Submitted:
06 September 2024
Posted:
09 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Olfactory Sensitivity Screening
2.3. Dynamic Headspace Sampling
2.4. Mass Spectrometry/Gas Chromatography–Olfactometry (MS/GC-O) Analysis
2.5. Statistical Analysis
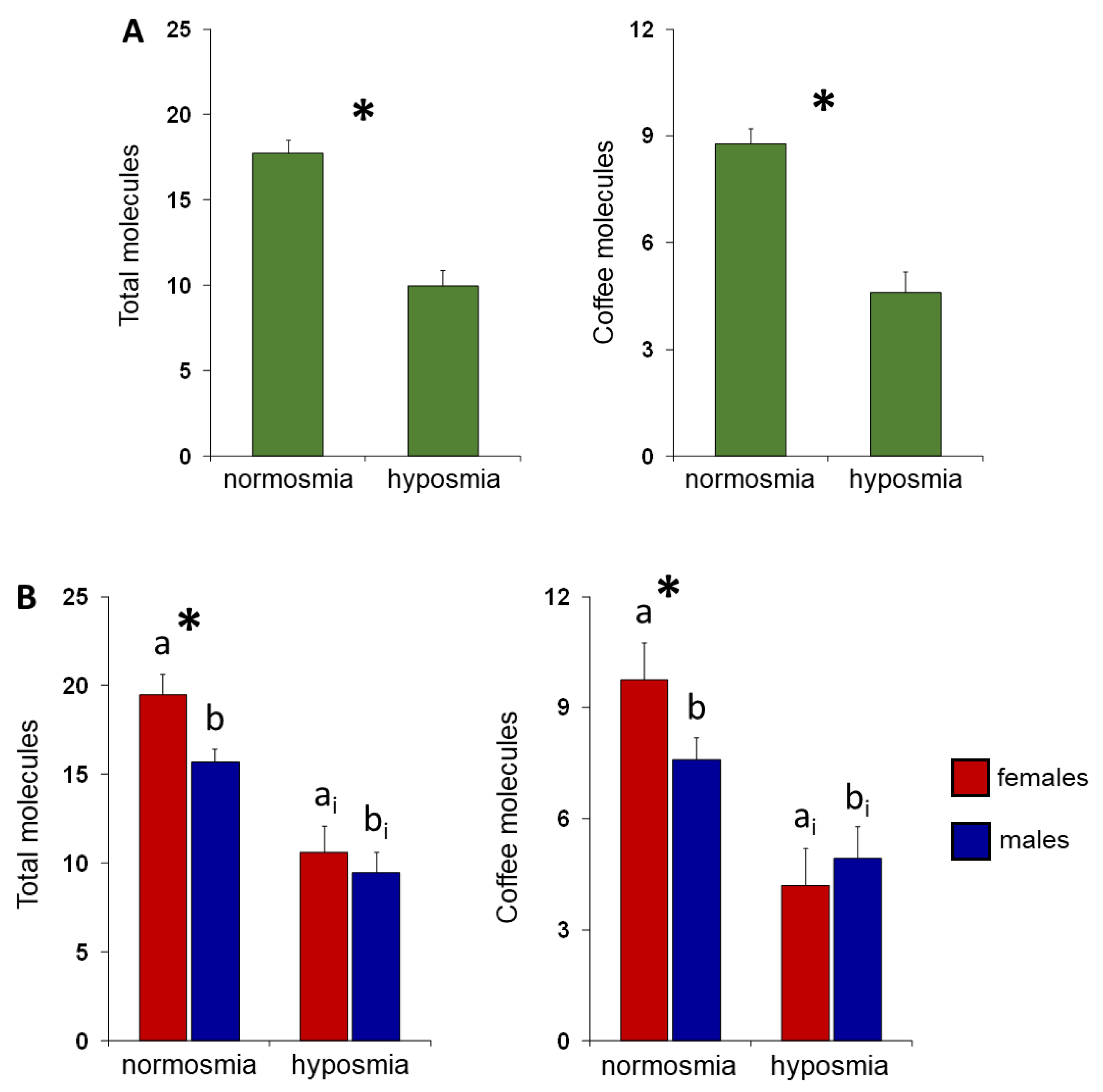
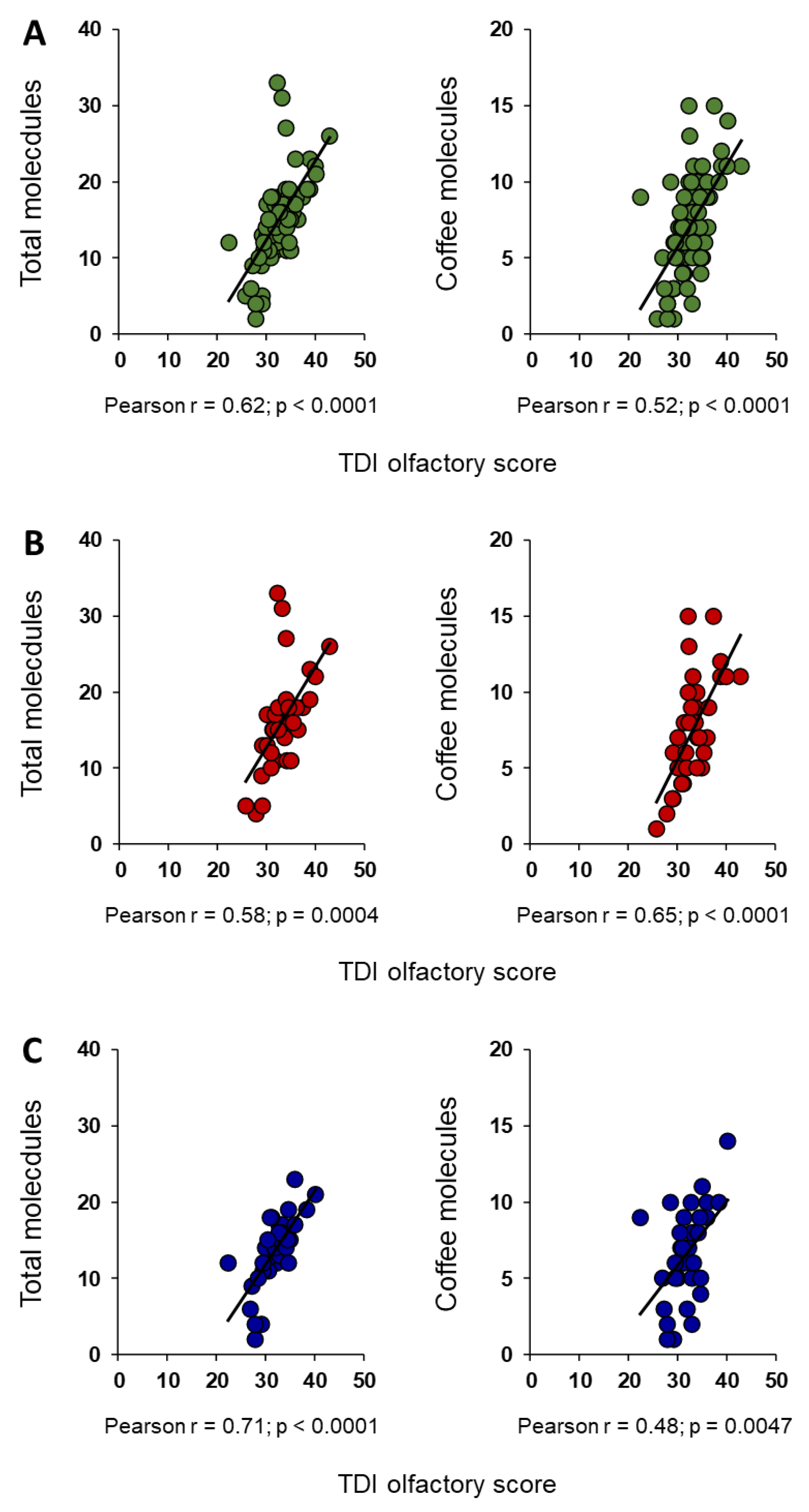
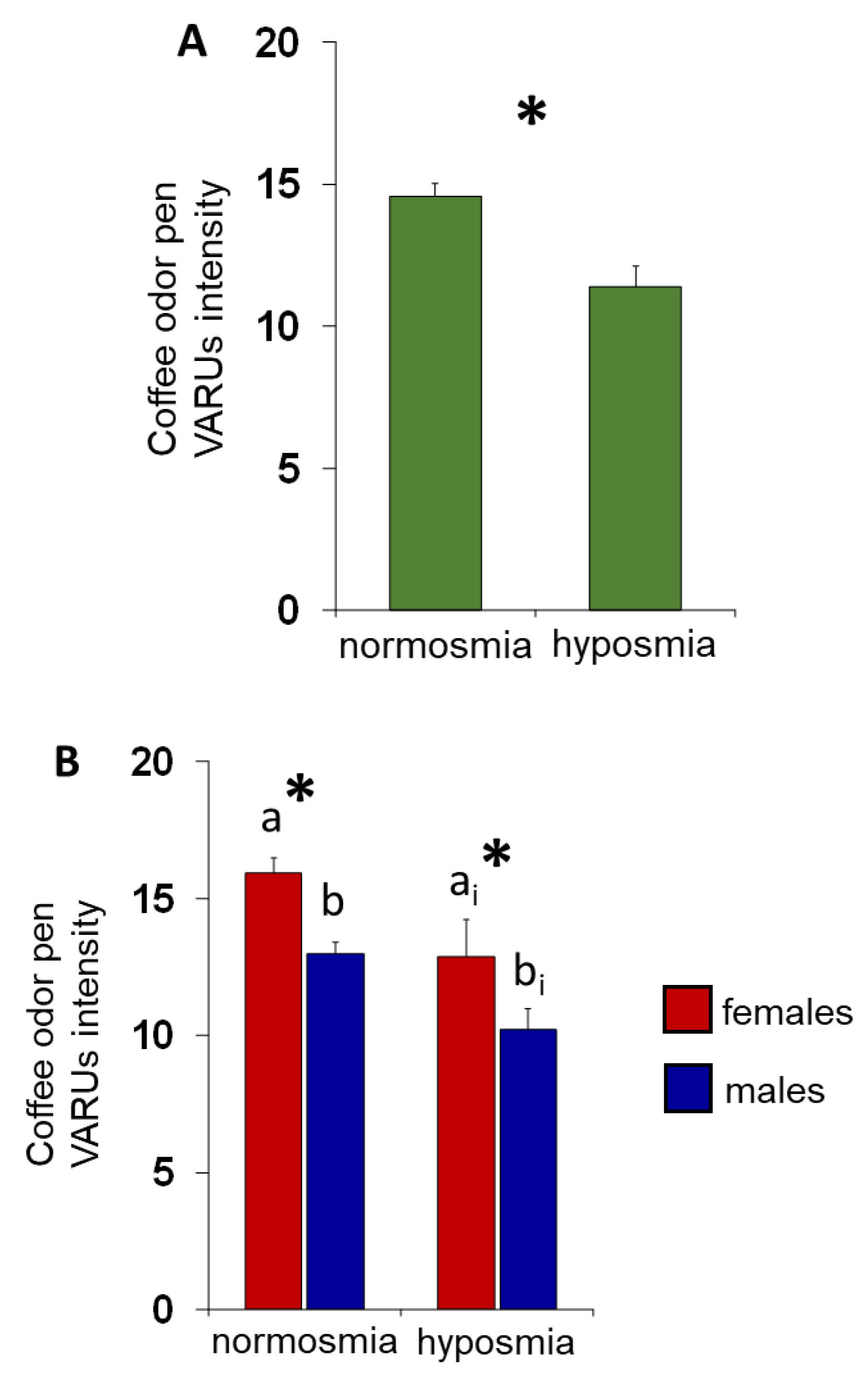
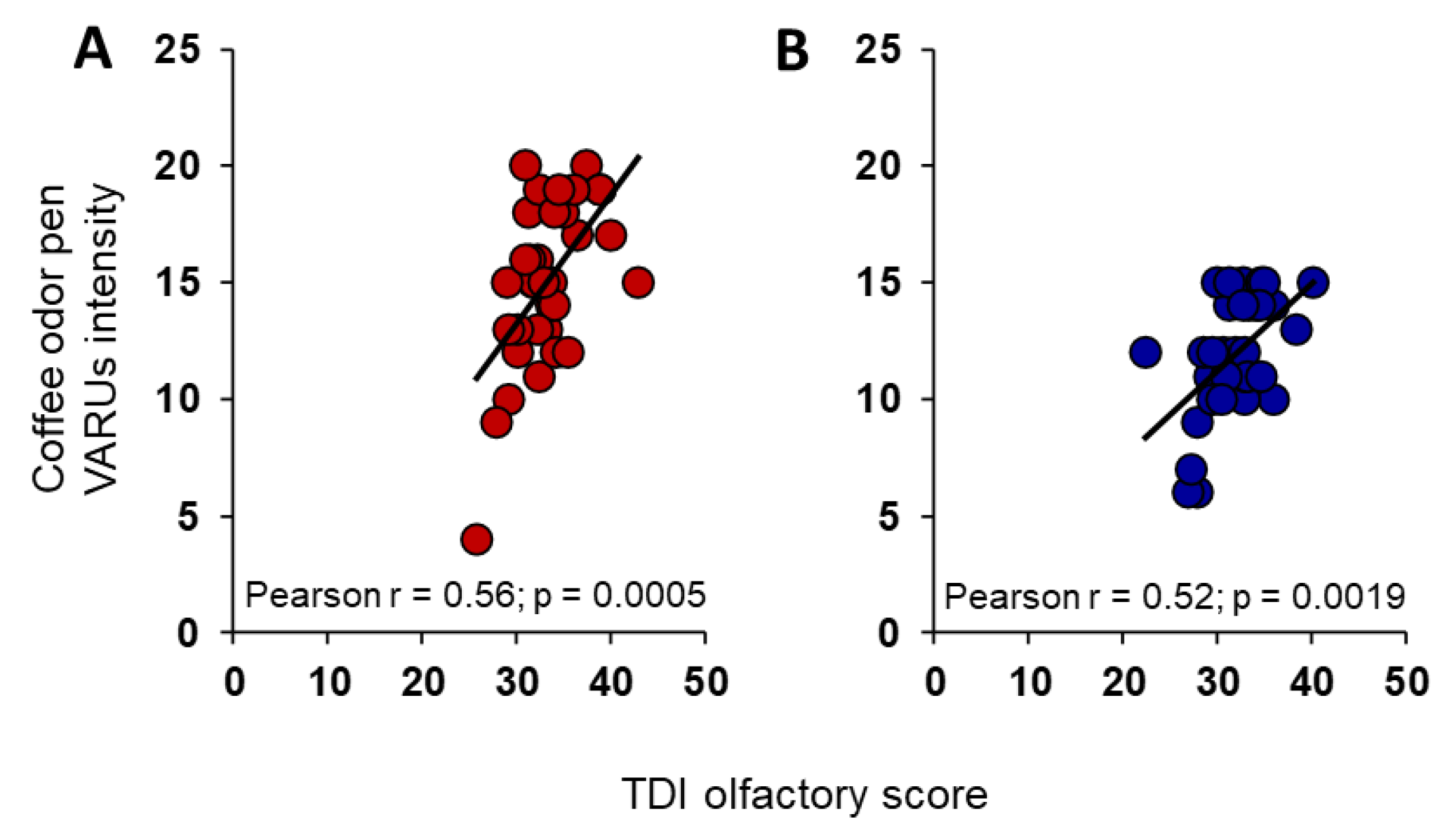
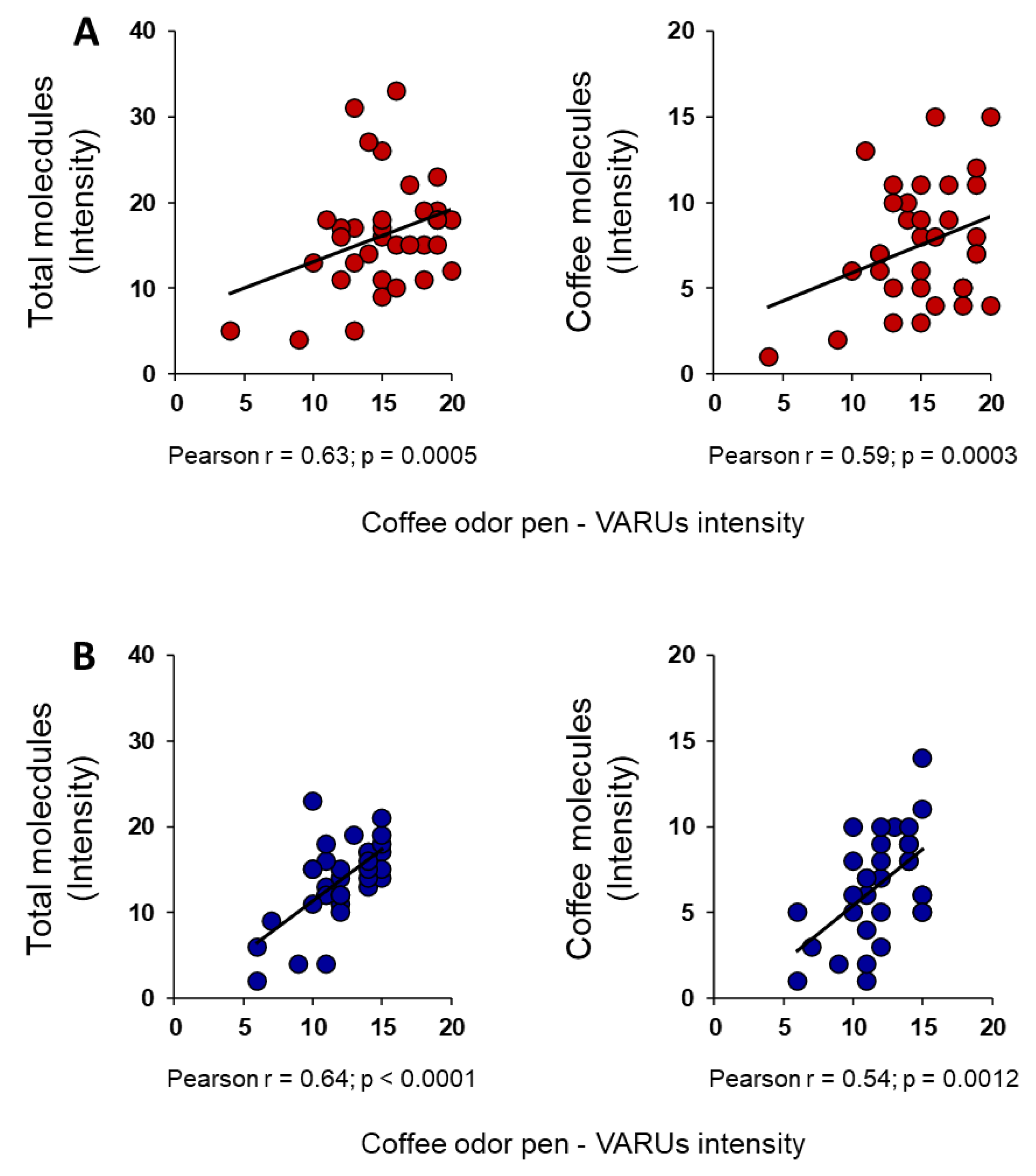
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aschenbrenner, K.; Hummel, C.; Teszmer, K.; Krone, F.; Ishimaru, T.; Seo, H.S.; Hummel, T. The influence of olfactory loss on dietary behaviors. The Laryngoscope 2008, 118, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.E.; Zhou, Y.; Liu, G.E. The essence of appetite: does olfactory receptor variation play a role? Journal of animal science 2018, 96, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Croy, I.; Nordin, S.; Hummel, T. Olfactory Disorders and Quality of Life—An Updated Review. Chemical Senses 2014, 39, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Nordin, S. Olfactory disorders and their consequences for quality of life. Acta oto-laryngologica 2005, 125, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Landolt, P.J.; Heath, R.R.; Chambers, D.L. Oriented flight responses of female Mediterranean fruit flies to calling males, odor of calling males, and a synthetic pheromone blend. Entomologia Experimentalis et Applicata 1992, 65, 259–266. [Google Scholar] [CrossRef]
- Lebreton, S.; Borrero-Echeverry, F.; Gonzalez, F.; Solum, M.; Wallin, E.A.; Hedenström, E.; Hansson, B.S.; Gustavsson, A.-L.; Bengtsson, M.; Birgersson, G.; et al. A Drosophila female pheromone elicits species-specific long-range attraction via an olfactory channel with dual specificity for sex and food. BMC Biology 2017, 15, 88. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Zhang, L.; Xu, X.; Cao, Z.; Zhang, L. Expressions of Olfactory Proteins in Locust Olfactory Organs and a Palp Odorant Receptor Involved in Plant Aldehydes Detection. Frontiers in physiology 2018, 9, 663. [Google Scholar] [CrossRef]
