1. Introduction
The control of balance requires a complex interplay between sensory information arising from visual, vestibular, and somatosensory systems for generation of motor commands to maintain an upright stance [
1]. Balance control predicts function and engagement of individuals in activities of daily living (ADLs) [
2,
3,
4]. Poor balance control, as it happens due to aging or neurological disorders, is a predictor of falls [
5,
6,
7,
8]. For neurotypical adults, standing balance is a seemingly trivial task, controlled primarily by subcortical brain structures and the spinal cord with very limited cortical involvement [
9]. Several studies have suggested the involvement of cortex [
10] when the stance is challenged through blindfolding the participant [
11], using a single support [
12,
13], standing on an unstable platform [
14], or standing on a platform capable of making unexpected translations or rotations [
15].
Activations across several fronto-parietal regions [
16,
17,
18,
19,
20], including anterior cingulate, supplementary motor area (SMA), and posterior parietal cortices [
21,
22,
23,
24], are commonly reported, yet causal relationships are not completely understood [
25]. Similar tasks often report the same regions of interest but may demonstrate different levels of activation. Researchers investigating postural stability often use a force plate capable of administering a translation of the standing platform (perturbation task; PT) or a force plate that is sway-referenced (sway reference task; SRT) in reference to deviations in subject’s center of pressure [
26]. The perturbation task (PT) is associated with a characteristic evoked potentials observed in the electroencephalographic (EEG) activity following the perturbation but preceding the initiation of a functional balance task [
27]. These perturbation-evoked potentials originate from fronto-central brain regions such as anterior cingulate or SMA [
21,
22]. The characteristics of these potentials such as the amplitude and latency are known to be affected by novelty [
24], predictability [
28,
29], attention [
30], and speed [
31] of the perturbation, observable on a single trial basis [
24,
32], and precedes postural instability and stepping to prevent imminent falls [
23]. For the sway reference task (SRT), on the other hand, these evoked potentials are smaller in magnitude, but can be identified during the task [
33]. PT and SRT differ in fundamental mechanisms underlying balance control [
34]. Platform translation is an imposed mechanical perturbation that can be considered an error signal unrelated to ongoing stance. However, sway-referencing the platform results in sensory manipulation and alters sensory feedback regarding motor outputs during ongoing control such that their effects are a function of the action of the participant. A smaller evoked response during SRT when compared with PT suggests that the cortical response is stronger in case of PT. None of the studies have performed a direct comparison of fronto-central cortical activation levels between the PT and SRT tasks. Such knowledge will lay a foundation for designing neurointerventions for balance improvements with effects generalizable across multiple tasks.
Furthermore, understanding the certainty of causal influence of cortical activation on the control of balance during challenging conditions is important for rehabilitation researchers. High frequency repetitive transcranial magnetic stimulation (cTBS) has been used for inducing virtual lesions of brain regions and understanding its effect on balance control [
35]. For example, our earlier work found that disruption of (SMA) using inhibitory cTBS altered EEG band power in lower frequency bands within brain areas involved in the control of balance during SRT, thus confirming the role of SMA in this behavior [
18]. The effects of repetitive cTBS over SMA on the fronto-central brain activity during PT are yet to be known.
In this study, we investigated differences in cortical activation measured using EEG across two commonly used balance tasks, the perturbation task and the sway reference task in neurotypical adults. We expected common EEG sources of brain activation across PT and SRT tasks but differences in their activation levels. Considering the known disruptive effects of cTBS over SMA on rhythmic synchronous cortical oscillations measured non-invasively using EEG during the SRT task, we expected that cTBS over SMA will alter EEG band power within the frontocentral brain regions during PT in neurotypical adults.
2. Materials and Methods
Twenty healthy, right-handed young adults (age: 26.0 ± 3.4 years; 8 females; height: 168.0 ± 11.8 cm; weight: 66.7 ± 13.8 kg, mean ± standard deviation (SD)) provided written, informed consent for participation. Two groups (n = 10, 4 females) were randomly selected to receive cTBS over SMA (cTBSSMA) or a sham control (cTBSSHAM). No participants reported history of balance, neurological, musculoskeletal, cardiovascular, or vestibular disorders, and were screened with the Physical Activity Readiness Questionnaire (PAR-Q) and TMS Adult Safety Screening Questionnaire. The Committee for the Protection of Human Subjects at the University of Houston approved this study.
Instrumentation
Electroencephalography (EEG):
Whole-scalp EEG with 64 active channel electrodes (Brain Products GmbH, Germany; 1000 Hz) was recorded at rest and during the balance tasks. An elastic cap constrained the movement of the electrodes during the balance task. A modified international 10-20 system was used, where GND and REF were attached to the earlobes, and replaced by T7 and T8 electrodes. However, four electrodes were repurposed for electrooculography (EOG), where the electrodes were placed around the participants’ eyes, to remove eye artifacts during EEG preprocessing [
18,
31].
Computerized dynamic posturography (CDP):
A standard, commercial CDP force platform (NeuroCom Balance Manager, Natus Medical Incorporated, Pleasanton, CA) was used for both balance tasks, as done previously [
18,
31]. During all posture tasks, subjects wore a safety harness to prevent falls or injury. The platform is equipped with a dynamic 45.72 cm x 45.72 cm dual force plate system. The ground reaction forces from under the feet of subjects were collected by four individual force transducers embedded within the force plate. Force platform data were collected at 100 Hz and processed by pre-installed software on a Windows-based desktop connected to the NeuroCom Balance Manager (Research module, NeuroCom software version 8.0, Natus Medical Incorporated, Pleasanton, CA). Our previous work has reported the analysis of center of pressure data during PT [
31] and SRT [
18]. An analog output signal of 5 V generated by the NeuroCom system was used to synchronize NeuroCom data with the EEG system [
31].
Balance Tasks
Sway Reference Task (SRT):
Participants were instructed to perform the sway reference task with varying sensory conditions. The goal of this task was to maintain a quiet upright stance with eyes closed. They completed nine trials of 20 second durations each, where the platform was referenced to their sway with different gain settings. The orientation of the platform of the balance manager was adjusted with respect to the gravitational vertical by rotating it in the sagittal plane about an axis through the subject’s ankle joint in some proportion (a preselected gain between -2 to +2) to the postural sway of the subject. The purpose of this task was to alter the relationships between postural sway and somatosensory inputs by randomly varying the gain of the support surface. We used a range of gains (-1.0, -0.4, 0, 0.4, 0.6, 1.0, 2.0) to expose subjects to different levels of postural difficulty. Subjects were not informed when the gain changed. These postural conditions allowed us to manipulate the difficulty of the postural control task progressively while concurrently monitoring variations in the EEG responses. Subjects were not allowed to practice at different gains of the sway-referenced support surface. The trials were continuous with a brief (<10 second) delay to save the data and begin the new trial, thus demarcating the start of each trial in the EEG and used in analysis.
Perturbation Task (PT):
Participants were instructed to maintain an upright stance with eyes closed during destabilizing postural perturbations. The perturbations were in the form of unexpected translation of the force platform upon which the subject stood. Six different perturbation conditions [
31] were used with direction (backward and forward), displacement (3.17 cm and 6.35 cm), speed (7.93 cm/s and 15.88 cm/s), and period (400 ms and 800 ms) varying from condition-to-condition, randomized in order. Participants completed a total of eighteen discrete trials, 3 trials per perturbation condition. Each trial lasted for 5000 ms, which included 1000 ms of “pre-perturbation phase,” 400 or 800 ms of “perturbation phase,” and 3600 or 3200 ms of “post-perturbation phase.” In the post-perturbation period, the force plate remained stationary at the translated position. The onset of perturbation occurred 1000 ms after the trial onset. Participants were not informed of the start of each trial, nor warned of perturbation onset. At the end of each trial, the platform translated back to the original position at 1.00 cm/s, which took 3500-6200 ms, and then the next trial was initiated after a brief (<10 s) delay to save the trial data. The effects of different perturbation characteristics have been studied in our previous work [
31]. All trials were grouped together for analysis. To capture a naïve response, no practice session was provided before the experimental trials. EEG activity was recorded for all the trials.
Transcranial Magnetic Stimulation Procedures
We estimated the active motor threshold (aMT) by placing a figure-of-eight TMS coil (Magstim Super Rapid
2 stimulator; Magstim, Whitland, UK) tangential to the scalp, oriented at 45° from the midsagittal line, with the handle pointing backward, inducing a current in the postero-anterior direction. The hand motor region of the left primary motor cortex (M1) that is associated with the right first dorsal interosseus (FDI) muscle was located using supra-threshold TMS pulses [
36,
37]. Electromyographic (EMG) activity in the FDI muscle of the right hand was recorded using a differential surface electrode (Bagnoli EMG system, Delsys, Natick, MA). The largest grip force exerted using the index finger and thumb on an instrumented force sensing handle over three trials was used as the maximum voluntary force (MVF) [
38]. The aMT was determined as the TMS intensity that induced 200 µV peak-to-peak motor evoked potentials (MEPs) in 5 of 10 trials in the FDI muscle during grip force exertion at 20% of MVF [
39], using visual feedback. The aMT was estimated to be 52 ± 5% (mean ± SD) of the maximum stimulator output [
18].
