Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potential life-saving form of adoptive immunotherapy for both malignant and non-malignant disorders [
1]. The hematopoietic stem cells are obtained from bone marrow, peripheral blood, or umbilical cord blood of suitable donors who are carefully selected by the transplant physicians according to patient, disease and donor characteristics.
Identifying the appropriate donor is critical for the outcome of HSCT and the final choice of the donor relies on several factors, with the primary determinant in donor being human leukocyte antigen (HLA) patient-donor matching [2, 3] and age thereafter [4-6]. HLA-identical sibling donors represent the first choice and further donor options are considered in patients who do not have such a related donor: indeed the inclusion of matched unrelated donors (MUDs), HLA-haploidentical related donors, and umbilical cord blood has increased the donor availability over time and nowadays the majority of HSCTs are performed from donors other than HLA-identical sibling [
1]. Additional factors such as donors’ age, gender, ABO matching, and CMV serostatus must be considered to ensure compatibility and minimize potential complications [3, 7].
However, there is currently a debate on the definition of the best stem cell donor when multiple suitable options exist. As an example, some reports suggest that a young unrelated donor might be preferred over an older HLA-identical sibling [8, 9] or that a haploidentical related donor is comparable to matched or even mismatched unrelated one [
10] in terms of expected patient outcome after HSCT. Moreover, other donor-specific or patient-donor variables such as AB0-matching, gender, parity for female donors, Cytomegalovirus serostatus are considered for the best donor selection but a defined hierarchy still lacks [4, 11-14]. Nonetheless, these are all significant elements that concur to patients’ outcome after HSCT.
To address this issue, we here developed a calculator able to provide the 2-year patients’ overall survival (OS) associated to each of the potential donor options that physicians face during the selection process. All the available local, own real data have been used to create predictive models that took into account the main patient, disease, transplant and donor characteristics, focusing on these latter. By using those real data from n=737 HSCTs performed at two transplant centers, the tool allowed to provide estimations associated with each donor type and therefore to support the transplant physician during the difficult process of stem cell donor selection.
Materials and Methods
Data Retrieval
We conducted a retrospective analysis on real-world data obtained from clinical electronic charts of two transplant centers (Istituto Clinico Humanitas, Rozzano, Italy and Azienda Ospedaliera Vittorio Emanuele, Catania, Italy). The study was conducted on adult HSCTs performed between 2010 and 2022 by these two centers, who provided data and allowed for the creation of a unique database including patients’ variables, disease characteristics, transplant procedures, donor variables, and post-transplant outcomes. All these data are regularly provided by each transplant center active in Europe to the EBMT registry after informed consent obtained from the patients. The variables were divided in three time groups: at day 0 (patients’ variables, disease characteristics, transplant procedures, donor variables), day +100 (post-transplant outcomes) and annual follow-up (post-transplant outcomes). Only first HSCTs were considered, transplantations with multiple donors were excluded as well as those HSCT that were part of a planned multiple graft protocol. Day 0 requested variables were as follows: patient’s age and gender, date of initial diagnosis, primary disease at diagnosis, disease status at HSCT, comorbidity index, patient CMV status, HLA match, degree of mismatch, donor age and gender, donor CMV status, source of stem cells, graft manipulation, intensity of conditioning regimen, graft-versus-host disease (GvHD) prophylaxis. Day +100 requested variables were: early graft loss, acute GvHD date of diagnosis and maximum extent, chronic GvHD date of diagnosis and maximum extent, first relapse or progression and date, survival status, main cause of death. Requested variables for annual follow-up were: acute GvHD date of diagnosis and maximum extent, chronic GvHD date of diagnosis and maximum extent, late graft failure, first relapse or progression and date, survival status, main cause of death.
