Submitted:
06 September 2024
Posted:
09 September 2024
You are already at the latest version
Abstract
Keywords:
Introduction
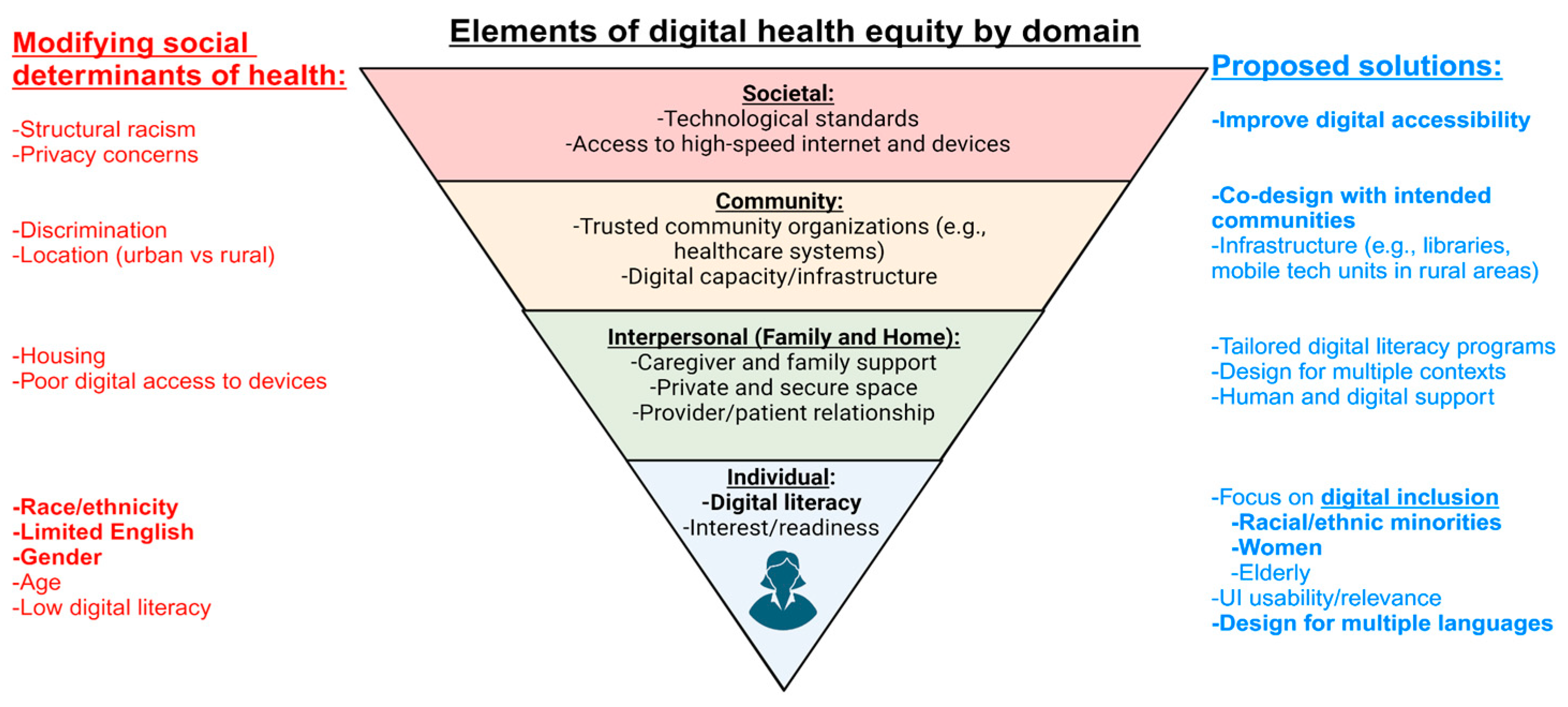
Components of Digital Health Equity
Social Determinants of Health Affect Health Outcomes in Cardiovascular Digital Health Studies:
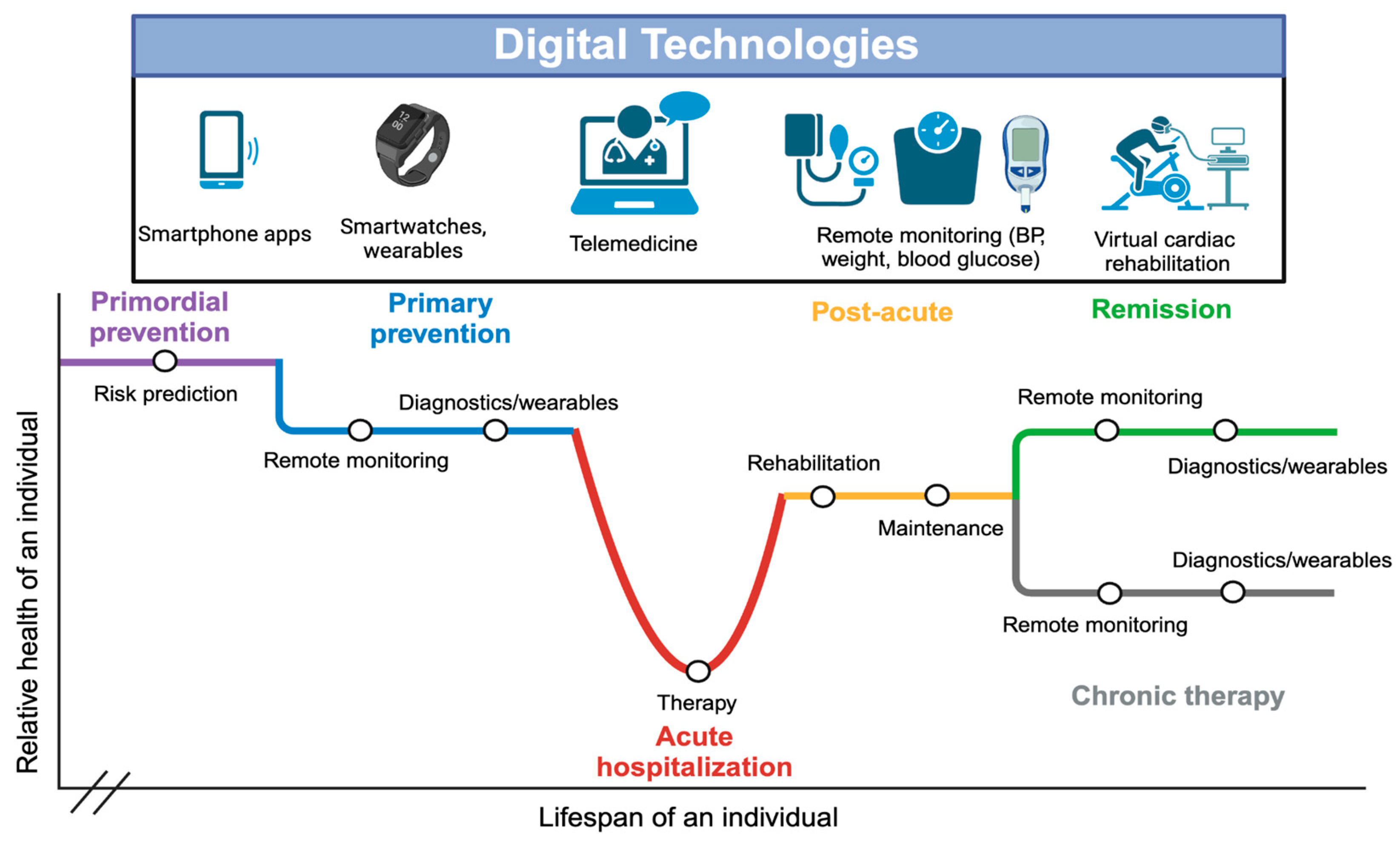
Online Patient Portals and Telemedicine:
Remote Monitoring of Cardiovascular Disease:
Smartphone Applications and Wearables:
Challenges and Barriers:
Future Directions/Recommendations:
Digital Accessibility through Societal and Governmental Policy:
Community Co-Design of Digital Health Interventions:
Focus on Digital Inclusion:
Multilingual Digital Health Technology Development:
Conclusions:
Funding
Conflicts of Interest
References
- Martin, S.S. Centennial Collection: Health Applications of Digital Technologies. Circulation 2024, 149, 1701–1703. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.; Shandhi, M.M.H.; Master, H.; Dunn, J.; Brittain, E. Wearable Devices in Cardiovascular Medicine. Circ Res. 2023, 132, 652–670. