1. Introduction
Spinal and bulbar muscular atrophy (SBMA), also called Kennedy’s disease, is a rare, X-linked, late-onset neuromuscular disorder, caused by a CAG repeat expansion in the first exon of the androgen receptor gene [
1,
2]. The main pathological features of SBMA are a progressive lower motor neuron degeneration in brainstem and spinal cord. Additional features include myogenic lesions and a multisystem involvement mainly due to androgen insensitivity (see Manzano et al. [
3] for review).
SBMA course is usually prolonged, with an average 20 years interval between the onset of symptoms and death [
4]. The main life-threatening in SBMA is ventilatory failure caused by weakness of the bulbar and respiratory musculature [
5].
Respiratory failure, such as the swallowing disorders, may occur at advanced stages of the disease [
4], however, the natural history of the respiratory impairment in SBMA has not been well characterized. The importance of this topic lies in the fact that the monitoring of respiratory function is indispensable for the long-term care of SBMA patients, being critical not only in the respiratory but also for the nutritional management of the patients.
Only one longitudinal study has previously characterized respiratory function in SBMA subjects, showing correlations between motor function and both forced vital capacity (FVC) and peak expiratory flow (PEF) has been demonstrated[
6]. In this context, the present study aimed to analyze the trajectories of respiratory function tests such as maximum inspiratory pressure (MIP), maximum expiratory pressure (MEP), and FVC across eleven years of observation. In addition, we have explored the correlation between pulmonary function tests and clinical and biochemical serum indices.
Improved insight into the characterization of natural history of respiratory function in SBMA could guide therapeutic management, improve the timing of supportive care and can be used as an outcome measure for follow-up of patients or future treatment efficacy assessments.
2. Materials and Methods
2.1. Patients
This is a longitudinal, retrospective study carried out on medical records of genetically confirmed SBMA patients, referring to the Motor Neuron Disease Center of Siena University Hospital over 11 years. Exclusion criteria were competing disorders that could interfere with the pulmonary function test results, physical or cognitive impairments that made the execution of respiratory function tests impossible, and other concomitant conditions that could affect the prognosis and/or the SBMA evolution. The Local Ethics Committee, approved the study.
2.2. Clinical and Serum Biochemical Analysis
We collected demographic and genetic data, age at symptom onset and comorbidities. For clinical analysis, we used a validated, disease-specific functional rating scale for SBMA (SBMAFRS) [
7,
8]. As the SBMAFRS scale has been validated since 2015, the score in the first years of disease was obtained for each patient, by analyzing both the medical records and individual items of the Amyotrophic Lateral Sclerosis Functional Rating Scale. The latter scale was administered to patients before SBMAFRS validation.
The following serum biochemical indices were analyzed: creatinine, creatine phosphokinase(CPK), myoglobin (MYO), glycated haemoglobin (HbA1c), triglycerides(TG), total cholesterol (COL), alanine and aspartate aminotranferase (ALT, AST, respectively), gammaglutamyl transpeptidase (GGT), bilirubin, alkaline phosphatase (APh), uric acid.
2.3. Pulmonary Function Tests
All participants started with regular lung function tests, including FVC in upright seated positions and MIP and MEP in the upright position. The assessments were carried out by the respiratory physiotherapists in compliance with the American Thoracic Society/European Respiratory Society guidelines [
9]. FVC is the maximal volume of air exhaled with maximally forced effort from a maximal inspiration. MIP and MEP were measured by a non compressible face mask. The MIP was retrieved by exhaling to residual volume and then inhaling with as much effort as possible for at least 3 s. The MEP was retrieved similarly but the opposite way, by inhaling followed by a forced expiration. The maximum value of three maneuvers was used for final value of MIP and MEP.
All parameters were expressed as a percentage of predicted normal values [
10,
11,
12]; age, weight and height were recorded by the respiratory physiotherapist in occasion of every access.
2.4. Statistical Analysis
Data were tested for their normality (Kolmogorov- Smirnov test with Lilliefors correction) before choosing a parametric or non-parametric statistical test. Descriptive statistics for continuous variables are presented as the mean ± SD. Statistical significance was assumed if the p value was < 0.05.
