Submitted:
05 September 2024
Posted:
06 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
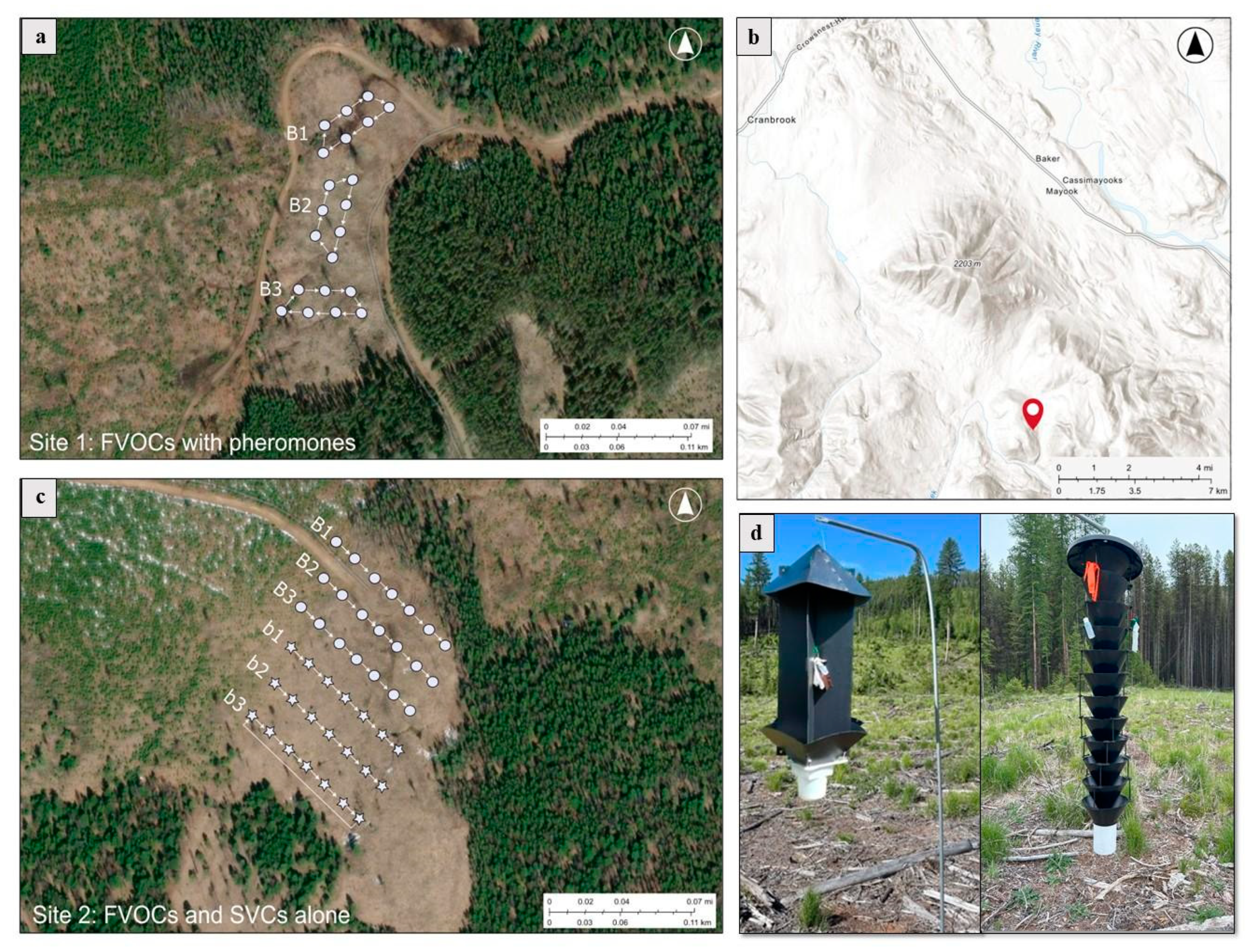
2.1. Experimental Design
2.1.1. Testing the Effects of FVOCs on the Attraction of Subcortical Beetles
2.1.2. Testing the Combined Effects of FVOCs and MPB Pheromones on Subcortical Beetle Attraction
2.1.3. Testing the Effects of SVCs on the Attraction of Subcortical Beetles
2.2. Statistical Analysis
3. Results
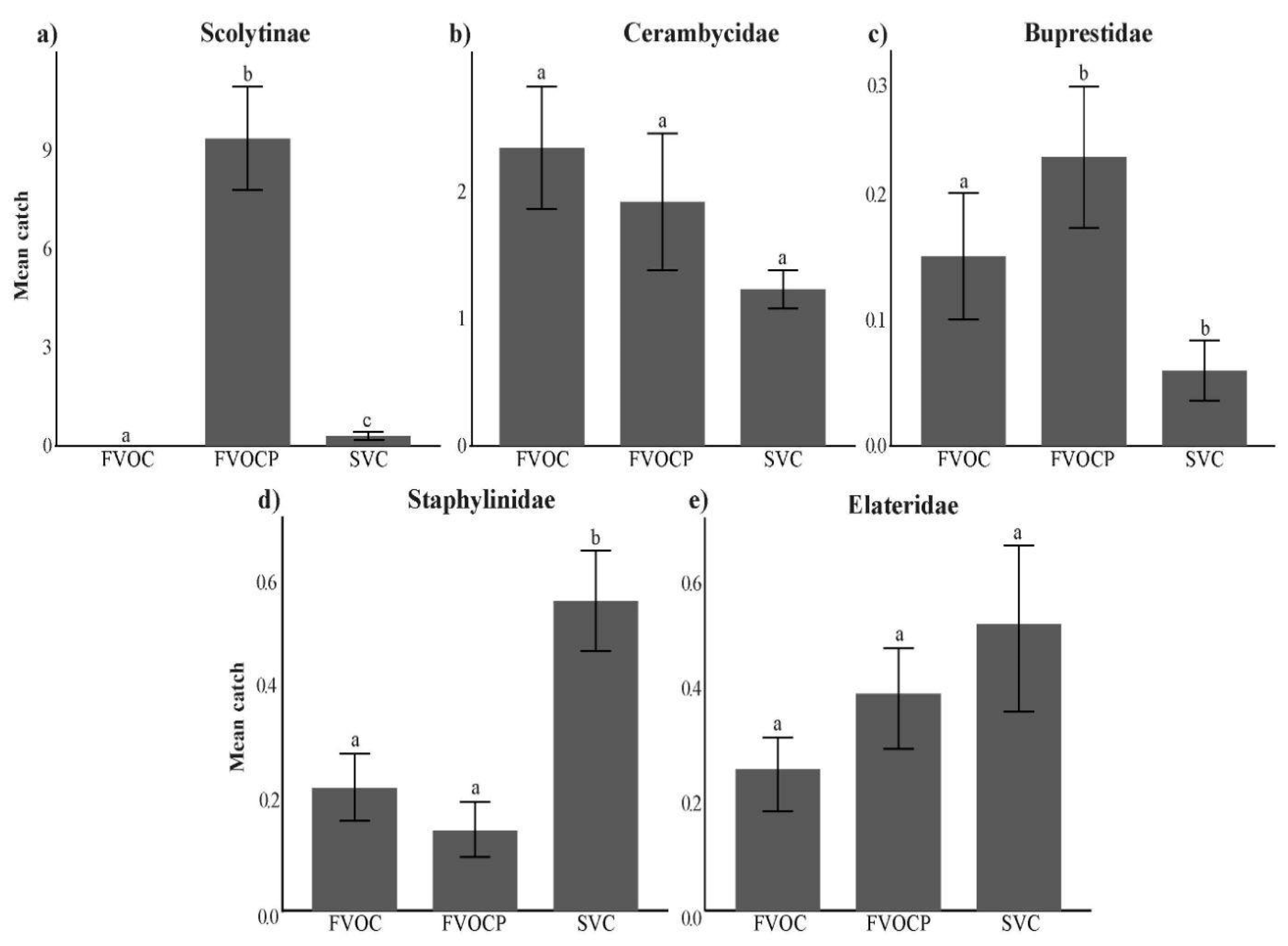
3.1. The Effects of FVOCs, SVCs and MPB Lure on the Attraction of Subcortical Beetles
3.1.1. Catches of Subcortical Beetles in FVOC Treatments
3.1.2. Catches of Subcortical Insects in FVOCs with MPB Lure
3.1.3. Catches of Subcortical Insects in SVC Treatments
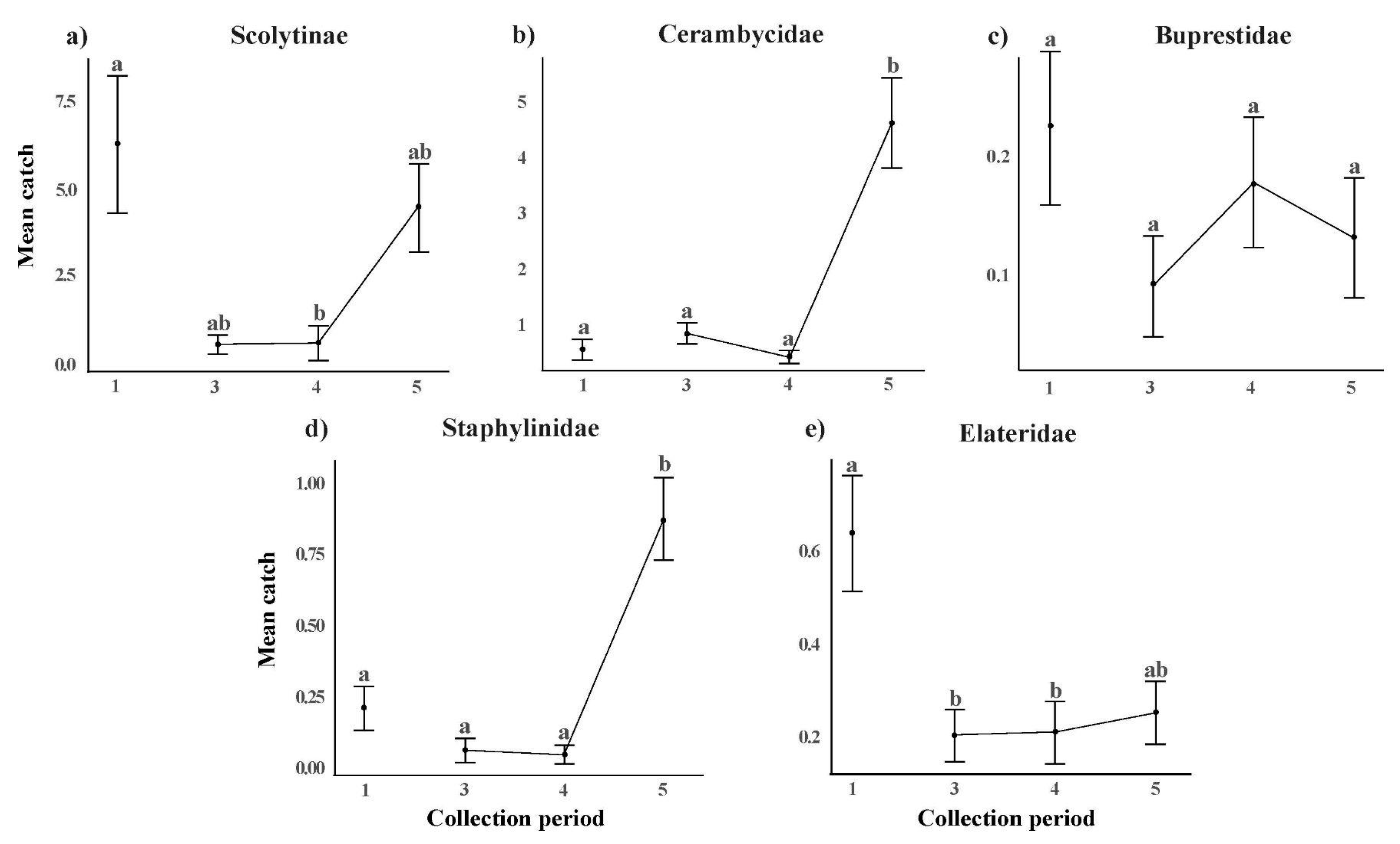
3.2. Temporal Variation in Subcortical Beetle Catches
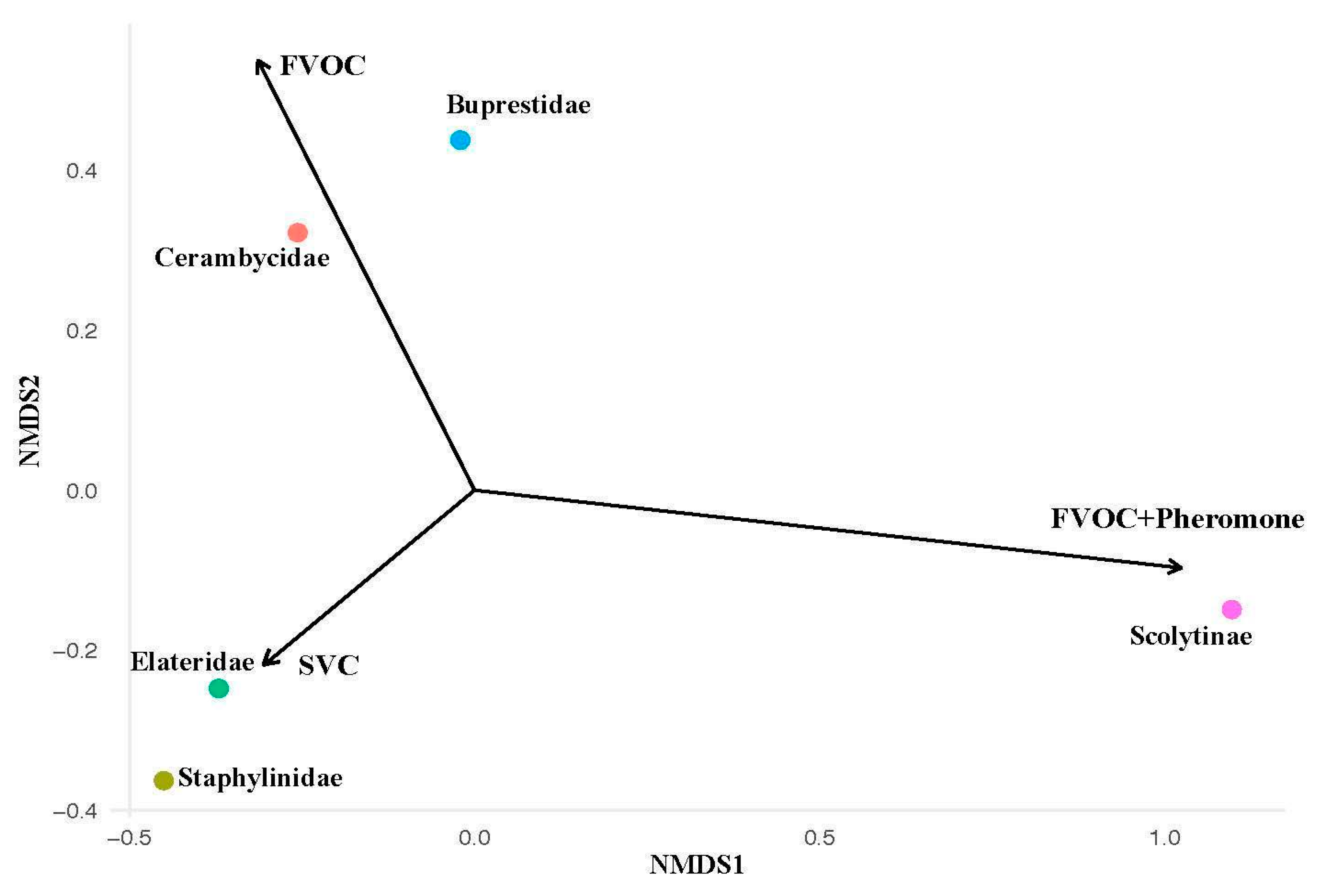
3.3. Differences in Subcortical Beetle Catches between Volatile Experiments
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byers, J. A. (2004). Chemical ecology of bark beetles in a complex olfactory landscape. Bark and wood boring insects in living trees in Europe, a synthesis, 89-134. [CrossRef]
- Erbilgin, N., Christiansen, E., Krokene, P. (2007). A host monoterpene influences Ips typographus responses (Coleoptera: Curculionidae, Scolytinae) to its aggregation pheromone: Implications for host colonization of bark beetles. Agricultural & Forest Entomology. 9: 135-140. [CrossRef]
- Davis, T.S., Crippen, T.L., Hofstetter, R.W., Tomberlin, J.K. (2013). Microbial volatile emissions as insect semiochemicals. Journal of Chemical Ecology, 39: 840–859. [CrossRef]
- Schlyter, F., Byers, J.A. & Löfqvist, J. (1987). Attraction to pheromone sources of different quantity, quality, and spacing: Density-regulation mechanisms in bark beetle Ips typographus. Journal of Chemical Ecology, 13: 1503–1523. [CrossRef]
- Wallin, K. F., & Raffa, K. F. (2000). Influences of host chemicals and internal physiology on the multiple steps of postlanding host acceptance behavior of Ips pini (Coleoptera: Scolytidae). Environmental Entomology, 29(3), 442-453. [CrossRef]
