1. Introduction
The circadian rhythm (CR) refers to organisms' ability to synchronize internal timekeeping with external cues, termed "zeitgebers," as coined by chronobiologist Jürgen Aschoff [
1]. Human physiological synchronization during the day is primarily triggered by 24-hour light fluctuations. These fluctuations convey diurnal cues to the suprachiasmatic nucleus (SCN), identified as the 'circadian pacemaker' located in the anterior hypothalamus [
4]. The cues are transmitted via the retino-hypothalamic pathway to the SCN, synchronizing biological rhythms with the 24-hour cycle and thereby impacting diverse physiological functions [
5,
6,
7]. This synchronization contributes to daily fluctuations in both physical and cognitive performance [
2,
3,
4,
5]. Recently, there has been a growing interest in understanding the impact of CR mainly on physical performance in both athletic and daily life contexts [
1,
6,
7]. In competitive situations, whether in a strenuous working environment or elite sports conditions, individuals are required to perform effectively both physically and mentally, as many athletic activities demand a combination of motor skills, as well as perceptual and cognitive functions [
8]. Several studies support this notion, exhibiting superior skill and sport-specific performance among athletes with high cognitive performance compared to low performers [
9,
10,
11]. Conventionally, the evaluation of functional tasks has been used as a surrogate for cognitive performance, including reaction time [
12], spatial memory [
13,
14] and sensorimotor tasks [
15,
16]. Additionally, circadian variations may influence neural factors such as nerve conduction velocity, cerebral perfusion, and the release of brain-derived neurotrophic factors (BDNF) [
4,
5], highlighting the potential link between circadian variations of physiological functioning and altered cognitive and neuromuscular performance [
17,
18].
Furthermore, in the sports world the hormonal and neuromuscular mechanisms of the biorhythmic regulation, can lead to impaired cognitive function due to the onset of physical fatigue. This complex multidimensional physiological state can detrimentally affect both peripheral and central aspects of the neuromuscular system [
19], depending on the mode and intensity of exercise. This is supported by observations that increased activation of the pre-frontal cortex (PFC) is associated with improved physical activity duration tolerance [
20], possibly due to the PFC's interaction with the basal ganglia and premotor cortex, which provide stimuli to overcome central fatigue and sustain central motor performance in the presence of inhibitory afferent signals [
21,
22]. Considering the role of the PFC in executive functioning, working memory and higher-order cognition, its function may be affected by neurotransmitter circadian variations [
23,
24]. Additionally, a state of physical fatigue resulting from exhaustive muscular activity has been shown to increase the activity of motor cortex and PFC, where the ability of cognition underlies, a function included in both mental and physical tasks [
25,
26]. This suggests a potential connection between CR, cognition, and physical performance, as they are all regulated by the frontal cortex [
24]. Little is known about the ability of physiological circadian variations to modulate the susceptibility of physical and cognitive functions following a state of provoked neuromuscular fatigue.
During real-life sports events, a prolonged game duration may contribute to create fatiguing conditions due to excessive neuromuscular effort, compromising athletic performance particularly in terms of decision making or sustained physical effort and as a result impact in-game quality and increase injury risk [
27]. A comprehensive body of research has examined cognitive tasks-modules of visual reaction time and visual memory response following various types of exercise (e.g handgrip dynamometer, Cycle Ergometer) [
28], providing though inconsistent findings with some cases reporting improvements [
29,
30], and other reporting deteriorated results [
31]. Moreover, numerous studies have investigated the impact of chronotype or sleep disturbance conditions on cognition or physical performance [
32,
33] and also separately explored the effect of physical fatigue on cognition or physical performance in athletes [
28]. However, there is currently a lack of studies evaluating the impact of both circadian variation and exercise induced fatigue on cognitive and physical performance [
34]. We argue that cognitive and physical performance indicators may be affected through intense physical activity during different times of the day, due to cyclical circadian variations of their underlying physiological components attributed by the multifaceted metabolic, hormonal, and neural nature of sustaining physical performance. Thus, the aim of the present study was to investigate the effect of time-dependent circadian variation on cognitive and physical performance after fatiguing exercise. Our main hypothesis was that there will be an interaction between circadian variation and physical fatigue state on selected cognitive and physical parameters, during three examined times of the day.
2. Materials and Methods
The study was approved by the Research Ethics Committee of University of Thessaly and carried out at the Laboratory of Biomechanics and Ergonomics, ErgoMech Lab – DPESS / Physical Education and Sport Science, of University of Thessaly. The experimental design was performed in accordance with the declaration of Helsinki. The participants consisted of recreational amateur athletes, men and women with ≥ 4 years of training experience in athletics and team sports, with a training frequency of ≥3/week. Data were collected from 18 participants (8 males, 10 females) (
Table 1), without any pre-existing musculoskeletal injuries, sleep disorders or other pathologies that may influence the findings of the study.