- Solari, P.; Corda, V.; Sollai, G.; Kreissl, S.; Galizia, C.G.; Crnjar, R. Morphological characterization of the antennal lobes in the Mediterranean fruit fly Ceratitis capitata. Journal of comparative physiology. A, Neuroethology, sensory, neural, and behavioral physiology 2016, 202, 131–146. [Google Scholar] [CrossRef]
- Sollai, G.; Solari, P.; Crnjar, R. Olfactory sensitivity to major, intermediate and trace components of sex pheromone in Ceratitis capitata is related to mating and circadian rhythm. Journal of insect physiology 2018, 110, 23–33. [Google Scholar] [CrossRef]
- Sollai, G.; Solari, P.; Crnjar, R. Differences in the Olfactory Sensitivity of Ceratitis capitata to Headspace of Some Host Plants in Relation to Sex, Mating Condition and Population. Diversity 2020, 12, 207. [Google Scholar] [CrossRef]
- Stevenson, R.J. An initial evaluation of the functions of human olfaction. Chem Senses 2010, 35, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Menuz, K.; Carlson, J.R. Olfactory perception: receptors, cells, and circuits. Cell 2009, 139, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Backstrand, J.R.; Ferris, A.M. Olfactory dysfunction and related nutritional risk in free-living, elderly women. Journal of the American Dietetic Association 1995, 95, 879–884, quiz 885-876. [Google Scholar] [CrossRef]
- Erskine, S.E.; Philpott, C.M. An unmet need: Patients with smell and taste disorders. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery 2020, 45, 197–203. [Google Scholar]
- Ferris, A.M.; Duffy, V.B. Effect of olfactory deficits on nutritional status. Does age predict persons at risk? Annals of the New York Academy of Sciences 1989, 561, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Gaillet-Torrent, M.; Sulmont-Rossé, C.; Issanchou, S.; Chabanet, C.; Chambaron, S. Impact of a non-attentively perceived odour on subsequent food choices. Appetite 2014, 76, 17–22. [Google Scholar] [CrossRef]
- Manesse, C.; Ferdenzi, C.; Sabri, M.; Bessy, M.; Rouby, C.; Faure, F.; Bellil, D.; Jomain, S.; Landis, B.N.; Hugentobler, M.; et al. Dysosmia-Associated Changes in Eating Behavior. Chemosensory Perception 2017, 10, 104–113. [Google Scholar] [CrossRef]