- Seybold, S. J., Bentz, B. J., Fettig, C. J., Lundquist, J. E., Progar, R. A., & Gillette, N. E. (2018). Management of western North American bark beetles with semiochemicals. Annual Review of Entomology, 63(1), 407-432. [CrossRef]
- Gandhi, K.J., Gilmore, D.W., Haack, R.A., Katovich, S.A., Krauth, S.J., Mattson, W.J., Zasada, J.C., Seybold, S.J. (2009). Application of semiochemicals to assess the biodiversity of subcortical insects following an ecosystem disturbance in a sub-boreal forest. Journal of Chemical Ecology, 35(12), 1384-410. [CrossRef] [PubMed]
- Afzal, S., Nahrung, H. F., Lawson, S. A., & Hayes, R. A. (2023). How effective are push–pull semiochemicals as deterrents for bark beetles? a global meta-analysis of thirty years of research. Insects, 14(10), 812. [CrossRef]
- Singh, V.V., Naseer, A., Mogilicherla, K., Trubin, A., Zabihi, K., Roy, A., Jakuš, R., Erbilgin, N. (2024). Understanding bark beetle outbreaks: exploring the impact of changing temperature regimes, droughts, forest structure, and prospects for future forest pest management. Reviews in Environmental Science Biotechnology, 23: 257–290. [CrossRef]
- Erbilgin, N., Powell, J.S., Raffa, K.S. (2003). Effect of varying monoterpene concentrations on the response of Ips pini (Coleoptera: Scolytidae) to its aggregation pheromone: implications for pest management and ecology of bark beetles. Agricultural & Forest Entomology, 5: 269-274. [CrossRef]
- Borden, J. H. (1989). Semiochemicals and bark beetle populations: exploitation of natural phenomena by pest management strategists. Ecography, 12(4), 501-510. [CrossRef]
- Erbilgin, N., Raffa, K.S. (2001a). Kairomonal range of generalist predators in specialized habitats: responses to multiple phloeophagous species emitting pheromones vs. host odors. Entomologia Experimentalis et Applicata, 99: 205-210. [CrossRef]
- Boone, C. K., Six, D. L., & Raffa, K. F. (2008). The enemy of my enemy is still my enemy: Competitors add to predator load of a tree-killing bark beetle. Agricultural and Forest Entomology, 10(4), 411-421. [CrossRef]
- Niinemets, Ü., Kännaste, A., & Copolovici, L. (2013). Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Frontiers in Plant Science, 4, 262. [CrossRef]