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N Engl J Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Prahalad, P.; Scheinker, D.; Desai, M.; Ding, V.Y.; Bishop, F.K.; Lee, M.Y.; et al. Equitable implementation of a precision digital health program for glucose management in individuals with newly diagnosed type 1 diabetes. Nat Med. 2024, 30, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Azizi, Z.; Broadwin, C.; Islam, S.; Schenk, J.; Din, N.; Hernandez, M.F.; et al. Digital Health Interventions for Heart Failure Management in Underserved Rural Areas of the United States: A Systematic Review of Randomized Trials. J Am Hear Assoc: Cardiovasc Cerebrovasc Dis. 2024, 13, e030956. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.E.; Mszar, R.; Grimshaw, A.A.; Gunderson, C.G.; Onuma, O.K.; Lu, Y.; et al. Digital Health Interventions for Hypertension Management in US Populations Experiencing Health Disparities. JAMA Netw Open. 2024, 7, e2356070. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Kim, D.S.; Hershman, S.G.; Shcherbina, A.; Johnson, A.; Tolas, A.; et al. Personalized digital behaviour interventions increase short-term physical activity: A randomized control crossover trial substudy of the MyHeart Counts Cardiovascular Health Study. Eur Hear J - Digit Heal. 2023, 4, 411–419. [Google Scholar] [CrossRef]
