1. Introduction
A hallmark of cancer is altered cellular metabolism. An early observation of such has been described as the Warburg effect, wherein rapidly dividing cancer cells shift their metabolism of glucose to lactate. For gliomas, the identification of isocitrate dehydrogenase (IDH) mutations as a critical marker of tumor behavior and prognosis has invigorated efforts to better understand glioma metabolism [
1]. Outside of the nervous system, systemic metabolic dysregulation manifesting as the metabolic syndrome has been associated with increased prevalence of systemic cancers [
2]. Recent studies have explored this association with gliomas but have had conflicting results [
3,
4,
5]. The purpose of this study is to test the hypothesis that patients with pre-existing metabolic syndrome have an increased prevalence of IDH-wild type glioblastoma and worse survival outcomes. This study emphasizes the examination of patients solely with IDH-wild type glioblastoma as IDH-mutant gliomas are known to have different metabolic features [
6].
2. Materials and Methods
A retrospective case-control study was conducted examining 73 patients with pathologically confirmed glioblastoma isocitrate dehydrogenase (IDH)-wild type treated at the University of California, Davis between 2018 and 2023. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of California, Davis, 95817 on April 23, 2023. All patients were adults, and they underwent surgical resection followed by standard of care chemoradiation [
7]—though some were unable to complete treatment due to poor functional status or decision to transition to hospice care. Electronic medical records were reviewed to identify whether these patients had developed the metabolic syndrome prior to diagnosis of the tumor. Records reviewed included progression free survival, overall survival, treatment, pathologic diagnosis, tumor molecular characteristics, blood pressure, body mass index, medications, and laboratory data.
Metabolic syndrome was defined based on the internationally agreed upon definition [
8]. Based on this definition, patients meet criteria for the metabolic syndrome if they have three of the five following criteria: obesity, hypertension, hyperglycemia, decreased high density lipoprotein (HDL), and hypertriglyceridemia. Obesity is defined as body mass index (BMI) greater than 30 kg/m
2. Hypertension is defined as systolic blood pressure greater that 130, diastolic blood pressure greater than 90, or currently taking antihypertensives. Hyperglycemia is determined by fasting blood glucose greater than 100, HgA1c greater than 6.0, or patient currently taking antihyperglycemics. Decreased HDL must be less than 40 mg/dL in males or less than 50mg/dL in females; it is also defined as patient currently taking a statin medication. Hypertriglyceridemia requires a triglyceride level greater than 150mg/dL. It is important to emphasize that these features were captured prior to tumor diagnosis and treatment, since certain treatments can exacerbate these metabolic risk factors (e.g., steroid use).
For calculation of overall survival, the date of diagnosis was defined as the date of the first surgery that led to tumor diagnosis. Date of death was determined as either the confirmed death date or the final electronic medical record (EMR) entry. All patients in this study died from this malignancy. Statistical analyses included unpaired t-tests when comparing two groups and linear regression models for comparison of two continuous variables. Kaplan-Meier curves were used to depict overall survival between groups over time. Statistical significance was drawn for p-values less than 0.05.
3. Results
3.1. Patient Characteristics
Table 1 depicts the characteristics of the patients involved in this study. All seventy-three patients had IDH-wild type glioblastoma. Metabolic syndrome was identified in 41% of the patients (30/73) (
Table 1), which is greater than the estimated prevalence of the metabolic syndrome of 33% in the general adult population in the United States [
9]. Patients with metabolic syndrome were significantly older than those without (69.7 vs 62.1 years, p = 0.01) (
Supplementary Figure S1). Thirty patients (41%) met the above criteria for metabolic syndrome, of which 10 (14%) were female and 20 (27%) were male. Among those without metabolic syndrome, 15 (21%) were female and 28 (38%) were male. There was a similar distribution of race between the two groups, with White race making up 77% of those with metabolic syndrome and 65% of those without. Those without the metabolic syndrome tended to have a higher prevalence of gross total resection, methylguanine methyltransferase (MGMT) methylation, and epidermal growth factor receptor (EGFR) amplification (
Table 1).