For each subject, we obtained high-resolution T1-weighted structural images [
18] using a 3T Siemens Trio whole-body MR scanner (Erlangen, Germany) before the experiment. A three-dimensional (3D) brain was reconstructed from the MRI slices to display the cortical surface (Brainsight software, Rogue Research, Montreal, QC, Canada). The location of the left SMA was demarcated for each subject. We chose left SMA because of postulated functional asymmetry between motor areas with the dominant brain hemisphere playing a more important role in the selection of appropriate postural strategies [
18]. The location of the left SMA was selected as the most medial part of the superior frontal gyrus, which was anterior and dorsal to the precentral gyrus [
40,
41,
42]. Because SMA is located directly anterior to the leg representation of M1 at the same depth on the interhemispheric surface [
43,
44], the optimal coil position to evoke MEPs in the right tibialis anterior (TA) muscle was determined first while subjects were instructed to exert ~20% of maximum voluntary contraction of right TA [
41,
45]. The coil was then moved anterior in small increments, and the final SMA location was chosen 1 cm anterior from the point where there were no MEPs in the right TA. If needed, the coil position was slightly adjusted based on the anatomical target. For the virtual lesion, the coil was placed and maintained horizontally with the handle pointing leftward [
44]. The Montreal Neurological Institute (MNI) coordinates of the stimulation site for the left SMA were -4.36 ± 2.61, -5.14 ± 1.88, 64.43 ± 4.04 mm (x, y, z, mean ± SD; n = 20) [
18] and were consistent with those reported in the literature [
40,
41,
46,
47,
48].
To disrupt the SMA activity, continuous theta burst stimulation (cTBS) was delivered at 80% of aMT of FDI (cTBS
SMA; n = 10). Repetitive CTBS pulses were delivered in the form of bursts of three pulses at 50 Hz at a rate of 5 Hz (total 600 pulses) [
49]. For the SHAM group (cTBS
SHAM; n = 10), the same stimulation parameters were used, but the coil was placed perpendicular over the SMA region such that no relevant current flow was induced in the cortical tissue [
50,
51,
52,
53]. The reduced corticospinal excitability and altered EEG measures lasts about 60 min [
49] and 30 min [
54,
55], respectively.
Experimental Procedures
Each subject participated in two sessions separated by ~14 days. In the first session, participants underwent a structural MRI of the brain, general assessment of the body, and familiarization trials with the force platform. In the second session, we performed EEG, cTBS, and the two balance tasks. Participants were instrumented with EEG, followed by assessment of the resting state EEG activity. Then, the cTBS procedures as detailed above were conducted. After which, the resting state EEG activity was reassessed. All participants performed SRT followed by PT. Following the two balance tasks, resting state EEG was reassessed. All procedures (including post tasks resting state EEG) were completed within 27.2 (± 1.4) minutes following cTBS.
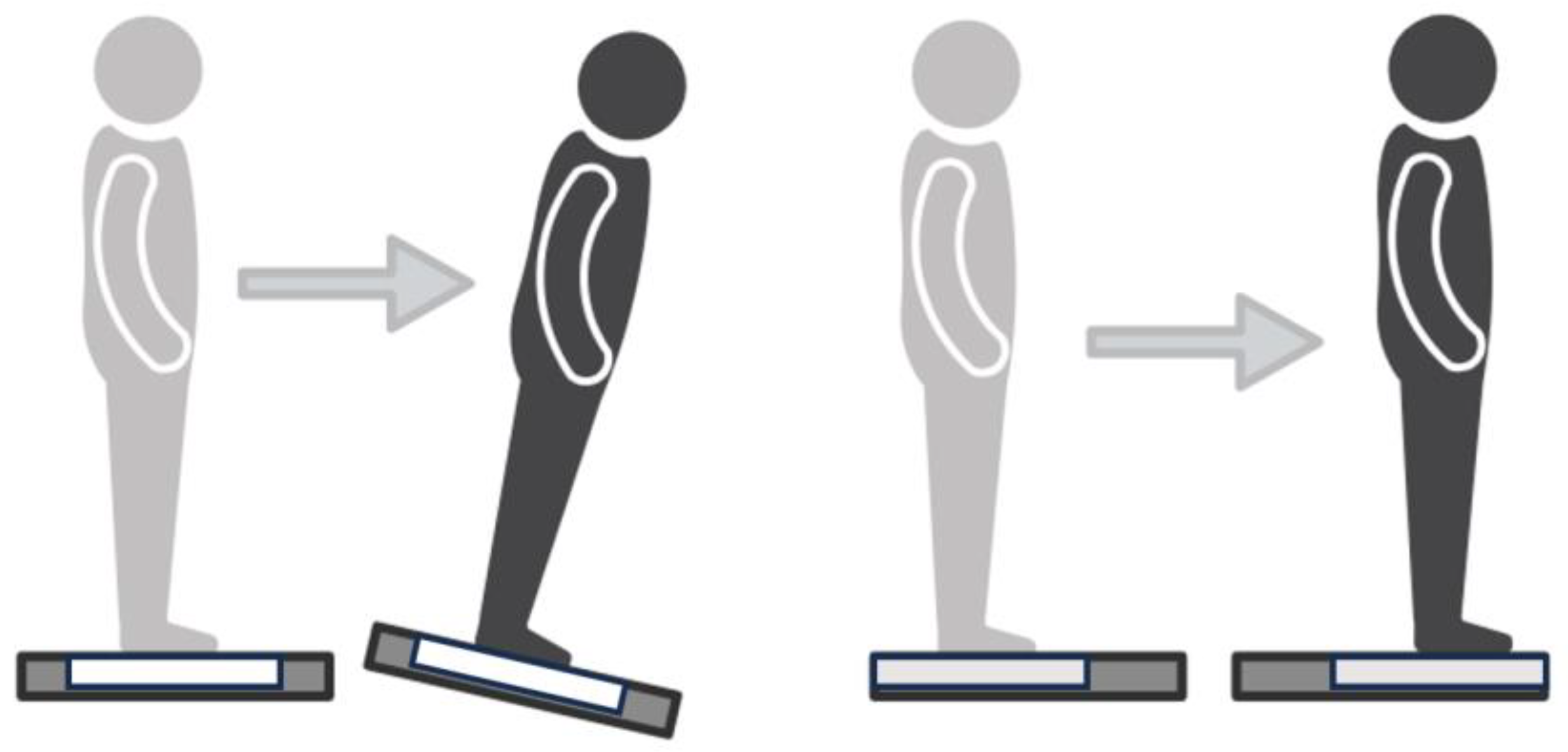
Figure 1.
Depiction of each task. (Left) Sway Reference Task, where the platform tilts in reference to the participants’ center of pressure. (Right) Perturbation Task, where the participant is translated unexpectedly forward.
Figure 1.
Depiction of each task. (Left) Sway Reference Task, where the platform tilts in reference to the participants’ center of pressure. (Right) Perturbation Task, where the participant is translated unexpectedly forward.
Data Analysis
EEG Preprocessing
All 64 channels passed through a zero-phase notch filter (60 Hz) to remove line noise, and a zero-phase bandpass filter (4th order, 0.1 Hz to 100 Hz, Butterworth). The electrooculography (EOG) channels were used in an H-Infinity filter (q = 1e-11, gamma = 1.15) to remove eye artifacts while retaining brain signals [
56]. The remaining 60 EEG channels were re-referenced to the common average reference. Artifact subspace reconstruction (ASR) was applied [
57] with a cutoff parameter of 30 [
58]. ASR utilized a clean EEG signal with a sliding window of 500ms to denoise the signal. Twenty second epochs, starting with trial onset, were used for SRT, and 1 second before and 3 seconds after perturbation onset was used to epoch PT. The potential sources of the cleaned EEG signal were determined through independent components analysis (ICA) with principal component analysis (PCA). DIPFIT in EEGLAB was used to fit dipoles to the provided independent components (ICs). ICA assumes the number of ICs to be equivalent to the number of given channels, but some ICs were removed due to their dipoles being outside of EEGLAB’s boundary element model (BEM) or ICs that were primarily noise due to channel, muscular, ocular, or bundle artifacts, determined through visual inspection.