- Shcherbina, A.; Hershman, S.G.; Lazzeroni, L.; King, A.C.; O’Sullivan, J.W.; Hekler, E.; et al. The effect of digital physical activity interventions on daily step count: A randomised controlled crossover substudy of the MyHeart Counts Cardiovascular Health Study. Lancet Digital Heal. 2019, 1, e344–e352. [Google Scholar] [CrossRef]
- Creber, R.M.; Dodson, J.A.; Bidwell, J.; Breathett, K.; Lyles, C.; Still, C.H.; et al. Telehealth and Health Equity in Older Adults With Heart Failure: A Scientific Statement From the American Heart Association. Circ: Cardiovasc Qual Outcomes. 2023, 16, e000123. [Google Scholar]
- Rodriguez, J.A.; Khoong, E.C.; Lipsitz, S.R.; Lyles, C.R.; Bates, D.W.; Samal, L. Telehealth Experience Among Patients With Limited English Proficiency. JAMA Netw Open. 2024, 7, e2410691. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [PubMed]
- Social determinants of health [Internet]. Available online: https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1 (accessed on 6 August 2024).
- Social Determinants of Health - Healthy People 2030 | health.gov [Internet]. Available online: https://health.gov/healthypeople/priority-areas/social-determinants-health (accessed on 6 August 2024).