- Postma, E.; Graaf, C.; Boesveldt, S. Food preferences and intake in a population of Dutch individuals with self-reported smell loss: An online survey. Food Quality and Preference 2019, 79, 103771. [Google Scholar] [CrossRef]
- Seo, H.S.; Guarneros, M.; Hudson, R.; Distel, H.; Min, B.C.; Kang, J.K.; Croy, I.; Vodicka, J.; Hummel, T. Attitudes toward Olfaction: A Cross-regional Study. Chem Senses 2011, 36, 177–187. [Google Scholar] [CrossRef]
- Stroebele, N.; De Castro, J.M. Effect of ambience on food intake and food choice. Nutrition 2004, 20, 821–838. [Google Scholar] [CrossRef]
- Albrecht, J.; Schreder, T.; Kleemann, A.M.; Schöpf, V.; Kopietz, R.; Anzinger, A.; Demmel, M.; Linn, J.; Kettenmann, B.; Wiesmann, M. Olfactory detection thresholds and pleasantness of a food-related and a non-food odour in hunger and satiety. Rhinology 2009, 47, 160–165. [Google Scholar] [PubMed]
- Bolhuis, D.P.; Lakemond, C.M.; de Wijk, R.A.; Luning, P.A.; de Graaf, C. Effect of salt intensity in soup on ad libitum intake and on subsequent food choice. Appetite 2012, 58, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Egecioglu, E.; Skibicka, K.P.; Hansson, C.; Alvarez-Crespo, M.; Friberg, P.A.; Jerlhag, E.; Engel, J.A.; Dickson, S.L. Hedonic and incentive signals for body weight control. Reviews in endocrine & metabolic disorders 2011, 12, 141–151. [Google Scholar]
- Power, M.L.; Schulkin, J. Anticipatory physiological regulation in feeding biology: cephalic phase responses. Appetite 2008, 50, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, M.G.; Boesveldt, S.; Lakemond, C.M.; van Boekel, M.A.; Luning, P.A. Odors: appetizing or satiating? Development of appetite during odor exposure over time. International journal of obesity (2005) 2014, 38, 650–656. [Google Scholar] [CrossRef]
- Stafford, L.D. Olfactory Specific Satiety depends on degree of association between odour and food. Appetite 2016, 98, 63–66. [Google Scholar] [CrossRef]