An average time-curve was used to evaluate the decline of both respiratory function tests and serum biochemical indices of the SBMA patients through 11 years. One-way repeated-measures ANOVA was applied to assess statistical differences.
The Spearman’s rank correlation coefficient was used to calculate the strength of association between the pulmonary function test and serum biochemical and clinical indices.
The relation between spirometric data and both clinical and serum biochemical results was then studied by comparing the serum biochemical indices obtained with a MIP and MEP values below and above 50% of the predicted value (respectively), and with an FVC below or above 75% of the predicted value. We chose an FVC of 75% based on the results obtained in amyotrophic lateral sclerosis, in which a FVC of 75% rather than 50% is discussed as the threshold for the initiation of noninvasive positive-pressure ventilation (NIPPV), based on the fact the nocturnal hypoventilation was observed in patients with FVC of >75%[
13].
The Mann–Whitney test and unpaired t- test were used to find differences between the values.
All tests were performed with GraphPad Prism version 7.04.
3. Results
Nine SBMA patients were included in the study. Their mean age at disease onset was 47.78 ±6.9 years and the mean disease duration (calculated from the symptom onset) was of 17.8±6.1 years. The CAG repeat number ranged from 42 to 50 (mean 45±2.5). As expected [
14], there was a significant inverse correlation between CAG repeat number and age at disease onset (p=0.0015; r=-0.91). At baseline, no patient complained of significant respiratory or swallowing deficits. All the patients had mild-moderate weakness but none needed walking support. Comorbidities were: bland steatosis in three patients, juvenile myoclonic epilepsy and Brugada