- Lyles, C.R.; Wachter, R.M.; Sarkar, U. Focusing on Digital Health Equity. JAMA 2021, 326, 1795–1796. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Clark, C.R.; Bates, D.W. Digital Health Equity as a Necessity in the 21st Century Cures Act Era. JAMA. 2020, 323, 2381–2382. [Google Scholar] [CrossRef] [PubMed]
- Lancet, T. 50 years of the inverse care law. Lancet. 2021, 397, 767. [Google Scholar] [CrossRef]
- Safavi, K.; Mathews, S.C.; Bates, D.W.; Dorsey, E.R.; Cohen, A.B. Top-Funded Digital Health Companies And Their Impact On High-Burden, High-Cost Conditions. Heal Aff. 2019, 38, 115–123. [Google Scholar] [CrossRef]
- Sieck, C.J.; Sheon, A.; Ancker, J.S.; Castek, J.; Callahan, B.; Siefer, A. Digital inclusion as a social determinant of health. npj Digit Med. 2021, 4, 52. [Google Scholar] [CrossRef]
- Richardson, S.; Lawrence, K.; Schoenthaler, A.M.; Mann, D. A framework for digital health equity. npj Digit Med. 2022, 5, 119. [Google Scholar] [CrossRef]
- Lyles, C.R.; Nguyen, O.K.; Khoong, E.C.; Aguilera, A.; Sarkar, U. Multilevel Determinants of Digital Health Equity: A Literature Synthesis to Advance the Field. Annu Rev Public Heal. 2022, 44, 383–405. [Google Scholar] [CrossRef]
- Chunara, R.; Zhao, Y.; Chen, J.; Lawrence, K.; Testa, P.A.; Nov, O.; et al. Telemedicine and Healthcare Disparities: A cohort study in a large healthcare system in New York City during COVID-19. J Am Méd Inform Assoc. 2020, 28, 33–41. [Google Scholar] [CrossRef]
- Ramsetty, A.; Adams, C. Impact of the digital divide in the age of COVID-19. J Am Méd Inform Assoc. 2020, 27, 1147–1148. [Google Scholar] [CrossRef]
- Crawford, A.; Serhal, E. Digital Health Equity and COVID-19, The Innovation Curve Cannot Reinforce the Social Gradient of Health. J Méd Internet Res. 2020, 22, e19361. [Google Scholar] [CrossRef]
- Budd, J.; Miller, B.S.; Manning, E.M.; Lampos, V.; Zhuang, M.; Edelstein, M.; et al. Digital technologies in the public-health response to COVID-19. Nat Med. 2020, 26, 1183–1192. [Google Scholar] [CrossRef]
- Lawrence, K. Digital Health. 2022, 121–130.
- Chidambaram, S.; Jain, B.; Jain, U.; Mwavu, R.; Baru, R.; Thomas, B.; et al. An introduction to digital determinants of health. PLOS Digit Heal. 2024, 3, e0000346. [Google Scholar] [CrossRef]
- Mahajan, S.; Lu, Y.; Spatz, E.S.; Nasir, K.; Krumholz, H.M. Trends and Predictors of Use of Digital Health Technology in the United States. Am J Med. 2021, 134, 129–134. [Google Scholar] [CrossRef]
- Hernandez, M.F.; Rodriguez, F. Health Techequity: Opportunities for Digital Health Innovations to Improve Equity and Diversity in Cardiovascular Care. Curr Cardiovasc Risk Rep. 2023, 17, 1–20. [Google Scholar] [CrossRef]
- Avoke, D.; Elshafeey, A.; Weinstein, R.; Kim, C.H.; Martin, S.S. Digital Health in Diabetes and Cardiovascular Disease. Endocr Res. 2024, 49, 124–136. [Google Scholar] [CrossRef]
- Mayberry, L.S.; Guy, C.; Hendrickson, C.D.; McCoy, A.B.; Elasy, T. Rates and Correlates of Uptake of Continuous Glucose Monitors Among Adults with Type 2 Diabetes in Primary Care and Endocrinology Settings. J Gen Intern Med. 2023, 38, 2546–2552. [Google Scholar] [CrossRef]