Statistical Analysis
We developed a calculator, using real-world data from these two transplant centers, to estimate the 2-year overall survival (OS) for each potential donor during the selection process, by investigating the significant factors influencing survival following HSCT. Parametric survival analysis was used to assess the relationship between the several covariates and the 2y-OS. Based on Anderson-Darling statistics, the Weibull distribution was selected as the most appropriate model for our data. This model enabled to evaluate the impact of different variables on survival duration following HSCT. Both main effects and interactions were considered. The categorical variables assessed included diagnosis, HLA match, and the presence of comorbidities. The continuous variables assessed were the age of patients and donors. Lastly, we evaluated interaction terms as patients’ age with diagnosis, patients’ age with HLA match, and donors’ age with HLA match. Statistical significance was defined as p value < 0.05, with 95% confidence interval. Minitab was used for statistical analyses (
https://www.minitab.com/en-us/).
Calculator Output
The present calculator is intended to be a predictive tool to estimate the 2y-OS and the respective 95% confidence interval of each single patient according to a pre-selected stem cell donor. It utilizes a modelled database containing patient and donor information alongside post-transplant outcomes obtained from real-world, local clinical experience. By filling the data from multiple donor options (i.e. an older HLA-identical sibling vs. a younger unrelated donor or an unrelated donor vs. a haploidentical) during the search, the tool is expected to provide the patient’s 2-y OS associated with each of these donors, thus supporting the selection of the best donor for that specific patient according to the center’s experience. A significant overlap between two or more donors may indicate that they are comparable in terms of patient’s post-transplant survival, however the calculator is intended to be a tool to support decision but not a decision-maker since the final choice will depend on multiple and somehow complex factors.
Results
Main Patient, Transplant and Donor Characteristics
The database originally contained n=851 HSCTs reflecting the inclusion criteria. N=114 were removed due to missing data, leading to n=737 patients transplanted between July 2010 to January 2022 at the two above cited transplant centers. N=285 items were available for each patient. HSCTs from HLA-mismatched unrelated donors and cord blood units were excluded due to their limited numbers not allowing a meaningful statistical analysis. The median patient age at HSCT was 48, while it was 40 for donors. Male patients were n=431 (58.5%) and female patients n=306 (41.5%). A total of n=218 HSCTs were performed from HLA-identical siblings, n=198 from matched unrelated donors (MUD) and n=321 from haploidentical donors. Patients without comorbidities were n=423 (57.4%), whereas n=256 (34.7%) and n=58 (7.8%) presented with one and >2 comorbidities, respectively. A total of n=294 patients (39.9%) died following HSCT. Main patients, transplant and donors characteristics are shown in Table 1.
Table 1.
Main patients and donor characteristics. .
Table 1.
Main patients and donor characteristics. .
| Patient characteristics |
Number of patients |
| Number of patients |
737 |
Gender
Male
Female |
431
306 |
| Median age at transplantation |
48 |
HLA match
Identical sibling
Matched unrelated
Haploidentical |
218
198
321 |
Diagnosis*
Group 1
Group 2