EEG Source Localization
A k-means clustering algorithm, with a k value of 5 [
18] was applied to the remaining ICs from both SRT and PT tasks within each group based on similarities in calculated features such as dipole 3D location. Only ICs that accounted for at least 85% of variance (less than 15% residual variance) and within 3 standard deviations of the cluster centroid were kept for source localization. The IC that accounted for most of the variance was selected for each participant in each cluster. The Brodmann Areas (BA) for each cluster were determined using the Yale Bioimaging Suite [
59] and a deviation of +/- 5mm of the cluster centroids’ Talairach coordinates. Clustering was computed using dipoles for cTBS
SMA from the SRT, from the PT, and from combined tasks (CT), and repeated for cTBS
SHAM. All clusters included independent components from at least 60% of subjects suggesting the clusters were representative of the tasks and most of the participants. Our region of interest was the fronto-central region, of which both groups had one cluster centroid during CT clustering, and these were used for further analysis. These CT clusters had over 80% of subjects contributing during both PT and SRT.
The power spectrum was calculated for each independent component using the pwelch function in MATLAB (MATLAB 2022a, Mathworks, Natick, MA) with non-overlapping Hamming windows with the default frequency resolution of π/256 rad/sample. Relative power for delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), and low gamma (30–50 Hz) frequency bands were calculated by summing the power over each frequency band and normalized with respect to the total power of the PSD (from 1-50 Hz) for each independent component.
cTBS over SMA and the fronto-central brain response during PT
Because our secondary aim was to investigate the effects of cTBS over SMA on the EEG activity in the fronto-central region, we selected an independent component (IC) over the fronto-central region by examining both the two-dimensional topoplots of all the ICs remaining after EEG data cleaning and the corresponding dipole projections in three-dimensions for each subject [
31]. A single IC was chosen in the fronto-central brain region for each subject, based on the latency of a large, consistent event related response for each subject [
23]. If the fronto-central response was unclear or distributed between a couple of ICs, of these, the IC which accounted for the largest amount of data variance was selected [
23]. The centroid of the 10 equivalent dipoles, one per subject, was determined through a single k-means clustering approach, separately for each group. To understand the effects of cTBS on the fronto-central region, we compared the relative power computed for each subject (individual ICs, as described above) between SMA and sham groups.
Statistical Analyses
We used maximum likelihood linear models because this approach avoids restrictive assumptions of other approaches such as repeated measures ANOVA and accommodates potential missing values. Our clustering step identified EEG sources with different locations across groups, thus we ran separate linear mixed models for each cluster within each group (cTBSSMA and cTBSSHAM) to investigate differences in EEG source power between SRT and PT. Linear mixed models with level 2 within-subject factor Task (SRT, PT), level 5 within-subject factor frequency band (delta, theta, alpha, beta, and low gamma), and interaction between task and frequency bands were included in the model.