syndrome in one patient, and epilepsy from childhood in one patient. Four out nine patients never reported difficulty with breathing during the eleven years of observation, two reported dyspnea by the fifth year, two by the eighth year, one by the seventh year. All five patients had mild dyspnea, not significantly affecting daily life. Patients experience dyspnea or during sustained exertion or occasionally during movements that involve bending the body such as tying shoelaces. Two patients used NIPPV, both from the eighth year of observation. One patient died at the end of the eleventh year due to complications during PEG placement. Pulmonary function tests, blood chemistry tests and SBMAFS scale were performed twice a year for 11 years.
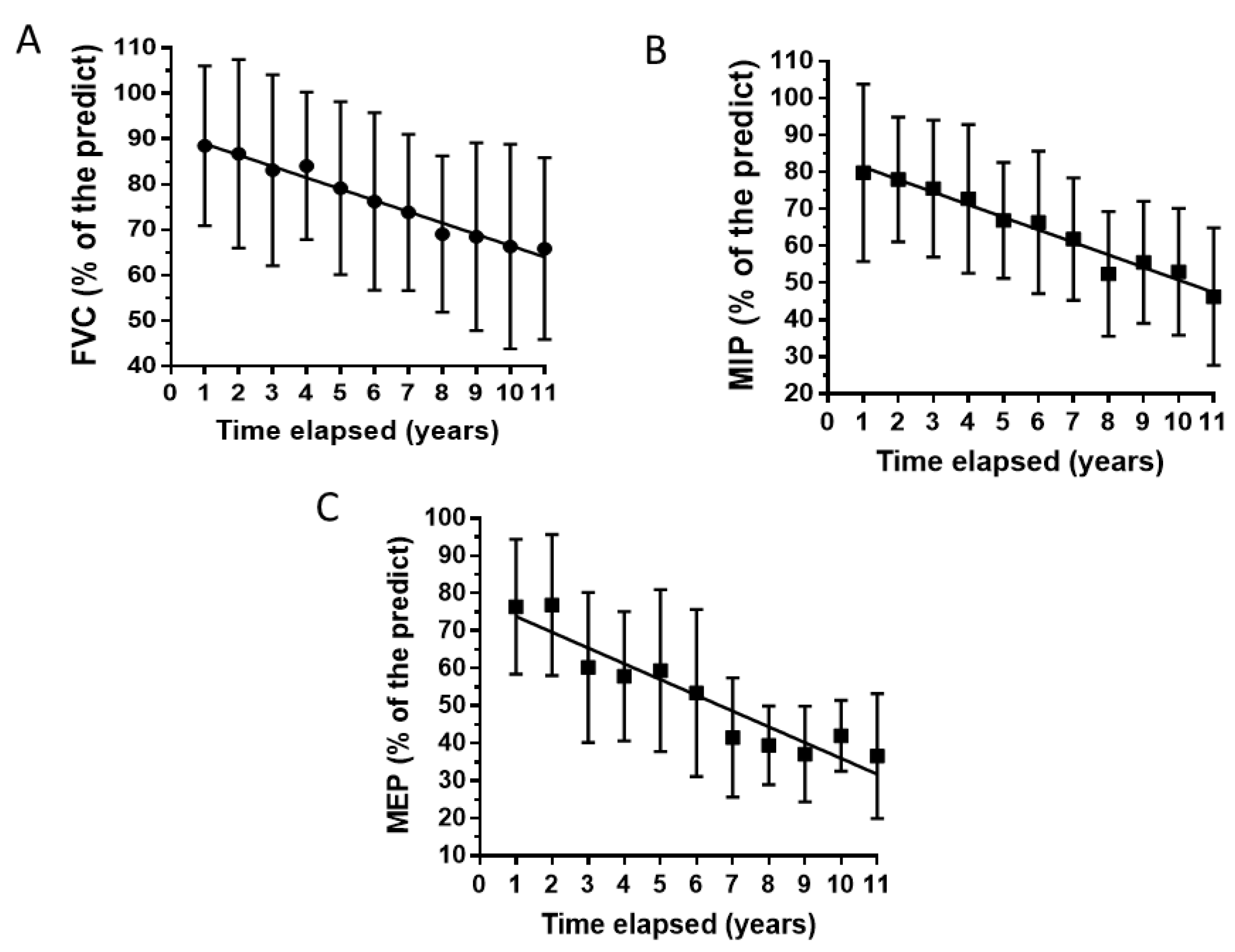
Figure 1 shows the average time-curves for FVC, MIP and MEP. All three respiratory function tests show a descending trend, with a significant statistical difference among the eleven years of measurements (One-way repeated-measures ANOVA: p values: 0.0036 for FVC and MEP and 0.0078 for MIP).
The average declines from the first to the eleventh year of observation were 25%, 42% and 52% for FVC, MIP and MEP respectively.
Table 1 reported the blood chemistry results.
The % of abnormal values are calculated considering all the measurement.s ALT, AST: alanine and aspartate aminotranferase respectively; CPK: creatine phosphokinase; GGT: gamma glutamyl transpeptidase; HbA1c: glycated haemoglobin; no: number; pt: patient
The values of CPK, MYO, ALT, AST were altered in all patients, whereas other markers of liver dysfunction were normal as well as the uric acid. HbA1 abnormality was a very rare event and a minority of patients had a moderate increase of TG. Creatinine values were below the reference values in 55.5% of cases, and total COL in 66%.
