Submitted:
05 September 2024
Posted:
05 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- Design of the compounds and their conformers for the study. The difference between conformers lies in both: i). the position of NO2 and fluorine containing groups; ii). The cis/trans position of -OCF3 group where it is possible.
- Performance of the Berny optimization without any symmetry constraints (all bond lengths, angles, and dihedral angles are changed) to find an equilibrium point. The vibration frequencies analysis was performed to verify that the energy minima were reached. The total energy of the conformers was compared to selecting the most abundant conformers. The stability and energetic properties of the designated conformers were only investigated.
- Evaluation of the binding energy per atom to compare the thermal stability of the compounds under study. This energy indicates the amount of energy required to separate an atom from a system of particles and its larger value shows higher thermal stability.
- Determination of the stability related to the chemical properties and aging of the compounds. HOMO-LUMO gap, chemical hardness, and softness are calculated for this purpose. It is known that compounds with larger HOMO-LUMO gap and chemical hardness are more resistant to undergoing a chemical reaction or to being transformed by an external perturbation, such as an applied electric field. On the other hand, a low chemical softness value denotes a high tendency of the molecule to degrade [57,58].
- Calculation of hardness index to evaluate the stability of the compounds.
3. Results
| Compound | BDE, eV | G, eV | H,eV | S, eV | Y |
|---|---|---|---|---|---|
| CF3N2 | 6.00 | 4.94 | 2.47 | 0.20 | 0.92 |
| CF3N3 | 5.43 | 4.73 | 2.36 | 0.21 | 0.91 |
| C2F6N2 | 5.42 | 5.24 | 2.62 | 0.19 | 0.93 |
| C2F6N3 | 5.38 | 5.02 | 2.51 | 0.20 | 0.92 |