Next, we studied the effects of cTBS over SMA on EEG source power over the fronto-central region. For EEG frontocentral source relative power, we ran linear mixed models with level 2 between-subject factor group (cTBSSMA, cTBSSHAM), level 5 within-subject factor frequency band (delta, theta, alpha, beta, and low gamma), with the interaction between group and frequency bands. Post-hoc comparisons were performed using Fisher LSD test with appropriate Bonferroni corrections. All the statistical analyses described thus far in this section were carried out using SPSS software (SPSS version 21, SPSS Inc.; Chicago, IL).
3. Results
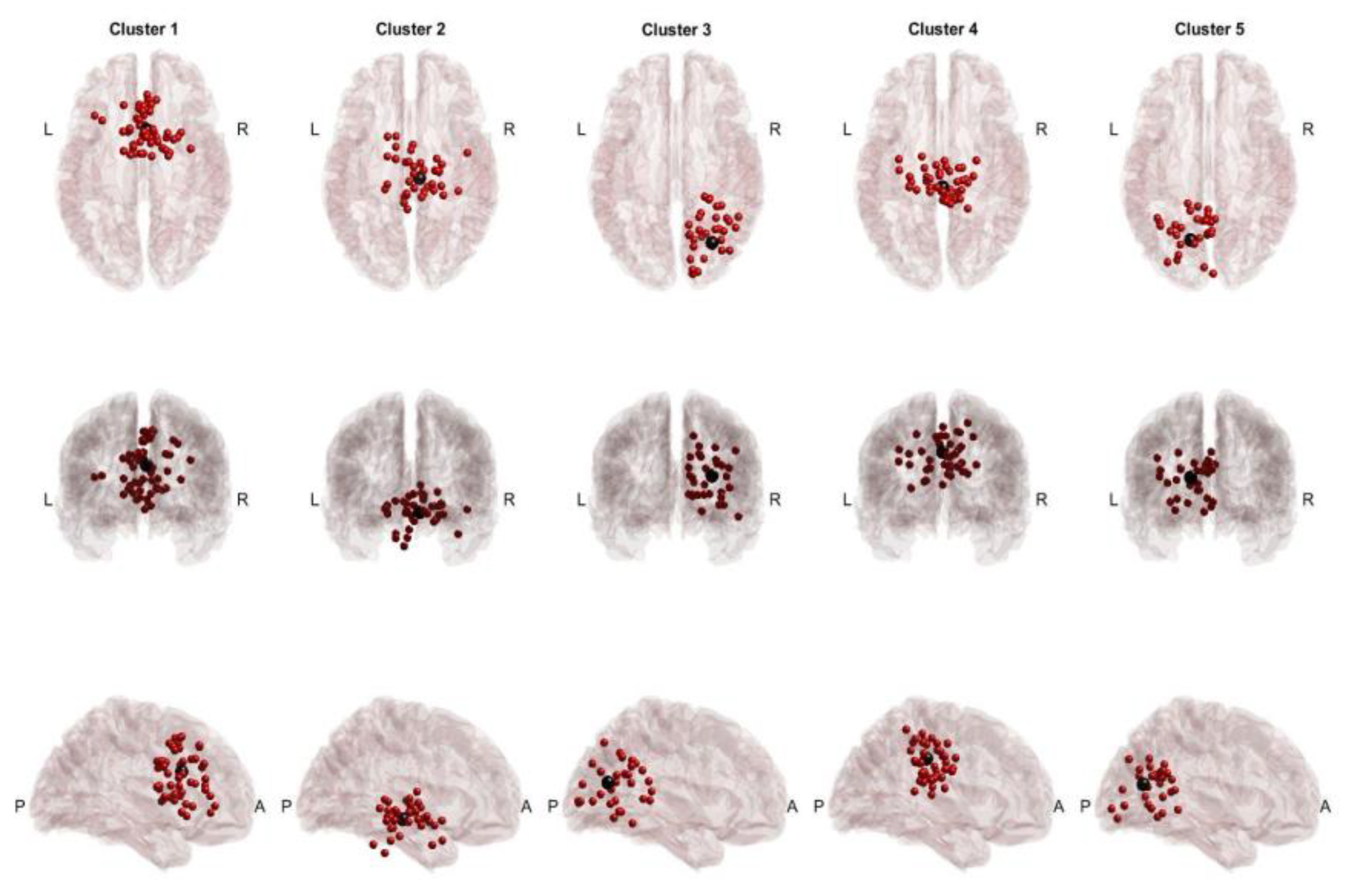
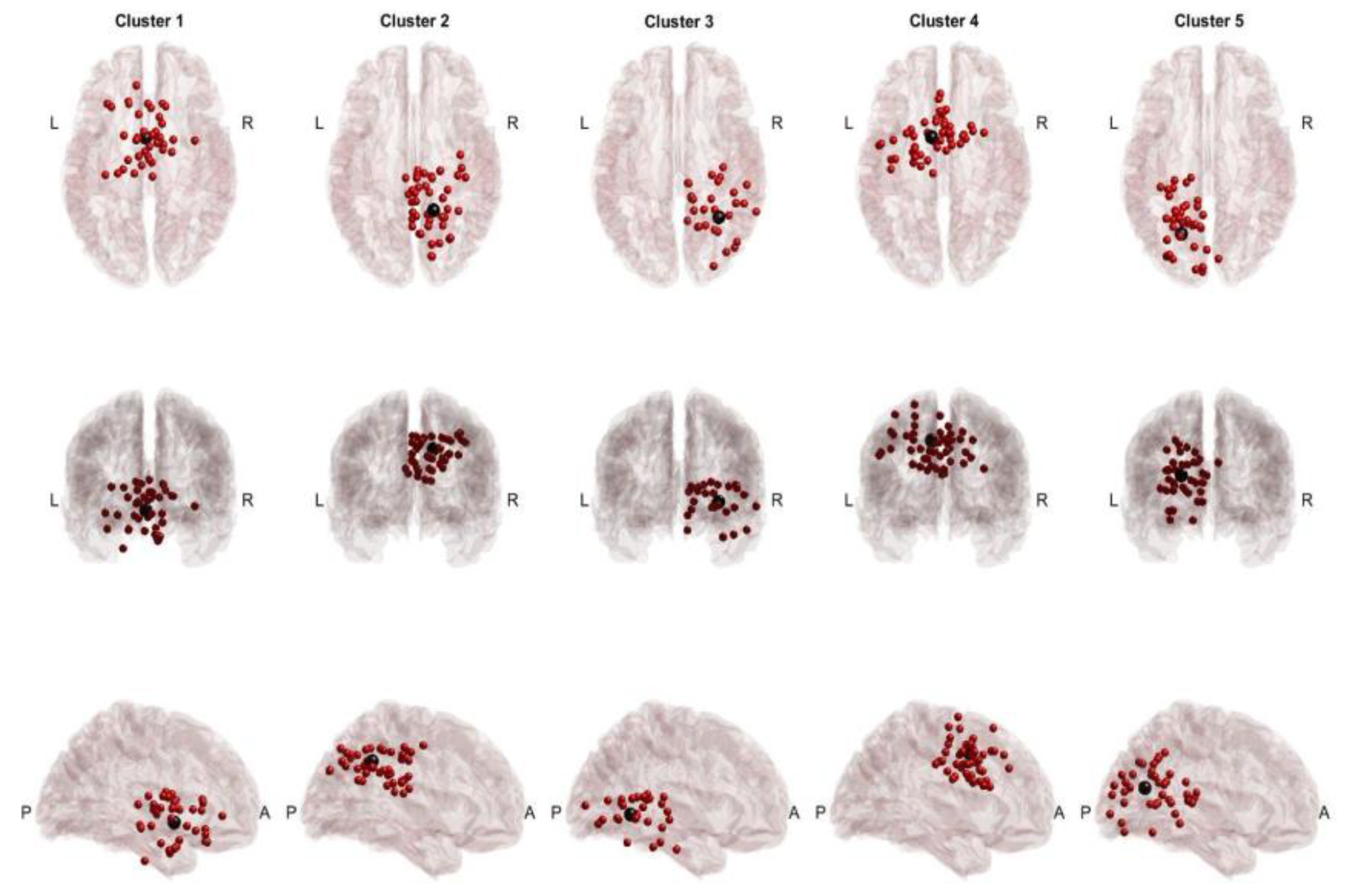
3.1. Across-Task Shared EEG Sources
Twenty participants successfully completed the protocol, 10 participants received sham cTBS (cTBS
SHAM) and another 10 participants received cTBS (inhibitory) over left SMA (cTBS
SMA ), before performance of SRT and PT. After preprocessing and artifactual source removal, the equivalent dipoles of the remaining sources were clustered using information from SRT and PT (cTBS
SHAM,
Figure 2; cTBS
SMA,
Figure 3), and the participant groups were computed separately. For the cTBS
SHAM group, common sources across tasks were found in the Cingulate Gyrus (BA 23 and 24) and the visual cortex (BA 19). In the cTBS
SMA group, common sources included the SMA (BA 6) and visual cortex. Because of our interest in the fronto-central region, a cluster in the ventral anterior cingulate (Talairach: [2, 10, 24]) for the cTBS
SHAM group and a cluster in the SMA region (Talairach: [-9, 1, 41]) for the cTBS
SMA group was selected as clusters of interest for further analysis.
3.2. Relative Power Comparison within Cross-Task Clusters
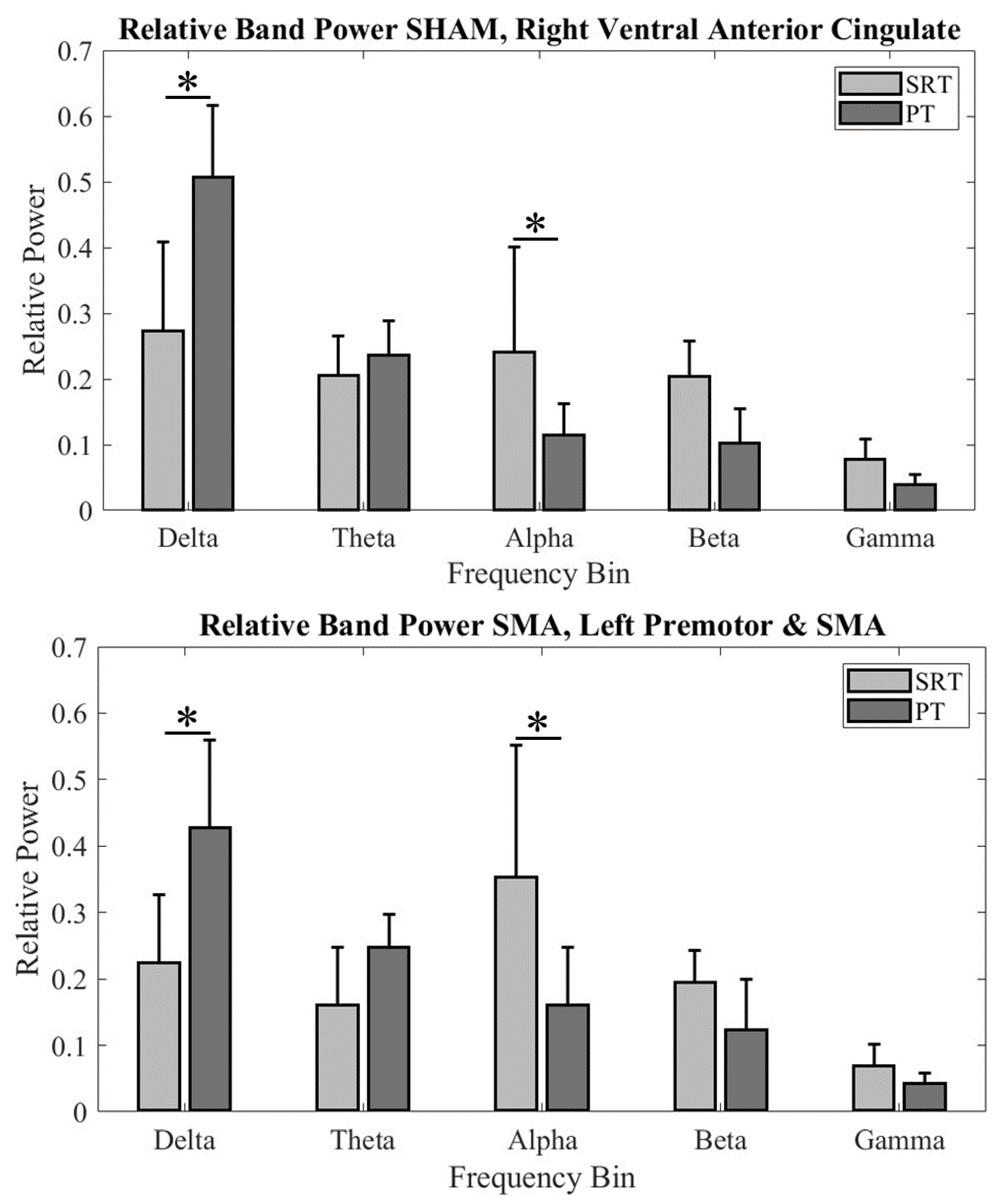
We compared relative EEG power at different frequency bands between tasks within each group’s fronto-central cluster. For the Ventral Anterior Cingulate (BA 24) in the cTBSSHAM group, we found significant difference in relative EEG power across two tasks (significant Task × Frequency bands interaction: F4, 94.352 = 21.352; p < 0.001; main effect of Frequency bands: F4, 94.352 = 60.958; p < 0.001). Post hoc comparisons found significantly higher delta band power (p < 0.000001) and lower alpha band power (p = 0.0098) during PT when compared SRT. No other comparisons were found to be significant (all p values>0.05).
Figure 4.
Band relative power across participants for (Top) cTBSSHAM group Cluster 1 and (Bottom) cTBSSMA group Cluster 4. An * denotes statistical significance of p < 0.05.
Figure 4.
Band relative power across participants for (Top) cTBSSHAM group Cluster 1 and (Bottom) cTBSSMA group Cluster 4. An * denotes statistical significance of p < 0.05.
Similarly, for the SMA (BA6) region in the cTBSSMA group, we found a significant difference in relative EEG power across two tasks (significant Task × Frequency bands interaction: F4, 94.352 = 9.537; p < 0.001). Post hoc comparisons found significantly higher delta band power (p < 0.00022) and lower alpha band power (p = 0.00052) during PT when compared to SRT. No other comparisons were found to be significant (all p values>0.05).