- Faiola, C., & Taipale, D. (2020). Impact of insect herbivory on plant stress volatile emissions from trees: A synthesis of quantitative measurements and recommendations for future research. Atmospheric Environment: X, 5. [CrossRef]
- Zaman, R., Antonioli, F., Shah, A., Ullah, A., May, C., Klutsch, J.G., Erbilgin, N. (2023a). A pine in distress: how infection by different pathogenic fungi affect lodgepole pine chemical defenses. Microbial Ecology, 86, 2666–2673. [CrossRef]
- Byers, J.A. (1992). Attraction of bark beetles, Tomicus piniperda, Hylurgops palliatus, and Trypodendron domesticum and other insects to short-chain alcohols and monoterpenes. Journal of Chemical Ecology 18: 2385–2402. [CrossRef]
- Erbilgin, N., Raffa, K.F. (2000). Opposing Effects of host monoterpenes on responses by two sympatric species of bark beetles to their aggregation pheromones. Journal of Chemical Ecology, 26: 2527–2548. [CrossRef]
- Kandasamy, D., Gershenzon, J. & Hammerbacher, A. (2016). Volatile organic compounds emitted by fungal associates of conifer bark beetles and their potential in bark beetle control. Journal of Chemical Ecology, 42, 952–969. [CrossRef]
- Kandasamy, D., Gershenzon, J., Andersson, M. N., & Hammerbacher, A. (2019). Volatile organic compounds influence the interaction of the Eurasian spruce bark beetle (Ips typographus) with its fungal symbionts. The ISME journal, 13(7); 1788-1800. [CrossRef]
- Jirošová, A., Modlinger, R., Hradecký, J., Ramakrishnan, R., Beránková, K., Kandasamy, D. (2022). Ophiostomatoid fungi synergize attraction of the Eurasian spruce bark beetle, Ips typographus to its aggregation pheromone in field traps. Frontiers in Microbiology.13:980251. [CrossRef]
- Zaman, R., May, C., Ullah, A., Erbilgin, N. (2023b). Bark beetles utilize ophiostomatoid fungi to circumvent host tree defenses. Metabolites 13(2), 239. [CrossRef]
- Miller, D.R., Asaro, C., Crowe, C.M., Duerr, D.A. (2011). Bark beetle pheromones and pine volatiles: attractant kairomone lure blend for longhorn beetles (Cerambycidae) in pine stands of the southeastern United States. Journal of Economic Entomology, 104(4), 1245–1257. [CrossRef]
- Grant, G. G., Poland, T. M., Ciaramitaro, T., Barry Lyons, D., & Jones, G. C. (2011). Comparison of male and female emerald ash borer (Coleoptera: Buprestidae) responses to phoebe oil and (Z)-3-hexenol lures in light green prism traps. Journal of Economic Entomology, 104(1), 173-179. [CrossRef]