Group 3 |
306
114
317 |
Median Karnofsky score
Karnofsky score >90% at HSCT |
87.7
446 |
Positive CMV serostatus
Negative CMV serostatu |
684
53 |
Total comorbidities
No comorbidity
1 comorbidity
>2 comorbidities |
423
256
58 |
| Donor characteristics |
Number of donors |
| Median age at donation |
40 |
Gender
Male
Female
missing
|
500
331
6
|
Positive CMV serostatus
Negative CMV serostatus |
556
181 |
Donor Type and Age
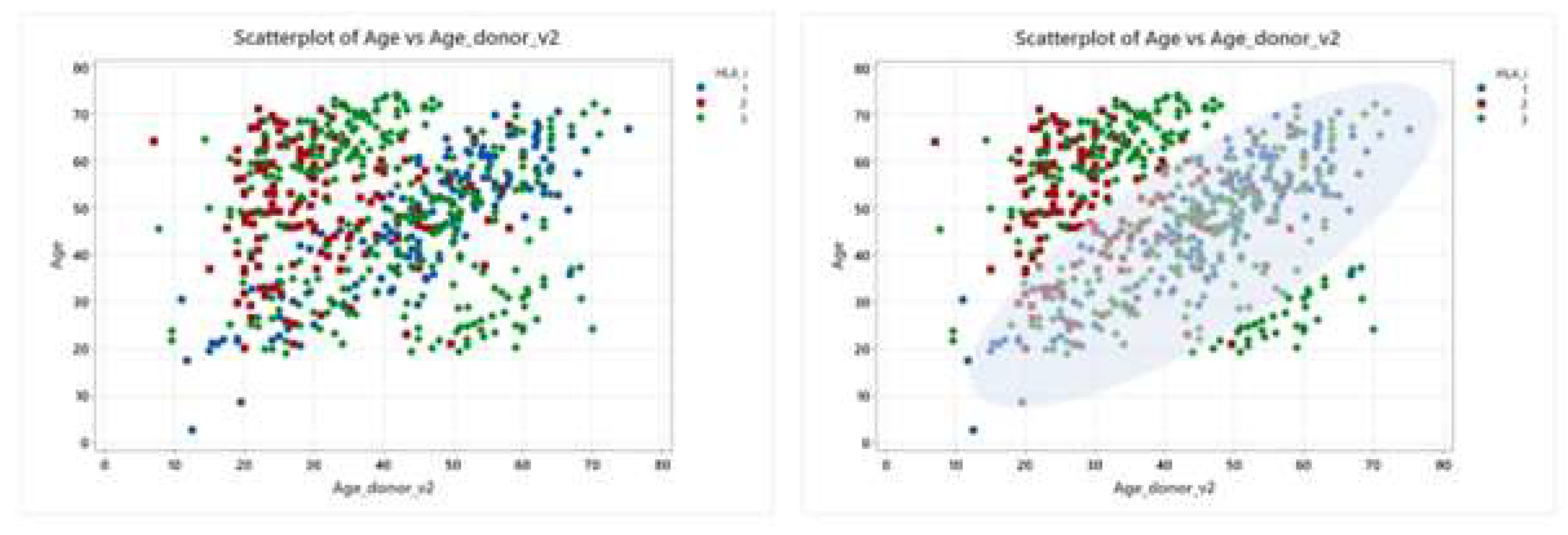
The type of stem cell donor and the donor age are variables that are connected and not fully independent, due to the three main reasons: a) an HLA-identical sibling has usually approximately the same patient’s age; b) a young MUD is mostly selected for donation; c) a haploidentical donor is usually a sibling, a parent or an offspring of patient and therefore belongs to three age periods. The scatterplot in Figure 1 illustrates the correlation between the patients and donors age in the different HLA match categories, corresponding to HLA-identical siblings, MUD and haploidentical donors, respectively. The scatterplot on the right includes a density estimation providing a visual indication of where data points are concentrated, that is the most common age combinations between patients and HLA identical siblings, represented by blue dots; as expected, the similar age distribution reflects the typical age gap between siblings. For MUD, depicted by red squares, the data are skewed towards younger ages, reflecting the broader age range found in unrelated donor registries. Haploidentical donors, represented by green diamonds, are broadly distributed but seem to be more concentrated in two points: there is a concentration of younger donors when the patients are older, and conversely, older donors when the patients are younger. This trend highlights the familial roles in donation: older patients often have their children as donors, while younger patients frequently receive donations from their parents.
Figure 1.
Scatterplots of patient and donors ages by HLA match types. The X axis represents the age of the donor, while the Y axis the age of the patient. Each point represents a donor-patient pair and is color-coded: HLA-identical sibling (blue), unrelated donor (red), haploidentical donor (green).
Figure 1.
Scatterplots of patient and donors ages by HLA match types. The X axis represents the age of the donor, while the Y axis the age of the patient. Each point represents a donor-patient pair and is color-coded: HLA-identical sibling (blue), unrelated donor (red), haploidentical donor (green).
Building the Model: Regression Analysis of Survival Predictors
Table 2 illustrates a regression analysis, based on the Weibull distribution, analyzing the impact of several variables, including the main factors (diagnosis, comorbidity, HLA match, patient’s age, and donor’s age) and interaction terms (Age patient*Diagnosis, HLA*Age patient, HLA*Age_donor, Diagnosis*Age_donor) on the 2y-OS following HSCT. Concerning the donor type (named here “HLA”), “HLA-1” is the reference category, representing HLA-identical sibling, whereas “HLA-2” and “HLA-3” are MUD and haploidentical donors, respectively. The negative coefficients would suggest a lower risk for MUD and haploidentical donors compared to HLA identical siblings. However, the p values of 0.450 for MUD and 0.804 for haploidentical donors, indicate that these findings are not statistically significant. Only the patient age has a statistically significant p-value, indicating a slight increase in mortaity risk as the age of the patient increases.