- Yin, W.; Hewson, L.; Linforth, R.; Taylor, M.; Fisk, I.D. Effects of aroma and taste, independently or in combination, on appetite sensation and subsequent food intake. Appetite 2017, 114, 265–274. [Google Scholar] [CrossRef]
- Ferreira, V. Revisiting psychophysical work on the quantitative and qualitative odour properties of simple odour mixtures: a flavour chemistry view. Part 2: qualitative aspects. A review. Flavour and Fragrance Journal 2012, 27, 201–215. [Google Scholar] [CrossRef]
- Ferreira, V. Revisiting psychophysical work on the quantitative and qualitative odour properties of simple odour mixtures: a flavour chemistry view. Part 1: intensity and detectability. A review. Flavour and Fragrance Journal 2012, 27, 124–140. [Google Scholar] [CrossRef]
- Frank, M.E.; Fletcher, D.B.; Hettinger, T.P. Recognition of the Component Odors in Mixtures. Chemical senses 2017, 42, 537–546. [Google Scholar] [CrossRef]
- Iannario, M.; Manisera, M.; Piccolo, D.; Zuccolotto, P. Sensory analysis in the food industry as a tool for marketing decisions. Advances in Data Analysis and Classification 2012, 6, 303–321. [Google Scholar] [CrossRef]
- Ruijschop, R.M.; Boelrijk, A.E.; de Ru, J.A.; de Graaf, C.; Westerterp-Plantenga, M.S. Effects of retro-nasal aroma release on satiation. The British journal of nutrition 2008, 99, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Schilling, B.; Kaiser, R.; Natsch, A.; Gautschi, M. Investigation of odors in the fragrance industry. Chemoecology 2010, 20, 135–147. [Google Scholar] [CrossRef]
- Delahunty, C.M.; Eyres, G.; Dufour, J.P. Gas chromatography-olfactometry. Journal of separation science 2006, 29, 2107–2125. [Google Scholar] [CrossRef] [PubMed]
- Jordán, M.J.; Tandon, K.; Shaw, P.E.; Goodner, K.L. Aromatic profile of aqueous banana essence and banana fruit by gas chromatography-mass spectrometry (GC-MS) and gas chromatography-olfactometry (GC-O). Journal of agricultural and food chemistry 2001, 49, 4813–4817. [Google Scholar] [CrossRef]
- Mayol, A.R.; Acree, T.E. Advances in Gas Chromatography-Olfactometry. In Gas Chromatography-Olfactometry; ACS Symposium Series; American Chemical Society: 2001; Volume 782, pp. 1-10.