- Noor, N.; Kamboj, M.K.; Triolo, T.; Polsky, S.; McDonough, R.J.; Demeterco-Berggren, C.; et al. Hybrid Closed-Loop Systems and Glycemic Outcomes in Children and Adults With Type 1 Diabetes: Real-World Evidence From a U.S.-Based Multicenter Collaborative. Diabetes Care. 2022, 45, e118–e119. [Google Scholar] [CrossRef]
- Odugbesan, O.; Addala, A.; Nelson, G.; Hopkins, R.; Cossen, K.; Schmitt, J.; et al. Implicit Racial–Ethnic and Insurance-Mediated Bias to Recommending Diabetes Technology: Insights from T1D Exchange Multicenter Pediatric and Adult Diabetes Provider Cohort. Diabetes Technol Ther. 2022, 24, 619–627. [Google Scholar] [CrossRef]
- Isaacs, D.; Bellini, N.J.; Biba, U.; Cai, A.; Close, K.L. Health Care Disparities in Use of Continuous Glucose Monitoring. Diabetes Technol Ther. 2021, 23, S-81–S-87. [Google Scholar] [CrossRef]
- Venkatesh, K.K.; Powe, C.E.; Buschur, E.; Wu, J.; Landon, M.B.; Gabbe, S.; et al. Disparities in Continuous Glucose Monitoring Use Among Women of Reproductive Age with Type 1 Diabetes in the T1D Exchange. Diabetes Technol Ther. 2023, 25, 201–205. [Google Scholar] [CrossRef]
- Feig, D.S.; Donovan, L.E.; Corcoy, R.; Murphy, K.E.; Amiel, S.A.; Hunt, K.F.; et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): A multicentre international randomised controlled trial. Lancet. 2017, 390, 2347–2359. [Google Scholar] [CrossRef]
- Aggarwal, R.; Chiu, N.; Wadhera, R.K.; Moran, A.E.; Raber, I.; Shen, C.; et al. Racial/Ethnic Disparities in Hypertension Prevalence, Awareness, Treatment, and Control in the United States, 2013 to 2018. Hypertension 2021, 78, 1719–1726. [Google Scholar] [CrossRef]
- Lu, X.; Yang, H.; Xia, X.; Lu, X.; Lin, J.; Liu, F.; et al. Interactive Mobile Health Intervention and Blood Pressure Management in Adults. Hypertension. 2019, 74, 697–704. [Google Scholar] [CrossRef]
- Stevenson, L.W.; Ross, H.J.; Rathman, L.D.; Boehmer, J.P. Remote Monitoring for Heart Failure Management at Home. J Am Coll Cardiol. 2023, 81, 2272–2291. [Google Scholar] [CrossRef]
- Chaudhry, S.I.; Mattera, J.A.; Curtis, J.P.; Spertus, J.A.; Herrin, J.; Lin, Z.; Phillips, C.O.; Hodshon, B.V.; et al. Telemonitoring in Patients with Heart Failure. N Engl J Med. 2010, 363, 2301–2309. [Google Scholar] [CrossRef]
- Ong, M.K.; Romano, P.S.; Edgington, S.; Aronow, H.U.; Auerbach, A.D.; Black, J.T.; et al. Effectiveness of Remote Patient Monitoring After Discharge of Hospitalized Patients With Heart Failure: The Better Effectiveness After Transition–Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016, 176, 310. [Google Scholar] [CrossRef]
- Koehler, F.; Winkler, S.; Schieber, M.; Sechtem, U.; Stangl, K.; Böhm, M.; et al. Telemedical Interventional Monitoring in Heart Failure (TIM-HF), a randomized, controlled intervention trial investigating the impact of telemedicine on mortality in ambulatory patients with heart failure: Study design. Eur J Hear Fail. 2010, 12, 1354–1362. [Google Scholar] [CrossRef]
- Takeda, A.; Martin, N.; Taylor, R.S.; Taylor, S.J. Disease management interventions for heart failure. Cochrane Database Syst Rev. 2019, 2019, CD002752. [Google Scholar] [CrossRef]
- Sammour, Y.; Main, M.L.; Austin, B.A.; Magalski, A.; Sperry, B.W. Outpatient Management of Guideline-Directed Medical Therapy for Heart Failure Using Telehealth: A Comparison of In-Office, Video, and Telephone Visits. J Card Fail. 2022, 28, 1222–1226. [Google Scholar] [CrossRef]