3.2. Metabolic Syndrome and Individual Risk Factors Are Associated with Worsened Overall Survival
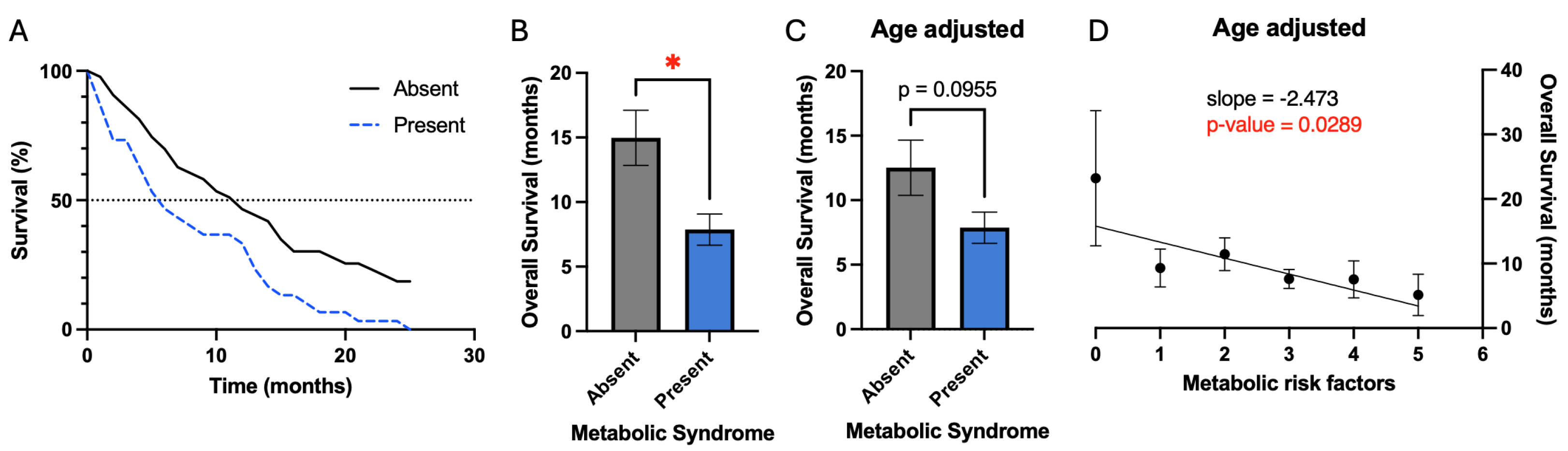
Overall survival was defined from the date of the first surgery to the date of death. Patients with metabolic syndrome had a significant reduction in mean overall survival (7.9 vs. 15.0 months, p-value 0.01) prior to correcting for confounding variables (
Figure 1B). Kaplan-Meier plot also demonstrates this result and demonstrates reduction in median overall survival for patients with metabolic syndrome (5.8 vs. 11.4 months) (
Figure 1A). Evaluation for confounding variables demonstrated that patients with metabolic syndrome were older on average (
Table 1). Age also demonstrated a significant negative association with overall survival (
Figure 3A). After correction for age differences between patients with and without metabolic syndrome, metabolic syndrome demonstrated a trend for worsened survival (7.9 vs. 12.5 months, p = 0.1) (
Figure 1C).
Given this trend, we conjectured that rather than evaluating survival simply based upon the criteria of metabolic syndrome, it may be valuable to evaluate metabolic risk factors individually and as patients accumulate additional risk factors. As patients accumulate additional risk factors, there is a significant negative association with overall survival (slope = -2.5 months per additional risk factor, p = 0.03) (
Figure 1D). However, the largest change in survival resulted from accumulation of the first risk factor, while subsequent risk factors had less of an effect on survival.