2.1. Experimental Design
Data collection took place at 09:00, 14:00, and 18:00 h in a randomized order with a minimum three-day interval between sessions to avoid exercise-induced muscle damage or mitigate the learning effect bias. Approximately 3 days before the initial session, participants underwent a familiarization session, received an overview of the study, provided written consent [
35], and completed a Pittsburgh Sleep Quality Index questionnaire [
36]. Participants refrained from physical exercise 24 h and abstained from caffeinated beverages 2 h before testing [
37]. Upon arrival at the laboratory, participants rested in a supine position for 20 minutes to establish baseline resting heart rate (HRrest) following Edwards et al. protocol [
38].
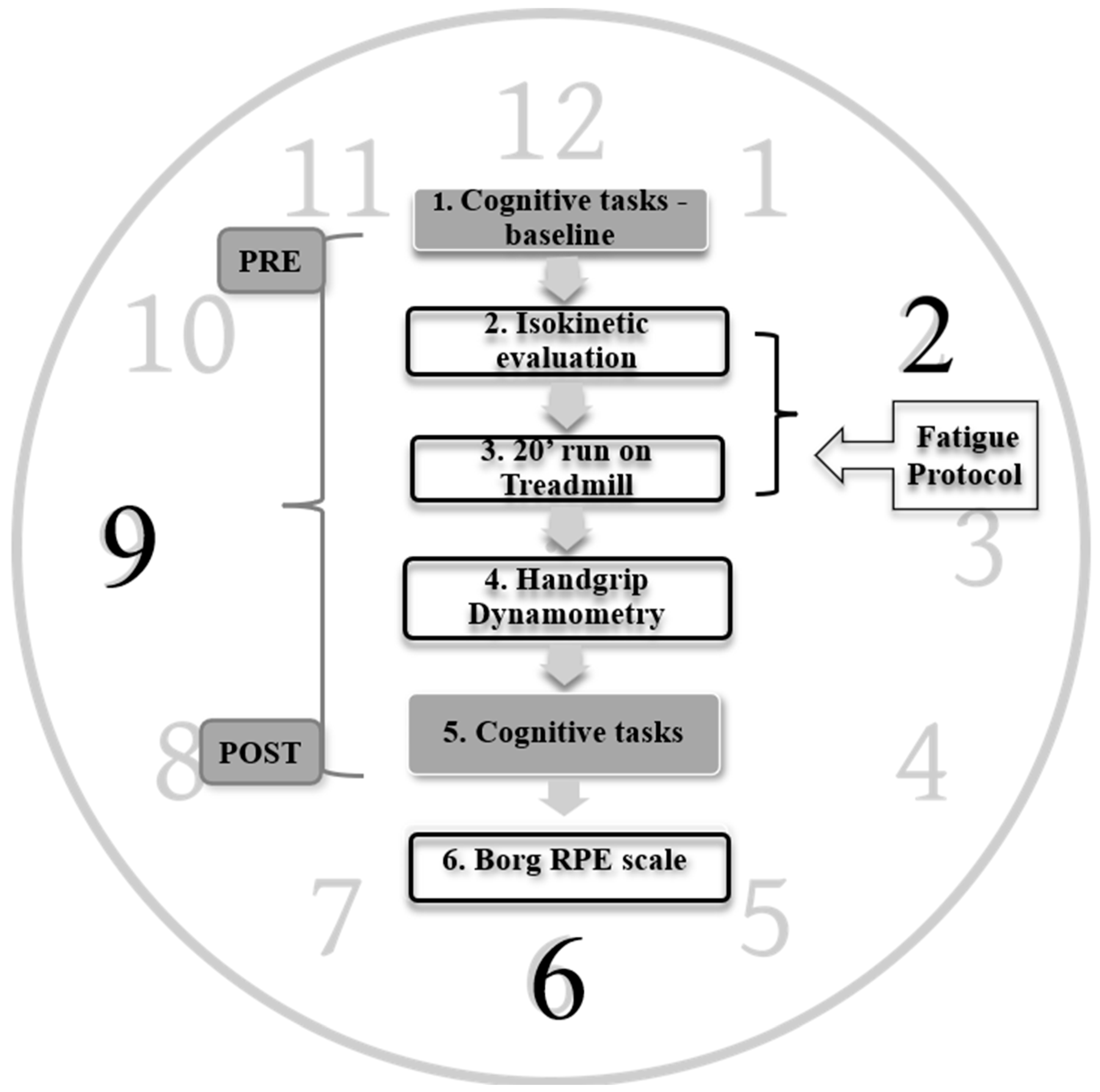
These resting heart rate recordings determined the aerobic zone of intensity during the subsequent running protocol. The fatigue experimental protocol comprised a continuous sequence of tasks designed to avoid any breaks between tasks, ensuring a continuous progression toward the primary objective of inducing a state of physical fatigue. The detailed sequence is illustrated in
Figure 1, encompassing the following steps: Step 1 involved the initial baseline measurement to assess cognitive performance in a relaxed state. Subsequently, Step 2 involved isokinetic strength evaluation, followed by Step 3, which involved a 20-minute running session on a treadmill. Steps 2 and 3 collectively constituted the fatigue protocol (refer to
Figure 1). Following fatigue inducement, Step 4 involved assessing hand grip strength. Lastly, Step 5 encompassed the assessment of cognitive performance and rate perceived exertion (RPE) during the fatigue state.
