1. Introduction
Pneumonia is a serious health concern since it has been connected to significant morbidity and mortality in patients. It is the primary infectious illness cause of death across the board in the entire world [
1,
2]. In critically ill patients, pneumonia may manifest as pneumonia contracted in the community (community acquired pneumonia [CAP], hospital acquired pneumonia [HAP]), or pneumonia connected to mechanical ventilation (ventilator associated pneumonia [VAP]). Pneumonia survivors frequently experience significant sequelae such altered lung function, a decline in mental and cognitive abilities, weakness and a reduction in motor function, and a loss of functional autonomy [
3,
4]. To increase the survival of critically ill patients, it is essential to make an accurate and early diagnosis of severe pneumonia.
For critically ill patients in the intensive care unit (ICU) who need mechanical breathing, enteral or parenteral feeding supplementation is crucial. To maintain gut integrity and intestinal permeability, enteral nutrition is the suggested feeding method for those with a functioning gastrointestinal (GI) tract [
5]. To reduce inflammatory reactions and decrease hypermetabolic status of the patients, guidelines on enteral nutrition in critical care settings recommended early enteral nutrition initiation, optimum delivery of prescribed nutrients, and strict glycemic control. Nutritional status deteriorates as a result of suboptimal nutrition delivery compared to predicted objectives, which is linked to worse patient outcomes such lengthened hospital stays (LOS), greater rates of infection, multiple organ failure, and death [
6].
Critically ill patients are more likely to experience a variety of eating intolerances, including abdominal distension, delayed gastric emptying, large gastric residual volumes (GRVs), diarrhea, and vomiting [
7,
8]. Due to anticipated procedures, routine nursing attention, and physical therapy, meal disruptions are common in critical care environments, posing a challenge to achieving dietary objectives. Furthermore, due to increased hepatic gluconeogenesis and catabolic hormone release, critically sick individuals may also have higher glycemic fluctuation [
9]. It is essential to implement a secure and efficient enteral feeding method in order to minimize potential hazards and complications, while enhancing the delivery of nutrition to this particular patient population.
There are various methods for delivering enteral nourishment. The latest clinical nutrition guidelines from the European Society for Clinical Nutrition and Metabolism suggest continuous enteral nutrition (CEN) as the preferred method, involving the controlled hourly administration of nutrients over an extended period using a feeding pump [
10]. Previous studies have shown that CEN may lower the risk of aspiration pneumonia and alleviate unpleasant GI symptoms. Other alternative enteral feeding approaches, such as bolus or intermittent enteral nutrition (IEN), involve providing larger amounts of feeds in short intervals of 4-6 hours, which may be more effective in meeting nutritional targets and enhancing muscle protein synthesis [
2]. However, there are still uncertainties about the optimal way to administer enteral nutrients to critically ill patients. However, there are still uncertainties about the optimal way to administer enteral nutrients to critically ill patients [
11]. Therefore, the present literature review aims to investigate the association between entreat feeding and the risk of pneumonia in critical care adults.
2. Method and Search Strategy
This updated systematic review complies with the PRISMA checklist guidelines for systematic reviews and meta-analyses [
12]. CINAHL, Cochrane, Embase, PubMed, Scopus, and Web of science were the databases that were searched. The studies were published between 2016 and 2023 were explored. The search process involved using different keywords, including “
enteral nutrition, feeding, association, aspiration, pneumonia, adults, and critical care”. These keywords were used in various combinations to find all the relevant articles. This initial exploration resulted in the revision of all titles.