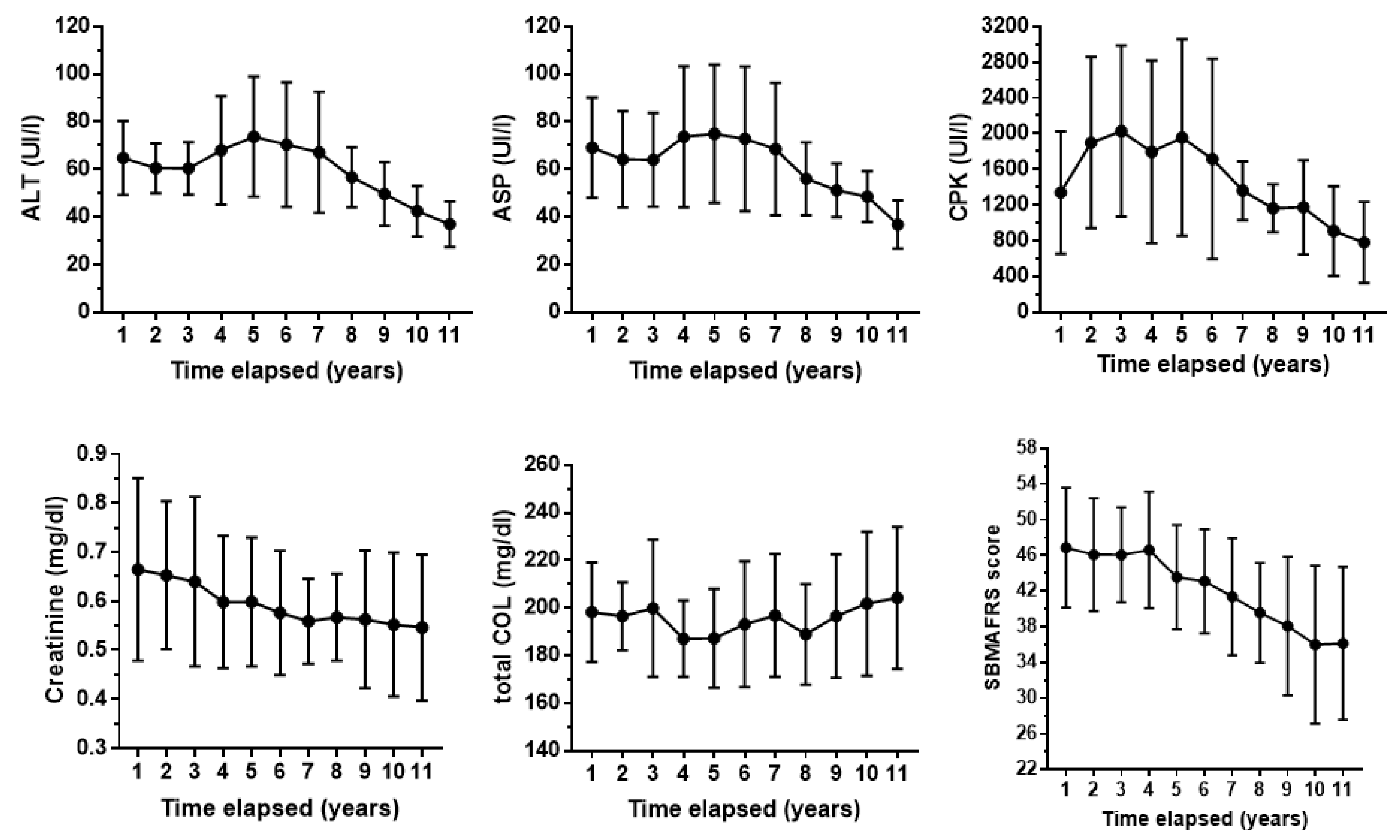
Figure 2 shows the average time-curves for some serum biochemical parameters and SBMAFRS.
At the eleventh year of evaluation, the average decline with respect to the first assessment was 17% for creatinine, 43% for ALT, 44% for ASP, 41% for CPK and 22% for SBMAFRS. The trend of creatinine is quite linear, while CPK and ALT/ AST decrease after a period of increase.
The correlations between serum parameters, clinical score and pulmonary function tests are reported in
Table 2. There is a significant correlation between SBMAFRS score and all three pulmonary function tests. MIP and MEP correlated with CPK and creatinine levels, and FVC correlates with ALT and AST. SBMAFRS score correlated with CPK and creatinine levels. ALT and AST correlated only with CPK levels.
Table 3 shows the clinical and serum biochemical indices obtained with a MIP and MEP values below and above 50% of the predicted value and with an FVC below or above 75% of the predicted value. SMBAFRS score, CPK and creatinine values were significantly lower in the case of MIP and MEP below the threshold reported above. SMBAFRS score was lower also for FVC below 75%. ALT and ASP were significantly lower in the case of MIP below 50%.