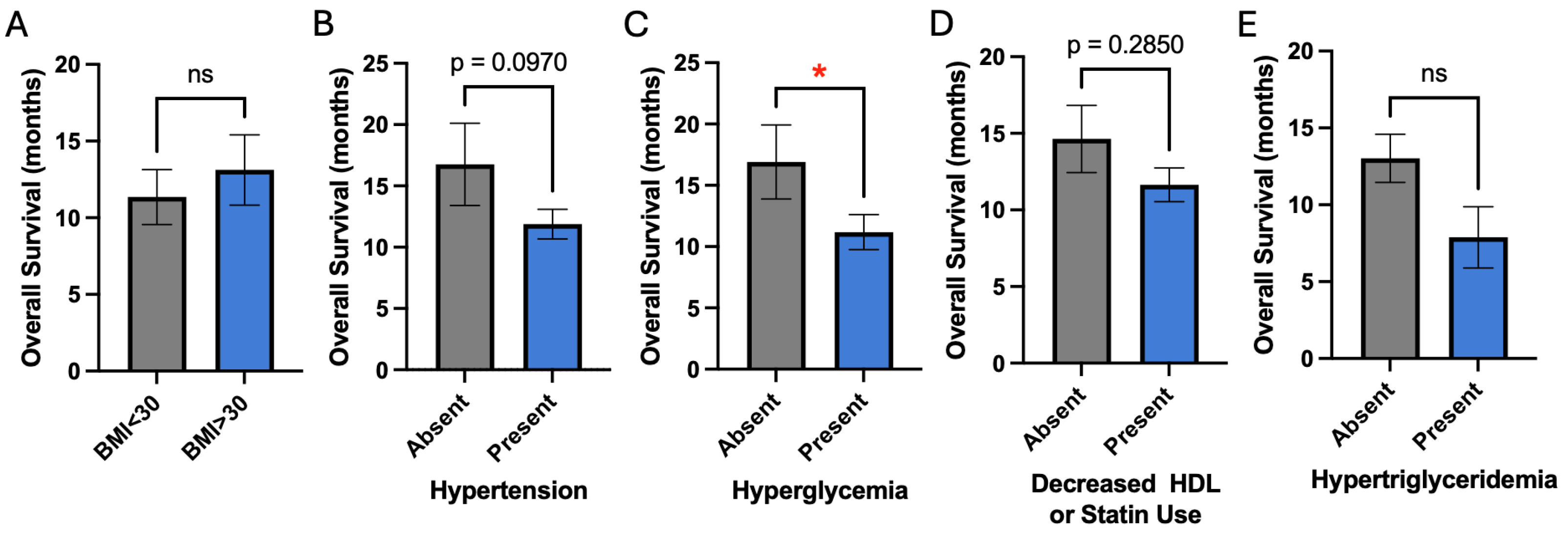
We next examined each metabolic risk factor individually as it affects overall survival in our patients. Given that we previously identified a strong negative association between age and overall survival, all subsequent analyses include a correction factor for age differences. Despite correction for age differences, hyperglycemia demonstrated a significant negative association with overall survival (11.2 vs. 16.9 years, p = 0.05) (
Figure 2C). Hypertension demonstrated a trend for worsened overall survival (11.9 vs. 16.8 years, p = 0.1) (
Figure 2B). Obesity, decreased HDL, and hypertriglyceridemia did not affect overall survival (
Figure 2A, D, E).
Figure 2.
Association of individual metabolic risk factors with overall survival. A, B, D, and E) BMI, hypertension, decreased HDL or statin use, and hypertriglyceridemia are not associated with survival. C) Hyperglycemia is significantly associated with worsened survival.
Figure 2.
Association of individual metabolic risk factors with overall survival. A, B, D, and E) BMI, hypertension, decreased HDL or statin use, and hypertriglyceridemia are not associated with survival. C) Hyperglycemia is significantly associated with worsened survival.