2.1. Eligibility Criteria
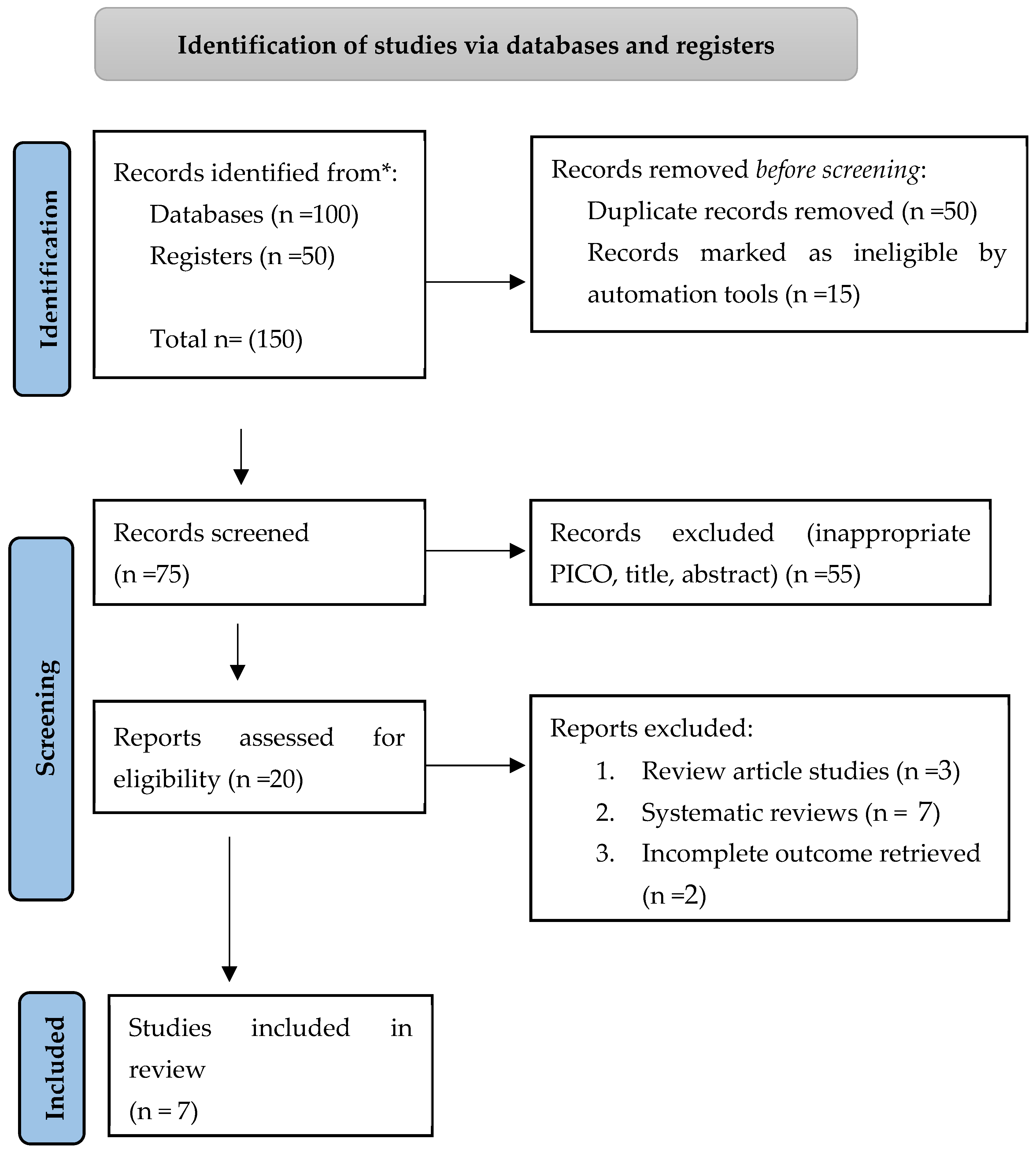
Only papers focusing on the association between enteral feeding and the risk of pneumonia in critical care adult patients were included. Then, involved only original, English language studies reporting the association between enteral feeding and the risk of pneumonia in critical care adults after evaluating the abstracts of the articles. On the other hand, review articles and editor letters were not included. These articles were further examined to exclude duplicates, non-full-text articles, and articles with unsatisfactory content, such as overlapped or incomplete data [
Figure 1] displays a detailed explanation of the search approach.