4. Discussion
Spirometry is the most widely used volitional test assessing lung volumes and capacity as a function of time. Given its role in diagnosis and management of several conditions affecting the respiratory system, spirometry is included in the care recommendations for several neuromuscular disorders [
15,
16,
17]. The lack of consideration for this issue in SBMA is likely due to the assumption that respiratory function is preserved in most patients with SBMA [
5]. The present longitudinal study shows a progressive decrease in the values of MIP, MEP and FVC over 11 years of observation in SBMA patients. Some of them (2 out of 9 patients) were severe enough to require the use of NIPPV. The SBMA group showed a close correlation between the three spirometric indices and the SBMAFRS score, suggesting that the clinical decline is closely linked to the worsening of spirometric data. The decrease in MEP and MIP values was severe, while it was mild for FVC. Indeed, measures of respiratory muscle strength decreased earlier than lung volumes in neuromuscular diseases [
18,
19], which is likely why they are more used than the measures of vital capacity in detecting respiratory muscle weakness [
19,
20,
21].
MIP is closely related to diaphragmatic strength, while MEP is generated through the abdominal and intercostals muscles. In our study, we observed a correlation between spirometry indices and CPK level for MIP and MEP but not for FVC. The lack of correlation was also confirmed by considering CPK values corresponding to FVC < 75%. As regards MIP and MEP, the correlations between CPK and these spirometric values are strengthened by the decrease of the latter (table 3). Among the other biochemical indices, the only other significant correlations were between ALT, ASP, creatinine and MIP < 50%. All the other serum indices did not correlate with spirometric parameters.
Most CPK are located in skeletal muscle and their elevation is affected by muscle mass and muscle damage; serum creatinine is a waste product from skeletal muscle creatine metabolism and is considered a predictive biomarker for SBMA [
22,
23]; finally, the increased transaminases are likely derived from muscle because almost all of these patients had normal GGT, Bilirubin, and APh. In this respect, the strong relationship between CPK and both transaminases and creatinine as well as between CPK and MIP/MEP, lend support to the hypothesis that the spirometric parameters assessed here are related to muscle denervation.
Our results support the findings of the previous study on spirometry in SBMA patients, which demonstrated the superiority of PEF over FVC in highlighting spirometric alterations in SBMA[
6]. PEF depends indeed upon the strength of abdominal and intercostals muscles. On this basis, one could assume that in SBMA, by using longitudinal data, spirometric measures relative to maximal strength of the respiratory muscles may have a better predictive value for pulmonary and muscular decline compared to FVC.
We believe that the temporal trend of serum values may be interesting since despite the relatively low number of patients studied, these values were analyzed longitudinally, twice a year for 11 years. Such an extensive evaluations are present in the literature for a maximum of 3 years [
24,
25,
26]. We showed that the serum levels of CPK, ALT, and AST are elevated in the early phase of the disease, while these levels gradually decline in the more advanced stages of the disease. In the last few years of observation, transaminases in some patients even reach normal values. Creatinine values have slightly declined through the years, while all the other biochemical indices did not show substantial changes, apart from a mild elevation of cholesterol. Our results are in line with those of Atsuta et al. [
4] obtained in 263 SBMA patients in which age-related changes of laboratory data were analyzed. In line with previous studies [
24,
26,
27] we confirmed that in SBMA the creatinine levels are unrelated to those of CPK, being CPK values a marker of muscle injury rather than muscle function.
In conclusions, we showed here that I) respiratory failure in SBMA it is not uncommon when analyzed through spirometry; 2) MIP and MEP may detect respiratory insufficiency earlier than FVC being likely related to muscle denervation.
Main limitation is due to the small patient sample size. However, the long period of observation and analysis of patients can be certainly a strong point of this monocentric study.