| C3F9N2a | 5.39 | 5.16 | -2.58 | 0.19 | 0.92 |
| C3F9N2b | 5.39 | 5.46 | 2.73 | 0.18 | 0.93 |
| C3F9N3 | 5.35 | 5.01 | 2.50 | 0.20 | 0.92 |
| OCF3N2/CF3ON2 | 5.41 | 5.10 | 2.55 | 0.20 | 0.92 |
| OCF3N3/CF3ON3 | 5.37 | 4.94 | 2.47 | 0.20 | 0.92 |
| 1O2C2F6N2a | 5.33 | 4.65 | 2.33 | 0.21 | 0.91 |
| 1O2C2F6N2b | 5.33 | 4.95 | 2.47 | 0.20 | 0.92 |
| 1O3C3F9N2b | 5.27 | 5.14 | 2.57 | 0.19 | 0.92 |
| 1O2C2F6N3a | 5.30 | 4.96 | 2.48 | 0.20 | 0.92 |
| 1O2C2F6N3b | 5.30 | 4.95 | 2.47 | 0.20 | 0.92 |
| 1O2C2F6N4a | 5.27 | 4.85 | 2.43 | 0.21 | 0.92 |
| 1O2C2F6N4b | 5.27 | 4.85 | 2.43 | 0.21 | 0.92 |
| 1O3C3F9N3a | 5.24 | 5.22 | 2.61 | 0.19 | 0.93 |
| 1O3C3F9N3b | 5.24 | 5.23 | 2.62 | 0.19 | 0.93 |
| 1CF2N2/1O2CF2N2 | 5.61 | 4.65 | 2.33 | 0.22 | 0.91 |
| 1CF2N3/1O2CF2N3 | 5.68 | 4.00 | 2.00 | 0.25 | 0.87 |
| 1CF2N4/1O2CF2N4 | 5.48 | 4.48 | 2.24 | 0.22 | 0.90 |
| 2CF4N2/1O2C2F4N2 | 5.66 | 4.73 | 2.37 | 0.21 | 0.91 |
| 12CF4N3 | 5.47 | 4.77 | 2.39 | 0.21 | 0.91 |
| 2CF4N4//O2C2F4N4 | 5.42 | 4.89 | 2.44 | 0.20 | 0.92 |
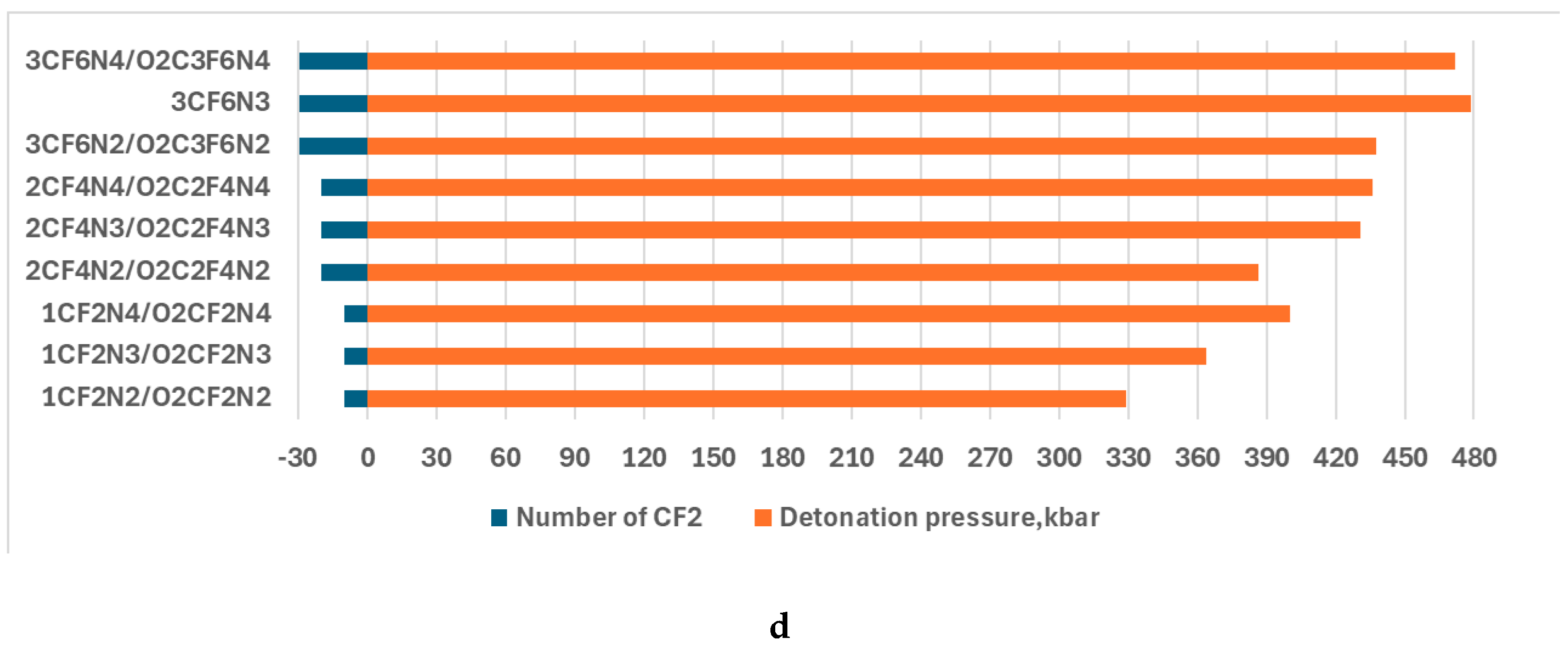
| 3CF6N2/O2C3F6N2 | 5.43 | 4.93 | 2.46 | 0.20 | 0.92 |
| 3CF6N3 | 5.53 | 4.46 | 2.23 | 0.22 | 0.90 |
| 3CF6N4/1O2C3F6N4 | 5.36 | 4.99 | 2.50 | 0.20 | 0.92 |
| TNT | 5.52 | 4.15 | 2.07 | 0.24 | 0.88 |
| APATO* | 5.70 | 3.21 | 1.60 | 0.31 | 0.81 |
| Compounds | ACD ρ ACD , g/cm3 |
Gaussian ρ , g/cm3 |
|---|---|---|
| CF3N3 | 1.77 | 2.07 |
| C2F6N2 | 1.69 | 2.07 |
| C2F6N3 | 1.82 | 2.34 |
| C3F9N2a | 1.74 | 2.09 |
| C3F9N2b | 1.74 | 1.96 |
| C3F9N3 | 1.85 | 2.01 |
| OCF3N2/CF3ON2 | 1.64 | 1.93 |
| OCF3N3/CF3ON3 | 1.80 | 2.11 |
| O2C2F6N2a | 1.74 | 2.42 |
| O2C2F6N2b | 1.73 | 1.99 |
| O3C3F9N2b | 1.79 | 2.11 |
| O2C2F6N3a | 1.85 | 1.98 |
| O2C2F6N3b | 1.85 | 2.01 |
| O2C2F6N4a | 1.96 | 2.14 |
| O2C2F6N4b | 1.96 | 2.14 |
| O3C3F9N3b | 1.89 | 2.13 |
| O3C3F9N3a | 1.89 | 2.05 |