- Adamson, P.B. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: New insights from continuous monitoring devices. Curr Hear Fail Rep. 2009, 6, 287. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet. 2011, 377, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Lindenfeld, J.; Zile, M.R.; Desai, A.S.; Bhatt, K.; Ducharme, A.; Horstmanshof, D.; et al. Haemodynamic-guided management of heart failure (GUIDE-HF): A randomised controlled trial. Lancet. 2021, 398, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Mastoris, I.; DeFilippis, E.M.; Martyn, T.; Morris, A.A.; Spall, H.G.V.; Sauer, A.J. Remote Patient Monitoring for Patients with Heart Failure: Sex- and Race-based Disparities and Opportunities. Card Fail Rev. 2023, 9, e02. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, E.M.; Henderson, J.; Axsom, K.M.; Costanzo, M.R.; Adamson, P.B.; Miller, A.B.; et al. Remote Hemodynamic Monitoring Equally Reduces Heart Failure Hospitalizations in Women and Men in Clinical Practice. Circ: Hear Fail. 2021, 14, e007892. [Google Scholar]
- Paul, L.D.; Moinul, S.; Urina-Jassir, M.; Gopal, D.M.; Ayalon, N. Expanding Pulmonary Artery Pressure Monitoring to Racially and Socially Diverse Populations: A Pilot CardioMEMS Program. Am J Méd Sci. 2024, 368, 408–410. [Google Scholar] [CrossRef]
- Markson, F.; Abe, T.A.; Adedinsewo, D.; Olanipekun, T.; Shamaki, G.R.; Kesiena, O.; et al. Sex Differences in CardioMEMS Utilization and Impact on Readmissions and Mortality in Heart Failure Patients. JACC: Hear Fail. 2023, 11, 1760–1762. [Google Scholar] [CrossRef]
- Center, P.R. Demographics of Mobile Device Ownership and Adoption in the United States [Internet]. Available online: https://www.pewresearch.org/internet/fact-sheet/mobile/.
- Aminorroaya, A.; Dhingra, L.S.; Nargesi, A.A.; Oikonomou, E.K.; Krumholz, H.M.; Khera, R. Use of Smart Devices to Track Cardiovascular Health Goals in the United States. JACC: Adv. 2023, 2, 100544. [Google Scholar] [CrossRef]
- Holko, M.; Litwin, T.R.; Munoz, F.; Theisz, K.I.; Salgin, L.; Jenks, N.P.; et al. Wearable fitness tracker use in federally qualified health center patients: Strategies to improve the health of all of us using digital health devices. npj Digit Med. 2022, 5, 53. [Google Scholar] [CrossRef]
- Victoria-Castro, A.M.; Martin, M.L.; Yamamoto, Y.; Melchinger, H.; Weinstein, J.; Nguyen, A.; et al. Impact of Digital Health Technology on Quality of Life in Patients With Heart Failure. JACC: Hear Fail. 2024, 12, 336–348. [Google Scholar] [CrossRef]
- Dorsch, M.P.; Farris, K.B.; Rowell, B.E.; Hummel, S.L.; Koelling, T.M. The Effects of the ManageHF4Life Mobile App on Patients With Chronic Heart Failure: Randomized Controlled Trial. JMIR mHealth uHealth. 2021, 9, e26185. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Maglaras, L.; Ferrag, M.A.; Almomani, I. Digitization of healthcare sector: A study on privacy and security concerns. ICT Express. 2023, 9, 571–588. [Google Scholar] [CrossRef]
- Alliance, N.D.I. Definitions [Internet]. Available online: https://www.digitalinclusion.org.
- Romm, T. Lacking a Lifeline: How a federal effort to help low-income Americans pay their phone bills failed amid the pandemic. Washington Post. 2021.