2.2. Outcomes
2.2.1. Cognitive Tasks – Baseline Measurements
Initially, two distinct mental cognitive tasks were conducted using the PEBL Launcher 2.1.1 Psychological Test Battery software (Sourceforge, CA, USA). The employed tests were as follows: (a) the "Match-to-sample test," designed to assess visual memory (VisM). This involved memorizing a sequence of 20 matrices of squared shapes and providing accurate answers within a time limit, and (b) the "Spatial Priming test," aimed at evaluating visual reaction time (VisRT). In this test, participants were required to click on a swiftly and continuously moving blue target, as quickly and accurately as possible within a squared frame, while ignoring the distraction of a yellow "flash" [
39]. Pre-measurements of VisM and VisRT were designated as VisM1 and VisRT1, respectively. Subsequently, post-measurements of the same type were conducted at the conclusion of the fatigue protocol, employing the same methodology as the pre-fatiguing test, and denoted as VisM2 and VisRT2.
2.2.2. Isokinetic Strength Evaluation Test – Fatigue Protocol
In accordance with the study protocol (refer to
Figure 1), an isokinetic strength evaluation test was incorporated into the fatigue provocation regimen. Following the completion of the mental task (refer to 2.3.1), the isokinetic test was immediately conducted. This testing protocol, as described by Tsaklis P. [
40], aimed to induce muscle fatigue and employed a Cybex-Norm isokinetic dynamometer (NY, USA). Participants started with a generalized warm-up protocol, including 10 minutes of low-intensity jogging on a treadmill (Runrace D140, Technogym, Cesena, IT). This warm-up also involved dynamic stretching the major lower limb muscle groups. Participants were then securely seated on the isokinetic dynamometer at a 90⁰ hip flexion angle. The knee joint's defined range of motion (ROM) was set at 0-120⁰ with a gravitational correction at 45⁰. The initial protocol encompassed an 8-repetition warm-up trial, involving concentric action of the knee extensors and flexors, with an angular velocity of 120⁰/s [
41].
Subsequently, to induce fatigue, a 30-repetition protocol was executed, utilizing concentric action of the knee extensors and flexors bilaterally at a fixed ROM of 120° and an angular velocity of 240⁰/s [
40]. Peak torque (PT) of the knee extensors of both limbs was calculated, to evaluate the maximum strength achieved throughout the 30 repetitions. Furthermore, the fatigue index (PTFI) was computed based on the percentage of PT loss over the total attempt duration [
40]. A 2-minute rest period was implemented between the warm-up and fatigue protocol. The fatigue protocol was conducted randomly on the dominant/non-dominant limb and then repeated on the contralateral side after a 3 min of recovery. The dominant leg was determined by asking the participant’s preferred limb when kicking a ball [
42].
2.2.3. Running Fatigue Protocol
Following, participants engaged in a 20-minute treadmill run (Runrace D140, Technogym, Cesena, Italy) in accordance with the outlined design (see
Figure 1). Heart rate monitoring, facilitated by a Polar T31 heart rate monitor (Kempele, Finland), was employed to track participants' heart rates, ensuring the effective monitoring of activity intensity. Utilizing the initial HRrest measured at the beginning of the experimental procedure, a medium intensity at 60% and a high intensity at 84% of the maximum heart rate (HRmax) were calculated to define the aerobic zone. During the initial 5 minutes of the test, participants aimed to achieve and keep at 60% of their HRmax, followed by the subsequent 15 minutes performed at 84% of their HRmax [
43]. This approach was designed to maintain participants within 84% of their HRmax for the majority of the 15-minute duration, thereby inducing the intended fatigue state.
2.2.4. Hand Grip Dynamometry
Subsequently, a hand grip strength test was conducted using the K-Force Grip® 2016 (Kinvent, Monpellier, France). The participants assumed a comfortable seated position, with elbows flexed at 90° and supported by a table. Forearm and wrist positioning were adjusted to individual neutral positions [
44]. Three attempts were performed with the dominant hand and the best attempt was calculated. Each trial comprised a single maximal isometric contraction lasting 10 s of the dominant hand, with a 60 s rest period between attempts. The rate force development (RFD) was computed based on the values obtained from the K-Force Grip® 2016 software, from the dominant hand during the 10 s trial [
45]. Additionally, fatigue index of the dominant hand (DynaFdh) was calculated, reflecting the decrement of isometric strength over the entire duration of the attempt.