| 1CF2N2/O2CF2N2 | 1.84 | 1.83 |
| 1CF2N3/O2CF2N3 | 1.95 | 1.80 |
| 1CF2N4/O2CF2N4 | 2.05 | 2.23 |
| 2CF4N2/O2C2F4N2 | 1.85 | 2.09 |
| 2CF4N3/O2C2F4N3 | 1.98 | 1.81 |
| 2CF4N4//O2C2F4N4 | 2.01 | 1.89 |
| 3CF6N2/O2C3F6N2 | 1.85 | 2.19 |
| 3CF6N3/O2C3F6N3 | 1.97 | 2.42 |
| 3*0CF6N4/O2C3F6N4 | 1.98 | 2.34 |
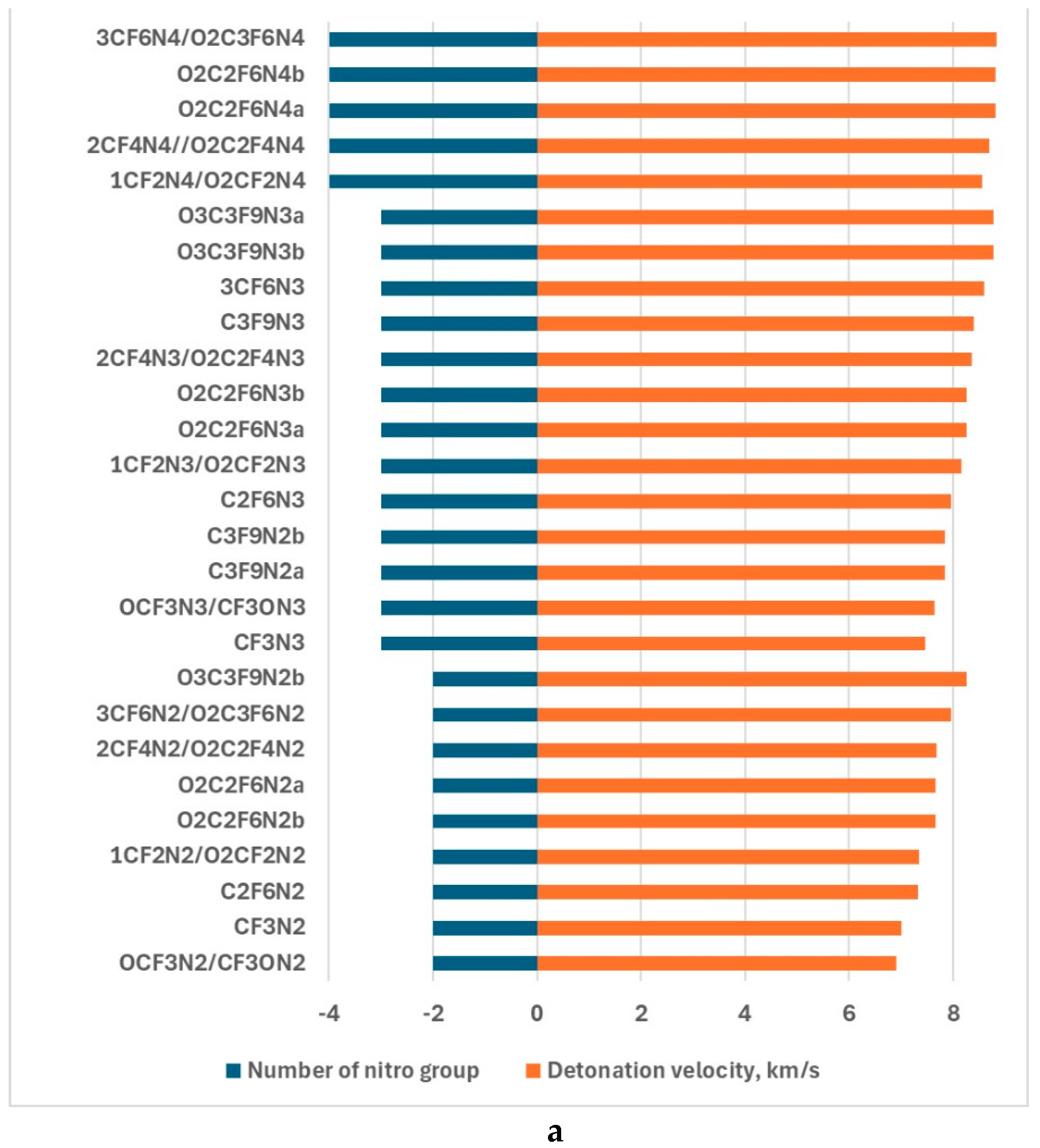
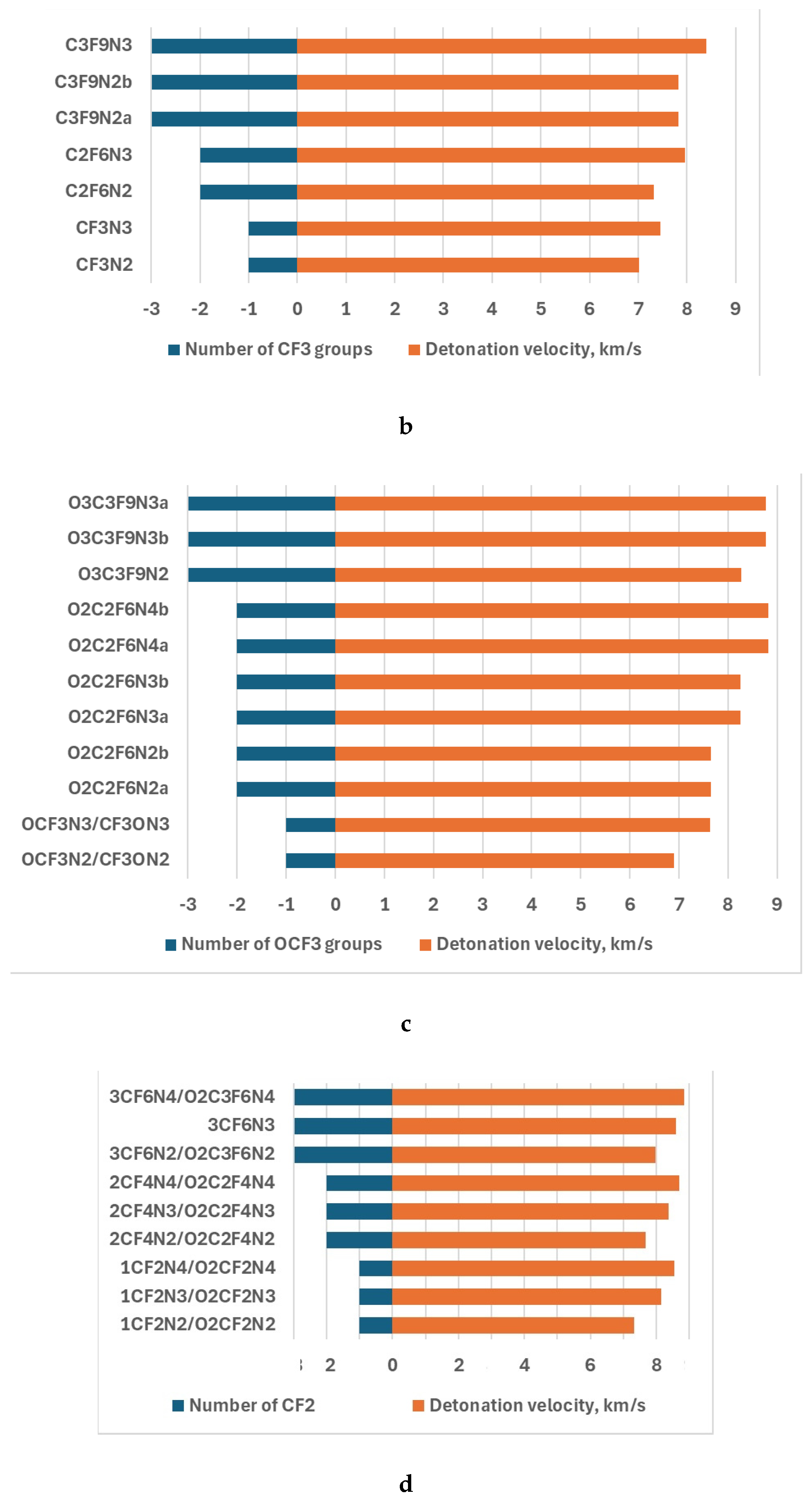
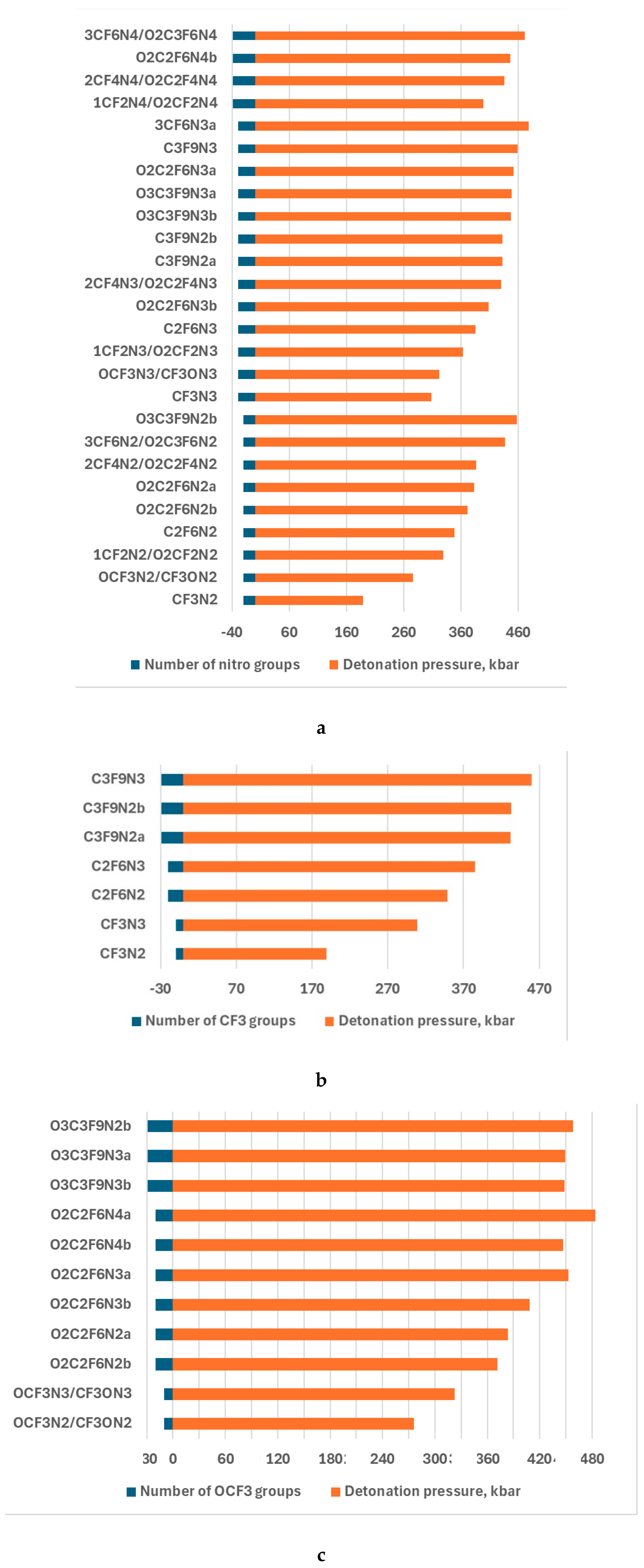
| Compounds | v,km/s | p,kba |
|---|---|---|
| CF3N2 | 7.01 | 189.13 |
| CF3N3 | 7.46 | 308.45 |
| C2F6N2 | 7.31 | 348.83 |
| C2F6N3 | 7.96 | 385.51 |
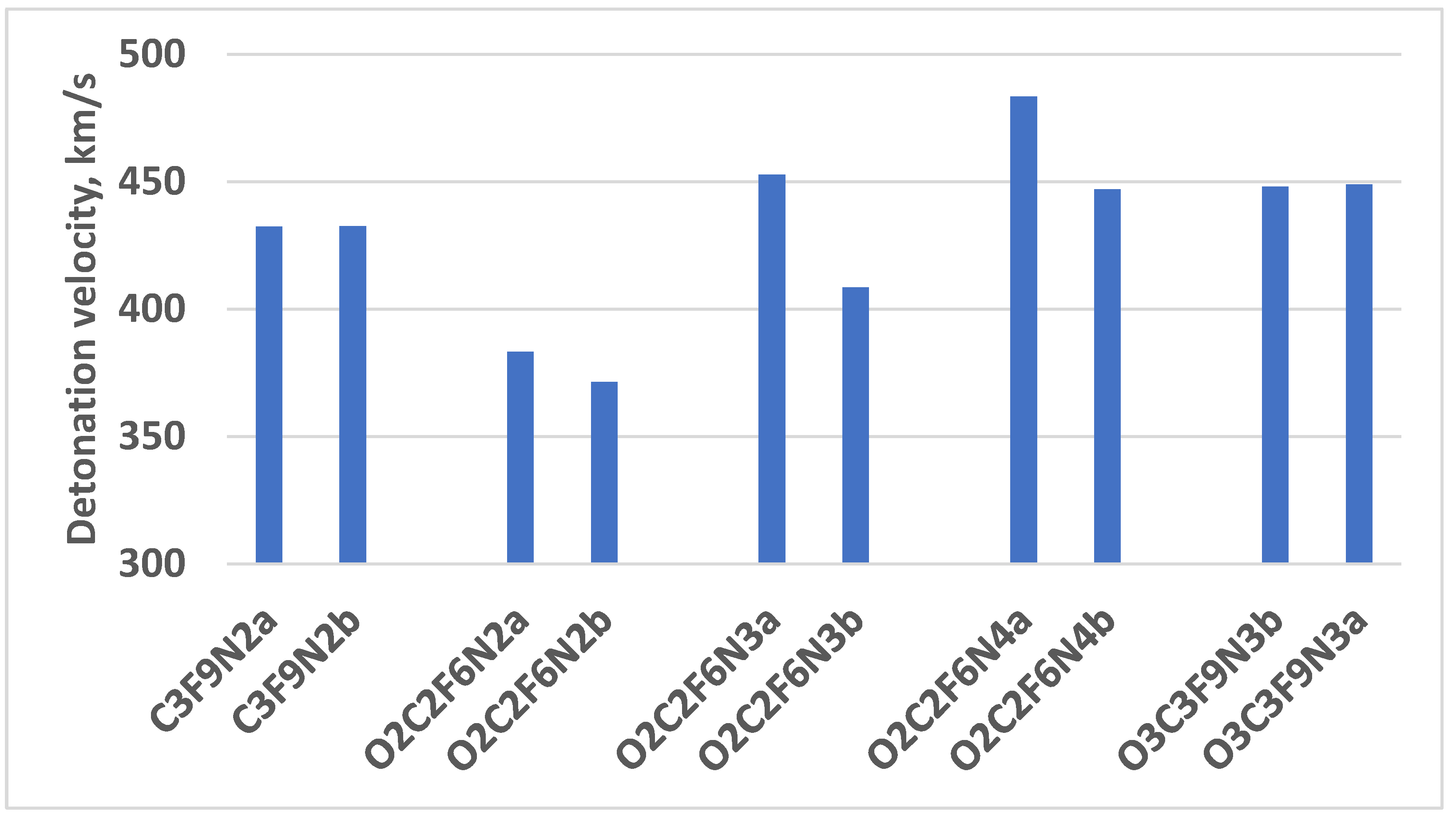
| C3F9N2a | 7.83 | 432.39 |
| C3F9N2b | 7.84 | 432.63 |
| C3F9N3 | 8.40 | 459.75 |
| OCF3N2/CF3ON2 | 6.91 | 276.32 |
| OCF3N3/CF3ON3 | 7.63 | 322.50 |
| O2C2F6N2a | 7.66 | 383.27 |
| O2C2F6N2b | 7.66 | 371.38 |
| O3C3F9N2b | 8.27 | 458.16 |
| O2C2F6N3a | 8.26 | 452.77 |
| O2C2F6N3b | 8.26 | 408.54 |
| O2C2F6N4a | 8.82 | 483.42 |
| O2C2F6N4b | 8.82 | 447.03 |
| O3C3F9N3b | 8.78 | 448.03 |
| O3C3F9N3a | 8.78 | 449.03 |
| 1CF2N2/O2CF2N2 | 7.34 | 329.08 |
| 1CF2N3/O2CF2N3 | 8.16 | 363.76 |
| 1CF2N4/O2CF2N4 | 8.55 | 400.03 |
| 2CF4N2/O2C2F4N2 | 7.69 | 386.62 |
| 2CF4N3/O2C2F4N3 | 8.36 | 430.63 |
| 2CF4N4//O2C2F4N4 | 8.70 | 436.06 |
| 3CF6N2/O2C3F6N2 | 7.97 | 437.87 |
| 3CF6N3 | 8.60 | 478.82 |
| 3CF6N4/O2C3F6N4 | 8.84 | 471.75 |
| TNT | 6.74 | 172.02 |
| APATO | 7.67 | 253.06 |
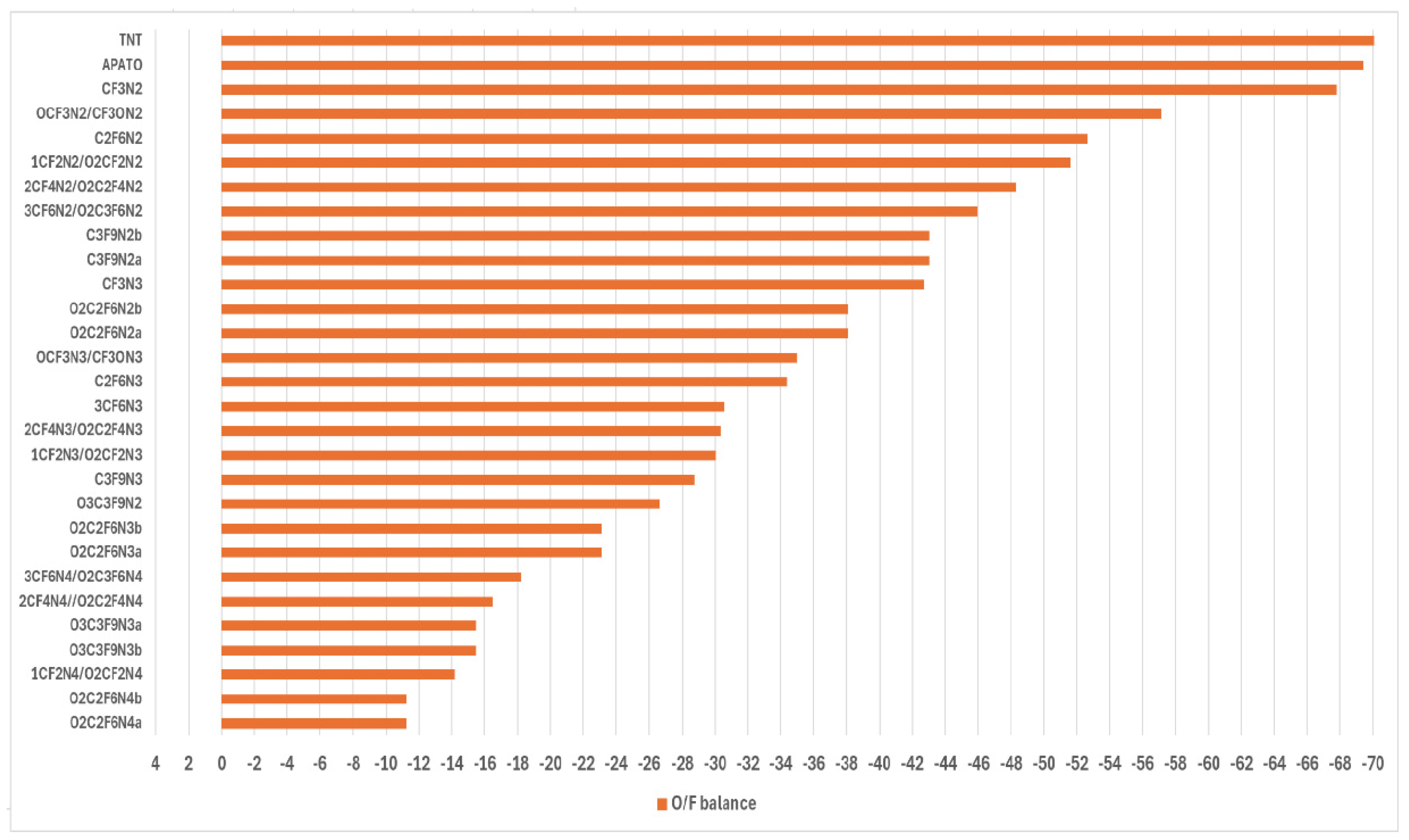
4. Discussion
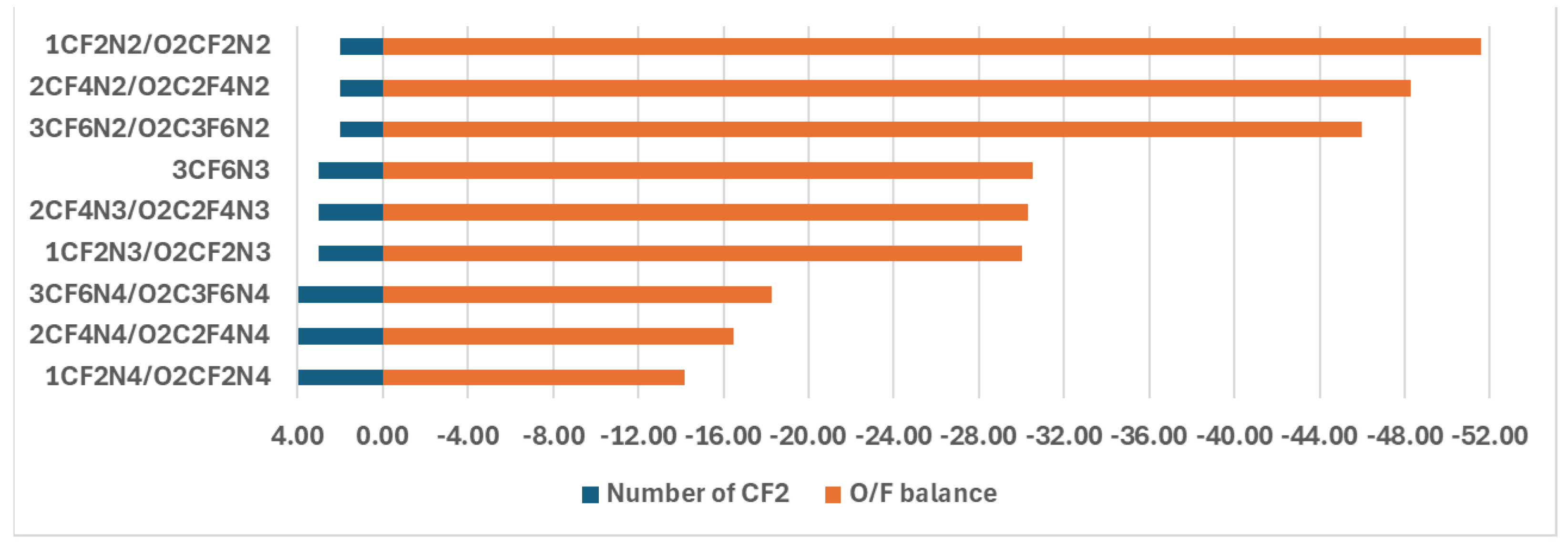
| N | m | OnCnF3nNm | CnF3nNm |
|---|---|---|---|
| 1 | -57.12 | -67.77 | |
| 2 | 2 | -38.08 | -52.61 |
| 3 | -26.66 | -43.00 | |
| 1 | -35.00 | -42.69 | |
| 2 | 3 | -23.09 | -34.37 |
| 3 | -15.48 | -28.77 |
| Compounds | Conformer a | Conformer, b | |
| cm3/mol | cm3/mol | ||
| C3F9N2 | 177.79 | 190.26 | |
| O2C2F6N2 | 167.02 | 174.62 | |
| O2C2F6N3 | 203.04 | 189.67 | |