- Brewer, L.C.; Jenkins, S.; Hayes, S.N.; Kumbamu, A.; Jones, C.; Burke, L.E.; et al. Community-Based, Cluster-Randomized Pilot Trial of a Cardiovascular Mobile Health Intervention: Preliminary Findings of the FAITH! Trial. Circulation 2022, 146, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Jr, H.A.T.; Francis, S.; Evans, C.R.; Harvey, M.; Newton, B.A.; Jones, C.P.; et al. Preventing Cardiovascular Disease Among Urban African Americans With a Mobile Health App (the MOYO App): Protocol for a Usability Study. JMIR Res Protoc. 2020, 9, e16699. [Google Scholar]
- Skolarus, L.E.; Cowdery, J.; Dome, M.; Bailey, S.; Baek, J.; Byrd, J.B.; et al. Reach Out Churches: A Community-Based Participatory Research Pilot Trial to Assess the Feasibility of a Mobile Health Technology Intervention to Reduce Blood Pressure Among African Americans. Heal Promot Pr. 2018, 19, 495–505. [Google Scholar] [CrossRef]
- Goodson, N.; Wicks, P.; Morgan, J.; Hashem, L.; Callinan, S.; Reites, J. Opportunities and counterintuitive challenges for decentralized clinical trials to broaden participant inclusion. npj Digit Med. 2022, 5, 58. [Google Scholar] [CrossRef]
- Rodriguez, F. Sex Disparities in Prevention of Atherosclerotic Cardiovascular Disease Across the Life Course. Circulation. 2023, 147, 523–525. [Google Scholar] [CrossRef]
- Smith, J.R.; Thomas, R.J.; Bonikowske, A.R.; Hammer, S.M.; Olson, T.P. Sex Differences in Cardiac Rehabilitation Outcomes. Circ Res. 2022, 130, 552–565. [Google Scholar] [CrossRef]
- Li, S.; Fonarow, G.C.; Mukamal, K.; Xu, H.; Matsouaka, R.A.; Devore, A.D.; et al. Sex and Racial Disparities in Cardiac Rehabilitation Referral at Hospital Discharge and Gaps in Long-Term Mortality. J Am Hear Assoc. 2018, 7, e008088. [Google Scholar] [CrossRef]
- Azizi, Z.; Adedinsewo, D.; Rodriguez, F.; Lewey, J.; Merchant, R.M.; Brewer, L.C. Leveraging Digital Health to Improve the Cardiovascular Health of Women. Curr Cardiovasc Risk Rep. 2023, 17, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Brewer, L.C.; Hayes, S.N.; Caron, A.R.; Derby, D.A.; Breutzman, N.S.; Wicks, A.; et al. Promoting cardiovascular health and wellness among African-Americans: Community participatory approach to design an innovative mobile-health intervention. PLoS ONE. 2019, 14, e0218724. [Google Scholar] [CrossRef] [PubMed]
- Miao, B.Y.; Sushil, M.; Xu, A.; Wang, M.; Arneson, D.; Berkley, E.; et al. Characterisation of digital therapeutic clinical trials: A systematic review with natural language processing. Lancet Digit Heal. 2024, 6, e222–e229. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Astudillo, K.; Velez, D.; Kelley, L.; Cobbs-Lomax, D.; Spatz, E.S. Use of Mobile Health Applications in Low-Income Populations. Circ: Cardiovasc Qual Outcomes. 2020, 13, e007031. [Google Scholar] [CrossRef]
- King, A.C.; Campero, M.I.; Sheats, J.L.; Sweet, C.M.C.; Hauser, M.E.; Garcia, D.; et al. Effects of Counseling by Peer Human Advisors vs Computers to Increase Walking in Underserved Populations. JAMA Intern Med. 2020, 180, 1481–1490. [Google Scholar] [CrossRef]
- King, A.C.; Bickmore, T.W.; Campero, M.I.; Pruitt, L.A.; Yin, J.L. Employing Virtual Advisors in Preventive Care for Underserved Communities: Results From the COMPASS Study. J Heal Commun. 2013, 18, 1449–1464. [Google Scholar] [CrossRef]
- King, A.C.; Campero, I.; Sheats, J.L.; Sweet, C.M.C.; Garcia, D.; Chazaro, A.; et al. Testing the comparative effects of physical activity advice by humans vs. computers in underserved populations: The COMPASS trial design, methods, and baseline characteristics. Contemp Clin Trials. 2017, 61, 115–125. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