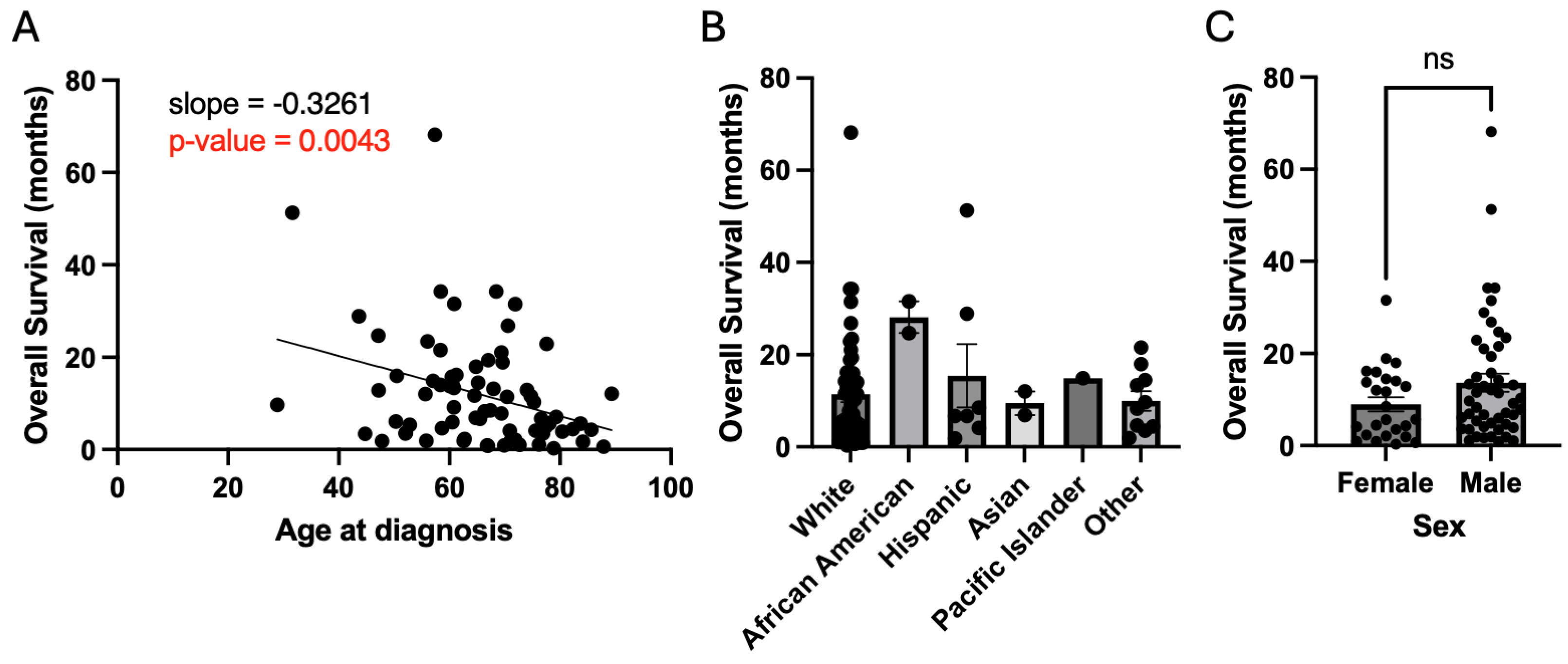
Analyses were performed to examine whether the following patient characteristics affected overall survival: age, race, sex, resection status, MGMT methylation status, and EGFR amplification status. As discussed previously, age demonstrated a strong negative association with overall survival (slope = -0.33 months of survival per year of age, p = 0.004) (
Figure 3A). Race and sex did not lead to a significant effect on overall survival (
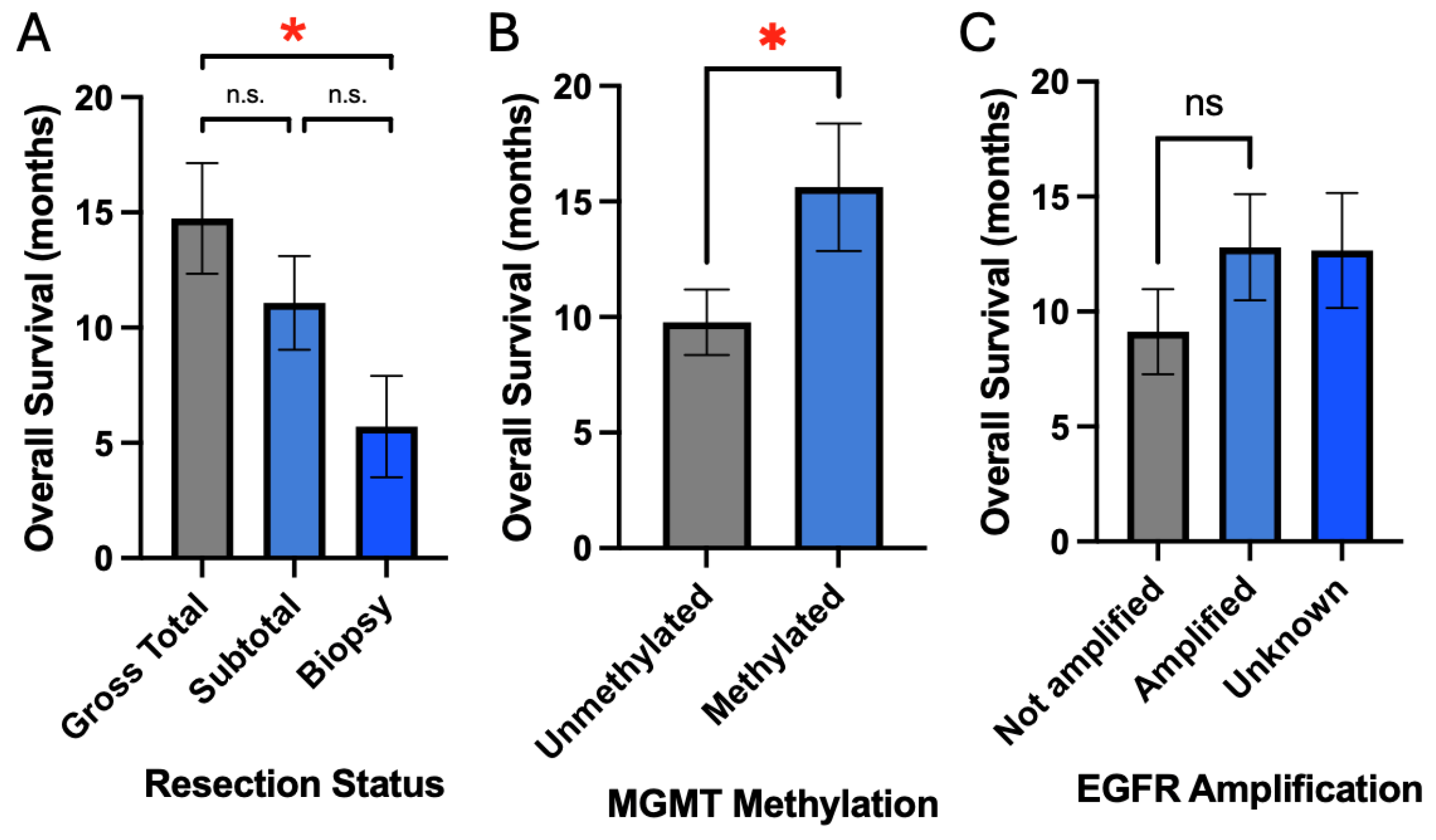
Figure 3B, C). Resection status did demonstrate a positive association with survival with gross total resection (GTR) having greater overall survival compared to biopsy (14.8 months vs. 5.7 months, p = 0.05) (
Figure 4A). MGMT methylation was also associated with improved survival compared to unmethylated status (15.6 months vs. 9.8 months, p = 0.04) (
Figure 4B). EGFR amplification was not associated with survival (12.8 months vs. 9.1 months, p = 0.29) (
Figure 4C).
Figure 3.
Patient demographics and overall survival. A) Older age at diagnosis has a directly proportional association with worsened survival. B and C) Race and sex are not associated with survival.
Figure 3.
Patient demographics and overall survival. A) Older age at diagnosis has a directly proportional association with worsened survival. B and C) Race and sex are not associated with survival.
Figure 4.
Extent of resection and tumor molecular markers are associated with overall survival. A) Increased extent of resection is associated with improved survival. B and C) MGMT methylation but not EGFR amplification is associated with improved survival.
Figure 4.
Extent of resection and tumor molecular markers are associated with overall survival. A) Increased extent of resection is associated with improved survival. B and C) MGMT methylation but not EGFR amplification is associated with improved survival.