| O2C2F6N4 | 166.04 | 198.60 | |
| O3C3F9N3 | 217.75 | 226.03 | |
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Structural formula | Compound Abbreviation |
|---|---|---|
| 1. | 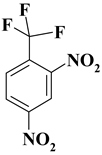 |
CF3N2 |
| 2. | 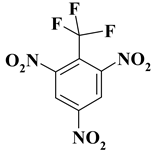 |
CF3N3 |
| 3. | 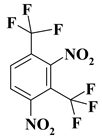 |
C2F6N2 |
| 4. | 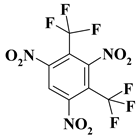 |
C2F6N3 |
| 5. | 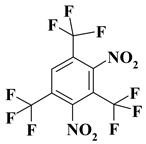 |
C3F9N2 |
| 6. | 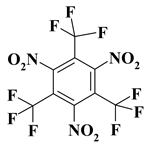 |
C3F9N3 |
| 7. | 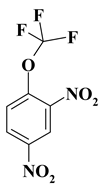 |
CF3ON2/OCF3N2 |
| 8. | 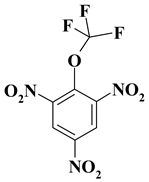 |
CF3ON3/OCF3N3 |
| 9. | 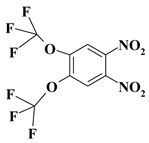 |
O2C2F6N2 |
| 10. | 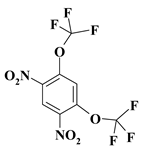 |
O2C2F6N2b/m |
| 11. | 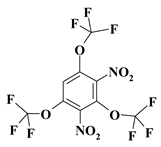 |
O3C3F9N2a |
| 12. | 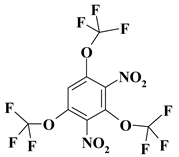 |
O3C3F9N2b |
| 13. | 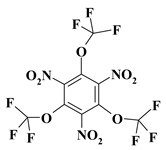 |
O3C3F9N3a |
| 14. | 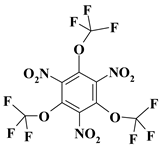 |
O3C3F9N3b |
| 15. | 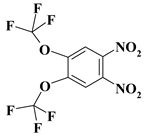 |
O2C2F6N2a |
| 16. | 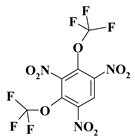 |
O2C2F6N2b |
| 17. | 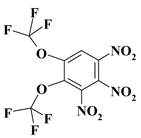 |
O2C2F6N3 |
| 18. | 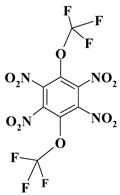 |
O2C2F6N4 |
| 19. | 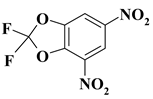 |
1CF2N2/O2CF2N2 |
| 20. | 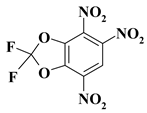 |
1CF2N3/O2CF2N3 |
| 21. | 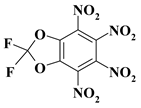 |
1CF2N4/O2CF2N4 |
| 22. |  |
2CF4N2/O2C2F4N2 |
| 23. |  |
2CF4N3/O2C2F4N3 |
| 24. |  |
2CF4N4/O2C2F4N4 |
Appendix B
| Compound | Conformer | ||
| a | B | ||
| C3F6N2 |  |
 |
|
| O2C2F6N2 |  |
 |
|
| O2C2F6N3 |  |
 |
|
| O2C2F6N4 |  |
 |
|
| O3C3F9N3 |  |
 |
|
References
- Koch, E.C. High Explosives, Propellants, Pyrotechnics; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2021; pp. 1-759. ISBN: 978-3-11-066052-4.
