicator |
MAC (no more than) |
K |
1A |
2A |
1B |
2B |
1C |
2C |
| Common coliform bacteria, CFU/100 ml |
500 |
160 |
50 |
0 |
50 |
0 |
50 |
0 |
|
Escherichia coli, CFU/100 ml |
100 |
160 |
Not found |
Not found. |
Not found |
Not found |
Not found |
Not found |
| Coliphages, BFU/100 ml |
100 |
100 |
Not found |
Not found |
Not found |
Not found |
Not found |
Not found |
| Viable eggs of helminths (ascaris, whipworm,threadworm, toxocara, fasciola, taeniidae, dwarf tapeworm), in 25 liters |
Not allowed |
Not found |
Not found |
Not found |
Not found |
Not found |
Not found |
Not found |
| Viable cysts of pathogenic intestinal protozoa, in 25 liters |
Not allowed |
Not found |
Not found |
Not found |
Not found |
Not found |
Not found |
Not found |
Table 3.
Result of chemical analysis of wastewater.
Table 3.
Result of chemical analysis of wastewater.
| Name of the indicator |
Control |
1 treatment cycle |
2 treatment cycles |
| 1 |
10.9 |
10.08 |
10.4 |
Density, g/cm3,
no more than |
995 |
993 |
995 |
| Carbonate (СО3) mg/dm3
|
66.0 |
48.0 |
24.0 |
| Hydrocarbonate (НСО3,)-mg/dm3
|
54.9 |
30.5 |
42.7 |
| Sulphate (SO42-), mg/dm3
|
0.06 |
62.53 |
42.03 |
| Chloride (Cl-), mg/dm3 |
70.9 |
0.0 |
0.0 |
| Calcium (Ca2+), mg/dm3
|
0.0 |
0.0 |
0.0 |
| Magnesium (Mg2+), mg/dm3
|
0.0 |
0.0 |
0.0 |
| Common iron (Fe), mg/dm3
|
0.25 |
0.35 |
0.30 |
| Iron (III), mg/dm3
|
0.4 |
0.16 |
0.2 |
| Н2S content, mg/dm3 |
44.3 |
17.04 |
0.0 |
Total alkalinity,
mg-eq/dm3
|
0.9 |
0.5 |
0.7 |
| Sum of sodium (Na), potassium (К), mg/dm3
|
117.0 |
78.0 |
101.0 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).