Table 2.
Variables affecting 2y-OS after HSCT and their interactions.
Table 2.
Variables affecting 2y-OS after HSCT and their interactions.
| Predictor |
Coefficient |
Standard error |
Z value |
P value |
Lower CI |
Upper CI |
| Intercept |
7.35820 |
0.239108 |
30.77 |
0.000 |
6.88956 |
7.82684 |
Diagnosis_1*
2
3 |
1.23406
0.603906 |
0.516391
0.271176 |
2.39
2.23 |
0.017
0.026 |
0.221949
0.0724101 |
2.24616
1.13540 |
HLA_1**
2
3 |
0.267212
-0.0726015 |
0.354110
0.292851 |
0.75
-0.25 |
0.450
0.804 |
-0.426830
-0.646578 |
0.961255
0.501375 |
| Age patient |
0.0164252 |
0.0076519 |
2.15 |
0.032 |
0.0014279 |
0.0314226 |
| Age donor |
-0.0090390 |
0.0077637 |
-1.16 |
0.244 |
-0.0242556 |
0.0061776 |
Age_patient*Diagnosis_1
2
3 |
-0.0189244
-0.0055234 |
0.0079260
0.0050316 |
-2.39
-1.10 |
0.017
0.272 |
-0.0344590
-0.0153852 |
-0.0033898
0.0043383 |
HLA_1*Age_patient
2
3 |
-0.0269508
-0.0150409 |
0.0092053
0.0078692 |
-2.93
-1.91 |
0.003
0.056 |
-0.0449929
-0.0304643 |
-0.0089097
0.0043383 |
HLA_1*Age_donor
2
3 |
0.0231561
0.0147905 |
0.0099341
0.0074005 |
2.33
2.00 |
0.020
0.046 |
0.0036856
0.0002859 |
0.0426266
0.0292951 |
Diagnosis_1*Age_donor
2
3 |
-0.0045711
-0.0023857 |
0.0072265
0.0050394 |
-0.63
-0.47 |
0.527
0.636 |
-0.0187347
-0.0122628 |
0.0095926
0.0074914 |
| Shape |
1.69843 |
0.0654468 |
|
|
1.57488 |
1.83167 |
Of note, the interaction term HLA*Age patient indicates how the effect of patient’s age on HSCT outcomes varies according to the HLA match, that is the stem cell donor type. HLA-2*Age patient (MUD) has a coefficient of -0.0269508 with a p-value of 0.003, which is statistically significant. The negative coefficient implies that as patient’s age increases, the risk of negative outcomes is lower with a MUD compared to an HLA-identical sibling. HLA-3*Age patient (haploidentical donors) has a coefficient of -0.01504909, but a p-value of 0.056, which is slightly above the threshold for statistical significance. This suggests a potential decreased risk of negative outcomes with haploidentical donors compared to HLA-identical siblings as patient’s age increases. Another important finding is the significant interaction term HLA*Age donor, indicating how the effect of donor’s age on HSCT outcomes varies depending on the HLA match, that is the stem cell donor type. HLA-2*Age_donor has a coefficient of 0.0231561, while HLA-3*Age_donor has a coefficient of 0.0147905. P-values are 0.020 and 0.046, indicating that both are statistically significant. The positive coefficients suggest that with increasing donor age, the survival following HSCT decreases, especially for HSCTs performed from MUD.