- Domingue, M. J., Baker, T. C., Blanco, J. J., & Fernandes, A. T. (2012). A multi-disciplinary approach for developing tools to monitor invasive buprestid beetle species. Invasive species: threats, ecological impact and control methods. Nova, Hauppauge, 3, 77-100.
- Allison, J.D., McKenney, J.L., Miller, D.R., Gimmel, M.L. (2013). Kairomonal responses of natural enemies and associates of the southern Ips (Coleoptera: Curculionidae: Scolytinae) to ipsdienol, ipsenol and cis-Verbenol. Journal of Insect Behavior 26, 321–335. [CrossRef]
- Miller, D. R. (2023). Coleopteran predators of bark and woodboring beetles attracted to traps baited with ethanol and α-pinene in pine (Pinaceae) forests of the southern United States of America. The Canadian Entomologist, 155, e5. [CrossRef]
- Wood, D. L. (1982). The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles.
- Erbilgin, N., Raffa, K.S. (2001b). Modulation of predator attraction to pheromones of two prey species by stereochemistry of plant volatiles. Oecologia, 127: 444-453. [CrossRef]
- Gitau, C. W., Bashford, R., Carnegie, A. J., & Gurr, G. M. (2013). A review of semiochemicals associated with bark beetle (Coleoptera: Curculionidae: Scolytinae) pests of coniferous trees: a focus on beetle interactions with other pests and their associates. Forest Ecology and Management, 297: 1-14. [CrossRef]
- Tóth, M. (2013). Pheromones and attractants of click beetles: an overview. Journal of Pest Science, 86: 3-17. [CrossRef]
- Payne, T.L., Coster, J.E., Richerson, J.V., Edson, L.J., Hart, E.R. (1978). Field response of the southern pine beetle to behavioral chemicals. Environmental Entomology 7(4): 578-582.
- Klutsch, J.G., Cale, J.A., Whitehouse, C., Kanekar, S.S., and Erbilgin, N. (2017). Trap trees: an effective method for monitoring mountain pine beetle activities in novel habitats. Canadian Journal of Forest Research. 47(10): 1432-1437. [CrossRef]
- Borden, J.H., Hunt, D.W.A., Miller, D.R., Slessor, K.N. (1986). Orientation in forest coleoptera: an uncertain outcome to responses by individual beetles to variable stimuli. Clarendon Press, Oxford, UK.