2.2.5. Cognitive Tasks – Post Fatigue Protocol and Perceived Exertion Scale
Finally, the participants completed the testing protocol by performing the cognitive tasks post fatigue measurements of VisM (VisM2) and VisRT (VisRT2) [
39]. Lastly, the participants were asked to visually indicate their subjective fatigue level using a CR-10 Borg rating of perceived exertion on a scale from 0 to 10 [
18,
46].
2.3. Statistics and Data Analysis
The sample size was determined using an a priori power analysis conducted with G*Power V 3.1.9.7 software from Heinrich-Heine-Universität, Düsseldorf, Germany. The following parameters were used within a repeated-measure ANOVA design: effect size (f) = 0.28, significance level (α) = 0.05, power = 0.80, and correlation between repeated measures (r) = 0.50. The initial power calculation indicated a sample size requirement of 15. To account for a projected ≈10% dropout rate, 19 individuals initially recruited to participate voluntarily in the study, with 18 participants completing the experimental procedure. Further statistical analyses were executed in SPSS 22.0 (IBM Inc., Chicago, IL, USA). Initially, parametric assumptions we examined by accessing data normality with the Shapiro-Wilk test. A two-way (2*3) (fatigue*time of day) repeated ANOVA on both factors was performed to evaluate the effect of the induced fatigue protocol and CR on mental cognition tasks (VisRT and VisM).
Post-hoc analysis of significant interactions and main effects were further investigated using Bonferroni adjustment. Additionally, a one-way repeated ANOVA was performed to evaluate CR effect comparing the mean values of Borg scale, PT, PTFI, RFD, DynaFdh measurements between the three different times of the day. Pearson correlation coefficient analysis was conducted on all examined parameters to evaluate their correlations. Data, presented in tables as mean and standard deviations values (M ± SD), considered significant at p < 0.05.
3. Results
3.1. Measures of Cognitive Performance
Repeated ANOVA 2*3 did not show an interaction between the examined factors for VisRT (F2.22= 0.181, p= 0.869) and VisM (F2.34= 0.111, p= 0.895). However, a main effect of the fatigue factor (F1.11 = 15,175, p= 0.002) was observed. Specifically, a decrease in the parameter of VisRT was noted across all three examined timepoint measurements, suggesting an influence of the induced fatigue state on VisRT performance. Additionally, the pre and post assessments of the VisM test exhibited non statistical significance (F1.17=0.459, p=0.507), suggesting no effect of the induced fatigue state on VisM performance in the three timepoint measurements (
Table 2).
3.2. Physical Parameters
One-way analysis of variance did not reveal a CR effect and differences between the three timepoint measurements of the day on Borg (F2,34 = 0.668 p = 0.519), RFD (F2,32 = 0.342 p = 0.713) and DynaFdh (F2,32 = 0.311 p = 0.735) post fatigue measurements. However, PT (F2,30 = 4.62 p = 0.018), and PTFI (F2,26 = 6.171 p = 0.006) showed a significant difference between the three different times of the day. Specifically, there was a significant difference between PT_9 (90.6 ± 28.1) and PT_18 (98.7 ± 31.8), where PT_18 (evening measurement) revealed a higher value than PT_9 (morning measurement) (p= 0.059). No other pairwise comparisons reached significance. Additionally, there was a significant difference between PTFI_9 (36.3 ± 1.8) and PTFI_18 (41.9 ± 1.3), where PowerF_18 (evening measurement) revealed a higher value than PTFI_9 (morning measurement) (p= 0.012). No other pairwise comparisons reached significance (
Table 3).
3.3. Correlation Analysis
There was a significant positive correlation between VisRT2_18 and DynaFdh_18 (r = 0.581, p <0.01), suggesting that as fatigue increases, cognition testing score increased as well. Additionally, a positive correlation was found between PT_14 and RFD_14 (r = 0.613, p <0.01), as well as PT_18 and RFD_18 (r = 0.599, p <0.01), indicating that both PT and RFD increase equally on the same time of the day (
Table 4).
4. Discussion
The existing literature has predominantly focused on the correlation between circadian variations and physical performance, revealing a range of diurnal alterations [
2]. However, minimal attention has been devoted to circadian variations in cognitive performance, particularly in the context of physical fatigue conditions. This study aimed to investigate the effects of the time of day and exercise-induced fatigue on both cognitive and physical performance. Our initial hypothesis was partially confirmed. While we found no significant interaction between circadian variation and physical fatigue state on selected cognitive and physical parameters at the three times of day examined, there was a significant main effect of fatigue on cognitive performance. Additionally, certain physical parameters related to strength exhibited circadian variations.