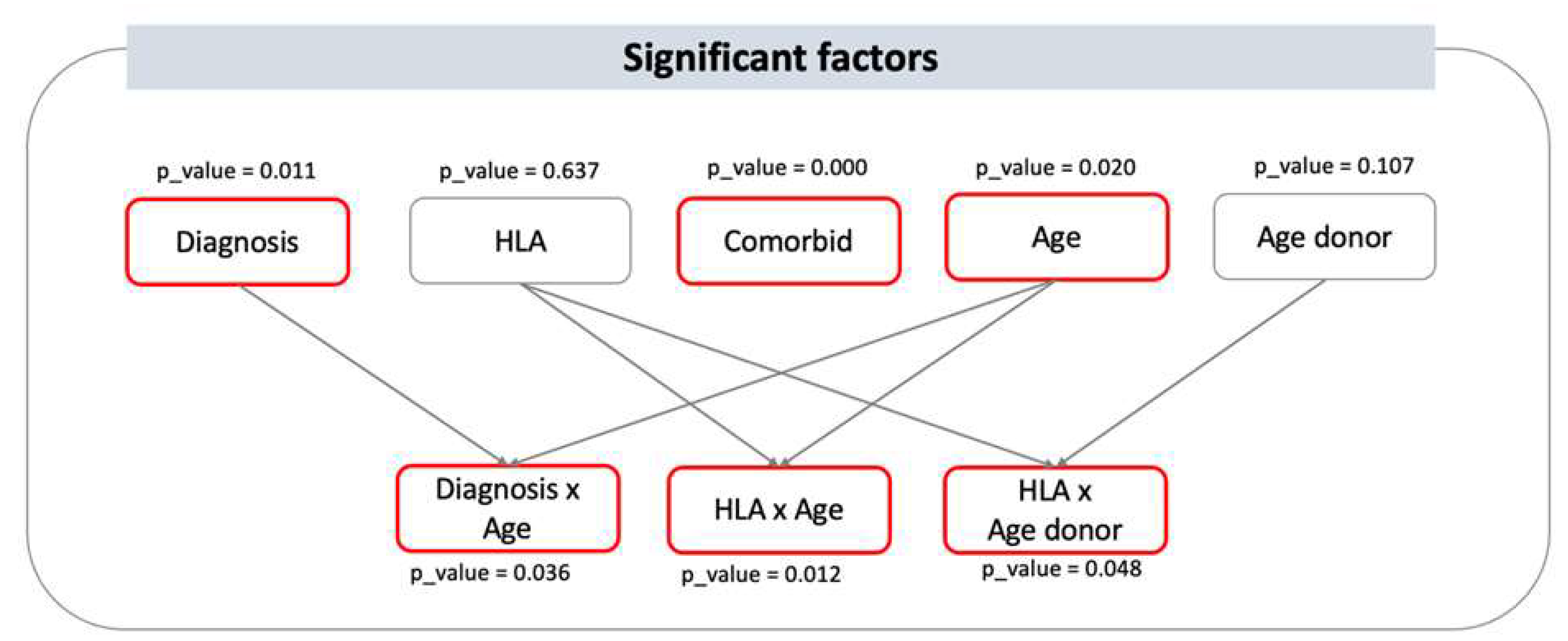
Overall, the significant factors having an impact on 2y-OS following HSCT are: HLA*Age patient, diagnosis, HLA*Age_donor, patient’s age, Age patient*Diagnosis. The HLA match, that is the type of stem cell donor, here demonstrates relevance to survival when analyzed in interaction with patient’s age and donor’s age, suggesting that the effect of age on outcome varies among the HLA match between patient and donor (Figure 2).
Figure 2.
Significant factors influencing 2-y OS and their interactions. visual representation of the statistically significant factors having an impact on 2y-OS following HSCT. Significant main variables are diagnosis, comorbidity, and age, with p-values of 0.011, 0.000, and 0.020, respectively. Donor type and donor age are not statistically significant as independent predictors of outcome, however, they are as interactions. Indeed, HLA*Age (i.e. patient age) and HLA*Age donor have p-values of 0.012 and 0.048, respectively, indicating that the effect of the patient and donor age depends on the HLA matching between patient and donor, that is here the donor type.
Figure 2.
Significant factors influencing 2-y OS and their interactions. visual representation of the statistically significant factors having an impact on 2y-OS following HSCT. Significant main variables are diagnosis, comorbidity, and age, with p-values of 0.011, 0.000, and 0.020, respectively. Donor type and donor age are not statistically significant as independent predictors of outcome, however, they are as interactions. Indeed, HLA*Age (i.e. patient age) and HLA*Age donor have p-values of 0.012 and 0.048, respectively, indicating that the effect of the patient and donor age depends on the HLA matching between patient and donor, that is here the donor type.