Author Contributions
Conceptualization: F.G.: S.C. Methodology, C.B.; F.C.; P.P. Formal analysis, F.G. E.E. Writing original draft preparation, F.G.; Writing review and editing, E.B.; N.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kennedy, W.R.; Alter, M.; Sung, J.H. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology 1968, 18, 671–680. [Google Scholar] [CrossRef] [PubMed]
- La Spada, A.R.; Wilson, E.M.; Lubahn, D.B.; Harding, A.E.; Fischbeck, K.H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature 1991, 352, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Manzano, R.; Sorarú, G.; Grunseich, C.; Fratta, P.; Zuccaro, E.; Pennuto, M.; Rinaldi, C. Beyond motor neurons: expanding the clinical spectrum in Kennedy's disease. J. Neurol. Neurosurg. Psychiatry 2018, 89, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, N.; Watanabe, H.; Ito, M.; Banno, H.; Suzuki, K.; Katsuno, M.; Tanaka, F.; Tamakoshi, A.; Sobue, G. Natural history of spinal and bulbar muscular atrophy (SBMA): a study of 223 Japanese patients. Brain 2006, 129, 1446–1455. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, A.; Katsuno, M.; Suzuki, K.; Hirakawa, A.; Hijikata, Y.; Yamada, S.; Inagaki, T.; Banno, H.; Sobue, G. Long-term treatment with leuprorelin for spinal and bulbar muscular atrophy: natural history-controlled study. J. Neurol. Neurosurg. Psychiatry. 2017, 88, 1026–1032. [Google Scholar] [CrossRef]
- Yamada, S.; Hashizume, A.; Hijikata, Y.; Inagaki, T.; Suzuki, K.; Kondo, N.; Kawai, K.; Noda, S.; Nakanishi, H.; Banno, H.; Hirakawa, A.; Koike, H.; Halievski, K.; Jordan, C.L.; Katsuno, M.; Sobue, G. Decreased Peak Expiratory Flow Associated with Muscle Fiber-Type Switching in Spinal and Bulbar Muscular Atrophy. PLoS One. 2016, 11, e0168846. [Google Scholar] [CrossRef]
- Hashizume, A.; Katsuno, M.; Suzuki, K.; Banno, H.; Suga, N.; Mano, T.; Araki, A.; Hijikata, Y.; Grunseich, C.; Kokkinis, A.; Hirakawa, A.; Watanabe, H.; Yamamoto, M.; Fischbeck, K.H.; Sobue, G. A functional scale for spinal and bulbar muscular atrophy: Cross-sectional and longitudinal study. Neuromuscul. Disord. 2015, 25, 554–62. [Google Scholar] [CrossRef]
- Querin, G.; DaRe, E.; Martinelli, I.; Bello, L.; Bertolin, C.; Pareyson, D.; Mariotti, C.; Pegoraro, E.; Sorarù, G. Validation of the Italian version of the SBMA Functional Rating Scale as outcome measure. Neurol. Sci. 2016, 37, 1815–1821. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; Jensen, R.; Johnson, D.C.; MacIntyre, N.; McKay, R.; Navajas, D.; Pedersen, O.F.; Pellegrino, R.; Viegi, G.; Wanger, J.; ATS/ERS Task Force. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–38. [Google Scholar] [CrossRef]
- American Thoracic Society/European Respiratory Society ATS/ERS Statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 2002, 66, 518–624.
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; Stocks, J. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.H.; Cooke, N.T.; Edwards, R.H.; Spiro, S.G. Predicted normal values for maximal respiratory pressures in caucasian adults and children. Thorax 1984, 39, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Prell, T.; Ringer, T.M.; Wullenkord, K.; Garrison, P.; Gunkel, A.; Stubendorff, B.; Witte, O.W.; Grosskreutz, J. Assessment of pulmonary function in amyotrophic lateral sclerosis: when can polygraphy help evaluate the need for non-invasive ventilation? J. Neurol. Neurosurg. Psychiatry. 2016, 87, 1022–1026. [Google Scholar] [CrossRef] [PubMed]
- Bellai, D.J.; Rae, M.G. A systematic review of the association between the age of onset of spinal bulbar muscular atrophy (Kennedy's disease) and the length of CAG repeats in the androgen receptor gene. eNeurologicalSci. 2024, 34, 100495. [Google Scholar] [CrossRef]