4.1. CR and Cognitive Performance
The current findings indicate that CR did not exert an effect on cognitive performance, specifically VisM and VisRT, even under conditions of induced physical fatigue. This observation is consistent with the study by Nogueira et al. (2021), which explored circadian variations in high-intensity motor tasks using the Grooved Pegboard test. Their research revealed no performance differences, suggesting that cognitive function, in the absence of physical exertion, may not be significantly influenced by circadian rhythms [
47]. This contrasts with the results reported by Rulleau and colleagues (2015), who previously identified circadian variations in cognitive parameters related to the temporal coupling between executed and simulated actions during motor imagery tasks in older adults. In their study, Rulleau et al. observed favorable performance during morning hours, even in the absence of physical fatigue [
48]. The different findings compared to our study may be due to the absence of fatigue conditions in the Rulleau et al. study and the age difference between the study samples. Moreover, it should be noted that due to the multifaceted nature of cognitive capacity, the literature presents a debatable stance on circadian variations. Cognition may be influenced by both endogenous factors (e.g., age, neural network malfunction) and exogenous factors (e.g., sleep deprivation, task difficulty, chronotype preference) [
49,
50] [
22]. Consequently, results may vary based on the applied protocol and potential study limitations [
51]. Thus, given the heterogeneity of our study design compared to the previously mentioned investigations, a direct comparison of results may not be appropriate.
4.2. Physical Fatigue and Cognitive Performance
Despite the non-significant interaction of CR and fatigue in the cognition tasks, the statistical outcome indicated a main effect of fatigue on VisRT, appearing to positively influence cognitive function after the fatigue protocol was applied (
Table 2). This outcome might vary in attribution, as according to the literature the effects of acute fatiguing exercise on cognition, range from strongly positive to negative [
21,
52,
53,
54]. This variability is primarily due to the lack of standardized evaluation methods for the influence of fatiguing exercise on cognitive functioning given the differing intensities and durations of physical activity protocols used [
54]. Additionally, participant bias during the second, post-fatigue cognitive measurement might have influenced the results. [
55]. However, substantial evidence supports the notion that acute fatiguing exercise generally enhances cognitive functioning, particularly tasks involving the prefrontal cortex. For instance, studies as Hendy et al. 2022, reveal that high-intensity aerobic exercise affects both excitability and inhibition in the upper limb motor cortex [
56]. This exercise modality is also linked to increased levels of circulating BDNF, a crucial element in neural plasticity and synaptic transmission. [
57], Neuroplasticity induced by aerobic exercise, even from a single session, can alter cortical excitability and impact neural circuits significantly [
58]. Furthermore, studies have reported improved cognitive performance at different times of the day related to strength training, attributed to a favorable neural metabolic environment at the neuromuscular junction [
59,
60]. This enhancement is primarily driven by increased blood flow, boosting cerebral cortex perfusion, and engaging BDNF regulation [
17,
18]. Consequently, resistance exercise modalities may enhance cognitive performance due to improved nerve conduction velocity, cerebral perfusion, and BDNF release [
61,
62]. Supporting this, the negative correlation between the VisRT2_18 variable and both RFD_18 and DynaFdh_18 suggests that, during evening measurements, higher strength development and fatigue index in strength-related modalities correspond to improved performance on cognitive reaction time tests.
4.3. CR and Physical Parameters
In the present study, we examined several physical performance indicators after applying a fatigue protocol at three different times of the day. The results showed no statistically significant differences in rate of perceived exertion (Borg), rate of force development (RFD), and Handgrip Dynamometry Fatigue Index (DynaFdh) measurements across these timepoints. However, a notable CR effect was observed for PT and PTFI, which both demonstrated higher outcomes at 18:00h compared to 09:00h. These findings suggest that PT and PTFI, which are crucial elements of strength capability, are influenced by the time of day, with improved performance in the evening. This could be attributed to physiological variations such as body temperature and hormonal levels that peak later in the day [
1]. Such circadian changes in body temperature could potentially affect nerve conduction velocity, explaining previously observed improvements for power output, coordination and reaction time performance shown in late afternoon [
63,
64]. Additionally, other studies have implicated circadian variations of cortisol and its effect on body temperature and muscle performance, displaying a higher PT and RFD during evening hours compared to morning outputs, evaluated via isokinetic or handgrip dynamometry tasks [
65,
66].
Notably, the findings of higher strength performance during evening hours and an increased fatigue index during the same period might be explained by the increased demand on anaerobic energy systems [
67], recruitment of fast-twitch muscle fibers [
23,
67], accumulation of metabolic waste products [
68], and potential central nervous system alterations, [
69], all of which contribute to muscle fatigue during high-intensity activities. Studies like those of Hammouda et. al. [
70] and Deschenes et al. [
65] have demonstrated significant diurnal effects, with improved performance for PT and total work performance among the participants performing maximal resistance exercise protocols using a isokinetic dynamometer. Thus, the observed peak in PT and PTFI in the evening indicates a direct relationship between increased power output and the simultaneous increase in fatigue development.