- Agrawal, J.P. High Energy Materials: Propellants, Explosives and Pyrotechnics. Wiley-VCH: Weinheim, Germany, 2010; pp.1-498. ISBN: 978-3-527-32610-5.
- Olah, G.A.; Squire, D.R. (Eds.). Chemistry of Energetic Materials; Academic Press: Cambridge, MA, US, 2012; pp. 1-212. ISBN: 978-0-1239-5897-6.
- Kominia, A.; Smith, J.L.; Sheehan, P.; Oxley, J.C. Thermal decomposition of fluorinated polymers used in plasticized explosives and munitions. Propellants, Explos. Pyrotech. 2024, 49, e202300207. [Google Scholar] [CrossRef]
- Grakauskas, V.; Baum, K. Aqueous fluorination of nitronate salts. J. Org. Chem. 1968, 33, 3080–3082. [Google Scholar] [CrossRef]
- Grakauskas, V. Direct liquid-phase fluorination of methyl trichloroacetate and acetic anhydride. J. Org. Chem. 1969, 34, 963–965. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Adolph, H.G. Fluoronitro aliphatics. II. Fluorodinitromethyl compounds. Synthetic approaches and general properties. J. Org. Chem. 1968, 33, 3073–3080. [Google Scholar] [CrossRef]
- Adolph, H.G.; Kamlet, M.J. Fluoronitroaliphatics. IV. Reactions of 2-fluoro-2, 2-dinitroethanol. J. Org. Chem. 1969, 34, 45–50. [Google Scholar] [CrossRef]
- Grakauskas, V.; Albert, A.H. Polynitroalkyltetrazoles. J. Het. Chem. 1981, 18, 1477–1479. [Google Scholar] [CrossRef]
- Pepekin, V.I.; Matyushin, Y.N.; Rozantsev, G.G.; Shevelev, S.A.; Apin, A.Y. Enthalpies of formation of dinitrophenylmethane, trinitrophenylethane, and fluorodinitrophenylmethane. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1972, 21, 2634–2636. [Google Scholar]
- Pepekin, V.I., Natsibullin, F.Y., Eremenko, L.T., Lebedev, Y.A. Enthalpy of formation of fluoronitromethyl radical and dissociation energy of CH and CF bonds in fluorodinitromethane. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1974, 23, 892-893.