- Pierce, R. Spirometry: an essential clinical measurement. Aust. Fam. Physician. 2005, 34, 535–539. [Google Scholar]
- Boentert, M.; Wenninger, S.; Sansone, V.A. Respiratory involvement in neuromuscular disorders. Current Opinion in Neurology. 2017, 30, 529–537. [Google Scholar] [CrossRef]
- Pirola, A.; De Mattia, E.; Lizio, A.; Sannicolò, G.; Carraro, E.; Rao, F.; Sansone, V.; Lunetta, C. The prognostic value of spirometric tests in Amyotrophic Lateral Sclerosis patients. Clin. Neurol. Neurosurg. 2019, 184, 105456. [Google Scholar] [CrossRef]
- Cattapan, S.E.; Laghi, F.; Tobin, M.J. Can diaphragmatic contractility be assessed by airway twitch pressure in mechanically ventilated patients? Thorax 2003, 58, 58–62. [Google Scholar] [CrossRef]
- Tilanus, T.B.M.; Groothuis, J.T; TenBroek-Pastoor, J.M.C.; Feuth, T.B.; Heijdra, Y.F.; Slenders, J.P.L.; Doorduin, J.; Van Engelen, B.G.; Kampelmacher, M.J.; Raaphorst, J. The predictive value of respiratory function tests for non-invasive ventilation in amyotrophic lateral sclerosis. Respir. Res. 2017, 18, 144. [Google Scholar] [CrossRef]
- De Troyer, A.; Borenstein, S.; Cordier, R. Analysis of lung volume restriction in patients with respiratory muscle weakness. Thorax. 1980, 35, 603–610. [Google Scholar] [CrossRef]
- Wenninger, S.; Stahl, K.; Wirner, C.; Einvag, K.; Thiele, S.; Walter, M.C.; Schoser, B. Utility of maximum inspiratory and expiratory pressures as a screening method for respiratory insufficiency in slowly progressive neuromuscular disorders. Neuromuscul. Disord. 2020, 30, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Hijikata, Y.; Katsuno, M.; Suzuki, K.; Hashizume, A.; Araki, A.; Yamada, S.; Inagaki, T.; Iida, M.; Noda, S.; Nakanishi, H.; Banno, H.; Mano, T.; Hirakawa, A.; Adachi, H.; Watanabe, H.; Yamamoto, M.; Sobue, G. Impaired muscle uptake of creatine in spinal and bulbar muscular atrophy. Ann. Clin. Transl. Neurol. 2016, 3, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Hijikata, Y.; Hashizume, A.; Yamada, S.; Inagaki, T.; Ito, D.; Hirakawa, A.; Suzuki, K.; Atsuta, N.; Tsuboi, T.; Hattori, M.; Hori, A.; Banno, H.; Sobue, G.; Katsuno, M. Biomarker-based analysis of preclinical progression in spinal and bulbar muscular atrophy. Neurology 2018, 90, e1501–e1509. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, A.; Katsuno, M.; Banno, H.; Suzuki, K.; Suga, N.; Mano, T.; Atsuta, N.; Oe, H.; Watanabe, H.; Tanaka, F.; Sobue, G. Longitudinal changes of outcome measures in spinal and bulbar muscular atrophy. Brain J. Neurol. 2012, 135, 2838–2848. [Google Scholar] [CrossRef]
- Dahlqvist, J.R.; Fornander, F.; de Stricker Borch, J.; Oestergaard, S.T.; Poulsen, N.S.; Vissing, J. Disease progression and outcome measures in spinobulbar muscular atrophy. Ann. Neurol. 2018, 84, 754–765. [Google Scholar] [CrossRef]
- Blasi, L.; Sabbatini, D.; Fortuna, A.; Querin, G.; Martinelli, I.; Vianello, S.; Bertolin, C.; Pareyson, D.; Pennuto, M.; Pegoraro, E.; Bello, L.; Sorarù, G. The value of serum creatinine as biomarker of disease progression in spinal and bulbar muscular atrophy (SBMA). Sci. Rep. 2023, 13, 17311. [Google Scholar] [CrossRef]
- Lombardi, V.; Querin, G.; Ziff, O.J.; Zampedri, L.; Martinelli, I.; Heller, C.; Foiani, M.; Bertolin, C.; Lu, C.H.; Malik, B.; Allen, K.; Rinaldi, C.; Zetterberg, H.; Heslegrave, A.; Greensmith, L.; Hanna, M.; Soraru, G.; Malaspina, A.; Fratta, P. Muscle and not neuronal biomarkers correlate with severity in spinal and bulbar muscular atrophy. Neurology 2019, 92, e1205–e1211. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).