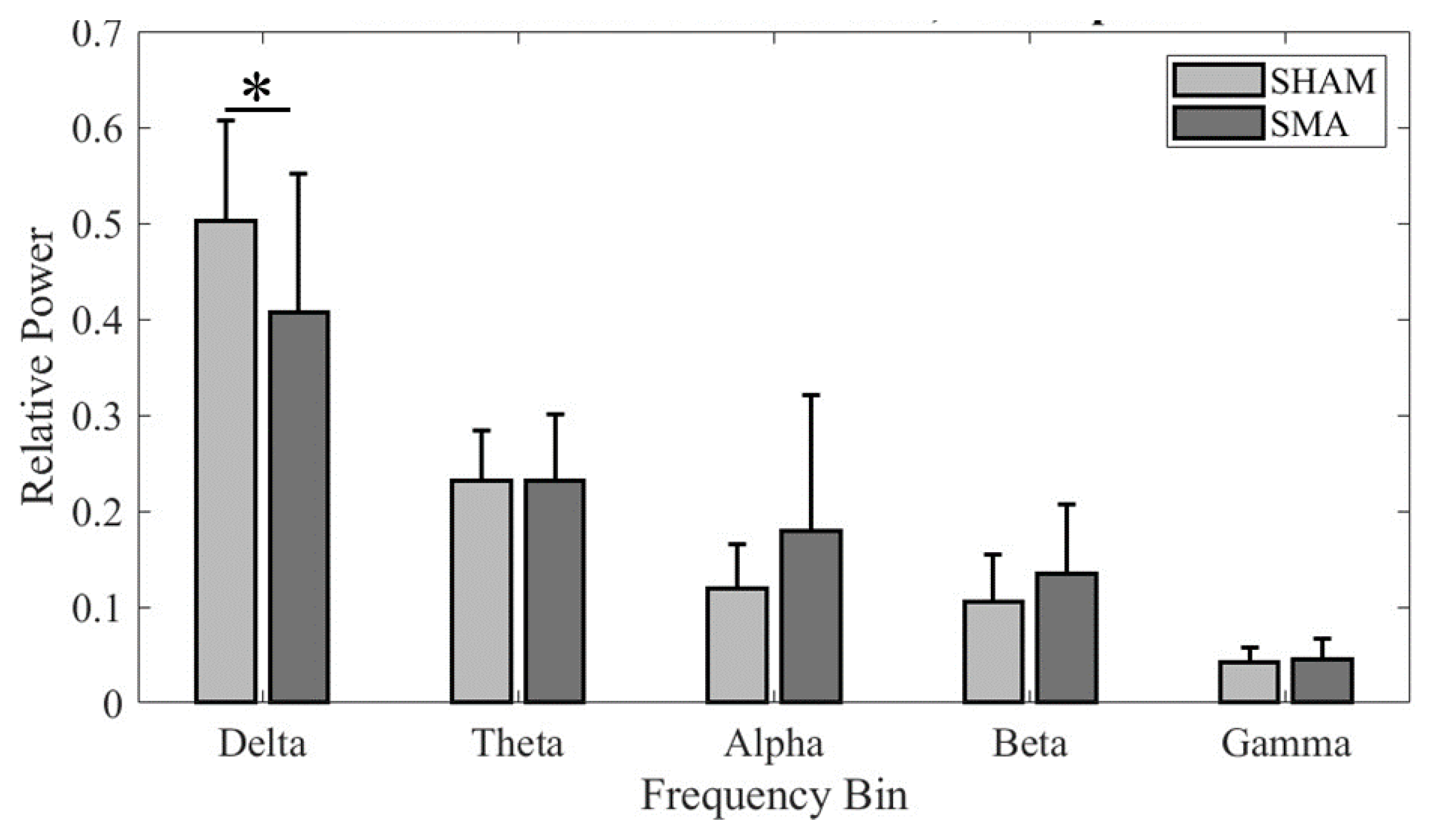
3.3. Fronto-Central Activation during PT Across-Group Localization
Because the secondary aim was to investigate the effects of cTBS over SMA on the EEG activity in the fronto-central region during PT, we focused our analysis on clusters formed using PT-related ICs in this region for each cTBS
SMA and cTBS
SHAM group. The Talairach coordinates of the cluster centroids in the fronto-central region were (0, 12, 42) for the cTBS
SHAM group, which suggests activation within BA 6 (Left SMA), and (2, -2, 48) for the cTBS
SMA group, which suggests activation within BA 6 (Right SMA). Band relative powers were computed for each IC from a participant contributing to these clusters and compared across groups (
Figure 5). We found a significant difference in frequency band power across the two groups (significant Group × Frequency bands interaction: F
4, 195.187 = 3.932; p = 0.004; main effect of Frequency bands: F
4, 195.187 = 114.41; p < 0.001). Post hoc comparison found a significantly lower delta frequency band power following cTBS over SMA (cTBS
SMA) when compared with the cTBS
SHAM group (p = 0.038). No other comparisons were found to be significant (all p values > 0.05).
4. Discussion
In this study, we compared the relative power of cortical sources identified using EEG between two commonly studied challenging balance tasks, SRT and PT in neurotypical adults. We also investigated whether cTBS over SMA can alter the EEG activations during PT in neurotypical adults. The novel findings from this study are: (1) For the ventral anterior cingulate region in the cTBSSHAM group, we found higher delta band relative power and lower alpha band relative power during PT when compared to SRT. (2) Similar differences were observed in delta and alpha band relative power between PT and SRT for the SMA region in the cTBSSMA group. (3) For the perturbation task, cTBS (inhibitory) over SMA significantly reduced the delta band relative EEG power over the frontoparietal region when compared with sham stimulation.
In our study, two groups of individuals performed both the perturbation task and the sway reference task. We identified EEG sources of activations and found commonalities and differences in the location of EEG clusters between the two groups. Mainly, we found EEG clusters localized within the frontocentral regions (cingulate gyrus, SMA), posterior cingulate cortex (PCC; BA 23 and 31), and visual cortex (VC; BA 17, 18 and 19), a finding consistent with previous studies [
16,
17,
18,
21,
60]. However, the locations of these clusters identified in two groups were distinct. For example, in the cTBS
SHAM and cTBS
SMA groups, our analysis found a cluster in the frontocentral regions but these clusters were localized within ventral anterior cingulate and supplementary motor area, respectively. As the purpose of this study was to compare EEG activations between two tasks, we performed within-group analysis for each cluster.
Lower Alpha Band Relative Power during PT When Compared to SRT
We found a significant decrease in alpha frequency band in the fronto-central region for PT as compared to SRT in both groups. Lower alpha frequency spectral power during a standing balance task has been argued to reflect higher task difficulty, i.e., more challenging balance task [
10,
14,
18,
61,
62]. For example, Kahya et al. (2022) increased difficulty and diverted attention through a standing balance task with mental arithmetic and found a decrease in alpha-band power as compared to quiet standing [
62]. Lower alpha power during performance of PT than SRT might be related to increased information processing necessary to process and/or respond to the platform translation resulting from reduced inhibition by higher centers [
10,
61,
63,
64].
Higher Delta Band Relative Power during PT When Compared to SRT
We found a significant increase in delta frequency band relative power during PT as compared to SRT in the frontocentral region for both groups. Delta oscillations have been shown to be involved in cognitive processes such as decision-making and attentional processes [
65] and are known to impact behavioral outcomes [
66], where the difficulty of a postural task has been associated with increased delta band activity [
67]. Slower brain oscillations due to their longer temporal window of processing information suggest involvement of neurons in widespread brain areas [
68,
69]. Primate work has shown interactions between parietal sensory areas and motor areas in the frontal region in the delta frequency range during a somatosensory discrimination task [
68]. The presence of delta band power in the fronto-central brain region during both balance tasks might represent long-range integrative processes with greater engagement of cognitive resources [
70] during PT (i.e., greater delta band power) than SRT. Therefore, it is conceivable that greater task demand and challenge to the standing balance led to significantly higher delta band power for PT than SRT.