- Hurwitz, H. Computation of detonation properties of fluoroexplosives. Technical Report DTIC NOLTR 65-217, Naval Ordnance Laboratory, White Oak, ML, USA. 1966, pp. 1-54. URL: https://apps.dtic.mil/sti/citations/AD0374171 , accessed at 30-05-2024.
- Bogdanova,Yu.A.; Gubin, S.A.; Korsunskii, B.L.; Pepekin, V.I. Detonation Characteristics of Powerful Insensitive Explosives. Combust. Explos. Shock Waves. 2009, 45, 738 –743.
- Politzer, P.; Lane, P. Structures and Properties of Energetic Difluoramines. In: Hargittai, M.;Hargittai, I. Advances in Molecular Structure Research, Vol.3, 1997, pp.269-285. ISBN: 978-0-7623-0208-6.
- Dalinger, I.L.; Kormanov, A. V.; Suponitsky, K.Y.; Muravyev, N.V.; Sheremetev, A.B. Pyrazole–tetrazole hybrid with trinitromethyl, fluorodinitromethyl, or (difluoroamino) dinitromethyl groups: High-performance energetic materials. Chem. Asian J. 2018, 13, 1165–1172. [Google Scholar] [CrossRef]
- Palysaeva, N.V.; Gladyshkin, A.G.; Vatsadze, I.A.; Suponitsky, K.Y.; Dmitriev, D.E.; Sheremetev, A.B. N-(2-Fluoro-2, 2-dinitroethyl) azoles: a novel assembly of diverse explosophoric building blocks for energetic compound design. Org. Chem. Front. 2019, 6, 249–255. [Google Scholar] [CrossRef]
- Kettner, M.A.; Klapötke, T.M. New energetic polynitrotetrazoles. Chem. Eur. J. 2015, 21, 3755–3765. [Google Scholar] [CrossRef]
- Klapötke, T.M.; Krumm, B.; Moll, R.; Rest, S.F.; Schnick, W.; Seibald, M. Asymmetric fluorodinitromethyl derivatives of 2, 2, 2-trinitroethyl N-(2, 2, 2-trinitroethyl) carbamate. J. Fluor. Chem. 2013, 156, 253–261. [Google Scholar] [CrossRef]
- Zhai, L.; Zhang, J.; Wu, M.; Huo, H.; Bi, F.; Wang, B. Balancing good oxygen balance and high heat of formation by incorporating of-C (NO2) 2F Moiety and Tetrazole into Furoxan block. J. Mol. Struct. 2020, 1222, 128934. [Google Scholar] [CrossRef]
- Zhai, L.; Wang, B.; Xu, K.; Huo, H.; Liu, N.; Li, Y.; Li, H.; Lian, P.; Fan, X. A new synthetic route for 3, 3′-bis (fluorodinitromethyl) difurazanyl ether (FOF-13) and its energetic properties. J. Energ. Mater. 2016, 34, 92–102. [Google Scholar] [CrossRef]
- Sheremetev, A.B. 3, 3-Bis(1-fluoro-1, 1-dinitromethyl)difurazanyl ether.In Proc. 29th Int. Ann. Conf. ICT, Karlsruhe, Germany. June 30-July 3. 1998, 58, 1-6.
- Dalinger, I.L.; Vinogradov, V.M.; Shevelev, S.Y.A.; Kuz’min, V.S.; Arnautova, E.A.; Pivina, T.Y.S. Synthesis and Calculation of Properties of N-Difluoroaminoazoles, the Novel Type of Energetic Materials. Propellants Explos. Pyrotech. 1998, 23, 212–217. [Google Scholar] [CrossRef]
- Chapman, R.D. Organic difluoramine derivatives. In: Klapötke, T. M. (Ed.). High energy density materials. Springer Verlag, Berlin Heidelberg. Struct. Bond., Vol. 125. 2007, pp.123–151.
- Reddy, V.P. Synthetic methods for high-energy Organofluorine Compounds. In: Energetic Materials. Advanced Processing Technologies for Next-Generation Materials. Eds. Mezger, M.J.; Tindle, K.J.; Pantoya, M.; Groven, L.J.; Kalyon, D.M. Boca Raton, FL., Taylor&Francis, CRC Press, 2017, pp. 1-19. ISBN 978-1-3516-8125-4.
- Wu, Q.; Li, Q.; Yan, G.; Zhang, Z.; Zhu, W. Molecular design of novel super high energy compounds by incorporating the difluoramino group, N-oxide and different bridge groups into the 1H-tetrazole. J. Fluor. Chem. 2019, 218, 21–26. [Google Scholar] [CrossRef]
- Oxley, J.C.; Smith, J.L.; Zhang, J.; Bedford, C. A comparison of the thermal decomposition of nitramines and difluoramines. J. Phys. Chem. A. 2001, 105, 579–590. [Google Scholar] [CrossRef]
- Ye, C.; Gao, H.; Shreeve, J.M. Synthesis and thermochemical properties of NF2-containing energetic salts. J. Fluor. Chem. 2007, 128, 1410–1415. [Google Scholar] [CrossRef]
- Dalinger, I.L.; Shkineva, T.K.; Sheremetev, A.B. Ethyl butyrates bearing nitro and difluoroamino groups. Mendeleev Commun. 2023, 33, 841–843. [Google Scholar] [CrossRef]
- Ugrak, B.I., Shkineva, T.K., Sheremetev, A.B., Dalinger, I.L. (Difluoroamino) furazans. Russ. Chem. Bull. 2023, 72, 2706-2716.