5. Conclusions
Although we found no interaction between circadian variation and physical fatigue state on selected cognitive parameters at the three times of day examined, there was an effect of fatigue on cognitive performance. Additionally, physical parameters related to strength exhibited circadian variations, exhibiting peak values in the evening hours. Improving our understanding of how humans perform mentally concerning circadian variations, in both rested and physically fatigued states, could help to inform sound athletic practices, prevention of musculoskeletal injuries and promote psycho-physiological well-being. We encourage future studies to delve deeper into biochemical markers, diverse physical fatigue protocols, and neurological aspects of cognitive performance during different times of the day. Implementing these factors may allow future authors to expand upon the circadian underpinnings of cognitive performance across diverse populations.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, P.V.T and P.K.; methodology, P.V.T and P.K.; software, P.K., T.T. and P.G.; validation, P.V.T., T.T. and G.G.; formal analysis, P.K. and P.G.; investigation, P.K. and P.G.; resources, P.K., T.T. and G.G.; data curation, P.V.T., P.K. and G.G.; writing—original draft preparation, P.K.; writing—review and editing, P.K., T.T. and P.G.; visualization, P.V.T.; supervision, P.V.T. and G.G.; project administration, P.K.; funding acquisition, no founding. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of University of Thessaly, Department of Physical Education and Sport Science, of (protocol code 2089 and date: 08/02/2023).
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to extend our heartfelt gratitude to the volunteers who generously gave their time and effort to participate in this study. We are also deeply appreciative of our colleagues of Ergo-Mech Lab laboratory for their valuable insights and unwavering support throughout the research process.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Atkinson, G. and T.J.S.m. Reilly, Circadian variation in sports performance. 1996. 21: p. 292-312. [CrossRef]
- Teo, W., M. J. Newton, and M.R. McGuigan, Circadian rhythms in exercise performance: implications for hormonal and muscular adaptation. Journal of sports science & medicine, 2011. 10(4): p. 600-606.
- Hayes, L.D. G.F. Bickerstaff, and J.S.J.C.i. Baker, Interactions of cortisol, testosterone, and resistance training: influence of circadian rhythms. 2010. 27(4): p. 675-705. [CrossRef]
- Blatter, K., C. J.P. Cajochen, and behavior, Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings. 2007. 90(2-3): p. 196-208. [CrossRef]
- Petruzzello, S.J. M. Han, and P.J.J. Nowell, The influence of physical fitness and exercise upon cognitive functioning: A meta-analysis. 1997. 19: p. 249-277. [CrossRef]
- Drust, B. , et al., Circadian rhythms in sports performance—an update. 2005. 22(1): p. 21-44.
- Reilly, T. , Human circadian rhythms and exercise. Critical Reviews in Biomedical Engineering, 1990. 18(3): p. 165-180.
- Vitošević, B.J.B.o.N.S.R. , The circadian clock and human athletic performance. 2017. 7(1).
- Kalén, A. , et al., The role of domain-specific and domain-general cognitive functions and skills in sports performance: A meta-analysis. 2021. 147(12): p. 1290.
- Müller, S., B. Abernethy, and D.J.T.q.j.o.e.p. Farrow, How do world-class cricket batsmen anticipate a bowler's intention? 2006. 59(12): p. 2162-2186. [CrossRef]
- Williams, A.M. , et al., Anticipation skill in a real-world task: measurement, training, and transfer in tennis. 2002. 8(4): p. 259. [CrossRef]
- Wright Jr, K.P. , et al., Relationship between alertness, performance, and body temperature in humans. 2002. [CrossRef]
- Folkard, S. and T.H.J.B.j.o.p. Monk, Circadian rhythms in human memory. 1980. 71(2): p. 295-307. [CrossRef]
- Ramírez, C. , et al., Circadian rhythms in phonological and visuospatial storage components of working memory. 2006. 37(5): p. 433-441. [CrossRef]
- Lotze, M. , et al., Daily rhythm of temporal resolution in the auditory system. 1999. 35(1): p. 89-100.
- Edwards, B., J. Waterhouse, and T.J.C.I. Reilly, The effects of circadian rhythmicity and time-awake on a simple motor task. 2007. 24(6): p. 1109-1124. [CrossRef]
- Jo, D. , et al., A new paradigm in sarcopenia: Cognitive impairment caused by imbalanced myokine secretion and vascular dysfunction. Biomedicine & Pharmacotherapy, 2022. 147: p. 112636. [CrossRef]
- Gligoroska, J.P. and S. Manchevska, The effect of physical activity on cognition - physiological mechanisms. Mater Sociomed, 2012. 24(3): p. 198-202. [CrossRef]
- Tornero-Aguilera, J.F. , et al., Central and peripheral fatigue in physical exercise explained: A narrative review. 2022. 19(7): p. 3909.