- Nuzzi, M.; Lo Scalzo, R.; Testoni, A.; Rizzolo, A. Evaluation of Fruit Aroma Quality: Comparison Between Gas Chromatography–Olfactometry (GC–O) and Odour Activity Value (OAV) Aroma Patterns of Strawberries. Food Analytical Methods 2008, 1, 270–282. [Google Scholar] [CrossRef]
- van Ruth, S.M. Methods for gas chromatography-olfactometry: a review. Biomolecular engineering 2001, 17, 121–128. [Google Scholar] [CrossRef]
- Crnjar, R.; Solari, P.; Sollai, G. The Human Nose as a Chemical Sensor in the Perception of Coffee Aroma: Individual Variability. Chemosensors 2023, 11, 248. [Google Scholar] [CrossRef]
- Melis, M.; Tomassini Barbarossa, I.; Hummel, T.; Crnjar, R.; Sollai, G. Effect of the rs2890498 polymorphism of the OBPIIa gene on the human ability to smell single molecules. Behavioural brain research 2021, 402, 113127. [Google Scholar] [CrossRef]
- Sollai, G.; Tomassini Barbarossa, I.; Usai, P.; Hummel, T.; Crnjar, R. Association between human olfactory performance and ability to detect single compounds in complex chemical mixtures. Physiology & behavior 2020, 217, 112820. [Google Scholar]
- Cain, W.S.; Gent, J.F. Olfactory sensitivity: reliability, generality, and association with aging. Journal of experimental psychology. Human perception and performance 1991, 17, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Feldmesser, E.; Bercovich, D.; Avidan, N.; Halbertal, S.; Haim, L.; Gross-Isseroff, R.; Goshen, S.; Lancet, D. Mutations in olfactory signal transduction genes are not a major cause of human congenital general anosmia. Chem Senses 2007, 32, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Hasin-Brumshtein, Y.; Lancet, D.; Olender, T. Human olfaction: from genomic variation to phenotypic diversity. Trends in genetics : TIG 2009, 25, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Jafek, B.W.; Gordon, A.S.; Moran, D.T.; Eller, P.M. Congenital anosmia. Ear, nose, & throat journal 1990, 69, 331–337. [Google Scholar]
- Attems, J.; Walker, L.; Jellinger, K.A. Olfaction and Aging: A Mini-Review. Gerontology 2015, 61, 485–490. [Google Scholar] [CrossRef]
- Cain, W.S.; Stevens, J.C. Uniformity of olfactory loss in aging. Annals of the New York Academy of Sciences 1989, 561, 29–38. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Franco-Lira, M.; Henríquez-Roldán, C.; Osnaya, N.; González-Maciel, A.; Reynoso-Robles, R.; Villarreal-Calderon, R.; Herritt, L.; Brooks, D.; Keefe, S.; et al. Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Experimental and toxicologic pathology : official journal of the Gesellschaft fur Toxikologische Pathologie 2010, 62, 91–102. [Google Scholar] [CrossRef]
- Doty, R.L.; Shaman, P.; Applebaum, S.L.; Giberson, R.; Siksorski, L.; Rosenberg, L. Smell identification ability: changes with age. Science (New York, N.Y.) 1984, 226, 1441–1443. [Google Scholar] [CrossRef]
- Keller, A.; Zhuang, H.; Chi, Q.; Vosshall, L.B.; Matsunami, H. Genetic variation in a human odorant receptor alters odour perception. Nature 2007, 449, 468–472. [Google Scholar] [CrossRef]
- Melis, M.; Mastinu, M.; Sollai, G. Effect of the rs2821557 Polymorphism of the Human Kv1.3 Gene on Olfactory Function and BMI in Different Age Groups. Nutrients 2024, 16, 821. [Google Scholar] [CrossRef]
- Melis, M.; Tomassini Barbarossa, I.; Crnjar, R.; Sollai, G. Olfactory Sensitivity Is Associated with Body Mass Index and Polymorphism in the Voltage-Gated Potassium Channels Kv1.3. Nutrients 2022, 14, 4986. [Google Scholar] [CrossRef] [PubMed]
- Min, H.J.; Kim, S.M.; Han, D.H.; Kim, K.S. The sniffing bead system, an olfactory dysfunction screening tool for geriatric subjects: a cross-sectional study. BMC geriatrics 2021, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Öberg, C.; Larsson, M.; Bäckman, L. Differential sex effects in olfactory functioning: The role of verbal processing. Journal of the International Neuropsychological Society 2002, 8, 691–698. [Google Scholar] [CrossRef]
- Schubert, C.R.; Fischer, M.E.; Pinto, A.A.; Klein, B.E.K.; Klein, R.; Tweed, T.S.; Cruickshanks, K.J. Sensory Impairments and Risk of Mortality in Older Adults. The journals of gerontology. Series A, Biological sciences and medical sciences 2017, 72, 710–715. [Google Scholar] [CrossRef]
- Silva Teixeira, C.S.; Cerqueira, N.M.; Silva Ferreira, A.C. Unravelling the Olfactory Sense: From the Gene to Odor Perception. Chem Senses 2016, 41, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Sollai, G.; Crnjar, R. Age-Related Olfactory Decline Is Associated With Levels of Exercise and Non-exercise Physical Activities. Frontiers in aging neuroscience 2021, 13, 695115. [Google Scholar] [CrossRef]
- Sollai, G.; Crnjar, R. Association among Olfactory Function, Lifestyle and BMI in Female and Male Elderly Subjects: A Cross-Sectional Study. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Sollai, G.; Melis, M.; Magri, S.; Usai, P.; Hummel, T.; Tomassini Barbarossa, I.; Crnjar, R. Association between the rs2590498 polymorphism of Odorant Binding Protein (OBPIIa) gene and olfactory performance in healthy subjects. Behavioural brain research 2019, 372, 112030. [Google Scholar] [CrossRef] [PubMed]
- Sollai, G.; Melis, M.; Tomassini Barbarossa, I.; Crnjar, R. A polymorphism in the human gene encoding OBPIIa affects the perceived intensity of smelled odors. Behavioural brain research 2022, 427, 113860. [Google Scholar] [CrossRef]
- Sorokowska, A.; Sorokowski, P.; Frackowiak, T. Determinants of human olfactory performance: a cross-cultural study. The Science of the total environment 2015, 506-507, 196–200. [Google Scholar] [CrossRef]
- Sorokowska, A.; Sorokowski, P.; Hummel, T. Cross-Cultural Administration of an Odor Discrimination Test. Chemosens Percept 2014, 7, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Sorokowski, P.; Karwowski, M.; Misiak, M.; Marczak, M.K.; Dziekan, M.; Hummel, T.; Sorokowska, A. Sex Differences in Human Olfaction: A Meta-Analysis. Front Psychol 2019, 10, 242. [Google Scholar] [CrossRef]
- Doty, R.L.; Cameron, E.L. Sex differences and reproductive hormone influences on human odor perception. Physiology & behavior 2009, 97, 213–228. [Google Scholar]
- Olofsson, J.K.; Nordin, S. Gender Differences in Chemosensory Perception and Event-related Potentials. Chemical Senses 2004, 29, 629–637. [Google Scholar] [CrossRef]