4. Discussion
The metabolic syndrome has been associated with increased prevalence and decreased survival in multiple types of cancer, including breast, liver, colorectal, bladder, pancreatic, and endometrial [
2], though a mechanistic understanding is lacking. One hypothesized mechanism suggests that metabolic dysregulation leads to a systemic inflammatory state in which cytokines and growth factors can facilitate cancer cell proliferation [
10]. Another proposed mechanism is that the metabolic syndrome leads to insulin resistance and hyperinsulinemia that leads to increased insulin-like growth factor (IGF 1) activity, which may lead to the development and progression of tumors [
11].
4.1. Association between the Prevalence of the Metabolic Syndrome and the Development of Glioblastoma
On the other hand, the association between the metabolic syndrome and glioblastoma pathogenesis has not been sufficiently explored. Three prior studies have examined this association with conflicting results. Rogers et al. examined a cohort of patients from Cleveland, Ohio and demonstrated a slightly higher prevalence of the metabolic syndrome among patients who developed glioblastoma compared to the general population (35.6% vs 34.7%, respectively) [
3]. McManus et al. similarly examined this association in a cohort of patients from New Zealand and also showed a slight increase in prevalence of the metabolic syndrome among patients who developed glioblastoma (18.2% vs 16%) [
4]. Lucas et al. examined a cohort of patients from Portugal and demonstrated the opposite association, having observed a much lower prevalence of the metabolic syndrome among patients who developed glioblastoma (11.1% vs 32.7%) [
5]. Our results demonstrate a higher prevalence of the metabolic syndrome in patients who developed glioblastoma (41% vs 33%) (
Table 1). These findings are interesting but are limited to retrospective studies and as such future prospective studies are needed to better evaluate this association. If the association is validated, then metabolic risk factor modification could prove to be a promising approach to the treatment of patients with glioblastoma. Further, studies examining the mechanisms underlying this association could identify future targets for drug development.
4.2. Association between the Metabolic Syndrome and Overall Survival in Patients with Glioblastoma
Two of the previously referenced studies demonstrated that the metabolic syndrome was associated with worsened survival. McManus et al. demonstrated a significantly decreased overall survival in patients with the metabolic syndrome (8.0 months vs 13.0 months, p = 0.016) [
4]. Rogers et al. demonstrated only a trend towards worsened survival in patients with the metabolic syndrome (7.7 months vs 12.7 months, p = 0.22); however, they identified a significant association of the metabolic syndrome with decreased survival in a subset of patients who completed the full schedule of concurrent chemoradiation (12.4 months vs 17.9 months, p = 0.18). Lucas et al. on the other hand demonstrated a trend towards improved survival in patients with the metabolic syndrome who later developed glioblastoma (19.8 months vs 17.7 months, p = 0.085) [
5]. Our results in
Figure 1 demonstrate that the metabolic syndrome in patients who later developed glioblastoma is associated with a trend toward decreased survival (
7.9 vs 12.5 months, p = 0.1)—after correction for the confounding variable of age, which itself is a significant prognostic factor. Interestingly, there was a significant association between the accumulation of additional metabolic risk factors and decreased overall survival (
Figure 1d). To our knowledge, this is the first study to demonstrate this somewhat linear association between the number of metabolic risk factors a patient has with overall survival.
4.3. Association of Hyperglycemia with Worsened Overall Survival
In our analysis, hyperglycemia emerged as an independent risk factor associated with decreased overall survival (
11.2 vs. 16.9 years, p = 0.05) (
Figure 2), while hypertension demonstrated a trend toward worsened survival. Hyperglycemia in patients prior to the diagnosis of glioblastoma has been demonstrated by multiple studies to be associated with decreased overall survival [
12,
13,
14]. Multiple hypotheses have been proposed to explain why hyperglycemia may worsen survival. As gliomas are reliant on glycolysis instead of aerobic respiration even in the presence of oxygen, the excess availability of glucose to these cancer cells can facilitate tumor growth [
15]. Another mechanism suggests that insulin resistance and hyperinsulinemia can facilitate tumor growth via IGF-1-mediated signaling [
11,
16]. This association between hyperglycemia and worsened overall survival challenges the commonplace usage of corticosteroids for the treatment of post-operative and tumor-related cerebral edema [
17]. Further, multiple studies are beginning to try to target hyperglycemia and glucose metabolism in the treatment of glioblastoma, though results thus far have not been compelling [
18,
19,
20,
21].