- Gandevia, S.C. , Spinal and supraspinal factors in human muscle fatigue. Physiol Rev, 2001. 81(4): p. 1725-89. [CrossRef]
- Lambourne, K. and P. Tomporowski, The effect of exercise-induced arousal on cognitive task performance: a meta-regression analysis. Brain Res, 2010. 1341: p. 12-24. [CrossRef]
- Xu, S., M. Akioma, and Z. Yuan, Relationship between circadian rhythm and brain cognitive functions. Front Optoelectron, 2021. 14(3): p. 278-287.
- Amann, M. , et al., Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. 2011. 589(21): p. 5299-5309. [CrossRef]
- Thomas, R. and P.J.E.j.o.a.p. Stephane, Prefrontal cortex oxygenation and neuromuscular responses to exhaustive exercise. 2008. 102(2): p. 153-163. [CrossRef]
- Bigliassi, M.J.B.P. , Functional significance of the dorsolateral prefrontal cortex during exhaustive exercise. 2022: p. 108442.
- Kimura, D. , et al., Effects of different exercise intensities on prefrontal activity during a dual task. 2022. 12(1): p. 13008.
- Nassis, G.P. , et al., Injury prevention training in football: let’s bring it to the real world. 2019, BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine.
- Özdemir, R.A., S. Kirazcı, and A. Uğraş, Simple reaction time and decision making performance after different physical workloads: an examination with elite athletes. Journal of Human Sciences, 2010. 7(2): p. 655-670.
- Ozyemisci-Taskiran, O. , et al., The effect of a single session submaximal aerobic exercise on premotor fraction of reaction time: an electromyographic study. 2008. 23(2): p. 231-235.
- Ando, S. , et al., Increase in reaction time for the peripheral visual field during exercise above the ventilatory threshold. 2005. 94(4): p. 461-467. [CrossRef]
- Facer-Childs, E.R., S. Boiling, and G.M. Balanos, The effects of time of day and chronotype on cognitive and physical performance in healthy volunteers. Sports Medicine - Open, 2018. 4(1): p. 47. [CrossRef]
- Vidueira, V.F. , et al., Circadian preference and physical and cognitive performance in adolescence: A scoping review. 2023. 40(9): p. 1296-1331.
- Reid, K.J., L. L. McGee-Koch, and P.C.J.P.i.b.r. Zee, Cognition in circadian rhythm sleep disorders. 2011. 190: p. 3-20. [CrossRef]
- Dambroz, F., F. M. Clemente, and I.J.P.O. Teoldo, The effect of physical fatigue on the performance of soccer players: A systematic review. 2022. 17(7): p. e0270099. [CrossRef]
- Touitou, Y., M. H. Smolensky, and F.J.C.i. Portaluppi, Ethics, standards, and procedures of animal and human chronobiology research. 2006. 23(6): p. 1083-1096. [CrossRef]
- Carpenter, J.S. and M.A.J.J.o.p.r. Andrykowski, Psychometric evaluation of the Pittsburgh sleep quality index. 1998. 45(1): p. 5-13. [CrossRef]
- Reilly, T. , et al., Diurnal Variation in Temperature, Mental and Physical Performance, and Tasks Specifically Related to Football (Soccer). Chronobiology International, 2007. 24(3): p. 507-519. [CrossRef]
- Edwards, B.J., K. Lindsay, and J. Waterhouse, Effect of time of day on the accuracy and consistency of the badminton serve. Ergonomics, 2005. 48(11-14): p. 1488-98. [CrossRef]
- Mueller, S.T. and B.J.J.J.o.n.m. Piper, The psychology experiment building language (PEBL) and PEBL test battery. 2014. 222: p. 250-259. [CrossRef]
- Tsaklis, P.J.I. and e. science, Isokinetic evaluation of the knee extensors and flexors anaerobic capacity. 2002. 10(2): p. 69-72.
- Tsatalas, T. , et al., The effects of muscle damage on walking biomechanics are speed-dependent. Eur J Appl Physiol, 2010. 110(5): p. 977-88. [CrossRef]
- Tsatalas, T. , et al., Altered Drop Jump Landing Biomechanics Following Eccentric Exercise-Induced Muscle Damage. Sports, 2021. 9(2): p. 24. [CrossRef]
- Tsaklis P.PhD., A method for modification of aerobic training intensity for special and general populations. Gazzetta Medica Italiana, 2020. 179: p. 314-5.