- Bugaud, C.; Alter, P. Volatile and non-volatile compounds as odour and aroma predictors in dessert banana (Musa spp.). Postharvest Biology and Technology 2016, 112, 14–23. [Google Scholar] [CrossRef]
- Aydın, E.; Tekeli, H.; Karabacak, E.; Altunay İ, K.; Aydın, Ç.; Çerman, A.A.; Altundağ, A.; Salihoğlu, M.; Çayönü, M. Olfactory functions in patients with psoriasis vulgaris: correlations with the severity of the disease. Archives of dermatological research 2016, 308, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Besser, G.; Erlacher, B.; Aydinkoc-Tuzcu, K.; Liu, D.T.; Pablik, E.; Niebauer, V.; Koenighofer, M.; Renner, B.; Mueller, C.A. Body-Mass-Index Associated Differences in Ortho- and Retronasal Olfactory Function and the Individual Significance of Olfaction in Health and Disease. Journal of clinical medicine 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Croy, I.; Symmank, A.; Schellong, J.; Hummel, C.; Gerber, J.; Joraschky, P.; Hummel, T. Olfaction as a marker for depression in humans. Journal of affective disorders 2014, 160, 80–86. [Google Scholar] [CrossRef]
- Graves, A.B.; Bowen, J.D.; Rajaram, L.; McCormick, W.C.; McCurry, S.M.; Schellenberg, G.D.; Larson, E.B. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology 1999, 53, 1480–1487. [Google Scholar] [CrossRef]
- Palouzier-Paulignan, B.; Lacroix, M.C.; Aimé, P.; Baly, C.; Caillol, M.; Congar, P.; Julliard, A.K.; Tucker, K.; Fadool, D.A. Olfaction under metabolic influences. Chem Senses 2012, 37, 769–797. [Google Scholar] [CrossRef]
- Pastor, A.; Fernández-Aranda, F.; Fitó, M.; Jiménez-Murcia, S.; Botella, C.; Fernández-Real, J.M.; Frühbeck, G.; Tinahones, F.J.; Fagundo, A.B.; Rodriguez, J.; et al. A Lower Olfactory Capacity Is Related to Higher Circulating Concentrations of Endocannabinoid 2-Arachidonoylglycerol and Higher Body Mass Index in Women. PloS one 2016, 11, e0148734. [Google Scholar] [CrossRef] [PubMed]
- Patel, Z.M.; DelGaudio, J.M.; Wise, S.K. Higher Body Mass Index Is Associated with Subjective Olfactory Dysfunction. Behavioural neurology 2015, 2015, 675635. [Google Scholar] [CrossRef] [PubMed]
- Perricone, C.; Shoenfeld, N.; Agmon-Levin, N.; de Carolis, C.; Perricone, R.; Shoenfeld, Y. Smell and autoimmunity: a comprehensive review. Clinical reviews in allergy & immunology 2013, 45, 87–96. [Google Scholar]
- Pinto, J.M.; Wroblewski, K.E.; Kern, D.W.; Schumm, L.P.; McClintock, M.K. Olfactory dysfunction predicts 5-year mortality in older adults. PloS one 2014, 9, e107541. [Google Scholar] [CrossRef] [PubMed]
- Poessel, M.; Freiherr, J.; Wiencke, K.; Villringer, A.; Horstmann, A. Insulin Resistance Is Associated with Reduced Food Odor Sensitivity across a Wide Range of Body Weights. Nutrients 2020, 12, 2201. [Google Scholar] [CrossRef]
- Ross, G.W.; Petrovitch, H.; Abbott, R.D.; Tanner, C.M.; Popper, J.; Masaki, K.; Launer, L.; White, L.R. Association of olfactory dysfunction with risk for future Parkinson’s disease. Annals of neurology 2008, 63, 167–173. [Google Scholar] [CrossRef]
- Sollai, G.; Melis, M.; Mastinu, M.; Paduano, D.; Chicco, F.; Magri, S.; Usai, P.; Hummel, T.; Barbarossa, I.T.; Crnjar, R. Olfactory Function in Patients with Inflammatory Bowel Disease (IBD) Is Associated with Their Body Mass Index and Polymorphism in the Odor Binding-Protein (OBPIIa) Gene. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Steinbach, S.; Proft, F.; Schulze-Koops, H.; Hundt, W.; Heinrich, P.; Schulz, S.; Gruenke, M. Gustatory and olfactory function in rheumatoid arthritis. Scandinavian journal of rheumatology 2011, 40, 169–177. [Google Scholar] [CrossRef]
- Steinbach, S.; Reindl, W.; Dempfle, A.; Schuster, A.; Wolf, P.; Hundt, W.; Huber, W. Smell and taste in inflammatory bowel disease. PloS one 2013, 8, e73454. [Google Scholar] [CrossRef]
- Steinbach, S.; Reindl, W.; Kessel, C.; Ott, R.; Zahnert, T.; Hundt, W.; Heinrich, P.; Saur, D.; Huber, W. Olfactory and gustatory function in irritable bowel syndrome. Eur Arch Otorhinolaryngol 2010, 267, 1081–1087. [Google Scholar] [CrossRef]
- Sun, C.; Tang, K.; Wu, J.; Xu, H.; Zhang, W.; Cao, T.; Zhou, Y.; Yu, T.; Li, A. Leptin modulates olfactory discrimination and neural activity in the olfactory bulb. Acta physiologica (Oxford, England) 2019, 227, e13319. [Google Scholar] [CrossRef] [PubMed]
- Tekeli, H.; Senol, M.G.; Altundag, A.; Yalcınkaya, E.; Kendirli, M.T.; Yaşar, H.; Salihoglu, M.; Saglam, O.; Cayonu, M.; Cesmeci, E.; et al. Olfactory and gustatory dysfunction in Myasthenia gravis: A study in Turkish patients. Journal of the neurological sciences 2015, 356, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Velluzzi, F.; Deledda, A.; Lombardo, M.; Fosci, M.; Crnjar, R.; Grossi, E.; Sollai, G. Application of Artificial Neural Networks (ANN) to Elucidate the Connections among Smell, Obesity with Related Metabolic Alterations, and Eating Habit in Patients with Weight Excess. Metabolites 2023, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Velluzzi, F.; Deledda, A.; Onida, M.; Loviselli, A.; Crnjar, R.; Sollai, G. Relationship between Olfactory Function and BMI in Normal Weight Healthy Subjects and Patients with Overweight or Obesity. Nutrients 2022, 14, 1262. [Google Scholar] [CrossRef] [PubMed]
- Walliczek-Dworschak, U.; Wendler, J.; Khan, T.; Aringer, M.; Hähner, A.; Hummel, T. Chemosensory function is decreased in rheumatoid arthritis. Eur Arch Otorhinolaryngol 2020, 277, 1675–1680. [Google Scholar] [CrossRef]
- Wilson, R.S.; Arnold, S.E.; Schneider, J.A.; Boyle, P.A.; Buchman, A.S.; Bennett, D.A. Olfactory impairment in presymptomatic Alzheimer’s disease. Annals of the New York Academy of Sciences 2009, 1170, 730–735. [Google Scholar] [CrossRef]
- Wilson, R.S.; Schneider, J.A.; Arnold, S.E.; Tang, Y.; Boyle, P.A.; Bennett, D.A. Olfactory Identification and Incidence of Mild Cognitive Impairment in Older Age. Archives of General Psychiatry 2007, 64, 802–808. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; de Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas chromatography analysis with olfactometric detection (GC-O) as a useful methodology for chemical characterization of odorous compounds. Sensors (Basel, Switzerland) 2013, 13, 16759–16800. [Google Scholar] [CrossRef]