2.2. Data Reviewing and Analysis
The full text and abstracts of the articles were evaluated to extract the relevant data and transfer it to a pre-made excel sheet. The chosen data were then amended in the excel sheet, and the data were combined to summarize the data to facilitate data analysis.
3. Result
Seven studies fulfilled the inclusion criteria [
13,
14,
15,
16,
17,
18,
19]. They were all conducted retrospectively. The study involved 2063 adult patients in critical care units. A summary of the findings from these studies is presented in Table 1.
Lee and colleagues conducted a retrospective observational study on trauma adult patients admitted to the ICU. They found that the intervention group received a higher amount of calories (94% vs. 75%), and protein (104% vs. 74%) compared to the control group. Additionally, they observed a significant correlation between early enteral feeding within 48 hours and a lower incidence of pneumonia and a greater number of ventilator-free days within 28 days [
13]. Mizuma et al, evaluated all individuals admitted within a 14-day period to determine the occurrence of stroke-associated pneumonia. They discovered that delayed enteral feeding exceeding 48 hours could result in prolonged hospitalization (median of 13 days) and ICU stay (median of 2 days). Conversely, early enteral feeding was associated with a reduction in pneumonia infections among stroke patients [
14]. The research conducted by Su et al. revealed a lower incidence of premature VAP and VAP, decreased mechanical ventilation days, shorter ICU stay, and better prognosis in patients receiving early enteral nutrition [
15]. Nakayama and colleagues conducted a study on the early enteral nutrition provided to patients diagnosed with cardiovascular disease within 5 days of admission to the ICU. Their findings indicate that none of the subjects experienced aspiration pneumonia [
16].
Furthermore, a study by Hamilton et al examined Adult ICU patients who were on enteral nutrition and had a risk factor for stress-related mucosal injury. The findings showed that patients in the pharmacologic prophylaxis with enteral nutrition (PPEN) group had a higher incidence of pneumonia (42.2% vs 15%, P=.0194). Nevertheless, there was no disparity between the groups when patients with pneumonia at admission were excluded (20.6% vs 10.5%, P=.5254) [
17].
Conversely, enteral feeding was linked to a higher occurrence of pneumonia. A study by Gaitanidis and team examined the differences between enteral and parental feeding in individuals suffering from acute pancreatitis. The findings indicated a heightened susceptibility to pneumonia and a higher fatality rate from infection among those receiving enteral feeding [
18]. A study by Patel et al. examined septic shock patients who were mechanically ventilated and admitted to the ICU, and were initiated on enteral nutrition within 48 hours. Among the patients receiving less than 600 kcal per day, no cases of aspiration pneumonia were reported. In contrast, 6.7% of patients not receiving enteral nutrition and 7.2% of patients receiving more than 600 kcal per day developed aspiration pneumonia [
19].
4. Discussion
Nutrition support therapy plays a vital role in the care of critically ill patients. There are established guidelines that can be utilized to administer EN in order to improve overall patient outcomes [
20,
21]. EN has an immunomodulation effects, such as preserving the integrity of the gut mucosa, stimulating intestinal motility, thus reducing bacterial overgrowth, and may increase splanchnic blood flow. As a result, bacterial translocation from the gut may be prevented [23]. In an ICU setting, EN is the recommended primary choice for nutritional therapy unless there is a contraindication [
20,
21].
Our literature review indicates that early initiation of enteral nutrition within 48 hours can result in numerous benefits as supported with other studies, such as a lower rate of infectious complications, shorter ICU stays, and fewer days spent in the hospital [
22,24]. Patients receiving EN demonstrated a notable reduction in adverse events such as aspiration pneumonia, emesis, and the need for increased respiratory support. Additionally, they achieved improved nutritional targets, better clinical outcomes, shorter periods of high-flow nasal cannula (HFNC) and oxygen supplementation, as well as a decreased length of hospital stay and mechanical ventilation. The effectiveness of EN has been linked to a decreased risk of infection and a shorter duration of ICU or hospitalization in various research studies [25,26]. It was found that even within 5 days of the initiation of enteral nutrition (EN), as studied by Nakayama et al, there was a decrease in the incidence of aspiration pneumonia. Therefore, guidelines recommend enteral nutrition over parenteral nutrition [27].