4.4. Strengths and Limitations
The prior studies included both IDH wild type and IDH mutant tumors in their analyses, though the proportion of patients with IDH mutant tumors was generally small and likely not to have affected their results considerably [
3,
4,
5]. As IDH mutant tumors have been shown to undergo different metabolism [
6], this study focused solely on IDH wild type tumors, which improves the generalizability of our findings.
This study also demonstrated that extent of resection and MGMT promoter methylation status were important prognostic factors that were significantly associated with overall survival. These results also validate the generalizability of our dataset by re-demonstrating these associations with overall survival [
22,
23]
.
There are limitations to the results presented in this study. This study had a limited sample size leading to relatively low power of the statistical results. Further, as a retrospective study, the results presented here are associations from which causality should not be assumed. Additionally, the retrospective nature of this study meant that there were group differences for which correction had to be made in order to make broader inferences. For example, patients with the metabolic syndrome were significantly older and age was significantly associated with worsened survival; thus, correction for age had to be performed to examine the effect of the metabolic syndrome on survival. Future prospective clinical studies and further mechanistic research are critical to clarify whether there is a meaningful association between the metabolic syndrome and survival in patients who develop glioblastoma.
5. Conclusions
The metabolic syndrome has been associated with tumorigenesis and decreased survival in multiple cancer types, though its association with glioma pathogenesis remains unclear. This study demonstrates that the metabolic syndrome has a higher prevalence in patients with glioblastoma compared to the general population and is associated with decreased overall survival. Hyperglycemia appears to be the strongest driver of this finding, which has been supported by multiple prior studies. Additional prospective clinical studies and mechanistic research are needed to better evaluate these associations. If confirmed, clinical strategies to target metabolic risk factors may play an important role in the treatment of patients with glioblastoma.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Figure S1. A) Patients with the metabolic syndrome were significantly older than patients without the metabolic syndrome. B) Patients with the metabolic syndrome demonstrated a trend towards higher KI67 index than those without the metabolic syndrome.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, JPA and OA; methodology and design, JPA and OA; data acquisition, JPA ; data analysis and interpretation, JPA; drafting of manuscript, JPA and OA; revision of manuscript and approval of final version, JPA and OA. Both listed authors participated in the writing of the manuscript and have read and approved the final version.
Funding
Dr. Aboud is supported in part by the UC Davis Paul Calabresi Career Development Award for Clinical Oncology as funded by the National Cancer Institute, National Institutes of Health through grant #2K12CA138464-11.
Institutional Review Board Statement
This project was reviewed by the Institutional Review Board of the University of California, Davis and was given an “exempt” status on April 11, 2023.
Data Availability Statement
All data can be made available upon request.
Acknowledgments
The authors would like to thank Dr. Robert O’Donnell for his critical review of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yan, H.; Parsons, D.W.; Jin, G.; McLendon, R.; Rasheed, B.A.; Yuan, W.; Kos, I.; Batinic-Haberle, I.; Jones, S.; Riggins, G.J.; et al. IDH1 and IDH2 Mutations in Gliomas. The New England journal of medicine 2009, 360, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Mili, N.; Paschou, S.A.; Goulis, D.G.; Dimopoulos, M.A.; Lambrinoudaki, I.; Psaltopoulou, T. Obesity, metabolic syndrome, and cancer: pathophysiological and therapeutic associations. Endocrine 2021, 74, 478–497. [Google Scholar] [CrossRef]