- d’Acampora Zellner, B.; Dugo, P.; Dugo, G.; Mondello, L. Gas chromatography-olfactometry in food flavour analysis. J Chromatogr A 2008, 1186, 123–143. [Google Scholar] [CrossRef]
- Dussort, P.; Depretre, N.; Bou-Maroun, E.; Fant, C.; GUICHARD, E.; Brunerie, P.; Le Fur, Y., Y.; Le Quéré, J.-L. An original approach for gas chromatography-olfactometry detection frequency analysis: Application to gin. Food Research International 2012, 49, 253–262. [Google Scholar] [CrossRef]
- Plutowska, B.; Wardencki, W. Application of gas chromatography–olfactometry (GC–O) in analysis and quality assessment of alcoholic beverages – A review. Food Chemistry 2008, 107, 449–463. [Google Scholar] [CrossRef]
- Pollien, P.; Ott, A.; Montigon, F.; Baumgartner, M.; Muñoz-Box, R.; Chaintreau, A. Hyphenated Headspace-Gas Chromatography-Sniffing Technique: Screening of Impact Odorants and Quantitative Aromagram Comparisons. Journal of agricultural and food chemistry 1997, 45, 2630–2637. [Google Scholar] [CrossRef]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Hummel, T.; Kobal, G.; Gudziol, H.; Mackay-Sim, A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol 2007, 264, 237–243. [Google Scholar] [CrossRef]
- Fischer, M.; Zopf, Y.; Elm, C.; Pechmann, G.; Hahn, E.G.; Schwab, D.; Kornhuber, J.; Thuerauf, N.J. Subjective and objective olfactory abnormalities in Crohn’s disease. Chem Senses 2014, 39, 529–538. [Google Scholar] [CrossRef]
- Rizzolo, A.; Polesello, A.; Polesello, S. Use of headspace capillary GC to study the development of volatile compounds in fresh fruit. Journal of High Resolution Chromatography 1992, 15, 472–477. [Google Scholar] [CrossRef]
- van Den Dool, H.; Dec. Kratz, P. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. Journal of Chromatography A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Murakami, K.; Ohtani, N.; Iwatsuki, K.; Sotoyama, K.; Wada, A.; Tokuno, K.; Iwabuchi, H.; Tanaka, K. Analysis of volatile compounds released during the grinding of roasted coffee beans using solid-phase microextraction. Journal of agricultural and food chemistry 2003, 51, 1961–1969. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and robusta roasted coffees analysed by SPME-GC-MS. Food research international (Ottawa, Ont.) 2018, 108, 628–640. [Google Scholar] [CrossRef]
- Gloess, A.N.; Yeretzian, C.; Knochenmuss, R.; Groessl, M. On-line analysis of coffee roasting with ion mobility spectrometry–mass spectrometry (IMS–MS). International Journal of Mass Spectrometry 2018, 424, 49–57. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M.; Lee, K.-G. Effect of reversed coffee grinding and roasting process on physicochemical properties including volatile compound profiles. Innovative Food Science & Emerging Technologies 2017, 44. [Google Scholar]
- López-Galilea, I.; Fournier, N.; Cid, C.; Guichard, E. Changes in headspace volatile concentrations of coffee brews caused by the roasting process and the brewing procedure. Journal of agricultural and food chemistry 2006, 54, 8560–8566. [Google Scholar] [CrossRef]
- Majcher, M.A.; Klensporf-Pawlik, D.; Dziadas, M.; Jeleń, H.H. Identification of aroma active compounds of cereal coffee brew and its roasted ingredients. Journal of agricultural and food chemistry 2013, 61, 2648–2654. [Google Scholar] [CrossRef]
- Sunarharum, W.; Williams, D.; Smyth, H. Complexity of coffee flavor: A compositional and sensory perspective. Food Research International 2014, 62, 315–325. [Google Scholar] [CrossRef]
- Yang, N.; Liu, C.; Liu, X.; Degn, T.K.; Munchow, M.; Fisk, I. Determination of volatile marker compounds of common coffee roast defects. Food Chemistry 2016, 211, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Zapata, J.; Londoño, V.; Naranjo, M.; Osorio, J.; Lopez, C.; Quintero, M. Characterization of aroma compounds present in an industrial recovery concentrate of coffee flavour. CyTA - Journal of Food 2018, 16, 367–372. [Google Scholar] [CrossRef]
- Gonzalez-Kristeller, D.C.; do Nascimento, J.B.; Galante, P.A.; Malnic, B. Identification of agonists for a group of human odorant receptors. Frontiers in pharmacology 2015, 6, 35. [Google Scholar] [CrossRef]
- Julliard, A.K.; Al Koborssy, D.; Fadool, D.A.; Palouzier-Paulignan, B. Nutrient Sensing: Another Chemosensitivity of the Olfactory System. Frontiers in physiology 2017, 8, 468. [Google Scholar] [CrossRef]
- Sollai, G.; Biolchini, M.; Crnjar, R. Taste receptor plasticity in relation to feeding history in two congeneric species of Papilionidae (Lepidoptera). Journal of insect physiology 2018, 107, 41–56. [Google Scholar] [CrossRef]
- Gaillard, I.; Rouquier, S.; Giorgi, D. Olfactory receptors. Cellular and molecular life sciences : CMLS 2004, 61, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, I.; Rouquier, S.; Pin, J.P.; Mollard, P.; Richard, S.; Barnabé, C.; Demaille, J.; Giorgi, D. A single olfactory receptor specifically binds a set of odorant molecules. The European journal of neuroscience 2002, 15, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Gerkin, R.C.; Castro, J.B. The number of olfactory stimuli that humans can discriminate is still unknown. eLife 2015, 4, e08127. [Google Scholar] [CrossRef] [PubMed]
- Malnic, B.; Godfrey, P.A.; Buck, L.B. The human olfactory receptor gene family. Proceedings of the National Academy of Sciences of the United States of America 2004, 101, 2584. [Google Scholar] [CrossRef]
- Malnic, B.; Hirono, J.; Sato, T.; Buck, L.B. Combinatorial receptor codes for odors. Cell 1999, 96, 713–723. [Google Scholar] [CrossRef]
- Mombaerts, P. Odorant receptor gene choice in olfactory sensory neurons: the one receptor-one neuron hypothesis revisited. Current opinion in neurobiology 2004, 14, 31–36. [Google Scholar] [CrossRef]
- Mombaerts, P.; Wang, F.; Dulac, C.; Chao, S.K.; Nemes, A.; Mendelsohn, M.; Edmondson, J.; Axel, R. Visualizing an olfactory sensory map. Cell 1996, 87, 675–686. [Google Scholar] [CrossRef]
- Shepherd, G.M. Outline of a theory of olfactory processing and its relevance to humans. Chem Senses 2005, 30 Suppl 1, i3–5. [Google Scholar] [CrossRef]
- Larsson, M.; Finkel, D.; Pedersen, N.L. Odor identification: influences of age, gender, cognition, and personality. The journals of gerontology. Series B, Psychological sciences and social sciences 2000, 55, P304–310. [Google Scholar] [CrossRef]