Example of Calculator
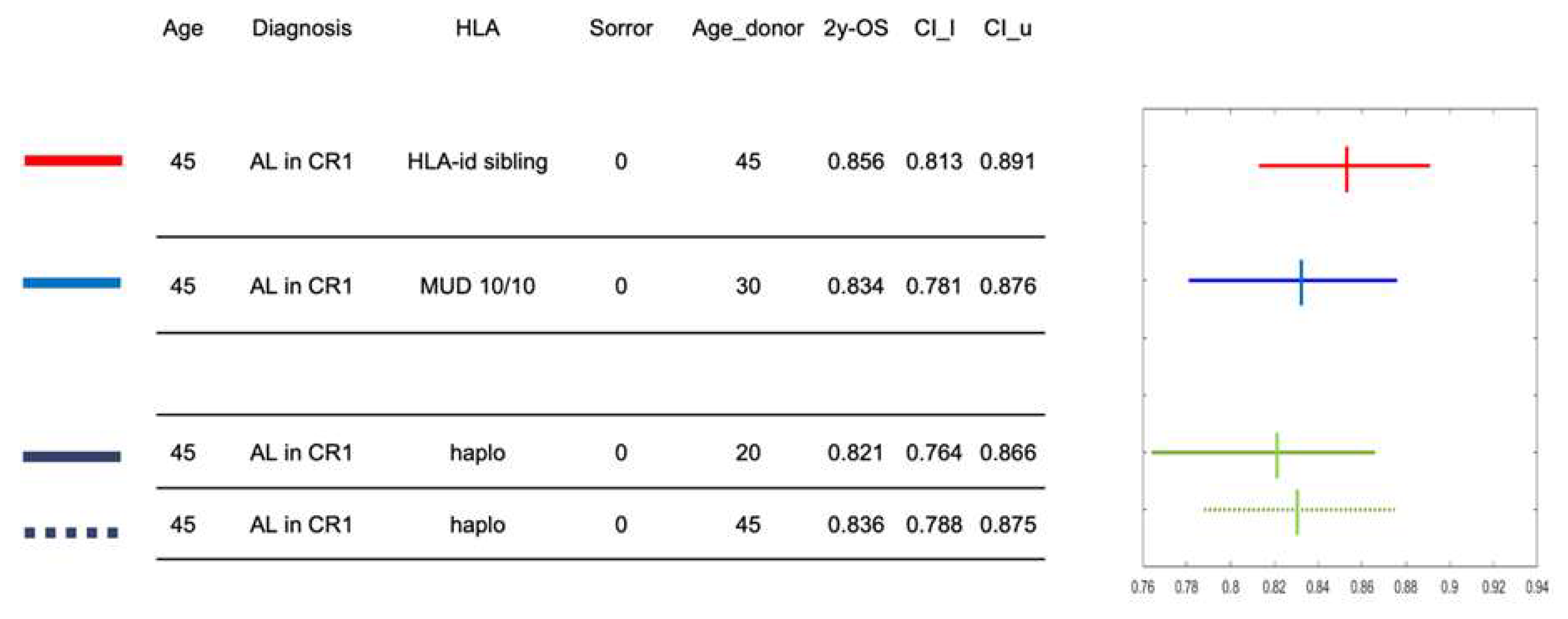
The Figure 3 reports an example of the calculator using a hypothetical, 45 year-old patient with a diagnosis of acute leukemia in first complete remission at HSCT and without any comorbidities (Sorror score of 0). Four possible donor options during the search occur: a) a 45 year-old HLA-identical sibling; b) a 30 year-old MUD; c) a 20 year-old haploidentical donor; d) a 45 years-old haploidentical donor. The calculator provides the 2y-OS of this patient associated to the four different donors and the graph on the right illustrates the confidence interval with the hazard ratio, providing guidance to the transplant physician in the decision-making process. As expected, the highest 2y-OS probability for this patient would be with a transplantation from an HLA identical sibling, as it is 0.856. Estimates of 2y-OS following HSCT from either the MUD or the haploidentical donors are quite comparable, although with large 95% confidence intervals. These results inform the final choice of the best donor and are intended to integrate local selection algorithms.