- Rogers, L.R.; Ostrom, Q.T.; Schroer, J.; Vengoechea, J.; Li, L.; Gerson, S.; Nock, C.J.; Machtay, M.; Selman, W.; Lo, S.; et al. Association of metabolic syndrome with glioblastoma: a retrospective cohort study and review. Neurooncol Pract 2020, 7, 541–548. [Google Scholar] [CrossRef]
- McManus, E.J.; Frampton, C.; Tan, A.; Phillips, M.C.L. Metabolics risk factors in a New Zealand glioblastoma cohort. Neurooncol Pract 2022, 9, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.; Carvalho, B.; Tuna, R.; Linhares, P. Metabolic Syndrome and Survival in Glioblastoma Patients: Retrospective Cohort Study and Review of the Literature. Cureus 2024, 16, e53641. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Hirode, G.; Wong, R.J. Trends in the Prevalence of Metabolic Syndrome in the United States, 2011-2016. Jama 2020, 323, 2526–2528. [Google Scholar] [CrossRef]
- Harvey, A.E.; Lashinger, L.M.; Hursting, S.D. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci 2011, 1229, 45–52. [Google Scholar] [CrossRef]
- Renehan, A.G.; Frystyk, J.; Flyvbjerg, A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab 2006, 17, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Derr, R.L.; Ye, X.; Islas, M.U.; Desideri, S.; Saudek, C.D.; Grossman, S.A. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol 2009, 27, 1082–1086. [Google Scholar] [CrossRef]
- Lu, V.M.; Goyal, A.; Vaughan, L.S.; McDonald, K.L. The impact of hyperglycemia on survival in glioblastoma: A systematic review and meta-analysis. Clin Neurol Neurosurg 2018, 170, 165–169. [Google Scholar] [CrossRef]
- Tieu, M.T.; Lovblom, L.E.; McNamara, M.G.; Mason, W.; Laperriere, N.; Millar, B.A.; Ménard, C.; Kiehl, T.R.; Perkins, B.A.; Chung, C. Impact of glycemia on survival of glioblastoma patients treated with radiation and temozolomide. J Neurooncol 2015, 124, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.A.; Asuthkar, S.; Guda, M.R.; Tsung, A.J.; Velpula, K.K. Cancer stem cell molecular reprogramming of the Warburg effect in glioblastomas: a new target gleaned from an old concept. CNS Oncol 2016, 5, 101–108. [Google Scholar] [CrossRef]
- Liu, E.K.; Vasudevaraja, V.; Sviderskiy, V.O.; Feng, Y.; Tran, I.; Serrano, J.; Cordova, C.; Kurz, S.C.; Golfinos, J.G.; Sulman, E.P.; et al. Association of hyperglycemia and molecular subclass on survival in IDH-wildtype glioblastoma. Neurooncol Adv 2022, 4, vdac163. [Google Scholar] [CrossRef]
- Pitter, K.L.; Tamagno, I.; Alikhanyan, K.; Hosni-Ahmed, A.; Pattwell, S.S.; Donnola, S.; Dai, C.; Ozawa, T.; Chang, M.; Chan, T.A.; et al. Corticosteroids compromise survival in glioblastoma. Brain 2016, 139, 1458–1471. [Google Scholar] [CrossRef]
- Adeberg, S.; Bernhardt, D.; Ben Harrabi, S.; Bostel, T.; Mohr, A.; Koelsche, C.; Diehl, C.; Rieken, S.; Debus, J. Metformin influences progression in diabetic glioblastoma patients. Strahlenther Onkol 2015, 191, 928–935. [Google Scholar] [CrossRef]
- Seliger, C.; Luber, C.; Gerken, M.; Schaertl, J.; Proescholdt, M.; Riemenschneider, M.J.; Meier, C.R.; Bogdahn, U.; Leitzmann, M.F.; Klinkhammer-Schalke, M.; Hau, P. Use of metformin and survival of patients with high-grade glioma. Int J Cancer 2019, 144, 273–280. [Google Scholar] [CrossRef]
- Thomas, J.G.; Veznedaroglu, E. Ketogenic Diet for Malignant Gliomas: a Review. Curr Nutr Rep 2020, 9, 258–263. [Google Scholar] [CrossRef]
- Rieger, J.; Bähr, O.; Maurer, G.D.; Hattingen, E.; Franz, K.; Brucker, D.; Walenta, S.; Kämmerer, U.; Coy, J.F.; Weller, M.; Steinbach, J.P. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol 2014, 44, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; Glantz, M. Association of the Extent of Resection With Survival in Glioblastoma: A Systematic Review and Meta-analysis. JAMA Oncol 2016, 2, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Esteller, M.; Garcia-Foncillas, J.; Andion, E.; Goodman, S.N.; Hidalgo, O.F.; Vanaclocha, V.; Baylin, S.B.; Herman, J.G. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. The New England journal of medicine 2000, 343, 1350–1354. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).