Relation between Brain Oscillations and the Cortical Potentials Following Balance Perturbations
The superimposition of oscillations in lower frequency bands such as delta, theta and alpha frequency bands may partly underlie the generation of cortical event-related potentials following balance perturbations [
71,
72,
73,
74,
75]. Imposed mechanical perturbations such as the one delivered in PT are associated with characteristic event-related potentials (ERPs) in the EEG activity. Mainly, a positive potential (P1) is generated over the parietal-central region within the first 50–70 ms after the onset of perturbation. This response is followed by a large negative potential (N1) generated over the fronto-central region with a peak latency of 100–200 ms after the onset of perturbation [
21,
31,
76,
77]. This N1 response has been suggested to indicate the involvement of higher-order processing in the form of error detection (i.e., difference between the actual balance state due to balance perturbation and the anticipated balance state) and signaling postural responses to the destabilizing effects of balance perturbations [
12,
31,
77]. The size of N1 potential is small during SRT where sensory feedback regarding motor outputs during ongoing balance control is altered such that the effects are a function of the action of the participant [
33]. Although the presence of a P1 response during SRT is debatable, our earlier work has shown involvement of bilateral posterior parietal cortices during SRT [
18]. The N1 response is followed by late evoked responses, the P2 and the N2 responses. There is a debate on the role of these late responses with some studies suggesting their involvement in cortical sensorimotor processing while others linked them with a shift in attention to novel perturbation events [
30,
78,
79]. In addition, the delta band power is known to increase while the alpha band power decreases in response to stimulus and task demands [
80,
81]. We suggest that larger relative delta band power (and smaller relative alpha power) for PT when compared with SRT might explain larger and clearly patterned ERPs observed following platform translation perturbations [
80,
81].
cTBS over SMA Altered Delta Band Power over the Fronto-Central Region during the PT Task
A cortical response in the fronto-parietal region is a well-documented phenomenon following mechanical perturbations of the standing platform (viz. translation). Clustering of fronto-central independent components for each participant showed activations of the SMA region (BA 6) for both groups. The study was designed to consider all trials together for clustering to provide better understanding of the changes on cortical activity measured as EEG power in five frequency bands following inhibitory cTBS protocol. We found a significant decrease in the delta frequency relative band power within the SMA region following cTBS over SMA when compared to sham stimulation. Previously, EEG frequency analysis of participants at rest reported significant decreased power in the delta band following cTBS [
82,
83]. Our results support this and report its occurrence during a standing balance task. cTBS might have increased neural noise in the stimulated area, thus disrupting the synchronicity of neural activities [
84,
85,
86]. The reduction in delta frequency band relative power within the SMA following cTBS might thus suggest disruption in long-range integrative processes between frontal and parietal regions necessary for the performance of PT [
16,
17,
18,
19,
20,
21,
22,
23,
24]. The spread of TMS stimuli to distant regions through cortico-cortical connections is well known [
87,
88]. The fronto-parietal regions are known to have long-range cortico-cortical connections with Broadman area 4, premotor areas, cingulate areas, and parietal cortices [
89,
90]. Also, smaller amplitude of delta oscillations following cTBS might have influenced the generation of ERPs in the fronto-central region affecting the functional postural response following platform translation. However, this remains to be known. Our study included only three trials per perturbation condition thus limiting the statistical power to investigate the effects of cTBS over SMA on the amplitude and latency of ERPs and behavioral implications [
91]. Our study raises the possibility of using cTBS to alter ERP-components by interfering with the underlying oscillations.
Our previous work using the sway reference task [
18], found changes in EEG band power within bilateral parietal cortices and cingulate gyrus following cTBS over SMA, a fronto-central region, when compared with sham stimulation. Although this earlier study did not study the effects of cTBS on delta band power, it found modulation in theta frequency band power across different levels of task difficulty following cTBS than sham stimulation. It also found a decrease in alpha band power following cTBS when compared to sham stimulation during the sway referencing task, a finding not observed in the current study that used a perturbation task to challenge the balance. The lack of an alpha effect in this study might be due to already lower alpha band power during PT (see
Figure 4), and the inability of cTBS to lower it further, i.e., a floor effect.
5. Conclusions
Our findings suggest PT is a more challenging balance task when compared with SRT requiring greater cognitive function and attentional control as indicated by higher delta and lower alpha relative frequency power. Thus, the results of our study could be relevant to researchers and clinicians to select between SRT and PT, depending on the individual’s need. Furthermore, a training paradigm targeting the respective mechanisms should account for the differences in expected cortical activations. cTBS modulated delta power in the fronto-central cortical regions during PT. Additional research is needed to determine how such an interference with delta oscillations affects the ERPs following platform perturbations. By isolating circuits that exhibit a favorable response to cTBS intervention, we will advance understanding of precise brain circuits associated with balance control. Mechanistic findings may lead to cortical targets for effective neuromodulation strategies for rehabilitation of balance control in clinical populations.
Author Contributions
Conceptualization, R.D.M. and P.J.P.; methodology, R.D.M.; K.K.K.; J.C-V.; P.J.P.; formal analysis, R.D.M. and K.K.K.; investigation, R.D.M.; resources, J.C-V. and P.J.P.; data curation, R.D.M.; writing—original draft preparation, R.D.M.; writing—review and editing, R.D.M.; K.K.K.; J.C-V.; P.J.P.; visualization, R.D.M.; supervision, P.J.P.; project administration, P.J.P.; funding acquisition, J.C.V and P.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant to PJP from the NIH National Center of Neuromodulation for Rehabilitation, the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/NICHD) under Grant P2CHD086844 to PJP; NIH/NICHD R25HD106896 to PJP and JC-V, and National Science Foundation IUCRC BRAIN Award # 2137255 to JC-V.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of Houston (STUDY00001590 and 04/16/2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The processed data presented in the study are openly available in Mendeley Data at 10.17632/vdk5yr2m85.1.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Shumway-Cook, A.; Woollacott, M. Attentional demands and postural control: the effect of sensory context. J Gerontol A Biol Sci Med Sci. 2000, 55, M10–M16. [Google Scholar] [CrossRef] [PubMed]
- Keenan M., A.; Perry, J.; Jordan, C. Factors affecting balance and ambulation following stroke. Clin Orthop Relat Res, 1984, 182, 165–171. [Google Scholar] [CrossRef]
- Fong K. N., K.; Chan C. C., H.; Au D. K., S. Relationship of motor and cognitive abilities to functional performance in stroke rehabilitation. Brain Injury, 2001, 15, 443–453. [Google Scholar] [CrossRef]
- Sandin K., J.; Smith B., S. The measure of balance in sitting in stroke rehabilitation prognosis. Stroke, 1990, 21, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.; Wong J., S.; McIlroy W., E.; Biasin, L.; Brunton, K.; Bayley, M.; Inness E., L. Do measures of reactive balance control predict falls in people with stroke returning to the community? Physiotherapy, 2015, 101, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Urabe, Y.; Murakami, M.; Itotani, K.; Kato, J. Discriminant analysis for predictor of falls in stroke patients by using the Berg Balance Scale. Singapore Med J, 2015, 56, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Lee, G. Impaired dynamic balance is associated with falling in post-stroke patients. Tohoku J Exp Med, 2013, 230, 233–239. [Google Scholar] [CrossRef]
- Bower, K.; Thilarajah, S.; Pua Y., H.; Williams, G.; Tan, D.; Mentiplay, B.; Denehy, L.; Clark, R. Dynamic balance and instrumented gait variables are independent predictors of falls following stroke. J NeuroEngineering and Rehabilitation, 2019, 16, 1–9. [Google Scholar] [CrossRef]
- Jahn, K.; Deutschländer, A.; Stephan, T.; Strupp, M.; Wiesmann, M.; Brandt, T. Brain activation patterns during imagined stance and locomotion in functional magnetic resonance imaging. NeuroImage, 2004, 22, 1722–1731. [Google Scholar] [CrossRef]
- Hülsdünker, T.; Mierau, A.; Strüder H., K. Higher Balance Task Demands are Associated with an Increase in Individual Alpha Peak Frequency. Front Hum Neurosci, 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Oliveira A., S.; Schlink B., R.; Hairston W., D.; König, P.; Ferris D., P. Restricted vision increases sensorimotor cortex involvement in human walking. J Neurophysiol, 2017, 118, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Slobounov, S.; Cao, C.; Jaiswal, N. Newell K. M. Neural basis of postural instability identified by VTC and EEG. Exp Brain Res, 2009, 199, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Qureshi N., K.; Yazidi, A.; Engell, H.; Mirtaheri, P. Single-leg stance on a challenging surface can enhance cortical activation in the right hemisphere – A case study. Heliyon, 1362. [Google Scholar] [CrossRef]