- Payne, T.L., Andryszak, N.A., Weiser, H., Dixon, E.A., Ibrahim, N., Coers, L. (1988). Antennal olfactory and behavioral response of the southern pine beetle, Dendroctonus frontalis, to analogs of its aggregation pheromone frontalin. Journal of Chemical Ecology, 14: 1217–1225. [CrossRef]
- Sullivan, B.T., Shepherd, W.P., Pureswaran, D.S., Tashiro, T., Mori, K. (2007). Evidence that (+)-endo-brevicomin is a male-produced component of the southern pine beetle aggregation pheromone. Journal of Chemical Ecology, 33: 1510–1527. [CrossRef]
- Francke, W., Bartels, J., Meyer, H., Schröder, F., Kohnle, U., Baader, E., Pierre Vité, J. (1995). Semiochemicals from bark beetles: New results, remarks, and reflections. Journal of Chemical Ecology, 21: 1043–1063. [CrossRef]
- Coster, J.E., Vité, J.P. (1972). Effects of feeding and mating on pheromone release in the southern pine beetle. Annals of the Entomological Society of America, 65: 263–266. [CrossRef]
- McCarty, F.A., Billings, P., Richerson, J.V., Payne, T.L., Edson, L.J. (1980). Response of the southern pine beetle to behavioral chemicals in the laboratory. Journal of Entomological Science,15: 307–317.
- Vité, J.P., Bakke, A., Renwick, J.A.A. (1972). Pheromones in Ips (Coleoptera: Scolytidae): occurrence and production. The Canadian Entomologist, 104: 1967–1975. [CrossRef]
- Byers, J.A., Birgersson, G. (1990). Pheromone production in a bark beetle independent of myrcene precursor in host pine species. Naturwissenschaften, 77: 385–387. [CrossRef]
- Vité, J.P., Renwick, J.A.A. (1971). Population aggregating pheromone in the bark beetle, Ips grandicollis. Journal of Insect Physiology, 17: 1699–1704. [CrossRef]
- Byers, J.A., Lanne, B.S., Schlyter, F., Lofqvist, J., Bergstrom, G. (1985). Olfactory recognition host-tree susceptibility by pine shoot beetles. Naturwissenschaften, 72, 324–326. [CrossRef]
- Hofstetter, R. W., Chen, Z., Gaylord, M. L., McMillin, J. D., & Wagner, M. R. (2008). Synergistic effects of α-pinene and exo-brevicomin on pine bark beetles and associated insects in Arizona. Journal of Applied Entomology, 132(5): 387-397.
- Miller, D. R. (2006). Ethanol and (−)-α-pinene: attractant kairomones for some large wood-boring beetles in southeastern USA. Journal of Chemical Ecology, 32: 779-794. [CrossRef]
- Andersson, M. N., Larsson, M. C., & Schlyter, F. (2009). Specificity and redundancy in the olfactory system of the bark beetle Ips typographus: single-cell responses to ecologically relevant odors. Journal of Insect Physiology, 55(6); 556-567.
- Hanks, L.M., Millar, J.G., Moreira, J.A., Barbour, J.D., Lacey, E.S., McElfresh, S., Reuter, F.R., Ray, A.M. (2007). Using generic pheromone lures to expedite identification of aggregation pheromones for the cerambycid beetles Xylotrechus nauticus, Phymatodes lecontei, and Neoclytus modestus modestus. Journal of Chemical Ecology, 33, 889–907. [CrossRef]
- Mitchell, R. F., Hughes, D. T., Luetje, C. W., Millar, J. G., Soriano-Agatón, F., Hanks, L. M., & Robertson, H. M. (2012). Sequencing and characterizing odorant receptors of the cerambycid beetle Megacyllene caryae. Insect biochemistry and molecular biology, 42(7), 499-505. [CrossRef]
- Molander, M.A., Winde, I.B., Burman, J., Nyabuga, F.N., Lindblom, T.U.T., Hanks, L.M., Millar, J.G., Larsson, M.C. (2019). Common cerambycid pheromone components as attractants for longhorn beetles (Cerambycidae) breeding in ephemeral oak substrates in northern Europe. Journal of Chemical Ecology, 45, 537–548. [CrossRef]
- Pajares, J. A., Álvarez, G., Ibeas, F., Gallego, D., Hall, D. R., & Farman, D. I. (2010). Identification and field activity of a male-produced aggregation pheromone in the pine sawyer beetle, Monochamus galloprovincialis. Journal of Chemical Ecology, 36: 570-583. [CrossRef]
- Teale, S. A., Wickham, J. D., Zhang, F., Su, J., Chen, Y., Xiao, W., Hanks, L.M. & Millar, J. G. (2011). A male-produced aggregation pheromone of Monochamus alternatus (Coleoptera: Cerambycidae), a major vector of pine wood nematode. Journal of Economic Entomology, 104(5): 1592-1598. [CrossRef]
- Miller, D.R., Allison, J.D., Crowe, C.M., Dickinson, D.M., Eglitis, A., Hofstetter, R.W., Munson, A.S., Poland, T.M., Reid, L.S., Steed, B.E., Sweeney, J.D. (2016). Pine sawyers (Coleoptera: Cerambycidae) attracted to α-Pinene, monochamol, and ipsenol in North America. Journal of Economic Entomology, 109(3): 1205–1214. [CrossRef]
- Schröder, F., Fettköther, R., Noldt, U., Dettner, K., König, W.A., Francke, W. (1994). Synthesis of (3R)-3-hydroxy-2-hexanone, (2R,3R)-2,3-hexanediol and (2S,3R)-2,3-hexanediol, the male sex pheromone of Hylotrupes bajulus and Pyrrhidium sanguineum (Cerambycidae). Liebigs Annalen der Chemie: 1211–1218. [CrossRef]
- Miller, D. R., Dodds, K. J., Eglitis, A., Fettig, C. J., Hofstetter, R. W., Langor, D. W., Mayfield, A. E., Munson, A. S., Poland, T. M., Raffa, K. F. (2013). Trap lure blend of pine volatiles and bark beetle pheromones for Monochamus spp. (Coleoptera: Cerambycidae) in pine forests of Canada and the United States. Journal of Economic Entomology, 106 : 1684 – 1692.