- Gerodimos, V.J.J.o.h.k. , Reliability of handgrip strength test in basketball players. 2012. 31: p. 25.
- Feng, L.R. , et al., Cognitive and motor aspects of cancer-related fatigue. 2019. 8(13): p. 5840-5849. [CrossRef]
- Borg, G. , Borg's perceived exertion and pain scales. 1998: Human kinetics.
- Nogueira, N.l.G.n.d.H.M. , et al., Influence of Chronotype on Motor Behavior in Healthy Individuals: Analyses of Manual Dexterity in Different Times of the Day. Motor Control, 2021: p. 1. [CrossRef]
- Rulleau, T., B. Mauvieux, and L. Toussaint, Influence of Circadian Rhythms on the Temporal Features of Motor Imagery for Older Adult Inpatients. Archives of Physical Medicine and Rehabilitation, 2015. 96(7): p. 1229-1234. [CrossRef]
- Matchock, R.L. and J.T. Mordkoff, Chronotype and time-of-day influences on the alerting, orienting, and executive components of attention. Exp Brain Res, 2009. 192(2): p. 189-98. [CrossRef]
- Bonnefond, A. , et al., Interaction of age with time of day and mental load in different cognitive tasks. 2003. 96(3_suppl): p. 1223-1236.
- Ramírez, C. , et al., Circadian rhythms in phonological and visuospatial storage components of working memory. Biological Rhythm Research, 2006. 37(5): p. 433-441. [CrossRef]
- Chang, Y.K. , et al., The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res, 2012. 1453: p. 87-101. [CrossRef]
- McMorris, T. and B.J. Hale, Differential effects of differing intensities of acute exercise on speed and accuracy of cognition: a meta-analytical investigation. Brain Cogn, 2012. 80(3): p. 338-51. [CrossRef]
- Basso, J.C. and W.A. Suzuki, The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. Brain Plast, 2017. 2(2): p. 127-152. [CrossRef]
- Schmiedek, F., M. Lövdén, and U.J.F.i.a.n. Lindenberger, Hundred days of cognitive training enhance broad cognitive abilities in adulthood: Findings from the COGITO study. 2010. 2: p. 27. [CrossRef]
- Hendy, A.M. , et al., Acute Effects of High-Intensity Aerobic Exercise on Motor Cortical Excitability and Inhibition in Sedentary Adults. Front Psychol, 2022. 13: p. 814633. [CrossRef]
- Yen, S. , et al., Revisiting the effects of exercise on cerebral neurovascular functions in rats using multimodal assessment techniques. iScience, 2023. 26(4): p. 106354. [CrossRef]
- Kuo, H.I. , et al., A single bout of aerobic exercise modulates motor learning performance and cortical excitability in humans. Int J Clin Health Psychol, 2023. 23(1): p. 100333. [CrossRef]
- Smolarek, A.d.C. , et al., The effects of strength training on cognitive performance in elderly women. Clinical Interventions in Aging, 2016. 11: p. 749-754.
- Chang, Y.-K. , et al., Effect of resistance-exercise training on cognitive function in healthy older adults: a review. 2012. 20(4): p. 497-517. [CrossRef]
- Huang, T. , et al., The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand J Med Sci Sports, 2014. 24(1): p. 1-10. [CrossRef]
- Helm, E.E. , et al., The influence of high intensity exercise and the Val66Met polymorphism on circulating BDNF and locomotor learning. 2017. 144: p. 77-85. [CrossRef]
- Shibata, S., Y. J.T.J.o.P.F. Tahara, and S. Medicine, Circadian rhythm and exercise. 2014. 3(1): p. 65-72. [CrossRef]
- Racinais, S. , et al., Morning versus evening power output and repeated-sprint ability. 2005. 22(6): p. 1029-1039. [CrossRef]
- Deschenes, M.R. , et al., Biorhythmic influences on functional capacity of human muscle and physiological responses. Med Sci Sports Exerc, 1998. 30(9): p. 1399-407. [CrossRef]
- Jasper, I. , et al., Circadian variations in the kinematics of handwriting and grip strength. 2009. 26(3): p. 576-594. [CrossRef]
- Wan, J.-j. , et al., Muscle fatigue: general understanding and treatment. 2017. 49(10): p. e384-e384. [CrossRef]
- Aquino, M. , et al., The impact of fatigue on performance and biomechanical variables—A narrative review with prospective methodology. Biomechanics, 2022. 2(4): p. 513-524.
- Tanaka, M., A. Ishii, and Y.J.B.r. Watanabe, Neural mechanism of central inhibition during physical fatigue: a magnetoencephalography study. 2013. 1537: p. 117-124. [CrossRef]
- Hammouda, O. , et al., High intensity exercise affects diurnal variation of some biological markers in trained subjects. Int J Sports Med, 2012. 33(11): p. 886-91. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).