- Gebel, A.; Lehmann, T.; Granacher, U. Balance task difficulty affects postural sway and cortical activity in healthy adolescents. Exp Brain Res, 2020, 238, 1323–1333. [Google Scholar] [CrossRef]
- Lm, N. Computerized dynamic posturography. Handbook of Balance Function Testing, 1993; 208–307. [Google Scholar]
- Mihara, M.; Miyai, I.; Hatakenaka, M.; Kubota, K.; Sakoda, S. Role of the prefrontal cortex in human balance control. NeuroImage, 2008, 43, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Solis-Escalante, T.; van der Cruijsen, J.; de Kam, D.; van Kordelaar, J.; Weerdesteyn, V.; Schouten A., C. Cortical dynamics during preparation and execution of reactive balance responses with distinct postural demands. NeuroImage, 2019, 188, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Nakagome, S.; Rao, N.; Paloski W., H.; Contreras-Vidal J., L.; Parikh P., J. Fronto-Parietal Brain Areas Contribute to the Online Control of Posture during a Continuous Balance Task. Neuroscience, 2019, 413, 135–153. [Google Scholar] [CrossRef]
- Surgent O., J.; Dadalko O., I.; Pickett K., A.; Travers B., G. Balance and the brain: a review of structural brain correlates of postural balance and balance training in humans. Gait Posture, 2019, 71, 245–252. [Google Scholar] [CrossRef]
- Wittenberg, E.; Thompson, J.; Nam C., S.; Franz J., R. Neuroimaging of Human Balance Control: A Systematic Review. Frontiers in Human Neuroscience. [CrossRef]
- Marlin, A.; Mochizuki, G.; Staines W., R.; McIlroy W., E. Localizing evoked cortical activity associated with balance reactions: does the anterior cingulate play a role? Journal of Neurophysiology, 2014, 111, 2634–2643. [Google Scholar] [CrossRef]
- Mierau, A.; Hülsdünker, T.; Strüder H., K. Changes in cortical activity associated with adaptive behavior during repeated balance perturbation of unpredictable timing. Frontiers in Behavioral Neuroscience, 2015; 9. [Google Scholar] [CrossRef]
- Solis-Escalante, T.; Stokkermans, M.; Cohen M., X.; Weerdesteyn, V. Cortical responses to whole-body balance perturbations index perturbation magnitude and predict reactive stepping behavior. European Journal of Neuroscience, 2020, 54, 8120–8138. [Google Scholar] [CrossRef]
- Payne A., M.; Hajcak, G.; Ting L., H. Dissociation of muscle and cortical response scaling to balance perturbation acceleration. J Neurophysiol, 2019; 121, 867–880. [Google Scholar] [CrossRef]
- Weber M., J.; Thompson-Schill S., L. Functional neuroimaging can support causal claims about brain function. J Cogn Neurosci, 2010; 22, 2415–2416. [Google Scholar] [CrossRef]
- Chaudhry, H.; Bukiet, B.; Ji, Z.; Findley, T. Measurement of balance in computer posturography: Comparison of methods—A brief review. Journal of Bodywork and Movement Therapies, 2011, 15, 82–91. [Google Scholar] [CrossRef]
- Bolton D. A., E. The role of the cerebral cortex in postural responses to externally induced perturbations. Neuroscience & Biobehavioral Reviews, 2015, 57, 142–155. [Google Scholar] [CrossRef]
- Adkin A., L.; Quant, S.; Maki B., E.; McIlroy W., E. Cortical responses associated with predictable and unpredictable compensatory balance reactions. Exp Brain Res, 2006, 172, 85–93. [Google Scholar] [CrossRef] [PubMed]
- . Mochizuki G, Sibley K. M.; Esposito J. G.; Camilleri J. M.; McIlroy W. E. Cortical responses associated with the preparation and reaction to full-body perturbations to upright stability. Clinical Neurophysiology, 2008, 119, 1626–1637. [Google Scholar] [CrossRef] [PubMed]
- Quant, S.; Adkin A., L.; Staines W., R.; Maki B., E.; McIlroy W., E. The effect of a concurrent cognitive task on cortical potentials evoked by unpredictable balance perturbations. BMC Neuroscience, 2004, 5, 1–12. [Google Scholar] [CrossRef]
- Goel, R.; Ozdemir R., A.; Nakagome, S.; Contreras-Vidal J., L.; Paloski W., H.; Parikh P., J. Effects of speed and direction of perturbation on electroencephalographic and balance responses. Exp Brain Res, 2018, 236, 2073–2083. [Google Scholar] [CrossRef]
- Ravindran A., S.; Malaya C., A.; John, I.; Francisco G., E.; Layne, C.; Contreras-Vidal J., L. Decoding neural activity preceding balance loss during standing with a lower-limb exoskeleton using an interpretable deep learning model. J. Neural Eng. 2022, 19. [Google Scholar] [CrossRef]
- Goel, R.; Nakagome, S.; Paloski W., H.; Contreras-Vidal J., L.; Parikh P., J. Assessment of Biomechanical Predictors of Occurrence of Low-Amplitude N1 Potentials Evoked by Naturally Occurring Postural Instabilities. IEEE Trans. Neural Syst. Rehabil. Eng.; 2022, 30, 476–485. [Google Scholar] [CrossRef]
- Rasman B., G.; Forbes P., A.; Tisserand, R.; Blouin, J.-S. Sensorimotor manipulations of the balance control loop–beyond imposed external perturbations. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef]
- Egger, S.; Wälchli, M.; Rüeger, E.; Taube, W. Short-term balance consolidation relies on the primary motor cortex: a rCTBS study. Sci Rep, 2023, 13, 1–6. [Google Scholar] [CrossRef]
- Parikh, P.; Davare, M.; McGurrin, P.; Santello, M. Corticospinal excitability underlying digit force planning for grasping in humans. J Neurophysiology, 2014, 111, 2560–2569. [Google Scholar] [CrossRef]
- Davare, M.; Parikh P., J.; Santello, M. Sensorimotor uncertainty modulates corticospinal excitability during skilled object manipulation. J Neurophysiol, 2019, 121, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Parikh P., J.; Santello, M. Role of human premotor dorsal region in learning a conditional visuomotor task. J Neurophysiol, 2017, 117, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Rossini P., M.; Burke, D.; Chen, R.; Cohen L., G.; Daskalakis, Z.; Di lorio, R.; Di Lazzaro, V.; Ferreri, F.; Fitzgerald P., B.; George M., S.; Hallett, M.; Lefaucheur J., P.; Langguth, B.; Matsumoto, H.; Miniussi, C.; Nitsche M., A.; Pascual-Leone, A.; Paulus, W.; Rossi, S.; Rothwell J., C.; Siebner H., R.; Ugawa Y; Walsh V. ; Ziemann U. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology, 1994, 91, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Picard, N. and Strick P. L. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex, 1996, 6, 342–353. [Google Scholar] [CrossRef]
- White, O.; Davare, M.; Andres, M.; Olivier, E. The role of left supplementary motor area in grip force scaling. PLOS ONE, 2013, 8, 1–9. [Google Scholar] [CrossRef]
- Zénon, A.; Sidibé, M.; Olivier, E. Disrupting the supplementary motor area makes physical effort appear less effortful. J Neurosci, 2015, 35, 8737–8744. [Google Scholar] [CrossRef]
- Picard, N.; Strick P., L. Imaging the premotor areas. Current Opinion in Neurobiology, 2001, 11, 663–672. [Google Scholar] [CrossRef]
- Matsunaga, K.; Maruyama, A.; Fujiwara, T.; Nakanishi, R.; Tsuji, S.; Rothwell J., C. Increased corticospinal excitability after 5 Hz rCTBS over the human supplementary motor area. J Physiology, 2005, 562, 295–306. [Google Scholar] [CrossRef]
- Steyvers, M.; Etoh, S.; Sauner, D.; Levin, O.; Siebner H., R.; Swinnen S., P.; Rothwell J., C. High-frequency transcranial magnetic stimulation of the supplementary motor area reduces bimanual coupling during anti-phase but not in-phase movements. Exp Brain Res, 2003, 151, 309–317. [Google Scholar] [CrossRef]
- Binkofski, F.; Buccino, G.; Stephan K., M.; Rizzolatti, G.; Seitz R., J.; Freund H., J. A parieto-premotor network for object manipulation: evidence from neuroimaging. Exp Brain Res, 1999; 128, 210–213. [Google Scholar] [CrossRef]
- Bursztyn L. L. C., D.; Ganesh, G.; Imamizu, H.; Kawato, M.; Flanagan J., R. Neural correlates of internal-model loading. Curr Biol, 2006, 16, 2440–2445. [Google Scholar] [CrossRef]
- Buch E., R.; Mars R., B.; Boorman E., D.; Rushworth M. F., S. A network centered on ventral premotor cortex exerts both facilitatory and inhibitory control over primary motor cortex during action reprogramming. J Neurosci, 2010, 30, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Z.; Edwards M., J.; Rounis, E.; Bhatia K., P.; Rothwell J., C. Theta burst stimulation of the human motor cortex. Neuron, 2005, 45, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Lisanby S., H.; Gutman, D.; Luber, B.; Schroeder, C.; Sackeim H., A. Sham CTBS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biological Psychiatry, 2001, 49, 460–463. [Google Scholar] [CrossRef]
- Davare, M.; Andres, M.; Cosnard, G.; Thonnard, J.-L.; Olivier, E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci, 2006, 26, 2260–2268. [Google Scholar] [CrossRef]
- Eggers, C.; Günther, M.; Rothwell, J.; Timmermann, L.; Ruge, D. Theta burst stimulation over the supplementary motor area in Parkinson’s disease. J Neurol, 2015, 262, 357–364. [Google Scholar] [CrossRef]
- Koch, G.; Bonni, S.; Giacobbe, V.; Bucchi, G.; Basile, B.; Lupo, F.; Versace, V.; Bozzali, M.; Caltagirone, C. Theta-burst stimulation of the left hemisphere accelerates recovery of hemispatial neglect. Neurology, 2012, 78, 24–30. [Google Scholar] [CrossRef]
- Noh N., A.; Fuggetta, G.; Manganotti, P.; Fiaschi, A. Long lasting modulation of cortical oscillations after continuous theta burst transcranial magnetic stimulation. PLOS ONE, 2012, 7, 1–12. [Google Scholar] [CrossRef]
- Vernet, M.; Bashir, S.; Yoo, W.-K.; Perez J., M.; Najib, U.; Pascual-Leone, A. Insights on the neural basis of motor plasticity induced by theta burst stimulation from CTBS–EEG. European J Neuroscience, 2013, 37, 598–606. [Google Scholar] [CrossRef]
- Kilicarslan, A.; Grossman R., G.; Contreras-Vidal J., L. A robust adaptive denoising framework for real-time artifact removal in scalp EEG measurements. J Neural Eng. 2016, 13. [Google Scholar] [CrossRef]
- Mullen, T.; Kothe, C.; Chi Y., M.; Ojeda, A.; Kerth, T.; Makeig, S.; Cauwenberghs, G.; Jung, T.-P. Real-time modeling and 3D visualization of source dynamics and connectivity using wearable EEG. 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2184. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Hsu, S.-H.; Pion-Tonachini, L.; Jung, T.-P. Evaluation of artifact subspace reconstruction for automatic artifact components removal in multi-channel EEG recordings. IEEE Transactions on Biomedical Engineering, 2020, 67, 1114–1121. [Google Scholar] [CrossRef]
- “Yale BioImage Suite MNI<->TAL.” 2008 Accessed: Mar. 15, 2024. [Online]. Available: https://bioimagesuiteweb.github.io/webapp/mni2tal.html.