- Silk, P. J., Sweeney, J., Wu, J., Price, J., Gutowski, J. M., & Kettela, E. G. (2007). Evidence for a male-produced pheromone in Tetropium fuscum (F.) and Tetropium cinnamopterum (Kirby)(Coleoptera: Cerambycidae). Naturwissenschaften, 94:697-701. [CrossRef]
- Sweeney, J. D., Silk, P. J., Gutowski, J. M., Wu, J., Lemay, M. A., Mayo, P. D., & Magee, D. I. (2010). Effect of chirality, release rate, and host volatiles on response of Tetropium fuscum (F.), Tetropium cinnamopterum Kirby, and Tetropium castaneum (L.) to the aggregation pheromone, fuscumol. Journal of Chemical Ecology, 36: 1309-1321. [CrossRef]
- Lacey, E. S., Ginzel, M. D., Millar, J. G., & Hanks, L. M. (2004). Male-produced aggregation pheromone of the cerambycid beetle Neoclytus acuminatus acuminatus. Journal of Chemical Ecology, 30: 1493-1507.
- Allison, J. D., Borden, J. H., McIntosh, R. L., De Groot, P., & Gries, R. (2001). Kairomonal response by four Monochamus species (Coleoptera: Cerambycidae) to bark beetle pheromones. Journal of Chemical Ecology, 27: 633-646. [CrossRef]
- Morewood, W. D., Simmonds, K. E., Wilson, I. M., Borden, J. H., & McIntosh, R. L. (2002). α-Pinene and ethanol: key host volatiles for Xylotrechus longitarsis (Coleoptera: Cerambycidae). Journal of the Entomological Society of British Columbia, 99: 117-122.
- Miller, D. R., & Asaro, C. (2023). Predators attracted to combination of bark beetle pheromones and host kairomones in pine forests of southeastern United States. Environmental Entomology, 52(5): 787-794. [CrossRef]
- Peschke, K., Friedrich, P., Kaiser, U., Franke, S., & Francke, W. (1999). Isopropyl (Z9)-hexadecenoate as a male attractant pheromone from the sternal gland of the rove beetle Aleochara curtala (Coleoptera: Staphylinidae). Chemoecology, 9: 47-54. [CrossRef]
- Avşar, İ., & Turan, Y. (2024). An overview of the pheromones of staphylinidae (Coleoptera). Transactions of the American Entomological Society, 150(2): 205-215.
- Faly, L., Brygadyrenko, V., & Paulauskas, A. (2024). Repellent and attractant activities of organic compounds on female and male Philonthus decorus (Coleoptera, Staphylinidae). Biology, 13(5), 294. [CrossRef]
- Tolasch, T., Von Fragstein, M., & Steidle, J. L. (2010). Sex pheromone of Agriotes acuminatus (Stephens, 1830)(Coleoptera: Elateridae). Journal of Chemical Ecology, 36: 314-318. [CrossRef]
- Tóth, M., Furlan, L., Yatsynin, V. G., Ujváry, I., Szarukán, I., Imrei, Z., Subchev, M., Tolasch, T. & Francke, W. (2002). Identification of sex pheromone composition of click beetle Agriotes brevis candeze. Journal of chemical ecology, 28: 1641-1652. [CrossRef]
- Toth, M., Furlan, L., Yatsynin, V. G., Ujvary, I., Szarukan, I., Imrei, Z., Tolasch, T., Francke, W. & Jossi, W. (2003). Identification of pheromones and optimization of bait composition for click beetle pests (Coleoptera: Elateridae) in Central and Western Europe. Pest Management Science, 59(4): 417-425. [CrossRef]
- Tolasch, T., von Fragstein, M., & Steidle, J. L. (2007). Sex pheromone of Elater ferrugineus L.(Coleoptera: Elateridae). Journal of chemical ecology, 33: 2156-2166. [CrossRef]
- Amman, G. D. (1977). The role of the mountain pine beetle in lodgepole pine ecosystems: impact on succession. In The role of arthropods in forest ecosystems (pp. 3-18). Berlin, Heidelberg: Springer Berlin Heidelberg.
- Safranyik, L. and Carroll, A.L. (2006). The biology and epidemiology of the mountain pine beetle in lodgepole pine forests. The Mountain Pine Beetle: A Synthesis of its Biology, Management and Impacts on Lodgepole Pine. 3-66. Natural Resources Canada.
- Cudmore, T. J., Björklund, N., Carroll, A. L., & Staffan Lindgren, B. (2010). Climate change and range expansion of an aggressive bark beetle: evidence of higher beetle reproduction in naïve host tree populations. Journal of Applied Ecology, 47(5), 1036-1043. [CrossRef]
- Sambaraju, K. R., & Goodsman, D. W. (2021). Mountain pine beetle: an example of a climate-driven eruptive insect impacting conifer forest ecosystems. CABI Reviews, (2021). [CrossRef]
- Amman, G. D. (1970). Prey consumption and variations in larval biology of Enoclerus spegeus (Coleoptera: Cleridae). The Canadian Entomologist, 102(11), 1374–1379. [CrossRef]
- Krause, A.M., Townsend, P.A., Lee, Y., Raffa, K.F. (2018). Predators and competitors of the mountain pine beetle Dendroctonus ponderosae (Coleoptera: Curculionidae) in stands of changing forest composition associated with elevation. Agricultural and Forest Entomology, 20.3, 402-413. [CrossRef]
- Carroll, A. L., Aukema, B. H., Raffa, K. F., Linton, D. A., Smith, G. D., & Lindgren, B. S. (2006). Mountain pine beetle outbreak development: the endemic—incipient epidemic transition. Canadian Forest Service, Mountain Pine Beetle Initiative Project, 1, 22.