Figure 3.
An example of calculator output for a defined patient and four stem cell donor options. An example of calculator output for a defined patient and four stem cell donor options is reported here. The hypothetical patient is 45-years old and is affected by acute leukemia in first complete remission. There are four donor options during the search: a) a 45 year-old HLA-identical sibling; b) a 30-year old unrelated donor; c) a 20-year old haploidentical donor; d) a 45-year old haploidentical donor. Hazard ratios of 2y-OS and 95% confidence intervals are shown on the right panel for each of the four donors.
Figure 3.
An example of calculator output for a defined patient and four stem cell donor options. An example of calculator output for a defined patient and four stem cell donor options is reported here. The hypothetical patient is 45-years old and is affected by acute leukemia in first complete remission. There are four donor options during the search: a) a 45 year-old HLA-identical sibling; b) a 30-year old unrelated donor; c) a 20-year old haploidentical donor; d) a 45-year old haploidentical donor. Hazard ratios of 2y-OS and 95% confidence intervals are shown on the right panel for each of the four donors.
Discussion
Allogeneic hematopoietic stem cell transplantation is a complex therapeutic procedure, where patient-, disease-, transplant- and stem cell donor-related variables determine the final outcome. As part of HSCT, the stem cell donor is usually selected by transplant physicians among multiple options, that are carefully evaluated under local algorithms built on evidence from literature and clinical experience.
We here developed a calculator able to provide the patient survival estimates associated to each one of multiple donor options, in order to support the transplant physicians during the selection process. By modelling a dataset from n=737 HSCTs from two centers, the proposed tool included the significant variables affecting post-transplant outcome and relied on local own real-world data to provide 2y-OS estimates according to HLA-identical, unrelated and haploidentical stem cell donor options. The tool calculates and prospectively suggests which would be the best donor based on the center’s experience, since the results come from a model based on real data of the same centers, representing a novelty and an original element of our proposed tool. To our knowledge, at least two calculators have already been developed; however, they use aggregated data from the registry [16, 17]. Moreover, there is currently no specific study focusing on donor selection and donor characteristics across all transplant types, which instead is the focus of our study that takes into account HLA-identical siblings, unrelated donors and haploidentical donors at the same time. Although the choice of the stem cell donor is mainly based on HLA matching and donor age, our model demonstrates that these two variables interact between them and that the impact of HLA matching vary according to both patient and donor age. This is in line with the evidence that young unrelated donors are preferred over older ones and might be a better option when compared with older HLA-identical siblings [
9]. Moreover, a timely haploidentical donor may be a suitable option if an unrelated donor requires a long delay negatively affecting patient prognosis or if an HLA-mismatched unrelated donor is solely available [
18]. For these reasons such a calculator may be useful to support decision making among multiple donor options and it is more true whenever the modelling come from local own real data, reflecting the clinical experience of the center.
We show here the feasibility of developing a tool that may help the transplant physicians in the decision-making process of selecting the most suitable stem cell donor for transplantation, even when the results between potential donors are statistically similar. Our main purpose was to effectively use real data information by comparing survival outcomes associated with different donors, to inform for a hopefully optimal selection.
We acknowledge some limitations, as the existence of missing data and the final number of HSCTs, possible preventing from more definite estimates and narrower confidence intervals. In addition, we cannot exclude that the partial knowledge of the complex inter-relation between variables, the existence of unknown variables or of variables not captured by current models, have affected the performance of our model. However, since the main aim of the present study was to build a prototype of calculator, we focused on few transplant centers (namely two) and believe that our scope has been fulfilled, showing the feasibility of this approach and the novelty of including different donor types in the same model. Moreover, we explored multiple and significant interactions between variables and this probably deserves further investigation since most regression models do not frequently include interaction terms, although they may be clinically meaningful. Of course, caution is needed when extrapolating our results due to the lack of internal or external validation; nonetheless, our calculator represents a first attempt to provide prospective suggestion on the best stem cell donor during the delicate process of selection by providing the patient’s survival estimates associated with each of the donor options.