- Cornell Kärnekull, S.; Jönsson, F.U.; Willander, J.; Sikström, S.; Larsson, M. Long-Term Memory for Odors: Influences of Familiarity and Identification Across 64 Days. Chemical Senses 2015, 40, 259–267. [Google Scholar] [CrossRef]
- Schaal, B.; Marlier, L.; Soussignan, R. Olfactory function in the human fetus: evidence from selective neonatal responsiveness to the odor of amniotic fluid. Behavioral neuroscience 1998, 112, 1438–1449. [Google Scholar] [CrossRef]
- Le Fur, Y.; Mercurio, V.; Moio, L.; Blanquet, J.; Meunier, J.M. A new approach to examine the relationships between sensory and gas chromatography-olfactometry data using Generalized Procrustes analysis applied to six French Chardonnay wines. Journal of agricultural and food chemistry 2003, 51, 443–452. [Google Scholar] [CrossRef] [PubMed]
- van Ruth, S.M.; O’Connor, C.H. Evaluation of three gas chromatography-olfactometry methods: comparison of odour intensity-concentration relationships of eight volatile compounds with sensory headspace data. Food Chemistry 2001, 74, 341–347. [Google Scholar] [CrossRef]
- Fadool, D.A.; Tucker, K.; Perkins, R.; Fasciani, G.; Thompson, R.N.; Parsons, A.D.; Overton, J.M.; Koni, P.A.; Flavell, R.A.; Kaczmarek, L.K. Kv1.3 channel gene-targeted deletion produces “Super-Smeller Mice” with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron 2004, 41, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Guthoff, M.; Tschritter, O.; Berg, D.; Liepelt, I.; Schulte, C.; Machicao, F.; Haering, H.U.; Fritsche, A. Effect of genetic variation in Kv1.3 on olfactory function. Diabetes/metabolism research and reviews 2009, 25, 523–527. [Google Scholar] [CrossRef]
- Tucker, K.; Cavallin, M.A.; Jean-Baptiste, P.; Biju, K.C.; Overton, J.M.; Pedarzani, P.; Fadool, D.A. The Olfactory Bulb: A Metabolic Sensor of Brain Insulin and Glucose Concentrations via a Voltage-Gated Potassium Channel. Results and problems in cell differentiation 2010, 52, 147–157. [Google Scholar]
| N. | Odor-active compound | Odor description | df (F-M) |
|---|---|---|---|
| 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 |
Octane, 3,5-dimethyl- Oxalic acid, isobutyl nonyl ester Toluene β-Pinene Ethylbenzene p-Xylene Oxalic acid, isobutyl pentyl ester Pyridine* D-Limonene* Furan,2-pentyl-* Pyrazine, methyl-* Acetoin 2-Propanone, 1-hydroxy- Pyrazine, 2,5-dimethyl-* Pyrazine, ethyl-* Pyrazine, 2,3-dimethyl-* DL-2,3-Butanediol* Vinyl butyrate Hex-4-yn-3-one, 2,2-dimethyl- Pyrazine, 2-ethyl-6-methyl-* Pyrazine, 2-ethyl-3-methyl-* Pyrazine, 2-(n-propyl)-* Pyrazine, 2,6-diethyl-* Pyrazine, 3-ethyl-2,5-dimethyl- * 2-Propanone, 1-(acetyloxy)- Pyrazine, 2-ethyl-3,5-dimethyl- * Furfural* Pyrazine, tetramethyl- Pyrazine, 3,5-diethyl-2-methyl- * Pyrazine, 2-ethenyl-5-methyl- Furan, 2-acetyl-* 2,3-Pentanedione* 2-Butanone, 1-(acetyloxy)- 2-Furanmethanol, acetate* Pyrazine, 2-methyl-6-(2 -propenyl)- 2-Cyclopenten-1-one, 2,3- dimethyl- Acetic acid, diethyl-* Pentanoic acid, 4-oxo-, methyl ester 2-Furancarboxaldehyde, 5- methyl-* 2-Furanmethanol, propanoate* Furan, 2,2’-methylenebis-* 2-Furanmethanol* Butanoic acid, 3-methyl-* Furan, 2-(2-furanylmethyl)-5- methyl-* Pyrazine, 2-acetyl-6-methyl 4(H)-Pyridine, N-acetyl-* Octaethylene glycol monododecyl ether 2-Hexadecanol N-Furfurylpyrrole* 2-Acetylpyrrole* |
Woody, burnt, unknown Burnt, unknown Coffee, smoked, solvent, roasted, fruit Sweet, floral, vanilla, herbs, incense, sulfur, pungent Petrol Vanilla, medicinal, floral, gas, pungent Floral, fruity, vanilla, sweet Coffee, smoked, roasted, cheese Sweet, sour, citrus Smoked, plastic, herbs Coffee, nutty, roasted, smoke, caramellic Coffee, sweet, roasted, parfum, woody, caramellic Sweet, pungent, fish, solvent, wet, feet, medicinal Coffee, citrus, medicinal, sweet, cocoa, shoes Coffee, nutty, egg, pungent, shoes Coffee, burnt, caramellic, fruity Sweet, caramellic, rose, wet Floral, parfum, bitter, solvent, pungent, plastic Sweet, solvent, pungent Coffee, sweet, smoked, medicinal, solvent, parfum, roasted, balsamic, fruit Coffee, cocoa, solvent, bitter, nutty, roasted, burnt, medicinal, solvent, herbs Green, musty, woody, earthy, wet, herbs, floral, fruit Coffee, roasted, earthy, musty, burnt, mushrooms, vegetable Coffee, nutty, roasted, floral, bitter, woody, solvent, wet Pungent, parfum, wet Coffee, musty, roasted, wet, herbs, musty Coffee, sweet, solvent, floral, pungent Coffee, roasted, burnt, vanilla, bitter, solvent Floral, musty, wet, solvent, fresh Coffee, nutty, bitter, plastic, earthy, musty Parfum Floral, earthy, sweat, musk, cheese, pungent, woody ---------- Roasted, fruit, herb, woody, coffee, vegetable, fish Pungent, sour, bitter, herbs, spicy Sweet, floral, lavender Roasted, solvent, rotten, musty, wet earth, coffee Sweet, nutty Coffee, sweet, parfum, solvent Coffee, pungent, floral, musty, herb, sweet, burnt, vegetable Coffee, nutty, popcorn, roasted, fish, sour, plastic, smoke Coffee, smoke, popcorn, nutty, roasted, sweet Cheese, smoke, stinky feet, acidic, fruity, putrid Nutty, plastic, unknown Putrid, musty, cheese, medicinal Shoes, wet, sweat, plastic, cheese Sweat, acidic Cheese, musty, putrid, plastic, shoes, burnt Solvent, cheese, musty, coffee, caramellic, smoked Coffee, roasted, almond, sweet, burnt, parfum, fresh, popcorn |
0-3 2-1 14-6 6-6 0-1 4-5 5-3 14-3 1-6 3-3 3-5 10-10 7-15 7-10 4-2 4-3 2-4 5-4 2-3 19-25 24-22 18-16 23-21 18-16 6-3 17-19 15-6 13-13 11-20 15-11 2-0 26-24 0-0 22-12 8-1 3-1 20-13 5-2 5-4 14-9 22-12 22-12 14-19 1-2 4-6 9-7 2-2 24-23 16-20 25-15 |
| Molecule | Perception ability |
F n (%) |
M n (%) |
p-Value |
| Toluene | Yes | 14 | 6 | 0.039 |
| No | 20 | 27 | ||
| Pyridine | Yes No |
14 20 |
3 30 |
0.003 |
| D-limonene | Yes No |
1 33 |
6 27 |
0.041 |
| 2-Propanone, 1-hydroxy- | Yes No |
7 27 |
15 18 |
0.030 |
| Furfural | Yes No |
15 19 |
6 27 |
0.022 |
| Pyrazine, 3,5-diethyl-2-methyl- | Yes No |
11 23 |
20 13 |
0.020 |
| 2-Furanmethanol, acetate | Yes No |
22 12 |
12 21 |
0.020 |
| Pyrazine, 2-methyl-6-(2-propenyl)- | Yes No |
8 26 |
1 32 |
0.014 |
| Furan,2,2-methylenebis- | Yes No |
22 12 |
12 21 |
0.020 |
| 2-Furanmethanol | Yes No |
22 12 |
12 21 |
0.020 |
| 2-Acetylpyrrole | Yes No |
25 9 |
15 18 |
0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