A prospective observational study was conducted by Cintra et al on elderly patients with advanced dementia. The study revealed that the mortality rate at 3 months was 11.1% in the oral feeding group and 41.9% in the alternative feeding group (p = 0.004). By 6 months, the mortality rate had increased to 27.8% and 58.1%, respectively (p = 0.012). Furthermore, a higher incidence of aspiration pneumonia was observed in the alternative feeding group (p = 0.006) [28]. In a meta-analysis conducted by Moore et al, indicated that EN groups had significant reduction in septic complications observed among EN patients [29].
Parenteral nutrition was frequently administered in cases of acute pancreatitis to prevent pancreatic stimulation. Traditionally, it was thought that delivering nutrition near Treitz’s ligament could activate the pancreas and exacerbate the condition of acute pancreatitis. [30] Approximately 60% of patients with acute pancreatitis exhibit gut barrier dysfunction. Hence, parenteral nutrition appeared to be the optimal choice for providing sufficient nutritional support without stimulating the pancreas [31]. Gaitanidis et al, demonstrated a significant decrease in infection and pneumonia cases among acute pancreatitis patients who received PN feeding, compared to those who received EN or other feeding methods. Additionally, patients who were administered PN had a lower mortality rate. [
18] Conversely, three meta-analyses, enteral nutrition has been found to greatly lower the risk of infections, organ failure, and mortality in acute pancreatitis patients in comparison to parenteral nutrition [32,33,34]. Further studies are necessary to determine the optimal form and type for pancreatitis patients.
5. Conclusions
The current literature review has shown that enteral nutrition was associated with a reduced risk of aspiration pneumonia and overall infections in critically ill patients. It has been proven to be effective for with no major complications. Early enteral nutrition not only protects the gastric mucosa but also decreases the incidence of pneumonia, shortens the duration of mechanical ventilation, reduces the length of ICU stay, and improves the prognosis. These observations strongly support the need for the prospective evaluation of the impact of early enteral nutrition in critically ill adult patients. Further research is required to assess the optimal nutritional approaches in critically ill patients.
Author Contributions
Conceptualization, Ahmed O. Alenazi and Mashael Alharbi; methodology, Afnan A. Alsaab, Abdulhadi Alzahrani, Salem T. Khrnoob, Ghazi N. Almutairi; validation, Ahmed O. Alenazi and Mashael Alharbi Saleh M. Alhuwaiji, Abdulhadi Alzahrani; investigation, Rakan M. Alassaf, Metrek A. Aldossary, Waad Alharbi, Shouq Alahmadi, and Ghada A. Aloufi; resources, Faisal alzoabi, Saleh M. Alhuwaiji, Abdulhadi Alzahrani, Ghazi N. Almutairi; writing—original draft preparation, Ahmed O. Alenazi; Shouq Alahmadi; and Ghada A. Aloufi writing—review and editing, Ahmed O. Alenazi; Mashael Alharbi; Metrek A. Aldossary, Afnan A. Alsaab; and Waad Alharbi; visualization, Ahmed O. Alenazi and Rakan M. Alassaf; supervision, Ahmed O. Alenazi and Ghada A. Aloufi; project administration, Salem T. Khrnoob, Faisal alzoabi, Saleh M. Alhuwaiji, Mashael Alharbi; funding acquisition, NON. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Correia MI, Perman MI, Waitzberg DL. Hospital malnutrition in Latin America: A systematic review. Clin Nutr. 2017 Aug; 36(4):958-67. [CrossRef]