- Muravyev, N.V., Fershtat, L., Zhang, Q. Synthesis, design and development of energetic materials: Quo Vadis?. J. Chem. Eng. 2024, 150410. [CrossRef]
- Muravyev, N.V.; Meerov, D.B.; Monogarov, K.A.; Melnikov, I.N.; Kosareva, E.K.; Fershtat, L.L.; Sheremetev, A.B.; Dalinger, I.L.; Fomenkov, I.V.; Pivkina, A.N. Sensitivity of energetic materials: Evidence of thermodynamic factor on a large array of CHNOFCl compounds. J. Chem. Eng. 2021, 421, 129804. [Google Scholar] [CrossRef]
- Sitzmann, M.E.; Gilligan, W.H.; Ornellas, D.L.; Thrasher, J.S. Polynitroaliphatic explosives containing the pentafluorosulfanyl (SF5) group: The selection and study of a model compound. J. Energ. Mater. 1990, 8, 352–374. [Google Scholar] [CrossRef]
- Martinez, H.; Zheng, Z.; Dolbier Jr, W.R. Energetic materials containing fluorine. Design, synthesis and testing of furazan-containing energetic materials bearing a pentafluorosulfanyl group. J. Fluor. Chem. 2012, 143, 112–122. [Google Scholar] [CrossRef]
- Gao, H.; Ye, C.; Winter, R.W.; Gard, G.L.; Sitzmann, M.E.; Shreeve, J.N.M. Pentafluorosulfanyl (SF5) containing energetic salts. Eur. J. Inorg. Chem. 2006, 16, 3221–3226. [Google Scholar] [CrossRef]
- Xiao-Hong, L.; Hong-Ling, C.; Wei-Wei, J.; Tong-Wei, L.; Rui-Zhou, Z.; Yong-Liang, Y. Theoretical studies on energetic materials bearing pentaflurosulphyl (SF 5) groups. J. Chem. Sci. 2014, 126, 1163–1172. [Google Scholar]
- Sitzmann, M.E.; Gilardi, R.D. Polynitroalkyl derivatives of SF5N CCl2: nitrations of SF5 imines. J. Fluor. Chem. 1993, 63, 203–215. [Google Scholar] [CrossRef]
- Li, C.; Liu, M.; Li, T.; Wang, L.; Zhang, R.; Jing, S. Fluorine Added to Lead the Way to Future Energetic Materials: 3, 5-difluoro-2, 4, 6-trinitroaniline. J. Energ. Mater. 2022, 1–10. [Google Scholar] [CrossRef]
- Zhu, J.; Li, C.; Jing, S.; Yang, L.; Liu, Y.; Zhang, W.; Zhang, J. 3,5-difluoro-2,4,6-trinitrophenol: A high-energy compound born under the “NO2FNO2” construction strategy. Propellants Explos. Pyrotech. 2024, 49(2), e202300184. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Zhou, J.; Bi, F.; Wang, B. Effect of fluoro substituents on polynitroarylenes: design, synthesis and theoretical studies of fluorinated nitrotoluenes. ChemPlusChem 2019, 84, 92–97. [Google Scholar] [CrossRef]
- Hu, F.; Wang, L.J.; Zhao, W.; Liu, Y.C.; Jing, S.M.; Liu, P.; He, J.X. Thermal Decomposition Kinetics and Compatibility of 3, 5-difluoro-2, 4, 6-trinitroanisole (DFTNAN). Materials 2021, 14, 4186. [Google Scholar] [CrossRef]
- Jing, S.; Jiang, Z.; Jiao, Q.; Li, Z.; Liu, Y.; Yang, L. 3, 5-difluoro-2, 4, 6-trinitroanisole: Promising melt-cast insensitive explosives instead of TNT. J. Energ. Mater. 2022, 40, 206–217. [Google Scholar] [CrossRef]
- Jiao, Q.; Li, T.; Ou, Y.; Jing, S.; Wang, F. Probing the Reaction Mechanisms of 3,5-Difluoro-2,4,6-Trinitroanisole (DFTNAN) through a Comparative Study with Trinitroanisole (TNAN). Materials, 2022, 15(7), 2568. [CrossRef]
- Li, Y.; Xue, M.; Sun, B.; Tu, Z.; Wang, X. Regulating the melting point by non-covalent interaction toward a promising insensitive melt-castable energetic material: 1, 2-Difluoro-4, 5-dinitrobenzene. Chinese J. Struc. Chem. 2023, 42(4), 100002. [Google Scholar] [CrossRef]
- Guo, Z.; Yu, Q.; Chen, Y.; Liu, J.; Li, T.; Peng, Y.; Yi, W. Fluorine-Containing Functional Group-Based Energetic Materials. Chem. Rec., 2023, 23(9), e202300108. [CrossRef]
- Fokin, A.V.; Studnev, Y.N.; Rapkin, A.I.; Komarov, V.A.; Verenikin, O.V.; Potarina, T.M. Synthesis and some properties of 5-fluorodinitromethyl- and 5-difluoronitromethyltetrazoles. Izv. Akad. Nauk SSSR Ser. Khim. 1981, 7:1592–1595.
- Chapman, R. D. Halogenated explosives to defeat biological agents. Defense Threat Reduction Agency. Tech. Report DTRA-IR-14-81. 2015 Sept., pp.1-37.
- Muravyev, N.V.; Suponitsky, K.Y.; Fedyanin, I.V.; Fomenkov, I.V.; Pivkina, A.N.; Dalinger, I.L. Bis-(2-difluoroamino-2, 2-dinitroethyl) nitramine–Energetic oxidizer and high explosive. Chem. Eng. 2022, 449, 137816. [Google Scholar] [CrossRef]
- Zhai, L.; Zhang, J.; Zhang, J.; Wu, M.; Bi, F.; Wang, B. Progress in Synthesis and Properties of High Energy Density Compounds Regulated by N—F Bond. Chin. J. Org. Chem. 2020, 40, 1484. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Adolph, H.G. Some comments regarding the sensitivities, thermal stabilities, and explosive performance characteristics of fluorodinitromethyl compounds. In Proc. Seventh Internat. Symp. Detonation, Annapolis, Maryland, USA, Jun. 16-19, 1981, pp.84-92.
- Warner D.A. Bulk synthesis of fluoroexplosives. Tech. Report AFATL-TR-67-154/ DTIC AD0501781. Defense Technical Information Center, Eglin AFB, FL, USA, Oct. 1967, pp.1-35. URL: https://archive.org/details/DTIC_AD0501781, accessed at 30-05-2024.
- Kumar, A.S.; Kommu, N.; Ghule, V.D.; Sahoo, A.K. Synthesis of trifluoromethyl-substituted N-aryl-poly-1, 2, 3-triazole derivatives. J. Mater. Chem. A. 2014, 2, 7917–7926. [Google Scholar] [CrossRef]
- Yan, Z.; Lu, T.; Liu, Y.; Liu, W.; Zhao, B.; Wang, Y.; Ge, Z. High thermal stability and insensitive fused triazole-triazine trifluoromethyl-containing explosives (TFX). ACS Omega 2021, 6, 18591–18597. [Google Scholar] [CrossRef]
- Chinnam, A.K.; Staples, R.J.; Shreeve, J.N.M. Selective Synthesis of Bis (3-(3-(trifluoromethyl)-1 H-1, 2, 4-triazol-5-yl)-4, 4′-azo-and-azoxyfurazan Derivatives. J. Org. Chem. 2021, 86, 7781–7786. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Z.; Meng, Z.; Zhai, L. A Novel Synthesis, Characterization and Performances of 1, 3, 5-Trinitro-2, 2-bis (trifluoromethyl)-1, 3, 5-triazinane. ChemistrySelect 2019, 4, 6338–6341. [Google Scholar] [CrossRef]
- Liu, Q.; Yuan, M.; He, J.; Yu, P.; Guo, X.; Liu, Y.; Gao, H.; Yin, P. Exchanging of NH2/NHNH2/NHOH groups: An effective strategy for balancing the energy and safety of fused-ring energetic materials. J. Chem. Eng. 2023, 466, 143333. [Google Scholar] [CrossRef]
- Tamuliene, J.; Sarlauskas, J. Impact of Incremental Methylene Groups on the Energetic Properties of Aromatic Nitramines. Energies, 2023,. 16, 3117. [CrossRef]
- Parthasarathi, R.; Padmanabhan, J.; Subramanian, V.; Maiti, B.; Chattaraj, P.K. Toxicity analysis of 3,3’,4,4’,5-pentachloro biphenyl through chemical reactivity and selectivity profiles. Curr. Sci. 2004, 86, 535–542. Available online: https://www.jstor.org/stable/24107906 (accessed on 10 June 2024).
- Kaya, S.; Kaya, C. New equation based on ionization energies and electron affinities of atoms for calculating of group electronegativity. Comput. Theor. Chem. 2015, 1052, 42–46. [Google Scholar] [CrossRef]
- Becke, A.D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Dunning Jr., T. H, Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A.; Bloino, J.; Janesko, B.G.; Gomperts, R.; Mennucci, B.; Hratchian, H.P.; Ortiz, J.V.; Izmaylov, A.F.; Sonnenberg, J.L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V.G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehar, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J.A.Jr., Peralta, J.E.; Ogliaro, F.; Bearpark, M.; Heyd, J.J.; Brothers, E.; Kudin, K.N.; Staroverov, V.N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J.C.; Iyengar, S.S.; Tomasi, J.; Cossi, M.; Millam, J.M.;, Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J.W.; Martin, R.L.; Morokuma, K.; Farkas, O.; Foresman, J.B., Fox, D.J. Gaussian, Inc., Wallingford CT. 2016, pp. 1-139. URL: https://www.cwu.edu/chemistry/sites/cts.cwu.edu.chemistry/files/documents/Gaussian_09_ReferenceManual.pdf (accessed: 26.05.2024).