- Bogost M., D.; Burgos P., I.; Little C., E.; Woollacott M., H.; Dalton B., H. Electrocortical Sources Related to Whole-body surface translations during a single- and dual-task paradigm. Front. Hum. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.; Guven, O.; Furman M., D.; Arshad, Q.; Bronstein A., M. Electroencephalographic correlates of continuous postural tasks of increasing difficulty. Neuroscience, 2018, 395, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Kahya, M.; Gouskova N., A.; Lo, O.-Y.; Zhou, J.; Cappon, D.; Finnerty, E.; Pascual-Leone, A.; Lipsitz L., A.; Hausdorff J., M.; Manor, B. Brain activity during dual-task standing in older adults. J Neuroeng Rehabil, 2022, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.; Cole, H. EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science, 1985, 228, 750–752. [Google Scholar] [CrossRef]
- Petrofsky, J.; Khowailed, I. Postural sway and motor control in trans-tibial amputees as assessed by electroencephalography during eight balance training tasks. Med Sci Monit. 2014, 20, 2695–2704. [Google Scholar] [CrossRef]
- Güntekin, B. and Başar E. Review of evoked and event-related delta responses in the human brain. International Journal of Psychophysiology, 2016, 103, 43–52. [Google Scholar] [CrossRef]
- Morillon, B.; Arnal L., H.; Schroeder C., E.; Keitel, A. Prominence of delta oscillatory rhythms in the motor cortex and their relevance for auditory and speech perception. Neuroscience & Biobehavioral Reviews, 2019, 107, 136–142. [Google Scholar] [CrossRef]
- Ozdemir R., A.; Contreras-Vidal J., L.; Paloski W., H. Cortical control of upright stance in elderly. Mechanisms of Ageing and Development, 2018, 169, 19–31. [Google Scholar] [CrossRef]
- Nácher, V.; Ledberg, A.; Deco, G.; Romo, R. Coherent delta-band oscillations between cortical areas correlate with decision making. PNAS, 1508. [Google Scholar] [CrossRef]
- Harmony, T. The functional significance of delta oscillations in cognitive processing. Front. Integr. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Payne A., M.; McKay J., L.; Ting L., H. The cortical N1 response to balance perturbation is associated with balance and cognitive function in different ways between older adults with and without Parkinson’s disease. Cereb Cortex Commun. 2022, 3. [Google Scholar] [CrossRef]
- Basar, E. Brain function and oscillations. I: Principles and approaches. Berlin: Springer Verlag. 1999. [Google Scholar]
- Basar, E. Brain function and oscillations. II: Principles and approaches. Berlin: Springer Verlag. 1999. [Google Scholar]
- Sauseng, P.; Klimesch, W.; Gruber W., R.; Hanslmayr, S.; Freunberger, R.; Doppelmayr, M. Are event-related potential com-ponents generated by phase resetting of brain oscillations? A critical discussion. Neuroscience, 2007, 146, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Sauseng, P.; Klimesch, W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci Biobehav Rev, 2008; 32, 1001–1013. [Google Scholar] [CrossRef]
- Min. B.-K.; Busch N. A.; Debener S.; Kranczioch C.; Hanslmayr S.; Engel A. K.; Herrmann C. S. The best of both worlds: phase-reset of the human EEG alpha activity and additive power contribute to ERP generation. Int. J. Pscyhophsio, 2007, 65, 58–68. [Google Scholar] [CrossRef]
- Maki, B. McIlroy W. Cognitive demands and cortical control of human balance-recovery reactions. J. Neural Transm (Vienna), 2007, 114, 1279–1296. [Google Scholar] [CrossRef] [PubMed]
- Varghese J., P.; Marlin, A.; Beyer K., B.; Staines W., R.; Mochizuki, G.; McIlroy W., E. Frequency characteristics of cortical activity associated with perturbations to upright stability. Neurosci Lett. 2014. [Google Scholar] [CrossRef]
- Mochizuki, G.; Sibley K., M.; Cheung H., J.; McIlroy W., E. Cortical activity prior to predictable postural instability: is there a difference between self-initiated and externally-initiated perturbations? Brain Res. 2009. [Google Scholar] [CrossRef]
- Quant, S.; Maki B., E.; McIlroy W., E. The association between later cortical potentials and later phases of postural reactions evoked by perturbations to upright stance. Neurosci Lett. 2005, 381, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev.; 1999, 29, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Lopes da Silva, F. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Rocchi, L.; Ibáñez, J.; Benussi, A.; Hannah, R.; Rawji, V.; Casula, E.; Rothwell, J. Variability and predictors of response to con-tinuous theta burst stimulation: a CTBS-EEG study. Front Neurosci, 2018, 12, 1–11. [Google Scholar] [CrossRef]
- Woźniak-Kwaśniewska, A.; Szekely, D.; Aussedat, P.; Bougerol, T.; David, O. Changes of oscillatory brain activity induced by repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex in healthy subjects. NeuroImage, 2014, 88, 91–99. [Google Scholar] [CrossRef]
- Ruzzoli, M.; Marzi C., A.; Miniussi, C. The neural mechanisms of the effects of transcranial magnetic stimulation on perception. J. Neurophysiol, 2982. [Google Scholar] [CrossRef]
- Teo J., T.; Swayne O., B.; Cheeran, B.; Greenwood R., J.; Rothwell J., C. Human theta burst stimulation enhances subsequent motor learning and increases performance variability. Cereb. Cortex. 2011, 21, 1627–1638. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, L.; Casula, E.; Tocco, P.; Berardelli, A. Somatosensory temporal discrimination threshold involves inhibitory mechanisms in the primary somatosensory area. J. Neurosci, 2016; 36, 325–335. [Google Scholar] [CrossRef]
- Siebner H., R.; Hartwigsen, G.; Kassuba, T.; Rothwell J., C. How does transcranial magnetic stimulation modify neuronal activity in the brain? Implications for studies of cognition. Cortex. 2009, 45, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.-L; Santarnecchi, E.; Buch E., R.; Cohen L., G. Non-invasive brain stimulation in neurorehabilitation: local and distant effects for motor recovery. Front Hum. Neurosci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Luppino, G.; Matelli, M.; Camarda, R.; Rizzolatti, G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp. Neurology. 1993, 338, 114–140. [Google Scholar] [CrossRef]
- Potgieser A. R., E.; de Jong B., M.; Wagemakers, M.; Hoving E., W.; Groen R. J., M. Insights from supplementary motor area syndrome in balancing movement initiation and inhibition. Front Hum. Neurosci. 2014. [Google Scholar] [CrossRef]
- Boudewyn M., A.; Luck S., J.; Farrens J., L.; Kappenman E., S. How many trials does it take to get a significant ERP effect? It depends. Psychophysiology. 2017, 55. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).