- Lewis SR, Butler AR, Alderson P, Smith AF. Enteral versus parenteral nutrition for adults in the intensive care unit. Cochrane Database of Systematic Reviews 2016, Issue 7. Art. No.: CD012276. [CrossRef]
- Hiura G, Lebwohl B, Seres DS. Malnutrition diagnosis in critically ill patients using 2012 Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition standardized diagnostic characteristics is associated with longer hospital and intensive care unit length of stay and increased in-hospital mortality. J Parenter Enteral Nutr. 2020 Feb; 44(2):256-64. [CrossRef]
- Pash, E. Enteral nutrition: options for short-term access. Nutr Clin Pract. 2018 Apr; 33(2):170-6. [CrossRef]
- Lakananurak N, Gramlich L. Nutrition management in acute pancreatitis: Clinical practice consideration. World J Clin Cases. 2020 May; 8(9):1561. [CrossRef]
- Savio RD, Parasuraman R, Lovesly D, Shankar B, Ranganathan L, Ramakrishnan N, Venkataraman R. Feasibility, tolerance and effectiveness of enteral feeding in critically ill patients in prone position. JICS. 2021 Feb; 22(1):41-6. [CrossRef]
- Klompas M, Branson R, Cawcutt K, Crist M, Eichenwald EC, Greene LR, Lee G, Maragakis LL, Powell K, Priebe GP, Speck K. Strategies to prevent ventilator-associated pneumonia, ventilator-associated events, and nonventilator hospital-acquired pneumonia in acute-care hospitals: 2022 Update. Infect Control Hosp Epidemiol. 2022 Jun; 43(6):687-713. [CrossRef]
- Segaran E, Barker I, Hartle A. Optimising enteral nutrition in critically ill patients by reducing fasting times. J Intensive Care Soc. 2016 Feb; 17(1):38-43. [CrossRef]
- Worthington P, Balint J, Bechtold M, Bingham A, Chan LN, Durfee S, Jevenn AK, Malone A, Mascarenhas M, Robinson DT, Holcombe B. When is parenteral nutrition appropriate?. J Parenter Enteral Nutr. 2017 Mar; 41(3):324-77. [CrossRef]
- Vashi PG, Virginkar N, Popiel B, Edwin P, Gupta D. Incidence of and factors associated with catheter-related bloodstream infection in patients with advanced solid tumors on home parenteral nutrition managed using a standardized catheter care protocol. BMC infect Dis. 2017 Dec; 17(1):1-9. [CrossRef]
- Compher C, Bingham AL, McCall M, Patel J, Rice TW, Braunschweig C, McKeever L. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. J Parenter Enteral Nutr. 2022 Jan; 46(1):12-41. [CrossRef]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS medicine. 2009; 6(7):e1000100.
- Lee JC, Williams GW, Kozar RA, Kao LS, Mueck KM, Emerald AD, Villegas NC, Moore LJ. Multitargeted feeding strategies improve nutrition outcome and are associated with reduced pneumonia in a level 1 trauma intensive care unit. J Parenter Enteral Nutr. 2018 Mar; 42(3):529-37. [CrossRef]
- Mizuma A, Netsu S, Sakamoto M, Yutani S, Nagata E, Takizawa S. Effect of early enteral nutrition on critical care outcomes in patients with acute ischemic stroke. J Int Med Res. 2021 Nov;49(11). [CrossRef]
- Su S, Sun R, Liu R, Xu Z. Effect of enteral nutrition time on pH value of gastric juice and ventilator-associated pneumonia in critically ill patient. Zhonghua wei Zhong Bing Ji Jiu Yi Xue. 2018 Aug 1;30(8):768-70. [CrossRef]
- Nakayama H, Nishimoto Y, Hotta K, Sato Y. Safety of early enteral nutrition for cardiac medical critically ill patients―A retrospective observational study―. Circ Rep. 2020 Oct 9;2(10):560-4. [CrossRef]
- Hamilton LA, Darby SH, Rowe AS. A retrospective cohort analysis of the use of enteral nutrition plus pharmacologic prophylaxis or enteral nutrition alone. Hosp Pharm. 2021 Dec;56(6):729-36. [CrossRef]