- Bleiker, K.P., Six, D.L. (2007). Dietary benefits of fungal associates to an eruptive herbivore: potential implications of multiple associates on host population dynamics. Environmental Entomology, 36(6):1384-96. [CrossRef] [PubMed]
- Goodsman, D.W., Erbilgin, N., Lieffers, V.J. (2012). The impact of phloem nutrients on overwintering mountain pine beetles and their fungal symbionts. Environmental Entomology, 41(3), 478–486. [CrossRef]
- Therrien, J., Mason, C.J., Cale, J.A. et al. (2015). Bacteria influence mountain pine beetle brood development through interactions with symbiotic and antagonistic fungi: implications for climate-driven host range expansion. Oecologia 179, 467–485. [CrossRef]
- Cale, J.A., Muskens, M., Najar, A., Ishangulyyeva, G., Hussain, A., Kanekar, S.S., Klutsch, J.G., Taft, S., Erbilgin, N. (2017). Rapid monoterpene induction promotes the susceptibility of a novel host pine to mountain pine beetle colonization but not to beetle-vectored fungi, Tree Physiology 37(12), 1597–1610. [CrossRef]
- Guevara-Rozo, S., Hussain, A., Cale, J.A., Klutsch, J.G., Rajabzadeh, R., Erbilgin, N. (2020). Nitrogen and ergosterol concentration varied in live jack pine phloem following inoculations with fungal associates of mountain pine beetle. Frontiers in Microbiology 11(1703). [CrossRef]
- Cale, J.A., Collignon, R.M., Klutsch, J.G., Kanekar, S.S., Hussain, A., Erbilgin, N. (2016) Fungal volatiles can act as carbon sources and semiochemicals to mediate interspecific interactions among bark beetle-associated fungal symbionts. PLoS ONE 11(9): e0162197. [CrossRef]
- Chiu, C. & Bohlmann, J. (2022). Mountain pine beetle epidemic: an interplay of terpenoids in host defense and insect pheromones. Annual Review of Plant Biology, 73:1, 475-494. [CrossRef]
- Moore, M.L., Six, D.L. (2015). Effects of Temperature on growth, sporulation, and competition of mountain pine beetle fungal symbionts. Microbial Ecology 70, 336–347. [CrossRef]
- Aukema, B.H., Dahlsten, D.L., Raffa, K.F. (2000). Improved population monitoring of bark beetles and predators by incorporating disparate behavioral responses to semiochemicals. Environmental Entomology, 29(3), 618–629. [CrossRef]
- Hofstetter, R. W., Gaylord, M. L., Martinson, S., & Wagner, M. R. (2012). Attraction to monoterpenes and beetle-produced compounds by syntopic Ips and Dendroctonus bark beetles and their predators. Agricultural and Forest Entomology, 14(2), 207-215. [CrossRef]
- Amman, G. D. (1972). Mountain pine beetle brood production in relation to thickness of lodgepole pine phloem. Journal of Economic Entomology, 65(1), 138-140.
- Erbilgin, N., Ma, C., Whitehouse, C., Shan, B., Najar, A., & Evenden, M. (2014). Chemical similarity between historical and novel host plants promotes range and host expansion of the mountain pine beetle in a naïve host ecosystem. The New Phytologist, 201(3), 940–950. [CrossRef]
- Wijerathna, A., Evenden, M. (2020). Effect of environmental conditions on flight capacity in mountain pine beetle (Coleoptera: Curculionidae: Scolytinae). Journal of Insect Behaviour, 33; 201–215. [CrossRef]
| Subcortical beetle community | Known attractants | Source | Species attracted | References |
|---|---|---|---|---|
| Curculionidae, Scolytinae | Trans-verbenol | Female pheromone | Dendroctonus ponderosae | [32,33] |
| Exo-brevicomin | Male pheromone | Dendroctonus ponderosae; Dendroctonus terebrans; Dendroctonus brevicomis | [34] | |
| (-)-Endo-brevicomin | Male pheromone | Dendroctonus frontalis | [35,36] | |
| (+)-Sulcatol | Female pheromone |
Gnathotrichus sulcatus; Ips sexdentatus |
[37] | |
| 4,6,6- Lineatin | Female pheromone | Trypodendron lineatum | [37] | |
| Frontalin | Female pheromone | Dendroctonus rufipennis; Dendroctonus brevicomis; Dendroctonus pseudotsugae; Dendroctonus terebrans | [35,38,39] | |
| Ipsdienol | Male pheromone | Ip scalligraphus; Ips pini; Ips duplicatus; Ips avulses; Ips paraconfusus; Ips grandicollis; Ips perturbatus; Ips grandicollis | [26,40,41] | |
| Ipsenol | Male pheromone | Ips grandicollis; Ips paraconfusus; Ips duplicatus | [40,42] | |
| Terpinolene | Host tree volatile | Dendroctonus ponderosae | [17,33,43] | |
| Myrcene | Host tree volatile | Dendroctonus ponderosae | [17,33,44] | |
| Alpha-pinene | Phloem tissue of host trees |
Dendroctonus frontalis; Dendroctonus brevicomis; Ips grandicollis; Hylastes porculus; Hylobius pales; Pachylobius picivorus |
[18,32,44,45] | |
| p-Cymene | Host tree volatile | Ips typographus | [46] | |
| (+)-3-carene | Host tree volatile | Trypodendron domesticum; Hylurgops palliatus | [17,18] | |
| Cerambycidae | 2-Methyl-1-butanol | Pheromone components of several species |
Neoclytus acuminatus; Neoclytus mucronatus; Phymatodes lengi; Xylotrechus colonus; Aegomorphus modestus; Astyleiopus variegatus; Lepturges angulatus; Monochamus carolinensis; Megacyllene caryae; Pyrrhidium sanguineum; Phymatodes alni ssp. alni; Phymatodes testaceus |
[47,48,49] |
| Ethanol | Similar compound to the male pheromone 2-(undecyloxy)-ethanol | Monochamus; Acanthocinus nodosus; Acanthocinus obsoletus; Arhopalus r. nubilus; Xylotrechus s. sagittatus | [45,50,51] | |
| Monochamol | Male-produced aggregation pheromone (from Monochamus galloprovincialis; Monochamus alternatus; Monochamus scutellatus) | Monochamus | [52] | |
| 3-Hydroxy-2-hexanone | Male-produced aggregation pheromone (from Pyrrhidium sanguineum; Phymatodes alni spp. alni; Phymatodes testaceus) | Several species from the subfamily Cerambycinae | [49,53] |