- Cardia, R.; Malloci, G.; Mattoni, A.; Cappellini, G. Effects of TIPS-Functionalization and Perhalogenation on the Electronic, Optical, and Transport Properties of Angular and Compact Dibenzochrysene. J. Phys. Chem. A. 2014, 118, 5170–5177. [Google Scholar] [CrossRef]
- Cardia, R.; Malloci, G.; Rignanese, G.M.; Blasé, X.; Molteni, E.; Cappellini, G. Electronic and optical properties of hexathiapentacene in the gas and crystal phases. Phys. Rev. B. 2016, 93, 235132. [Google Scholar] [CrossRef]
- Dardenne, N.; Cardia, R.; Li, J.; Malloci, G.; Cappellini, G.; Blasé, X.; Charlier, J.C.; Rignanese, G. Tuning Optical Properties of Dibenzochrysenes by Functionalization: A Many-Body Perturbation Theory Study. Phys. Chem. C. 2017, 121, 24480–24488. [Google Scholar] [CrossRef]
- Antidormi, A.; Aprile, G.; Cappellini, G.; Cara, E.; Cardia, R.; Colombo, L.; Farris, R.; d’Ischia, M.; Mehrabanian, M.; Melis, C.; Mula, G.; Pezzella, A.; Pinna, E.; Riva, E.R. Physical and Chemical Control of In-terface Stability in Porous Si–Eumelanin Hybrids. J. Phys. Chem. C. 2018, 122, 28405–28415. [Google Scholar] [CrossRef]
- Mocci, P.; Cardia, R.; Cappellini, G. Inclusions of Si-atoms in Graphene nanostructures: A computational study on the ground-state electronic properties of Coronene and Ovalene. J. Phys. Conf. Ser. 2018, 956, 012020. [Google Scholar] [CrossRef]
- Mocci, P.; Cardia, R.; Cappellini, G. Si-atoms substitutions effects on the electronic and optical properties of coronene and ovalene. New J. Phys. 2018, 20, 113008. [Google Scholar] [CrossRef]
- Kumar, A.; Cardia, R.; Cappellini, G. Electronic and optical properties of chromophores from bacterial cellulose. Cellulose. 2018, 25, 2191–2203. [Google Scholar] [CrossRef]
- Szafran, M.; Koput, J. Ab initio and DFT calculations of structure and vibrational spectra of pyridine and its isotopomers. J. Mol. Struct. 2001, 565, 439–448. [Google Scholar] [CrossRef]
- Begue, D.; Carbonniere, P.; Pouchan, C. Calculations of Vibrational Energy Levels by Using a Hybrid ab Initio and DFT Quartic Force Field: Application to Acetonitrile. J. Phys. Chem. A. 2005, 109, 4611–4616. [Google Scholar] [CrossRef]
- Cooper, P.W. Explosives Engineering; Wiley-VCH: New York, USA. 1996; pp.1-480. ISBN: 0-471-18636-8.
- Shevchenko, A.A.; Dolgoborodov, A.Yu.; Brazhnikov, M.A.; Kirilenko, V.G. Pseudoideal detonation of mechanoactivated mixtures of ammonium perchlorate with nanoaluminum. J. Phys. Conf. Ser. (IOP Publ.) 2018, 946, 012055. [Google Scholar] [CrossRef]
- Kozak, G.D. Measurement and calculation of the ideal detonation velocity for liquid nitrocompounds. Combust. Explos. Shock Waves. 1998, 34, 581–586. [Google Scholar] [CrossRef]
- Bolton, O.; Simke, L.R.; Pagoria, P.F.; Matzger, A.J. High Power Explosive with Good Sensitivity: A 2:1 Cocrystal of CL-20:HMX. Cryst. Growth Des. 2012, 12, 4311–4314. [Google Scholar] [CrossRef]
- Viswanath, D.S.; Ghosh, T.K.; Boddu, V.M. 5-Nitro-2,4-dihydro-3H-1,2,4-Triazole-3-one (NTO). In Emerging Energetic Materials: Synthesis, Physicochemical, and Detonation Properties. Springer: Dordrecht, The Netherlands. 2018; pp. 163–211.
- Rajakumar, B.; Arathala, P.; Muthiah, B. Thermal Decomposition of 2-Methyltetrahydrofuran behind Reflected Shock Waves over the Temperature Range of 1179–1361 K. J. Phys. Chem. A. 2021, 125, 5406–54223. [Google Scholar]
- Šarlauskas, J.;Tamulienė, J. Preparation and Characterization of Cationic Energetic Salts of 5-Amino-3-[(2,4,6-trinitrophenyl)amino]-1H-1,2,4-triazole (APATO). Central European Journal of Energetic Materials. 2022, 19(3), 311–325. [CrossRef]
- Free Chemical Drawing Software. ChemSketch. Version 10.0. ACD/Labs. Available online: https://www.acdlabs.com/resources/free-chemistry-software-apps/chemsketch-freeware/ (accessed on 30 January 2023).
- Kaushik, M. A review of innovative chemical drawing and spectra prediction computer software. Mediterr. J. Chem. 2014, 3, 759–766. [Google Scholar] [CrossRef]
- Wen, L.; Wang, B.; Yu, T.; Lai, W.; Shi, J.; Liu, M.; Liu, Y. Accelerating the search of CHONF-containing highly energetic materials by combinatorial library design and high-throughput screening. Fuel 2022, 310, 122241. [Google Scholar] [CrossRef]
- Keshavarz, M.H.; Zamani, A. A simple and reliable method for predicting the detonation velocity of CHNOFCl and aluminized explosives. Cent. Eur. Energ. Mater. 2015, 12, 13–33. [Google Scholar]
- Keshavarz, M.H.; Pouretedal, H.R. An empirical method for predicting detonation pressure of CHNOFCl explosives. Thermochim. Acta 2004, 414, 203–208. [Google Scholar]
- Urizar, M.J.; James, E., Jr.; Smith, L.C. Detonation velocity of pressed TNT. Phys. Fluids. 1961, 4, 262–274. [Google Scholar] [CrossRef]
- Chandler, J.; Ferguson, R.E.; Forbes, J.; Kuhl, A.L.; Oppenheim, A.K.; Spektor, R. Confined combustion of TNT explosion products in air. In Proc. Conf. 8th international Colloquium on Dust Explosions, Schaumburg, IL, US. Tech. Rep. UCRL-JC-131748; ON: DE00003648; OSTI ID; 3648, Sept. 21-25, 1998. Accessed at URL: https://www.osti.gov/biblio/3648 (on 03-07-2024).
- Yang, J.; Bai, T.; Guan, J.; Li, M.; Zhen, Z.; Dong, X.; Wang, Y.; Wang, Y. Novel fluorine-containing energetic materials: how potential are they? A computational study of detonation performance. J. Mol. Model. 2023, 29(8), p.228. [CrossRef]
- Kang, Y.; Dong, Y.; Liu, Y.; Gao, H.; Wang, Y.; Shreeve, J.M. Halogen bonding (CF··· X) and its effect on creating ideal insensitive energetic materials. J. Chem. Eng. 2022, 440, 135969. [Google Scholar] [CrossRef]
- Wu, W.; You, Y.; Weng, Z. Recent advances in the synthesis of fluoroalkylated compounds using fluoroalkyl anhydrides. Chin. Chem. Lett. 2022, 33, 4517–4530. [Google Scholar] [CrossRef]
- Balachandar, K.G.; Thangamani, A. Novel high performance energetic materials of fluorine-containing 2, 6-dinitro-4-(trifluoromethyl) phenol derivatives with substituted azoles. J. Fluor. Chem. 2021, 247, 109801. [Google Scholar] [CrossRef]
- Koch, E.C.; Contini, A. Metal Fluorocarbon Pyrolants, XVI: Theoretical and Experimental Investigation of Poly [bis (2, 2, 2-trifluoroethoxy) phosphazene] (PTFEP) as Oxidizer in Magnesium Based Pyrolants. Propellants Explos. Pyrotech. 2014, 39, 761–767. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