- Gaitanidis A, Breen K, Mendoza A, Fawley J, Lee J, Parks J, Kaafarani HM, Velmahos G, Fagenholz PJ. Enteral nutrition is associated with high rates of pneumonia in intensive care unit (ICU) patients with acute pancreatitis. J Crit Care. 2022 Jun 1; 69:154012. [CrossRef]
- Patel JJ, Kozeniecki M, Biesboer A, Peppard W, Ray AS, Thomas S, Jacobs ER, Nanchal R, Kumar G. Early trophic enteral nutrition is associated with improved outcomes in mechanically ventilated patients with septic shock: a retrospective review. J Intensive Care Med. 2016 Aug; 31(7):471-7. [CrossRef]
- Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019; 38: 48–79. [CrossRef]
- Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P; Canadian Critical Care Clinical Practice Guidelines Committee. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. J Parenter Enteral Nutr 2003; 27: 355–373. [CrossRef]
- Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, et al. Trial of the route of early nutritional support in critically ill adults. N Engl J Med 2014; 371: 1673–1684. [CrossRef]
- Capurso G, Zerboni G, Signoretti M, Valente R, Stigliano S, Piciucchi M, Delle Fave G. Role of the gut barrier in acute pancreatitis. J Clin gastroenterol. 2012 Oct 1;46:S46-51. [CrossRef]
- Reignier J, Boisramé-Helms J, Brisard L, Lascarrou JB, Ait Hssain A, Anguel N, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: A randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet 2018; 391: 133–143. [CrossRef]
- Hilker R, Poetter C, Findeisen N, Sobesky J, Jacobs A, Neveling M, Heiss WD. Nosocomial pneumonia after acute stroke: implications for neurological intensive care medicine. Stroke. 2003 Apr 1;34(4):975-81. [CrossRef]
- Liu Y, Zhao W, Chen W, Shen X, Fu R, Zhao Y, Liu H. Effects of early enteral nutrition on immune function and prognosis of patients with sepsis on mechanical ventilation. J Intensive Care Med. 2020 Oct;35(10):1053-61. [CrossRef]
- IAP WG, Guidelines AA. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013 Jul-Aug;13(4):e1-5. [CrossRef]
- Cintra MT, De Rezende NA, De Moraes EN, Cunha LC, da Gama Torres HO. A comparison of survival, pneumonia, and hospitalization in patients with advanced dementia and dysphagia receiving either oral or enteral nutrition. J Nutr Health Aging. 2014 Dec;18:894-9. [CrossRef]
- Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg. 1992; 216: 172–183. [CrossRef]
- O’Keefe SJ, Broderick T, Turner M, Stevens S, O’Keefe JS. Nutrition in the management of necrotizing pancreatitis. Clin Gastroenterol Hepatol. 2003 Jul 1;1(4):315-21.
- Wu LM, Sankaran SJ, Plank LD, Windsor JA, Petrov MS. Meta-analysis of gut barrier dysfunction in patients with acute pancreatitis. Br J Surg. 2014 Dec;101(13):1644-56. [CrossRef]
- Yi F, Ge L, Zhao J, Lei Y, Zhou F, Chen Z, Zhu Y, Xia B. Meta-analysis: total parenteral nutrition versus total enteral nutrition in predicted severe acute pancreatitis. Intern Med. 2012;51(6):523-30. [CrossRef]
- Al-Omran M, AlBalawi ZH, Tashkandi MF, Al-Ansary LA. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev. 2010 Jan.(1): CD002837. [CrossRef]
- Hsieh PH, Su HY, Lin CY, Kang YN, Chang CC. Infection rate among nutritional therapies for acute pancreatitis: A systematic review with network meta-analysis of randomized controlled trials. PloS One. 2019 Jul 10;14(7):e0219151. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).