In conclusion, the present study showed the feasibility of using transplant centers’ retrospective data to prospectively suggest the best stem cell donor during the difficult process of selection, according to the center experience. We believe that this approach improves the performance and applicability over external models since our results are derived from local, own real-world data information and take into account multiple distinct donor types (HLA-identical sibling, unrelated and haploidentical donors) at the same time.
A collaborative multicenter study is underway with the aim of increasing the number of transplants, refining the impact of variables and their interactions and perform internal validation, to finally improve the model performance.
Statements and declarations
authors declare no conflicts of interests related to the present work.
References
- Passweg JR, Baldomero H, Ciceri F et al (2024) Hematopoietic cell transplantation and cellular therapies in Europe 2022. CAR-T activity continues to grow; transplant activity has slowed: a report from the EBMT. Bone Marrow Transplant. [Online ahead of print]. [CrossRef]
- Lee SJ, Klein J, Haagenson M et al (2007) High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood 110:4576-4583. [CrossRef]
- Shouval R, Fein JA, Labopin M et al (2019) Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: a European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol 6:e573–e584. [CrossRef]
- Shaw BE, Logan BR, Spellman SR et al (2018) Development of an Unrelated Donor Selection Score Predictive of Survival after HCT: Donor Age Matters Most. Biol Blood Marrow Transplant 24:1049–1056. [CrossRef]
- Fleischhauer K, Tran TH, Meisel R et al (2023) Donor Selection for Allogeneic Hematopoietic Cell Transplantation. Dtsch Arztebl Int 120:261–268. [CrossRef]
- Kollman C, Spellman SR, Zhang MJ et al (2016) The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood 127:260-267. [CrossRef]
- Mangum DS, Caywood E (2022) A clinician’s guide to HLA matching in allogeneic hematopoietic stem cell transplant. Hum Immunol 83:687–694. [CrossRef]
- Servais S, Porcher R, Xhaard A et al (2014) Pre-transplant prognostic factors of long-term survival after allogeneic peripheral blood stem cell transplantation with matched related/unrelated donors. Haematologica 99:519–526. [CrossRef]
- Kröger N, Zabelina T, de Wreede L et al (2013) Allogeneic stem cell transplantation for older advanced MDS patients: improved survival with young unrelated donor in comparison with HLA-identical siblings. Leukemia 27:604-609. [CrossRef]
- Schäfer HS, Finke J (2016) Mismatched unrelated alternative donors for hematological malignancies. Semin Hematol 53:77–81. [CrossRef]
- Timofeeva OA, Philogene MC, Zhang QJ (2022) Current donor selection strategies for allogeneic hematopoietic cell transplantation. Hum Immunol 83:674–686. [CrossRef]
- Azari M, Barkhordar M, Bahri T et al (2’24) Determining the predictive impact of donor parity on the outcomes of human leukocyte antigen matched hematopoietic stem cell transplants: a retrospective, single-center study. Front Oncol 14:1339605. [CrossRef]
- Boeckh M, Nichols WG (2004) The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood 103:2003–2008. [CrossRef]
- Ljungman P, de la Camara R, Robin C et al (2017) Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis 19:e260-e272. [CrossRef]
- Worel N (2016) AB0-mismatched allogeneic hematopoietic stem cell transplantation. Transfus Med Hemother 43:3-12. [CrossRef]
- Logan BR, Maiers MJ, Sparapani RA et al (2021) Optimal Donor Selection for Hematopoietic Cell Transplantation Using Bayesian Machine Learning. JCO Clin Cancer Inform 5:494-507. [CrossRef]
- Shouval R, Labopin M, Bondi O et al (2015) Prediction of allogeneic hematopoietic stem-cell transplantation mortality 100 days after transplantation using a machine learning algorithm: A European group for blood and marrow transplantation acute leukemia working party retrospective data mining study. J Clin Oncol 33:3144–3151. [CrossRef]
- Crocchiolo R, Cornacchini G, Lando G et al (2020) The number of HLA confirmatory tests during unrelated donor search as a driver for the evaluation of back-up haploidentical donor(s). Transfus Apher Sci 59: 102766. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).