|
| Ipsenol | Bark beetle pheromone (Ips species) | Monochamus carolinensis; Monochamus clamator, Monochamus mutator; Monochamus obtusus; Monochamus scutellatus; Monochamus titillator | [52,54] |
|
| 3-Hydroxy-2-octanone | Male-produced aggregation pheromone | Plagionotus arcuatus | [49] | |
| Fuscumol acetate | Male-produced aggregation pheromone (from | Tetropium | [55,56] | |
| C6 (anti) diols (2S,3R;2R,3S) hexanediol | Male-produced aggregation pheromone (from Neoclytus acuminatus acuminatus; Curius dentatus) | Neoclytus acuminatus acuminatus; Curius dentatus | [57] | |
| α-pinene | Monoterpene produced by many coniferous trees, including Pinus and Picea species | Acanthocinus nodosus, A. obsoletus, Arhopalus rusticus nubilus, Asemum striatum, Monochamus titillator, Prionus pocularis, Xylotrechus integer, and X. sagittatus sagittatus; Xylotrechus longitarsis; Monochamus scutellatus | [45,52,58,59] | |
| Buprestidae | α-pinene | Monoterpene produced by many coniferous trees, including Pinus and Picea species | Buprestis lineata | [45,60] |
| Staphylinidae | Isopropyl (Z9)-hexadecenoate | Female-produced aggregation pheromone | Aleochara curtula | [61,62] |
| Methyl alcohol | Tree derived volatile | Female Philonthus decorus | [63] | |
| Elateridae | Ethanol | Released by trees, especially stressed individuals | Alaus myops | [45] |
| Neryl butanoate | Female-produced aggregation pheromone | Agriotes acuminatus | [64] |
|
| Geranyl butanoate | Female-produced aggregation pheromone | Agriotes brevis; Agriotes lineatus; Agriotes sputator | [65,66] | |
| (E,E)- Farnesyl butanoate | Female-produced aggregation pheromone | Agriotes brevis | [65] |
|
| Geranyl octanoate | Female-produced aggregation pheromone | Agriotes lineatus; Agriotes obscurus | [66] |
|
| Geranyl isovalerate | Female-produced aggregation pheromone | Agriotes litigiosus fen. typicus & Agriotes litigosus var. laichartingi | [66] |
|
| Geranyl hexanoate | Female-produced aggregation pheromone | Agriotes obscurus; Agriotes sordidus | [66] |
|
| (E,E)-Farnesyl acetate | Female-produced aggregation pheromone | Agriotes ustulatus | [66] |
|
| 7-Methyloctyl-5-methylhexanoate | Female-produced aggregation pheromone | Elater ferrugineus | [67] |
|
| 7-Methyloctyl octanoate | Female-produced aggregation pheromone | Elater ferrugineus | [67] |
|
| 7-Methyloctyl-7-methyloctanoate | Female-produced aggregation pheromone | Elater ferrugineus | [67] |
|
| 7-Methyloctyl (Z)-4-decenoate | Female-produced aggregation pheromone | Elater ferrugineus | [67] |
| Treatments/ Chemical | Chemical purity (%) | Concentrations (uL mL-1) | Release rate* (mg day-1) |
|---|---|---|---|
| Acetoin | ≥96 | 61.87 | 1.48 |
| 3-Methyl-1-butanol | 98 | 39.87 | 0.98 |
| 2-Methyl-1-butanol | ≥99 | 20.92 | 0.56 |
| Isobutanol | ≥99 | 30.33 | 0.62 |
| 2-Methyl-2-butanol | 99 | 33.27 | 0.84 |
| FVOC mixture | 48.39 | 0.96 | |
| * Release rates determined by weight lost in the laboratory at 22°C | |||
| Compounds | Enantiomeric ratios | Chemical purity (%) | Source |
|---|---|---|---|
| Geranyl acetate | ≥97 | Sigma-Aldrich | |
| α-pinene | (-) | 98 | Sigma-Aldrich |
| Camphene | (-) | 90 | SAFC |
| β-Pinene | (+) | ≥94 | TCI Chemicals |
| 3-Carene | 90 | Sigma-Aldrich | |
| β-Myrcene | 90 | Sigma-Aldrich | |
| Limonene | (S)-(-) | 96 | Fluka Analytical |
| Terpinolene | ≥90 | SAFC | |
| Bornyl acetate | ≥99 | SAFC | |
| γ-Terpinene | 97 | Fluka Analytical | |
| Camphor | ≥95 | Fluka Analytical | |
| Borneol | (-) | 97 | Sigma-Aldrich |
| Group | Treatments | Cerambycidae | Scolytinae | Buprestidae | Elateridae | Staphylinidae |
|---|---|---|---|---|---|---|
| FVOC | 2-Methyl-1-butanol | 36 | 0 | 1 | 3 | 4 |
| 2-Methyl-2-butanol | 27 | 0 | 3 | 3 | 1 | |
| 3-Methyl-1-butanol | 31 | 0 | 1 | 1 | 2 | |
| Acetoin | 17 | 0 | 3 | 3 | 1 | |
| Isobutanol | 20 | 0 | 4 | 2 | 1 | |
| Control | 19 | 0 | 2 | 3 | 2 | |
| Mixture | 21 | 0 | 4 | 3 | 0 | |
| FVOC with pheromones | 2-Methyl-1-butanol | 15 | 148 | 4 | 3 | 2 |
| 2-Methyl-2-butanol | 8 | 118 | 1 | 7 | 2 | |
| 3-Methyl-1-butanol | 6 | 83 | 2 | 5 | 2 | |
| Acetoin | 45 | 95 | 3 | 2 | 2 | |
| Isobutanol | 15 | 85 | 1 | 1 | 4 | |
| Pheromone alone | 18 | 74 | 1 | 5 | 2 | |
| Mixture | 35 | 91 | 0 | 5 | 3 | |
| SVC | AP | 17 | 5 | 9 | 7 | 1 |
| Control | 14 | 6 | 9 | 6 | 2 | |
| GC | 16 | 4 | 15 | 2 | 0 | |
| Healthy | 16 | 3 | 13 | 15 | 0 | |
| LL | 21 | 5 | 5 | 0 | 2 | |
| OM | 13 | 4 | 6 | 7 | 1 | |
| EH | 26 | 3 | 5 | 13 | 0 | |
| Total | 436 | 724 | 92 | 96 | 34 |
| Subcortical beetle family | Known attractants | Key FVOCs | Key FVOCs when pheromones are added | Key SVCs |
|---|---|---|---|---|
| Curculionidae Sub-family: Scolytinae | Trans-verbenol, Exo-brevicomin, Terpinolene, Myrcene | N/A | 2-Methyl-1-butanol | N/A |
| Cerambycidae | 2-Methyl-1-butanol ⍺-Pinene | 2-Methyl-1-butanol | Acetoin, blend | Endocronartium harknessii |
| Buprestidae | Tree stress volatiles primarily | 2-Methyl-1-butanol | Isobutanol |
Leptographium longiclavatum, Endocronartium harknessii |
| Staphylinidae | Bark beetle pheromones, some monoterpenes | Isobutanol, blend | Acetoin | Grosmannia clavigera, Healthy tree |
| Elateridae | Bark beetle pheromones, some monoterpenes | N/A | 2-Methyl-2-butanol | Healthy tree, Endocronartium harknessii |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





